Abstract
Background
Congenital bicuspid aortic valves (BAVs) result from fusion of two valve cusps, resulting in left-noncoronary (L-N), right-left (R-L), and right-noncoronary (R-N) morphologies. BAVs predispose to ascending thoracic aortic aneurysms (ATAAs). This study hypothesized that ATAAs with each BAV morphology group possess unique signatures of matrix metalloproteinases (MMPs) and endogenous tissue inhibitors (TIMPs).
Methods
ATAA tissue from 46 BAV patients was examined for MMP/TIMP abundance and global MMP activity compared to normal aortic specimens (n=15). Proteolytic balance was calculated as the ratio of MMP abundance to a composite TIMP score (TS). Results were stratified by valve morphology group (L-N (n=6), R-L (n=31), and R-N(n=9)).
Results
The BAV specimens (p<0.05 vs. normal aorta, 100%) displayed elevated global MMP activity (273±63%), MMP-9 (263±47%), and decreased MMP -7 (56±10%), -8 (58±11%), TIMP -1 (63±7%) and -4 (38±3%). The R-L group showed increased global MMP activity (286±89%) and MMP-9 (267±55%) with reduced MMP -7 (45±7%) -8 (68±15%), TIMP -1 (58±7%) and -4 (35±3%). The L-N group showed elevated global MMP activity (284±71%), and decreased MMP-8 (37±17%) and TIMP-4 (48±14). In the R-N group, MMP -7 (46±13%) and -8 (36±17%), and TIMP -1 (59±10) and -4 (42±5%) were decreased. The R-L group demonstrated an increased proteolytic balance for MMP-1, MMP-9, and MMP-12 relative to L-N and R-N.
Conclusion
Each BAV morphology group possesses a unique signature of MMPs and TIMPs. MMP/TIMP score ratios suggest that the R-L group may be more aggressive, justifying earlier surgical intervention.
Introduction
Bicuspid aortic valves (BAVs), the most common congenital cardiac malformation, occur in 1–2% of the population and are believed to result from fusion of valve cusps in utero.(1) Three valve morphologies result: left coronary-non-coronary fusion (L-N), right coronary-left coronary fusion (R-L), and right coronary-non-coronary fusion (R-N). BAVs are prone to stenosis and regurgitation, and are associated with aortic pathology including ascending thoracic aortic aneurysms (ATAAs).(1, 2)
Histopathological changes within the BAV aorta include alterations in matrix proteins, smooth muscle cell density, elastic fragmentation, and lamellar thickness.(3, 4) The matrix metalloproteinase (MMP) family mediates aortic extracellular matrix remodeling in murine aneurysm models,(5, 6) and unique MMP profiles have been reported in patients with BAV-associated ATAAs.(7, 8) Proteolytic indices, the ratio of MMPs to TIMPs, are used as a functional measurement of aortic remodeling activity.(8, 9)
Of the three possible cusp fusions, the R-L configuration is the most common.(10) Aortic valve regurgitation predominates in these patients,(11, 12) and R-L aneurysms may possess more extensive aortic wall degeneration.(10) Alternatively, the R-N configuration is more prone to aortic valve stenosis(13) and trends toward elevated mid-ascending aortic diameter.(11)
Quantifying specific MMP and TIMP profiles in each BAV morphology could uncover mechanistic underpinnings for aortic remodeling in BAV patients. Accordingly, this project tested the hypothesis that differential MMP/TIMP profiles occur between BAV-ATAAs and normal aorta and that each BAV morphology group would possess a unique protein signature of key MMPs and TIMPs.
Material and Methods
Study Population
Ascending aortic tissue samples were obtained from 46 patients with known BAV during ascending aortic replacement, taken from the widest region of the ascending aorta. No patients had aortic dissection, inflammatory aortic disease, or known syndromic aortic disease. Normal aortic specimens were similarly harvested from the ascending aorta of heart transplant donors or recipients (n=15). Patient gender, age, aortic diameter, and BAV morphology were charted. This study was approved by the Institutional Review Board of the Medical University of South Carolina and The University of Pennsylvania. Informed consent was obtained from all patients.
Aortic Sample Preparation and Multiplex Analysis
Aortic tissue was snap frozen and stored at −80°C until analyzed. Thawed tissue was transferred to cold buffer (volume 1:6 w/v) containing 10mM cacodylic acid pH 5.0, 0.15M NaCl, 10mM ZnCl2, 1.5mM NaN3, and 0.01% Triton X-100 (v/v), homogenized in a Tissuelyser (Qiagen, Valencia, CA) and centrifuged (800 x g, 10 min, 4°C). The supernatant was analyzed using a MultiAnalyte Suspension Array system (BioRad Bio-Plex System, Hercules, CA) which measures multiple soluble MMPs and TIMPs simultaneously. Each well received 50 μL of diluted antibody-conjugated beads (multiplex base kit, Catalog# LMP000, for MMPs -1,-2,-3,-7,-8,-9,-11,-12; TIMPs -1,-2,-3,-4.; R&D Systems, Minneapolis, MN) and 10 μg of aortic homogenate. Samples were incubated (room temperature, 2 hours) on a microplate shaker, filtered, and washed 3 times with 100 μL of Wash Buffer (part # 895003). Diluted goat anti-human polyclonal biotinylated antibodies (50 μL, analyte-specific; included with antibody-conjugated bead kits; R&D Systems) were then added to each well, and incubated (room temperature, 1 hour) on a microplate shaker. The beads were again filtered and washed as before and dilute Streptavidin-PE (50 μL, R&D Systems) was added for 30 minutes at room temperature. After filtration and washing, the beads were analyzed using the Bio-Plex System, fluorescence was measured and then compared with standard curves for each analyte also run on the same plate. Protein quantities were calculated using Bio-Plex Manager™ Software 4.1 (BioRad, Hercules, CA) and normalized to GAPDH determined in each sample by ELISA assay (Catalog # 3401, Bioo Scientific, Austin, TX).
Global MMP Activity Assay
Aortic homogenates (15 μg) from normal and BAV patients were incubated (60 minutes, 37°C) with a quenched fluorogenic peptide containing a pan-specific MMP cleavage site (MCA-Pro-Leu-Gly-Leu-(Dpa)-Ala-Arg-NH2; Catalog # BML-P126-0001; Enzo life Sciences, Plymouth Meeting, PA). Peptide cleavage by active MMPs removes of the quenching group, permitting fluorescence to be measured and recorded on a fluorescent microplate reader (Fluorostar Galaxy BMG Labtechnologies, Cary, NC). Fluorescence was converted to active MMP units by comparison to a recombinant MMP standard curve and normalized to GAPDH as determined by ELISA assay (Bioo Scientific, Austin, TX).
Data Analysis
Global MMP activity and MMP/TIMP protein abundance was expressed as relative fluorescence units/hr/ng GAPDH, or ng analyte/ng GAPDH, respectively. The data were analyzed in three groups. First, the percent change in normalized MMP and TIMP abundance and global MMP activity of all BAV specimens was compared to the normal group. Second, the percent change in normalized MMP and TIMP abundance and global MMP activity was stratified by BAV valve morphology group, and compared to the normal group. Lastly, relative proteolytic balance was expressed as the ratio of normalized MMP abundance to a composite TIMP score (TS) comprised of the sum of TIMP-1, TIMP-3, and TIMP-4 abundance in each sample. Because TIMP-2 is known to have both activating and inhibitory functions,(19) the proteolytic index was calculated using two TS scores computed with and without TIMP-2.
Statistical calculations were made using the Stata v8.2 software package (Intercooled, College Station, TX). Demographic data was compared using a two-sample mean comparison test. Changes in MMP and TIMP abundance were determined using two-tailed one-sample mean comparisons versus the referent normal group set at 100%. Differences between groups were determined using one-way ANOVA (prcompw module) with Tukey’s wholly significant difference (wsd) post-hoc analysis for separation of means. For all calculations, p<0.05 was considered significant.
Results
Patient demographic information is summarized in Table 1. BAV patients were older than normals and the ascending aortic diameter was greater. Patients in the R-L group were younger than the L-N group with no aortic diameter differences.
Table 1.
Patient demographic data. Gender, age, and ascending aortic diameter of patients with normal aortic valves and aortas, or ATAA-associated with a BAV. Values are expressed as mean ± SEM
| Male | Female | Age (yrs) | Asc Aorta Diameter (cm) | |
|---|---|---|---|---|
| Normal | 13 | 2 | 48.3±4.5 | 3.2±0.2 |
| BAV | 39 | 7 | 57.5±1.8 * | 4.8±0.1 * |
|
| ||||
| L-N | 4 | 2 | 65.2±4.2 * | 4.9±0.4 * |
| R-L | 27 | 4 | 55.0±2.2 | 4.8±0.1 * |
| R-N | 8 | 1 | 60.9±4.2 * | 5.0±0.2 * |
p<0.05 versus patients with normal aorta
Global MMP activity and MMP/TIMP protein abundance were next measured. MMP abundance was largely unchanged in BAV versus normal aortas except for decreased MMP-7 and MMP-8 and increased MMP-9. For the TIMPs, TIMP-1 and TIMP-4 protein levels were significantly decreased (Table 2 and 3). Interestingly, when global MMP activity was measured, MMP activity was elevated in the BAV specimens (Table 3). When the BAV aortic specimens were stratified valve morphology, the L-N and R-L groups displayed elevated MMP activity compared to normal, while the R-N group trended toward increased activity (Table 3). MMP and TIMP protein stratified by valve morphology group showed decreased MMP-8 and TIMP-4 abundance in the L-N group compared to normal aorta (Table 2 and 3). In the R-L group MMP-7, MMP-8, TIMP-1, and TIMP-4 were decreased, while MMP-9 was elevated compared to normal aorta (Table 2 and 3). Lastly, the R-N group demonstrated decreased MMP-7, MMP-8, TIMP-1, and TIMP-4 compared to normal aorta (Table 2 and 3).
Table 2.
GAPDH normalized absolute MMP and TIMP concentrations (ng MMP or TIMP/ ng GAPDH) in normal and aneurysmal aorta from all BAV patients or BAV patients stratified by valve morphology (L-N, R-L, R-N). Values are expressed as the mean ± SEM
| MMP | Normal | BAV | L-N | R-L | R-N |
|---|---|---|---|---|---|
| MMP-1 (×10−5) | 1.16±0.30 | 1.03±0.19 | 1.20±0.71 | 1.09±0.24 | 0.75±0.29 |
| MMP-2 (×10−3) | 11.79±2.04 | 15.17±1.85 | 23.66±7.55 | 13.61±2.05 | 14.89±3.63 |
| MMP-3 (×10−5) | 15.52±3.66 | 13.96±1.80 | 25.50±7.30 | 12.38±1.95 | 11.72±3.10 |
| MMP-7 (×10−5) | 6.19±1.09 | 3.44±0.59 | 8.42±4.00 | 2.80±0.45 | 2.88±0.83 |
| MMP-8 (×10−3) | 8.96±3.13 | 5.15±1.01 | 3.32±1.56 | 6.06±1.38 | 3.24±1.54 |
| MMP-9 (×10−3) | 4.37±1.48 | 11.47±2.04 | 12.05±5.59 | 11.68±2.42 | 10.39±5.57 |
| MMP-12 (×10−5) | 1.00±0.38 | 1.31±0.29 | 1.95±0.71 | 1.30±0.40 | 0.86±0.35 |
| TIMP-1 (×10−3) | 25.10±4.35 | 15.82±1.68 | 23.33±8.22 | 14.57±1.70 | 14.69±2.51 |
| TIMP-2 (×10−3) | 31.63±4.82 | 34.45±3.53 | 45.99±14.46 | 32.91±4.18 | 31.41±4.42 |
| TIMP-3 (×10−3) | 6.11±0.90 | 5.68±0.63 | 5.56±1.43 | 5.68±0.87 | 5.75±0.92 |
| TIMP-4 (×10−5) | 9.06±1.43 | 3.47±0.27 | 4.30±1.25 | 3.19±0.29 | 3.81±0.41 |
Table 3.
MMP activity and MMP or TIMP protein abundance in normal and aneurysmal aorta from all BAV patients or BAV patients categorized by valve morphology (L-N, R-L, R-N). Values (mean ± SEM) are expressed as a percent of normal aorta
| Normal | BAV | L-N | R-L | R-N | |
|---|---|---|---|---|---|
| Global MMP Activity | 100±20 | 273±62 * | 284±71 * | 286±89 * | 223±85 |
|
| |||||
| MMP-1 | 100±26 | 89±17 | 103±61 | 93±21 | 64±25 |
| MMP-2 | 100±17 | 129±16 | 201±64 | 115±17 | 126±31 |
| MMP-3 | 100±24 | 90±12 | 164±47 | 80±13 | 75±20 |
| MMP-7 | 100±18 | 56±10 * | 136±65 | 45±7 * # | 46±13 * |
| MMP-8 | 100±35 | 58±11 * | 37±17 * | 68±15 * | 36±17 * |
| MMP-9 | 100±35 | 263±47 * | 276±128 | 267±55 * | 238±128 |
| MMP-12 | 100±37 | 130±29 | 194±71 | 130±39 | 85±35 |
|
| |||||
| TIMP-1 | 100±17 | 63±7 * | 93±33 | 58±7 * | 59±10 * |
| TIMP-2 | 100±15 | 109±11 | 145±46 | 104±13 | 99±14 |
| TIMP-3 | 100±15 | 93±10 | 91±23 | 93±14 | 94±15 |
| TIMP-4 | 100±16 | 38±3 * | 48±14 * | 35±3 * | 42±5 * |
p<0.05 versus reference normal set at 100%;
p<0.05 versus L-N group
Proteolytic indices were then determined as the ratios of MMP protein abundance normalized to a TIMP score (TS) determined by the sum of TIMPs -1, -3, and 4, or -1 through -4 for each specimen (Table 4 and 5, respectively). Relative to normal aorta, the BAV aortas showed increased proteolytic indices for MMP-2, MMP-9, and MMP-12, and decreased proteolytic indices for MMP-7 and MMP-8. By valve morphology, increased proteolytic indices were observed for MMP-2 and MMP-3 in the L-N group, and for MMP-2 in the R-N group, while the proteolytic index for MMP-8 was decreased in both groups. In the R-L group, the proteolytic index was significantly increased for MMP-1, MMP-2, MMP-9, and MMP-12, and decreased for MMP-7 and MMP-8 (Tables 4, 5, and Figure 1).
Table 4.
MMP protein abundance to TIMP score (TS = TIMP-1+TIMP-3+TIMP-4) ratios for normal and aneurysmal aorta from all BAV patients or BAV patients with different valve morphologies (L-N, R-L, R-N). Values (mean ± SEM) are expressed as a percent of normal aorta
| MMP/TS Ratio | Normal | BAV | L-N | R-L | R-N |
|---|---|---|---|---|---|
| MMP-1/TS | 100±17 | 136±21 | 75±30 | 164±28 * | 82±19 |
| MMP-2/TS | 100±13 | 180±16 * | 220±29 * | 167±20 * | 203±39 * |
| MMP-3/TS | 100±18 | 138±19 | 197±32 * | 130±26 | 123±34 |
| MMP-7/TS | 100±10 | 74±8 * | 124±41 | 67±6 *# | 65±17 |
| MMP-8/TS | 100±43 | 50±9 * | 21±8 * | 61±12 * | 31±8 * |
| MMP-9/TS | 100±41 | 360±76 * | 196±44 | 432±104 * | 178±66 |
| MMP-12/TS | 100±31 | 178±24 * | 213±60 | 168±28 * | 182±66 |
p<0.05 versus reference normal value of 100;
p<0.05 versus L-N group
Table 5.
MMP protein abundance to TIMP score (TS = TIMP-1+TIMP-2+TIMP-3+TIMP-4) ratios for normal and aneurysmal aorta from all BAV patients or BAV patients with different valve morphologies (L-N, R-L, R-N). Values (mean ± SEM) are expressed as a percent of normal aorta
| MMP/TS Ratio | Normal | BAV | L-N | R-L | R-N |
|---|---|---|---|---|---|
| MMP-1/TS | 100±20 | 123±21 | 49±11 * | 153±29 | 71±15 |
| MMP-2/TS | 100±9 | 172±12 * | 232±46 * | 155±12 * | 183±28 * |
| MMP-3/TS | 100±13 | 134±14 * | 237±47 * | 109±11 # | 139±34 |
| MMP-7/TS | 100±12 | 66±8 * | 139±51 | 59±4 *# | 41±9 *# |
| MMP-8/TS | 100±46 | 54±8 * | 45±19 * | 61±10 * | 34±9 * |
| MMP-9/TS | 100±41 | 343±61 * | 211±68 | 403±83 * | 199±60 |
| MMP-12/TS | 100±28 | 169±24 * | 218±92 | 172±28 * | 126±48 |
p<0.05 versus reference normal value of 100;
p<0.05 versus L-N group
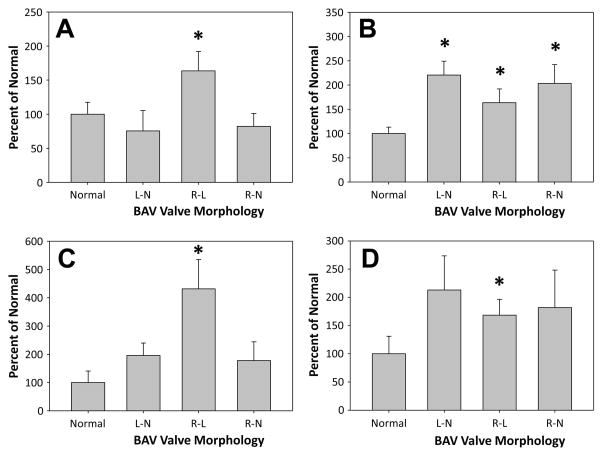
Figure 1. Analysis of MMP/TS in BAV specimens stratified by BAV valve morphology group.
MMP protein abundance corrected for changes TIMP score (TIMP-1 + TIMP-3 + TIMP-4) are shown for key mediators of matrix remodeling (MMP-1, MMP-2, MMP-9, and MMP-12). The results suggest that the R-L morphology may have a more aggressive phenotype as compared to the R-N and L-N groups. Results are expressed as a percent change from referent normal (mean ± SEM); * p<0.05 versus normal set at 100%.
A: MMP-1/TS; B: MMP-2/TS; C: MMP-9/TS; D: MMP-12/TS.
Comment
The essential findings of this study, which sought to correlate BAV cusp fusion to MMP and TIMP biology in BAV-associated ATAAs, are 3-fold. First, this study demonstrated that global MMP activity was increased in the BAV specimens. MMP protein levels were largely unchanged but protein abundance of TIMPs -1 and -4 was dramatically reduced, suggesting that loss of MMP inhibition alone can increase global MMP activity and ATAA development. This suggests that delivery of recombinant TIMP proteins or local viral-mediated TIMP gene expression may have therapeutic relevance in these patients. Second, the present report demonstrated differential proteolytic profiles in each BAV morphology group, suggesting that regional matrix remodeling may be valve morphology specific. Third, when MMP abundance was corrected for TIMP score, the results suggest that the R-L morphology group may be more aggressive, and may justify an earlier surgical intervention.
MMP expression
Aneurysmal dilation of the each segment of the aorta has been attributed to elevated MMP expression and activity, typically MMP-2 and MMP-9.(14, 15) Both MMP-2 and MMP-9 can degrade non-fibrillar collagens, elastin, and various types of ground substance, and their expression and activity can be modulated by changes in hemodynamic forces and elevated wall tension using ex vivo systems.(16, 17) The matrilysin MMP-7 cleaves numerous ECM proteins and releases matrix-bound cytokines, participating in tumor invasion, inflammation, apoptosis, and angiogenesis.(18) In normal aorta, MMP-7 is immunohistochemically associated with intracellular and extracellular spaces of medial smooth muscle cells.(19) It has recently been shown that MMP-7 cannot diffuse through the vascular wall and subsequently accumulates in mucoid pools.(19) Extraction of MMP-7 from this mucoid material can be difficult and incomplete; thus, its quantification in this study may be subject to sampling error. Additional investigation of MMP-7 utilizing specific protein extraction techniques may more accurately assess its contribution to BAV-associated aortic dilation.
MMP-8, known as neutrophil collagenase, may be synthesized and released by endothelial cells, smooth muscle cells, and macrophages stimulated by pro-inflammatory cytokines.(20) Elevated MMP-8 is identified in abdominal aortic aneurysms, a disease known to be highly inflammatory in nature.(21) BAV aneurysms, on the other hand, have negligible inflammatory infiltrate,(22) therefore minimal quantities of MMP-8 were expected in the BAV aortic samples examined in this study.
Elastin degeneration within the aortic media is a common characteristic of aneurysm formation.(23) The primary elastase implicated is MMP-12, also known as macrophage elastase.(24) MMP-12 is selectively expressed in macrophages and foam cells of aneurysmal and atherosclerotic lesions,(25) and immunolocalizes to residual elastic fiber fragments in aneurysmal tissue.(24) MMP-12 deficiency attenuates experimental abdominal aortic aneurysm formation with decreased macrophage recruitment.(25) The present report showed that while MMP-12 abundance was not significantly altered in BAV associated ATAAs, when normalized for TIMP score abundance was elevated versus normal aorta. This may suggest that while inflammatory infiltration is minimal, resident macrophages may still contribute to MMP-12 activity within the aortic wall.
TIMP expression
The four TIMPs are capable of inhibiting all of the MMPs, through a 1:1 stoichiometric interaction.(26) Results from the present study followed previous immunoblotting results from this laboratory, demonstrating decreased TIMP-1, TIMP-3, and TIMP-4 protein abundance in BAV aorta compared to non-diseased aorta.(8) TIMP-2, which remained unchanged in the present study, also confirmed pre-existing data.(7, 8) TIMP-2 plays an integral role in the activation of the latent-form of MMP-2 by a mechanism that employs membrane type-1 MMP, and at higher concentrations is able to inhibit MMP-2 activity directly.(27) The dual role of TIMP-2 in MMP-2 activation and inhibition complicates interpretation of its function in aneurysm disease.
The TIMPs may also contribute to the regulation of cell proliferation and apoptosis through other pathways which may influence aortic dilation.(28) The delivery of effective exogenous MMP inhibitors to compensate for increased MMP activity in the face of decreased TIMP abundance as reported in this study, may help to prevent or attenuate the progression of aortic dilation in BAV patients.
Decreased TIMPs Induce a Proteolytic Shift in the BAV Aorta
The relative balance between the MMPs and TIMPs provides a specific measure of the proteolytic environment in the aortic wall. In the present study, a multi-analyte profiling system allowed for absolute quantification of soluble MMP and TIMP protein levels. A TIMP score, derived by summating the absolute concentrations of TIMP -1, -3, and -4, was used to relate the amount of each MMP to its maximal potential inhibition. TIMP-2 was initially omitted from this calculation, though it had little overall impact when included (Table 5). In this study, increased proteolytic indices in the BAV ATAAs were primarily a function of decreased TIMP abundance and not elevated MMPs, underscoring the important consideration of net stoichiometry between MMPs and their endogenous inhibitors when estimating the proteolytic balance within an aortic aneurysm.
Histopathologic changes in the media of ATAAs have been well documented, and specifically delineated for the BAV-associated aneurysms.(3, 10, 29) The presence of thin, fragmented elastin fibers and decreased types I and III collagen have suggested elevated proteolytic activity.(3, 30) Importantly, histological grading noted more extensive degradation of the ascending aortic wall associated with BAVs secondary R-L cusp fusion.(10) In the present study when MMP abundance was normalized for TIMP score, the R-L morphology displayed elevated proteolytic indices for MMPs -1, -2, -9, and -12, while the L-N configuration displayed elevated indices for MMP-2, and -3, and the R-N configuration for MMP-2. The heightened proteolytic indices observed in the R-L morphology group, supports work by Russo et al., suggesting that the R-L morphology is more aggressive.(10) Surgeons are usually faced with aortopathies from this most common group, and hence a more aggressive approach with regard to early aortic replacement in BAV patients with R-L cusp fusion may be warranted.
While this study has identified a proteolytic imbalance in the BAV-associated ATAAs, some limitations must be addressed. First, MMP-2 levels were not significantly increased in BAV aortas as seen in previous investigations.(8, 22) This discrepancy can be attributed to the multiplex suspension array system which uses two independent antibodies (in a sandwich technique) capable of binding all forms of MMP-2 (latent, active, and TIMP bound), whereas previous studies from this laboratory evaluated the 64 kDa active MMP-2 band alone. Second, previous histopathologic studies of the BAV-associated aortic wall have selectively sampled the right anterolateral (convex) surface, believed to undergo the greatest hemodynamic stress.(4, 29, 30) In the present study, while specimen location characteristics with regard to sampling on the convex or concave surface were not available, care was taken that all specimens were harvested from the widest region of the ascending aorta. Thus, it is possible that quantification of MMP abundance and activity specifically from the outer ascending aortic convexity could have revealed an even greater divergence in the proteolytic profile of BAV-ATAAs versus normal aorta. Last, because a significant difference in patient age between normal and BAV patients was identified, age-dependent effects on MMP activity and abundance cannot be specifically ruled out. Interestingly, there was no significant age difference identified between normal patients and those in the R-L morphology group, in contrast to differences identified between normal patients and the R-N and L-N groups. This suggests that ATAAs associated with R-L valve morphology are treated earlier, supporting previous reports suggesting that the R-L morphology may be a more aggressive aneurysm phenotype.
Despite the limitations, this project has uniquely identified that the proteolytic shift promoting BAV-associated aortic dilation may be attributable, at least in part, to decreased endogenous MMP inhibition. Furthermore, when MMP abundance was corrected for TIMP score, the results suggest that the R-L morphology group may be more aggressive, and may justify an earlier surgical intervention. Thus, strategies to restore the balance between MMP activity and inhibition in BAV-associated aneurysms may present both prophylactic as well as therapeutic options for this patient population.
Acknowledgments
This study was supported by NIH/NHLBI grant R21 HL089170.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braverman AC, Guven H, Beardslee MA, Makan M, Kates AM, Moon MR. The bicuspid aortic valve. Curr Probl Cardiol. 2005;30(9):470–522. doi: 10.1016/j.cpcardiol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119(6):880–90. doi: 10.1161/CIRCULATIONAHA.108.795401. [DOI] [PubMed] [Google Scholar]
- 3.Bauer M, Pasic M, Meyer R, Goetze N, Bauer U, Siniawski H, et al. Morphometric analysis of aortic media in patients with bicuspid and tricuspid aortic valve. Ann Thorac Surg. 2002;74(1):58–62. doi: 10.1016/s0003-4975(02)03650-0. [DOI] [PubMed] [Google Scholar]
- 4.Della Corte A, Quarto C, Bancone C, Castaldo C, Di Meglio F, Nurzynska D, et al. Spatiotemporal patterns of smooth muscle cell changes in ascending aortic dilatation with bicuspid and tricuspid aortic valve stenosis: focus on cell-matrix signaling. J Thorac Cardiovasc Surg. 2008;135(1):8–18. e1–2. doi: 10.1016/j.jtcvs.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Barbour JR, Stroud RE, Lowry AS, Clark LL, Leone AM, Jones JA, et al. Temporal disparity in the induction of matrix metalloproteinases and tissue inhibitors of metalloproteinases after thoracic aortic aneurysm formation. J Thorac Cardiovasc Surg. 2006;132(4):788–95. doi: 10.1016/j.jtcvs.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 6.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110(5):625–32. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyum J, Fellinger EK, Schmoker JD, Trombley L, McPartland K, Ittleman FP, et al. Matrix metalloproteinase activity in thoracic aortic aneurysms associated with bicuspid and tricuspid aortic valves. J Thorac Cardiovasc Surg. 2004;127(3):686–91. doi: 10.1016/j.jtcvs.2003.11.049. [DOI] [PubMed] [Google Scholar]
- 8.Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg. 2007;133(4):1028–36. doi: 10.1016/j.jtcvs.2006.10.083. [DOI] [PubMed] [Google Scholar]
- 9.Tamarina NA, McMillan WD, Shively VP, Pearce WH. Expression of matrix metalloproteinases and their inhibitors in aneurysms and normal aorta. Surgery. 1997;122(2):264–71. doi: 10.1016/s0039-6060(97)90017-9. discussion 71–2. [DOI] [PubMed] [Google Scholar]
- 10.Russo CF, Cannata A, Lanfranconi M, Vitali E, Garatti A, Bonacina E. Is aortic wall degeneration related to bicuspid aortic valve anatomy in patients with valvular disease? J Thorac Cardiovasc Surg. 2008;136(4):937–42. doi: 10.1016/j.jtcvs.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 11.Novaro GM, Tiong IY, Pearce GL, Grimm RA, Smedira N, Griffin BP. Features and predictors of ascending aortic dilatation in association with a congenital bicuspid aortic valve. Am J Cardiol. 2003;92(1):99–101. doi: 10.1016/s0002-9149(03)00480-6. [DOI] [PubMed] [Google Scholar]
- 12.Schaefer BM, Lewin MB, Stout KK, Byers PH, Otto CM. Usefulness of bicuspid aortic valve phenotype to predict elastic properties of the ascending aorta. Am J Cardiol. 2007;99(5):686–90. doi: 10.1016/j.amjcard.2006.09.118. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes SM, Sanders SP, Khairy P, Jenkins KJ, Gauvreau K, Lang P, et al. Morphology of bicuspid aortic valve in children and adolescents. J Am Coll Cardiol. 2004;44(8):1648–51. doi: 10.1016/j.jacc.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 14.Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res. 2007;139(2):292–307. doi: 10.1016/j.jss.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1995;15(8):1145–51. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- 16.Lehoux S, Lemarie CA, Esposito B, Lijnen HR, Tedgui A. Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling. Circulation. 2004;109(8):1041–7. doi: 10.1161/01.CIR.0000115521.95662.7A. [DOI] [PubMed] [Google Scholar]
- 17.Ruddy JM, Jones JA, Stroud RE, Mukherjee R, Spinale FG, Ikonomidis JS. Differential effects of mechanical and biological stimuli on matrix metalloproteinase promoter activation in the thoracic aorta. Circulation. 2009;120(11 Suppl):S262–8. doi: 10.1161/CIRCULATIONAHA.108.843581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin) The Journal of biological chemistry. 2001;276(30):28261–7. doi: 10.1074/jbc.M103608200. [DOI] [PubMed] [Google Scholar]
- 19.Borges LF, Touat Z, Leclercq A, Zen AA, Jondeau G, Franc B, et al. Tissue diffusion and retention of metalloproteinases in ascending aortic aneurysms and dissections. Human pathology. 2009;40(3):306–13. doi: 10.1016/j.humpath.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Herman MP, Sukhova GK, Libby P, Gerdes N, Tang N, Horton DB, et al. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation. 2001;104(16):1899–904. doi: 10.1161/hc4101.097419. [DOI] [PubMed] [Google Scholar]
- 21.Wilson WR, Schwalbe EC, Jones JL, Bell PR, Thompson MM. Matrix metalloproteinase 8 (neutrophil collagenase) in the pathogenesis of abdominal aortic aneurysm. Br J Surg. 2005;92(7):828–33. doi: 10.1002/bjs.4993. [DOI] [PubMed] [Google Scholar]
- 22.LeMaire SA, Wang X, Wilks JA, Carter SA, Wen S, Won T, et al. Matrix metalloproteinases in ascending aortic aneurysms: bicuspid versus trileaflet aortic valves. J Surg Res. 2005;123(1):40–8. doi: 10.1016/j.jss.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Dobrin PB, Mrkvicka R. Failure of elastin or collagen as possible critical connective tissue alterations underlying aneurysmal dilatation. Cardiovasc Surg. 1994;2(4):484–8. [PubMed] [Google Scholar]
- 24.Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest. 1998;102(11):1900–10. doi: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longo GM, Buda SJ, Fiotta N, Xiong W, Griener T, Shapiro S, et al. MMP-12 has a role in abdominal aortic aneurysms in mice. Surgery. 2005;137(4):457–62. doi: 10.1016/j.surg.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 27.Karagiannis ED, Popel AS. A theoretical model of type I collagen proteolysis by matrix metalloproteinase (MMP) 2 and membrane type 1 MMP in the presence of tissue inhibitor of metalloproteinase 2. J Biol Chem. 2004;279(37):39105–14. doi: 10.1074/jbc.M403627200. [DOI] [PubMed] [Google Scholar]
- 28.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115(Pt 19):3719–27. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 29.Della Corte A, De Santo LS, Montagnani S, Quarto C, Romano G, Amarelli C, et al. Spatial patterns of matrix protein expression in dilated ascending aorta with aortic regurgitation: congenital bicuspid valve versus Marfan’s syndrome. J Heart Valve Dis. 2006;15(1):20–7. discussion 7. [PubMed] [Google Scholar]
- 30.Cotrufo M, Della Corte A, De Santo LS, Quarto C, De Feo M, Romano G, et al. Different patterns of extracellular matrix protein expression in the convexity and the concavity of the dilated aorta with bicuspid aortic valve: preliminary results. J Thorac Cardiovasc Surg. 2005;130(2):504–11. doi: 10.1016/j.jtcvs.2005.01.016. [DOI] [PubMed] [Google Scholar]