Abstract
Progesterone receptors (PR), in concert with peptide growth factor-initiated signaling pathways, initiate massive expansion of the epithelial cell compartment associated with the process of alveologenesis in the developing mammary gland. PR-dependent signaling events also contribute to inappropriate proliferation observed in breast cancer. Notably, PR-B isoform-specific cross talk with growth factor-driven pathways is required for the proliferative actions of progesterone. Indeed, PRs act as heavily phosphorylated transcription factor “sensors” for mitogenic protein kinases that are often elevated and/or constitutively activated in invasive breast cancers. In addition, phospho-PR-target genes frequently include the components of mitogenic signaling pathways, revealing a mechanism for feed-forward signaling that confers increased responsiveness of PR+ mammary epithelial cells to these same mitogenic stimuli. Understanding the mechanisms and isoform selectivity of PR/kinase interactions may yield further insight into targeting altered signaling networks in breast and other hormonally responsive cancers (i.e. lung, uterine, ovarian) in the clinic. This review focuses on PR phosphorylation by mitogenic protein kinases and mechanisms of PR-target gene selection that lead to increased cell proliferation.
PR structure and function
The ovarian steroid hormone, progesterone, acts by binding to and activating progesterone receptor (PR) A-, B-, and C-isoforms expressed in target tissues. Isoform-specific expression results from selection of among three independent translational start sites embedded within the same transcript, encoded by a single gene (Kastner, Krust, Turcotte et al., 1990). The full-length receptor, PR-B (116 kDa), contains a unique N-terminal segment, termed the B-upstream segment (BUS), that is not present in the truncated isoforms, PR-A (94 kDa), or PR-C (60 kDa). PR-C lacks both the BUS and a portion of the DNA-binding domain (DBD), rendering it transcriptionally inactive (Wei, Hawkins, Baker et al., 1996). In addition to intact DBDs, the two transcriptionally active isoforms, PR-B and PR-A, contain the following structural/functional domains: a flexible hinge region (H; also referred to as the carboxy terminal extension or CTE) that functions, in part, to aid DNA binding (Roemer, Adelman, Churchill et al., 2008), a ligand-binding domain (LBD), and multiple activating function (AF) domains required for transcriptional activity (Fig. 1). Studies from knockout-mice have shown that PR-B is necessary for the alveologenesis phase of normal mammary gland development, while PR-A is required for uterine development (Conneely, Mulac-Jericevic, Lydon et al., 2001,Lydon, DeMayo, Funk et al., 1995,Mulac-Jericevic, Lydon, DeMayo et al., 2003,Shyamala, Yang, Silberstein et al., 1998). PR-C, lacking transcription activity, has been shown to inhibit PR-B function in the uterus (Condon, Hardy, Kovaric et al., 2006), and conversely, appears to potentiate the transcriptional activity of the other PR isoforms in the breast (Wei, Norris and Baker, 1997).
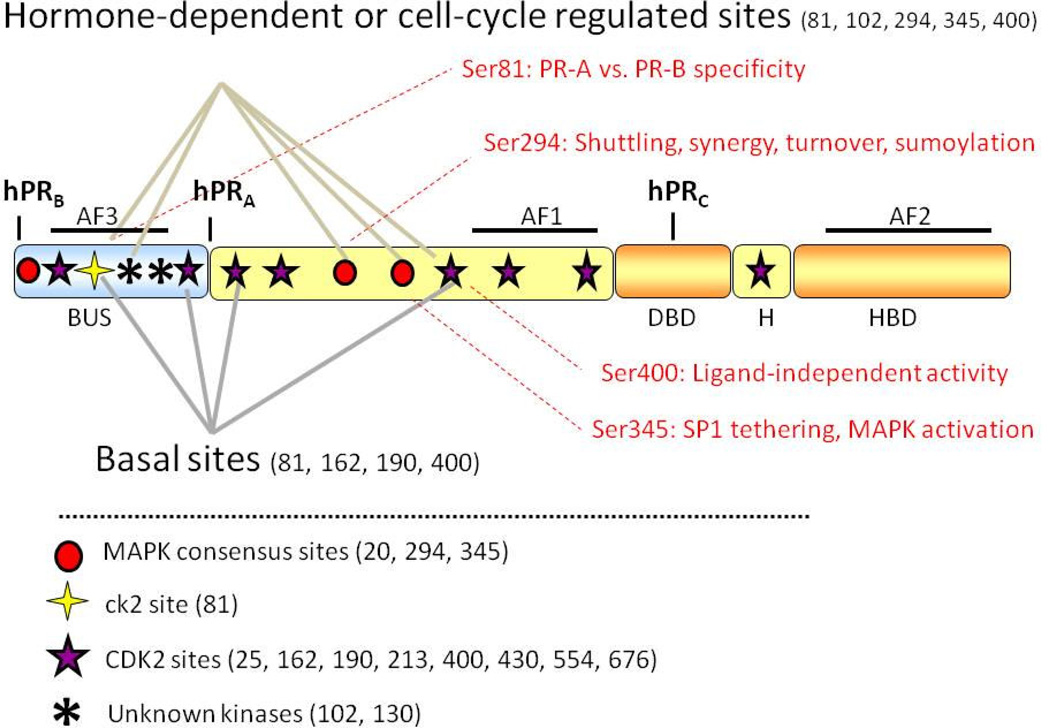
Figure 1. Progesterone receptor structure and phosphorylation sites.
All three human PR isoforms (hPRA, hPRB and hPRC) are transcribed from the same gene, containing distal and proximal promoters, and created via differential use of three internal translational start sites. Shown are three transcription activation function (AF) domains, the B-upstream segment (BUS), the DNA-binding domain (DBD), the hinge region (H) and the hormone-binding domain (HBD). PR is phosphorylated basally, as well as in response to hormone. Shown here are known PR phosphorylation sites as determined in vitro and in vivo, and the protein kinases that are likely responsible for direct phosphorylation at these sites.
PR isoforms rapidly shuttle between the cytoplasm and the nucleus; unliganded receptors reside in both compartments and exist as part of multi-protein complexes in association with heat-shock protein chaperone molecules, such as Hsp70 and Hsp90 (Pratt, Sanchez, Bresnick et al., 1989,Kost, Smith, Sullivan et al., 1989). Additionally, unliganded and liganded PRs (primarily PR-B; (Boonyaratanakornkit, McGowan, Sherman et al., 2007)) participate in cytoplasmic or membrane-associated signaling complexes that activate mitogenic protein kinases, such as c-Src, MAPK and PI3K (Boonyaratanakornkit, Scott, Ribon et al., 2001,Migliaccio, Piccolo, Castoria et al., 1998,Bagowski, Myers and Ferrell, 2001,Faivre and Lange, 2007,Carnevale, Proietti, Salatino et al., 2007). In response to progesterone-binding, membrane-tethered PRs rapidly activate these kinases and can also transactivate EGFR (Faivre, Daniel, Hillard et al., 2008); this PR-dependent activity has been termed a “non-genomic” action because it occurs independently of the transcriptional activity of PRs (Fig. 2). In the classical or genomic model of PR action, ligand binding induces dissociation of PR from chaperone complexes; dimerized (hetero or homo) PRs are largely retained in the nucleus where they bind to DNA either directly through progesterone response elements (PRE), or indirectly through tethering interactions with other transcription factors (AP1, SP1, STATs) (Owen, Richer, Tung et al., 1998,Stoecklin, Wissler, Schaetzle et al., 1999,Cicatiello, Addeo, Sasso et al., 2004).
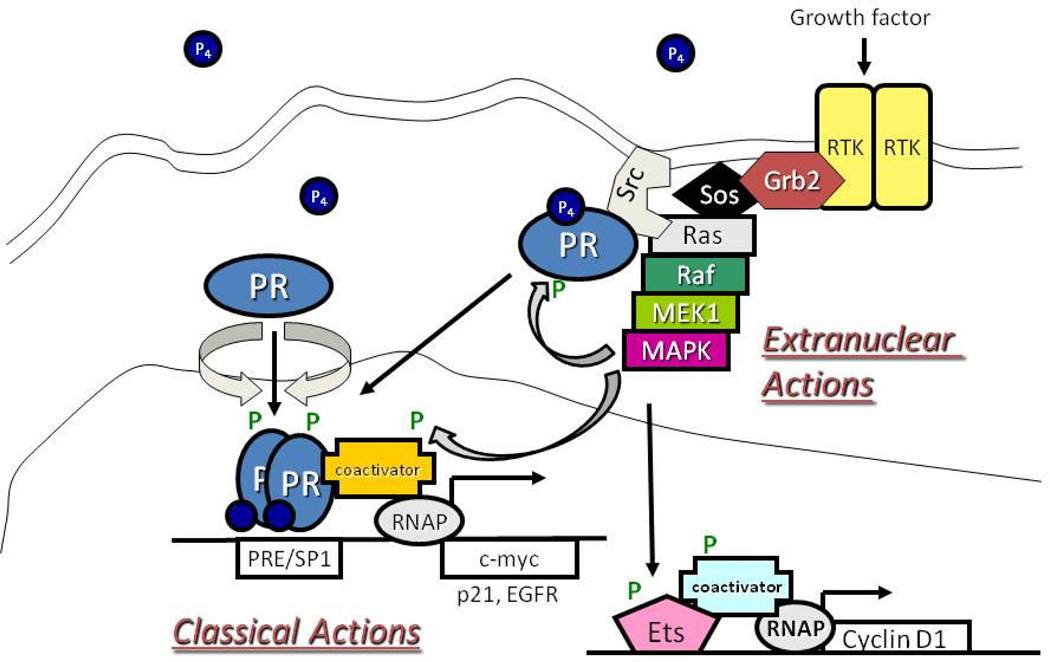
Figure 2. Integration of PR rapid signaling and transcriptional activities.
Progesterone (P4) binding to PR induces the rapid association of PR and c-Src. This interaction leads to a c-Src-dependent activation of the MAPK module through Ras/Raf signaling. This MAPK activation can lead to phosphorylation (P) of PR, transcriptional coactivators, and/or activation of downstream MAPK target genes (i.e. Cyclin D1). Phosphorylated PRs can activate transcription directly by binding to progesterone response elements (PREs) or indirectly though tethering interactions (i.e. SP1). Extranuclear and classical actions of PR are likely highly integrated actions, rather than separable events mediated by discrete populations of receptors.
Notably, PR-A and PR-B isoforms are highly post-translationally modified, primarily on serine (Ser; phosphorylation) and lysine (Lys; acetylation, ubiquitination, and sumoylation) residues located in the N-terminal region (Lange, Shen and Horwitz, 2000,Weigel, Bai, Zhang et al., 1995,Daniel, Gaviglio, Czaplicki et al.,Abdel-Hafiz, Takimoto, Tung et al., 2002,Hagan, Regan, Dressing et al., 2011). These modifications are frequently ligand-dependent, but can also occur independently of progestin-binding (i.e. in response to kinase activation), and significantly alter receptor stability, localization, tethering interactions, transcriptional activity, and promoter selectivity (Daniel, Knutson and Lange, 2009,Ward and Weigel, 2009). For example, PR phospho-species exhibit differential activities on a given promoter, but also appear to select different promoters (reviewed in (Daniel et al., 2009)). Although the mechanisms are not entirely clear, unique PR phospho-species are likely directed to distinct PR target gene subsets in part via phosphorylation-dependent protein-protein interactions with the same set of protein kinases that may occur within so-called “rapid signaling complexes”. Thus, the non-genomic and genomic actions of PRs are highly integrated functions that serve to coordinate isoform-specific PR actions and mediate PR-target gene promoter selection; mechanisms of PR integration with signaling pathways is the topic of this review (Fig. 2).
MAPK activation alters PR function
MAPK signaling modulates PR activity directly by phosphorylating the receptor on consensus site serine residues Ser294 and Ser345 (Faivre et al., 2008,Lange et al., 2000). These distinctly regulated phosphorylation events have unique functional consequences for PR that ultimately regulate cell fate. Upon growth factor stimulation, PR phosphorylation of Ser294 primes the receptor for robust transcriptional activation in response to ligand by ensuring retention in the nucleus (discussed below) (Qiu, Olsen, Faivre et al., 2003), association with DNA (Daniel, Qiu, Faivre et al., 2007), and removal of repressive modification by sumoylation (Daniel, Faivre and Lange, 2007). Ser294-phosphorylated receptors are transcriptionally hypersensitive to low concentrations of ligand on a select set of promoters (Qiu and Lange, 2003); the mechanism of growth factor-induced PR hypersensitivity maps to phospho-Ser294 antagonism of Lys388 sumoylation (Daniel et al., 2007). Likewise, phosphorylation on Ser294 increases PR ubiquitinylation, an activation step for many transcription factors (Salghetti, Caudy, Chenoweth et al., 2001), and also augments its downregulation (Lange et al., 2000). Therefore Ser294 phosphorylation in response to MAPK activation (by either progestins or growth factors) generates receptors that are hyperactive on select promoters in response to ligand and serves to couple this activity to rapid proteasome-dependent turnover. In addition, phosphorylated/desumoylated receptors are active on a subset of ligand-independent PR-target gene promoters whose expressed protein products (IRS-1 and STC1) contribute to breast cancer cell proliferation and pro-survival (Daniel and Lange, 2009). Furthermore, Ser294 appears to be a “hot-spot” for the regulation of PR-B transcriptional activity as phospho-mutant PR-B (Ser294 to alanine) is virtually transcriptionally inactive when measured on endogenous genes (i.e. in cells stably expressing S294A PR-B relative to cells containing wild-type PR) (Shen, Horwitz and Lange, 2001). Notably, PR-A is not appreciably phosphorylated on Ser294 in intact cells, while this site in PR-A can be phosphorylated in vitro using recombinant PR-A proteins (Clemm, Sherman, Boonyaratanakornkit et al., 2000). This finding underscores the role of protein-protein interactions between PRs and associated signaling complexes that contain protein kinases as major determinants of PR isoform specificity.
In an alternative route to phosphorylation-dependent PR promoter selection, rapid progestin-mediated MAPK activation drives specific phosphorylation of PR-B on Ser345, a site shown to be critical for PR tethering to SP1 transcription factors (Faivre et al., 2008). PR/c-Src/EGFR rapid signaling complex formation precedes PR Ser345 phosphorylation and PR/SP1 association with non-classical promoters (lacking PREs), such as p21 and EGFR (Fig. 2). This unique mechanism of steroid receptor activation by MAPK signaling (i.e. non-genomic/genomic signaling integration) is required for progestin-induced breast cancer cell entry into S-phase (Faivre et al., 2008).
Cyclin dependent kinase 2 (CDK2) regulation of PR function
Studies using both in vitro and in vivo techniques have identified multiple CDK2-dependent phosphorylation sites on PR (reviewed in (Moore, Narayanan and Weigel, 2007)). These sites include PR serines 25, 162, 190, 213, 400, 554, 676 (Zhang, Beck, Poletti et al., 1997,Knotts, Orkiszewski, Cook et al., 2001) and threonine 430 (Knotts et al., 2001). Additionally, while Ser294 is phosphorylated by MAPK (discussed earlier), it can also be phosphorylated by CDK2 (Daniel and Lange, 2009). Although only a fraction of these CDK2 sites have been studied in depth, PR phosphorylation by CDK2 has specific implications for PR function and activity. Phosphorylation of PR serines 190, 294, 554 and 676 clearly contributes to PR hormone-dependent transcriptional activity (Shen et al., 2001,Takimoto, Hovland, Tasset et al., 1996). Individual mutation of each of these sites results in significant decreases (20–90%) in overall PR transcriptional activity, as measured using PRE-reporter gene constructs. While Sers 190, 554 or 676- phospho-mutant PRs exhibit significant decreases in transcriptional activity, these mutant PR species are each able to bind DNA similar to wild-type PR, suggesting that phosphorylation at these serines may contribute to recruitment of co-activators to PR-containing transcriptional complexes (Takimoto et al., 1996).
Phosphorylation of PR Ser400 by CDK2 has been linked to enhanced ligand-independent PR transcriptional activity, as measured using PRE-reporter gene constructs (Pierson-Mullany and Lange, 2004). In the presence of high CDK2 kinase activity and/or low cell cycle inhibitors (namely, p27), PR Ser400 is constitutively phosphorylated and thereby drives heightened PR transcriptional activity in the absence of progestins (Pierson-Mullany and Lange, 2004). This particular interaction of CDK2 with PR has important implications for deregulated PR activity in the context of breast cancer, as transformed cells often exhibit loss of cell cycle control that is characterized by Rb-inactivation, elevated CDK4/6 activity, high expression of cyclins D, E, or A, and/or low expression of cell cycle inhibitors (Slingerland and Pagano, 2000,Cariou, Catzavelos and Slingerland, 1998,Musgrove, Davison and Ormandy, 2004,Alkarain, Jordan and Slingerland, 2004,Tawfic, Yu, Wang et al., 2001,Wilson, Cramer, Welman et al., 2006) ultimately leading to increased, deregulated CDK2 activity. Notably, PR-target genes include key cell cycle mediators (reviewed in (Dressing and Lange, 2009)) such as D-type cyclins and cyclin E, the regulatory subunits of CDK4/6 and CDK2, respectively. Thus, activation of unliganded PRs in this setting (cell cycle deregulation leading to high CDK2 activity) may produce a “feed forward” mechanism of persistent CDK2 activation early in breast tumor development. This unliganded activity of PR can be blocked by anti-progestins (Pierson-Mullany and Lange, 2004), suggesting that selective PR modulators could be used to block CDK2-driven cell proliferation and pro-survival in PR+ tumor cells.
Phosphorylation events also contribute to PR nuclear localization. Recent studies suggest that mutant PRs unable to enter the nucleus (devoid of nuclear localizations signals; ΔNLS PR) are phosphorylated on Ser190, but not on Sers 81, 294, 345 and 400 ((Daniel, Gaviglio, Czaplicki et al.) and data not shown). However, ΔNLS PR is phosphorylated on these sites upon coexpression and dimerization with wt PR, forcing ΔNLS PR nuclear entry. Other studies showed that Ser400 phosphorylation (CDK2-dependent) enhanced ligand-induced nuclear accumulation (Pierson-Mullany and Lange, 2004), while Ser294 phosphorylation was required for growth factor (EGF, MAPK), but not progestin-mediated nuclear accumulation (Qiu et al., 2003). These data suggest that phosphorylation of PR on these residues occurs upon nuclear entry and serves to promote nuclear retention. Phosphorylation on Ser190, another CDK2 site, likely occurs in the cytoplasm and does not contribute to nuclear entry or retention (Daniel et al.). Together, these data demonstrate that CDK2 is able to phosphorylate PR in both the cytoplasm and the nucleus and that, once in the nucleus, phosphorylation at some CDK2 sites (Sers 294 and 400) promotes PR nuclear retention, perhaps via protein-protein interactions requiring these specific phosphorylation events. Moreover, rapid nuclear translocation and retention of PR appears to be critical for proper execution of rapidly-activated (i.e. c-myc mRNA expression is induced by liganded PR within minutes) PR-target genes (Daniel et al.). Properly timed PR nuclear entry/retention in response to phosphorylation events likely ensures robust execution of PR transcriptional activity at such “early genes” perhaps, in part, by ensuring that both PR and its co-regulators are activated (i.e. appropriately phosphorylated) and co-localized in the nucleus. Indeed, latent nuclear localization of PR is associated with delays in PR-induced immediate early genes (i.e. c-myc) but not in overall PR transcriptional activity measured at late time points (i.e. on reporter genes) and/or on endogenous genes that are not particularly sensitive to changes in phosphorylation events (Daniel et al.).
Interestingly, CDK2 not only acts to phosphorylate PR but may also act as an integral part of PR transcriptional complexes. Cyclins A and E, the regulatory subunits of CDK2, bind to both unliganded and liganded PRs; these constitutive interactions may serve to recruit and sustain CDK2 activity at active sites of transcription (reviewed in (Dressing and Lange, 2009)). Although endogenous genes have not been extensively studied, Cyclin A is clearly recruited along with PR to stably embedded (i.e. in chromatin) MMTV promoter regions (Narayanan, Adigun, Edwards et al., 2005). Thus, CDK2 (a cyclin A-binding partner) is also likely present at PR-bound PRE-containing enhancers (Moore et al., 2007,Narayanan et al., 2005,Weigel and Moore, 2007). Inhibition of CDK2 activity using a small molecule CDK2 inhibitor, roscovitine, decreased phosphorylation of SRC-1 (steroid receptor co-activator-1) and blocked recruitment of both PR and SRC-1 to the PR transcriptional complex on the MMTV promoter (Narayanan et al., 2005). In these studies, mutation of PR at multiple CDK2 phosphorylation sites had no effect on reporter gene transcription. Thus, CDK2 appears to mediate SRC-1 co-activator phosphorylation (independently of PR phosphorylation). The scaffolding function of PR/cyclin interactions likely serves to recruit and sustain CDK2 activity (wherein the primary substrate is SRC-1); this model awaits confirmation on endogenous genes and during cell cycle traverse.
ck2 modification of PR
Initial in vitro work showed that PR Ser81 (unique to the BUS region of PR-B) was phosphorylated by ck2, a ubiquitously expressed, constitutively active protein kinase (Zhang, Beck, Poletti et al., 1994). Recent published work from the Lange lab has shown in breast cancer cells that basal levels of PR Ser81 phosphorylation are rapidly increased in response to either agonist or antagonist ligands (Hagan et al., 2011); an effect shown to be dependent on ck2. However, unlike other PR phosphorylation sites (i.e. Ser294), PR Ser81 phosphorylation is unresponsive to growth-factor or serum treatment of cells. Interestingly, in the absence of ligand, PR Ser81 phosphorylation is increased in cells that are synchronized at the G1/S phase border, suggesting that phosphorylation at this site is regulated in a cell-cycle dependent manner (Hagan et al., 2011). In line with this finding, ligand-independent cell survival, as measured by soft-agar colony formation, was decreased in cells expressing a PR phospho-mutant (S79/81A PR) that cannot be phosphorylated at Ser81 (Hagan et al., 2011). Moreover, this mutant displayed defects in recruitment to selected PR-B-target genes important for proliferation and pro-survival, and was impaired in its ability to recruit ck2 to PR-associated enhancer sites (Fig. 3)(Hagan et al., 2011). ck2, a kinase shown to be upregulated in every cancer studied thus far, including breast cancer, is not thought to be oncogenic on its own, but appears to increase the oncogenic potential of cancer-promoting proteins and pro-growth signals that are its substrate molecules (Tawfic et al., 2001,Trembley, Wang, Unger et al., 2009). In the context of breast cancer, where progestins have been implicated as a risk factor for tumor development and early progression (Beral, 2003,Anderson, Limacher, Assaf et al., 2004,Chlebowski, Hendrix, Langer et al., 2003), overexpressed ck2 could further enhance the oncogenic potential of PR through inappropriate phosphorylation (on Ser81), thereby directing phospho-Ser81 PR-B to growth-promoting genes.
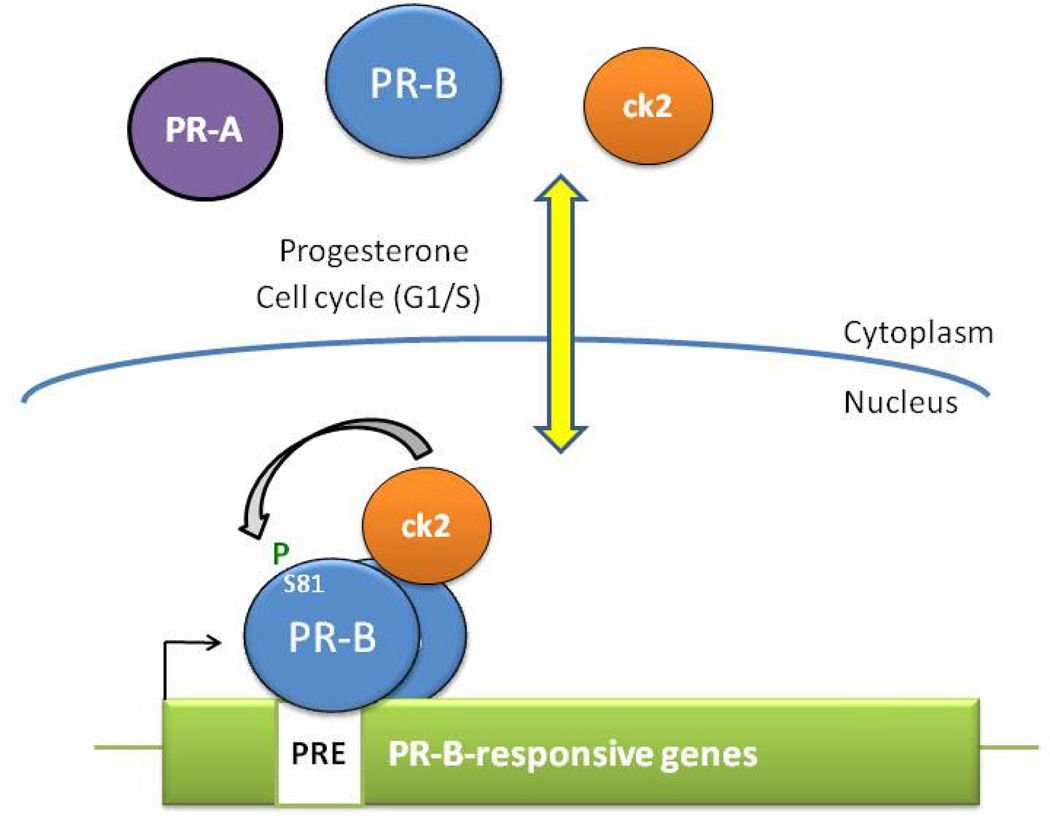
Figure 3. Ck2-dependent PR-B Ser81 phosphorylation mediates isoform-specfic target gene selection.
In response to progesterone binding or cell cycling (G1/S), PR-B is phosphorylated at Ser81 by ck2. Phospho-Ser81-PR-B/ck2 complexes are recruited to promoter/enhancer regions of Ser81-responsive PR-target genes. Phosphorylation at PR-B Ser81 (not present in PR-A) is a major determinant of PR isoform-specific target-gene selectivity.
PR-dependent activation and amplification of kinase signaling pathways
Several studies illustrate the emerging concept that PRs and its associated signaling pathways are fully integrated, from membrane-initiated events to genomic actions (Fig. 2). Upon progestin treatment, PR rapidly associates with signaling complexes via two distinct domains: a consensus poly-proline rich region (PR amino acids 396–456) known to interact with consensus SH3 domains (Boonyaratanakornkit et al., 2001) and unique (to PR) regions termed Estrogen Receptor Interacting Domains or ERIDs; ERID1 (amino acids 165–345) and ERID2 (amino acids 456–546) are located in the PR N-terminus (Ballare, Uhrig, Bechtold et al., 2003). Progestin-binding induces direct interaction of PR with the SH3 domain of c-Src, or to ER (via the ERID domains), causing rapid (5–10 min) activation of the EGFR/c-Src/Ras/Erk pathway (Boonyaratanakornkit et al., 2001,Migliaccio et al., 1998,Faivre et al., 2008,Ballare et al., 2003) and the PI3K/Akt pathway (Carnevale et al., 2007). These signals, shown to be critical for progestin-induced proliferation of breast cancer cells (Boonyaratanakornkit et al., 2007), provide a feed forward signaling mechanism for PR/progestin-dependent genomic events in addition to activating other transcription factors (Faivre et al., 2008). Phosphorylation of PR and co-activator molecules enhances PR transcriptional activity on classical (Qiu and Lange, 2003) and non-classical promoters (Faivre et al., 2008). Progestin-activated Erk is recruited to PR-containing transcriptional complexes in chromatin (Vicent, Ballare, Nacht et al., 2006) and PR devoid of ERIDs activates a gene expression profile distinct from wt PR (Quiles, Millan-Arino, Subtil-Rodriguez et al., 2009), indicating that PR-induced kinase signaling contributes directly to promoter activation and selectivity. Notably, progestin treatment also elicits delayed (18h) and sustained activation of MAPK signaling, whereby MAPK-dependent upregulation of PR-target genes (Wnt1, MMPs, and EGFR) completes an autocrine signaling pathway that culminates in high cyclin D levels and breast cancer cell growth/survival in soft agar (Faivre and Lange, 2007). Thus, progestin/PR-mediated rapid activation of MAPK signaling ultimately functions to amplify PR genomic actions, modulate PR target gene selectivity (i.e. by directing phospho-PRs to selected promoters), and induce sustained MAPK signaling (i.e. downstream of activated EGFR) capable of activating multiple (PR-independent) transcription factors that serve to perpetuate the proliferative signal (long after liganded PRs have been downregulated). In this manner, progesterone/PRs may confer greatly increased sensitivity of target tissues to the actions of peptide growth factors. These interactions clearly allow for rapid expansion of the mammary epithelium during puberty and pregnancy (in preparation for lactation), but may inappropriately drive early breast cancer progression of steroid hormone receptor positive tumors.
In addition to scaffolding MAPK pathway signaling events, PR also participates in signaling complexes with cell cycle regulators. PR contains numerous consensus CDK binding motifs, and has been shown to associate with CDK2, perhaps mediating its interactions with cyclins E and A (discussed above) (Narayanan et al., 2005,Faivre, Skildum, Pierson-Mullany et al., 2005). This complex formation, in addition to PR transcriptional upregulation of cyclins and CDK inhibitors (p21, p27) that appears to be required for initiating CDK kinase activity, may account for the rapid (15 min) and sustained (days) activation of CDK2 observed in breast cancer cells upon a single treatment with progestin (Pierson-Mullany and Lange, 2004). Again, these studies indicate that phospho-PRs are capable of robust positive feed forward or self-regulation of the very same signaling pathways that they rapidly activate.
A number of studies have illustrated further cross-talk between PRs and Signal Transducers and Activators of Transcription (STATs), involving PR-mediated activation of both STAT3 and STAT5. Cumulative work from the Elizalde lab has shown that STAT3 activation by the heregulin/ErbB-2 pathway is mediated by ligand-independent functions of PR, and requires phosphorylation of PR Ser294 (in response to growth factor stimulation) (Proietti, Rosemblit, Beguelin et al., 2009). Further work has defined a bi-directional transcriptional co-activator relationship between PR and STAT3, each appearing to activate the transcriptional capacity of the other (Beguelin, Diaz Flaque, Proietti et al.,Proietti, Beguelin, Flaque et al.). A similar story has emerged for STAT5 and PR. Progesterone treatment induces PR-dependent STAT5 nuclear translocation and transcriptional activity, potentially mediated by a direct interaction between PR and STAT5 (Richer, Lange, Manning et al., 1998), at times involving other signaling molecules that serve as co-regulators like FGFR-2 (Fibroblast growth factor receptor-2) (Cerliani, Guillardoy, Giulianelli et al.). PR-dependent regulation of (downstream) STAT5 activity is well established as critical for normal mammary gland development (Santos, Haslam and Conrad,Santos, Haslam and Conrad, 2008).
Indeed, the end point of mitogenic signaling pathway activation is often the regulation of transcription factor substrates. Notably, phospho-PR target genes most often include the components of signal transduction pathways (T. Knutson and C. Lange, unpublished results). Thus, PR is directly responsible for modulating/maintaining kinase signaling in cells via transcriptional upregulation of growth factor receptors, their ligands, and their downstream effectors and associated adaptor molecules. Direct PR target genes include EGFR, IRS1, STAT5A, numerous Ras pathway members (including adaptors and exchange factors), many kinases, as well as peptide growth factors (Hb-EGF, Wnt1) and other secreted signaling molecules (Daniel et al., 2007,Jacobsen, Richer, Sartorius et al., 2003). Ultimately, kinase pathway “restructuring” by PR may serve to prime mammary epithelial cells for the rapid proliferation stage associated with massive expansion of the (pregnant) mammary gland that occurs in preparation for lactation. Similarly, the deregulation of these events during breast cancer development and/or early progression is suspected to contribute to advanced malignant breast cancer phenotypes.
PR significance in breast cancer
Highly publicized and controversial clinical data has demonstrated that women taking hormone-replacement therapy (HRT) whose regimens included estrogen and synthetic progesterone, but not estrogen alone, experienced increased breast tumor number, size, and aggressiveness (Beral, 2003,Anderson et al., 2004); increased breast cancer risk was reversed upon cessation of HRT (Beral, 2003,Chlebowski, Kuller, Prentice et al., 2009). Significantly, nearly 70% of breast cancers express both ER and PR at diagnosis, in contrast to PR/ER expression in just 7–10% of normal (non-pregnant) breast luminal epithelium (Seagroves, Lydon, Hovey et al., 2000). As these steroid receptor (SR)-positive tumors progress, many of them become hormone-independent (refractory to estrogen- or ER-targeted endocrine treatments) while retaining high SR expression, suggesting an early switch to autocrine and/or paracrine growth factor signaling (Osborne, Schiff, Arpino et al., 2005). In addition, a majority of these cancers have upregulated and activated protein kinases, such as MAPK, Akt, c-Src, cyclin/CDKs, and ck2, all of which modify and/or activate PR and/or its co-regulators (discussed in detail above) (Tawfic et al., 2001,Wilson et al., 2006,Gregory, Fei, Ponguta et al., 2004,Steeg and Zhou, 1998). In breast cancer cells, PR-B action clearly drives proliferation and pro-survival signaling. Interestingly, PR (mRNA expression) was recently identified as an independent-(single-gene) predictor of poor outcome in non-small cell lung cancer, implicating PR and hormone-responsiveness in cancers other than breast (Jeong, Xie, Xiao et al.). In an environment where progesterone is no longer required to drive cellular proliferation (i.e. ligand-independence), constitutive activation of PR-activating protein kinases may promote uncontrolled cell growth that is primarily driven by deregulated phospho-PR-target genes. Most recently, progesterone was shown to mediate mammary gland stem cell self-renewal via paracrine mechanisms in which secreted factors (Wnt, RANKL) derived from PR-positive cells influenced the PR-null stem cell niche (Joshi, Jackson, Beristain et al.,Asselin-Labat, Shackleton, Stingl et al., 2006). Progesterone/progestins may alter breast cancer stem cell behavior by similar mechanisms. In sum, in light of the cumulative data discussed herein, understanding how mitogenic protein kinases alter PR (and vice-versa) is critical to fully understanding breast tumor etiology with the goal of developing superior approaches for the prevention or treatment of endocrine resistance in SR-positive breast cancers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. Embo J. 1990:9. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei LL, Hawkins P, Baker C, Norris B, Sheridan PL, Quinn PG. An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol Endocrinol. 1996;10:1379–1387. doi: 10.1210/mend.10.11.8923464. [DOI] [PubMed] [Google Scholar]
- 3.Roemer SC, Adelman J, Churchill ME, Edwards DP. Mechanism of high-mobility group protein B enhancement of progesterone receptor sequence-specific DNA binding. Nucleic Acids Res. 2008;36:3655–3666. doi: 10.1093/nar/gkn249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol. 2001;179:97–103. doi: 10.1016/s0303-7207(01)00465-8. [DOI] [PubMed] [Google Scholar]
- 5.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 6.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shyamala G, Yang X, Silberstein G, Barcellos-Hoff MH, Dale E. Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc Natl Acad Sci U S A. 1998;95:696–701. doi: 10.1073/pnas.95.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–775. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 9.Wei LL, Norris BM, Baker CJ. An N-terminally truncated third progesterone receptor protein, PR(C), forms heterodimers with PR(B) but interferes in PR(B)-DNA binding. J Steroid Biochem Mol Biol. 1997;62:287–297. doi: 10.1016/s0960-0760(97)00044-7. [DOI] [PubMed] [Google Scholar]
- 10.Pratt WB, Sanchez ER, Bresnick EH, Meshinchi S, Scherrer LC, Dalman FC, Welsh MJ. Interaction of the glucocorticoid receptor with the Mr 90,000 heat shock protein: an evolving model of ligand-mediated receptor transformation and translocation. Cancer Res. 1989;49:2222s–2229s. [PubMed] [Google Scholar]
- 11.Kost SL, Smith DF, Sullivan WP, Welch WJ, Toft DO. Binding of heat shock proteins to the avian progesterone receptor. Mol Cell Biol. 1989;9:3829–3838. doi: 10.1128/mcb.9.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol. 2007;21:359–375. doi: 10.1210/me.2006-0337. [DOI] [PubMed] [Google Scholar]
- 13.Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 14.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. Embo J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bagowski CP, Myers JW, Ferrell JE. The classical progesterone receptor associates with p42 MAPK and is involved in PI3-K signaling in Xenopus oocytes. J Biol Chem. 2001;276:37708–37714. doi: 10.1074/jbc.M104582200. [DOI] [PubMed] [Google Scholar]
- 16.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27:466–480. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carnevale RP, Proietti CJ, Salatino M, Urtreger A, Peluffo G, Edwards DP, Boonyaratanakornkit V, Charreau EH, Bal de Kier Joffe E, Schillaci R, Elizalde PV. Progestin effects on breast cancer cell proliferation, proteases activation, and in vivo development of metastatic phenotype all depend on progesterone receptor capacity to activate cytoplasmic signaling pathways. Mol Endocrinol. 2007;21:1335–1358. doi: 10.1210/me.2006-0304. [DOI] [PubMed] [Google Scholar]
- 18.Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol. 2008;22:823–837. doi: 10.1210/me.2007-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owen GI, Richer JK, Tung L, Takimoto G, Horwitz KB. Progesterone regulates transcription of the p21(WAF1) cyclin- dependent kinase inhibitor gene through Sp1 and CBP/p300. J Biol Chem. 1998;273:10696–10701. doi: 10.1074/jbc.273.17.10696. [DOI] [PubMed] [Google Scholar]
- 20.Stoecklin E, Wissler M, Schaetzle D, Pfitzner E, Groner B. Interactions in the transcriptional regulation exerted by Stat5 and by members of the steroid hormone receptor family. J Steroid Biochem Mol Biol. 1999;69:195–204. doi: 10.1016/s0960-0760(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 21.Cicatiello L, Addeo R, Sasso A, Altucci L, Petrizzi VB, Borgo R, Cancemi M, Caporali S, Caristi S, Scafoglio C, Teti D, Bresciani F, Perillo B, Weisz A. Estrogens and progesterone promote persistent CCND1 gene activation during G1 by inducing transcriptional derepression via c-Jun/c-Fos/estrogen receptor (progesterone receptor) complex assembly to a distal regulatory element and recruitment of cyclin D1 to its own gene promoter. Mol Cell Biol. 2004;24:7260–7274. doi: 10.1128/MCB.24.16.7260-7274.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 97:1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weigel NL, Bai W, Zhang Y, Beck CA, Edwards DP, Poletti A. Phosphorylation and progesterone receptor function. J Steroid Biochem Mol Biol. 1995;53:509–514. doi: 10.1016/0960-0760(95)00098-k. [DOI] [PubMed] [Google Scholar]
- 24.Daniel AR, Gaviglio AL, Czaplicki LM, Hillard CJ, Housa D, Lange CA. The Progesterone Receptor Hinge Region Regulates the Kinetics of Transcriptional Responses Through Acetylation, Phosphorylation, and Nuclear Retention. Mol Endocrinol. doi: 10.1210/me.2010-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdel-Hafiz H, Takimoto GS, Tung L, Horwitz KB. The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J Biol Chem. 2002;277:33950–33956. doi: 10.1074/jbc.M204573200. [DOI] [PubMed] [Google Scholar]
- 26.Hagan CR, Regan TM, Dressing GE, Lange CA. ck2-Dependent Phosphorylation of Progesterone Receptors (PR) on Ser81 Regulates PR-B Isoform-Specific Target Gene Expression in Breast Cancer Cells. Mol Cell Biol. 2011;31:2439–2452. doi: 10.1128/MCB.01246-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniel AR, Knutson TP, Lange CA. Signaling inputs to progesterone receptor gene regulation and promoter selectivity. Mol Cell Endocrinol. 2009;308:47–52. doi: 10.1016/j.mce.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward RD, Weigel NL. Steroid receptor phosphorylation: Assigning function to site-specific phosphorylation. Biofactors. 2009;35:528–536. doi: 10.1002/biof.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu M, Olsen A, Faivre E, Horwitz KB, Lange CA. Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endocrinol. 2003;17:628–642. doi: 10.1210/me.2002-0378. [DOI] [PubMed] [Google Scholar]
- 30.Daniel AR, Qiu M, Faivre EJ, Ostrander JH, Skildum A, Lange CA. Linkage of progestin and epidermal growth factor signaling: phosphorylation of progesterone receptors mediates transcriptional hypersensitivity and increased ligand-independent breast cancer cell growth. Steroids. 2007;72:188–201. doi: 10.1016/j.steroids.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniel AR, Faivre EJ, Lange CA. Phosphorylation-dependent antagonism of sumoylation derepresses progesterone receptor action in breast cancer cells. Mol Endocrinol. 2007;21:2890–2906. doi: 10.1210/me.2007-0248. [DOI] [PubMed] [Google Scholar]
- 32.Qiu M, Lange CA. MAP kinases couple multiple functions of human progesterone receptors: degradation, transcriptional synergy, and nuclear association. J Steroid Biochem Mol Biol. 2003;85:147–157. doi: 10.1016/s0960-0760(03)00221-8. [DOI] [PubMed] [Google Scholar]
- 33.Salghetti SE, Caudy AA, Chenoweth JG, Tansey WP. Regulation of transcriptional activation domain function by ubiquitin. Science. 2001;293:1651–1653. doi: 10.1126/science.1062079. [DOI] [PubMed] [Google Scholar]
- 34.Daniel AR, Lange CA. Protein kinases mediate ligand-independent derepression of sumoylated progesterone receptors in breast cancer cells. Proc Natl Acad Sci U S A. 2009;106:14287–14292. doi: 10.1073/pnas.0905118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen T, Horwitz KB, Lange CA. Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol Cell Biol. 2001;21:6122–6131. doi: 10.1128/MCB.21.18.6122-6131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clemm DL, Sherman L, Boonyaratanakornkit V, Schrader WT, Weigel NL, Edwards DP. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol Endocrinol. 2000;14:52–65. doi: 10.1210/mend.14.1.0413. [DOI] [PubMed] [Google Scholar]
- 37.Moore NL, Narayanan R, Weigel NL. Cyclin dependent kinase 2 and the regulation of human progesterone receptor activity. Steroids. 2007;72:202–209. doi: 10.1016/j.steroids.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Beck CA, Poletti A, Clement JPt, Prendergast P, Yip TT, Hutchens TW, Edwards DP, Weigel NL. Phosphorylation of human progesterone receptor by cyclin-dependent kinase 2 on three sites that are authentic basal phosphorylation sites in vivo. Mol Endocrinol. 1997;11:823–832. doi: 10.1210/mend.11.6.0006. [DOI] [PubMed] [Google Scholar]
- 39.Knotts TA, Orkiszewski RS, Cook RG, Edwards DP, Weigel NL. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J Biol Chem. 2001;276:8475–8483. doi: 10.1074/jbc.M009805200. [DOI] [PubMed] [Google Scholar]
- 40.Takimoto GS, Hovland AR, Tasset DM, Melville MY, Tung L, Horwitz KB. Role of phosphorylation on DNA binding and transcriptional functions of human progesterone receptors. J Biol Chem. 1996;271:13308–13316. doi: 10.1074/jbc.271.23.13308. [DOI] [PubMed] [Google Scholar]
- 41.Pierson-Mullany LK, Lange CA. Phosphorylation of Progesterone Receptor Serine 400 Mediates Ligand-Independent Transcriptional Activity in Response to Activatoin of Cyclin-Dependent Protein Kinase2. Mol. Cell. Biol. 2004;24 doi: 10.1128/MCB.24.24.10542-10557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 43.Cariou S, Catzavelos C, Slingerland JM. Prognostic implications of expression of the cell cycle inhibitor protein p27Kip1. Breast Cancer Res Treat. 1998;52:29–41. doi: 10.1023/a:1006154900130. [DOI] [PubMed] [Google Scholar]
- 44.Musgrove EA, Davison EA, Ormandy CJ. Role of the CDK Inhibitor p27 (Kip1) in Mammary Development and Carcinogenesis: Insights from Knockout Mice. J Mammary Gland Biol Neoplasia. 2004;9:55–66. doi: 10.1023/B:JOMG.0000023588.55733.84. [DOI] [PubMed] [Google Scholar]
- 45.Alkarain A, Jordan R, Slingerland J. p27 deregulation in breast cancer: prognostic significance and implications for therapy. J Mammary Gland Biol Neoplasia. 2004;9:67–80. doi: 10.1023/B:JOMG.0000023589.00994.5e. [DOI] [PubMed] [Google Scholar]
- 46.Tawfic S, Yu S, Wang H, Faust R, Davis A, Ahmed K. Protein kinase CK2 signal in neoplasia. Histol Histopathol. 2001;16:573–582. doi: 10.14670/HH-16.573. [DOI] [PubMed] [Google Scholar]
- 47.Wilson GR, Cramer A, Welman A, Knox F, Swindell R, Kawakatsu H, Clarke RB, Dive C, Bundred NJ. Activated c-SRC in ductal carcinoma in situ correlates with high tumour grade, high proliferation and HER2 positivity. Br J Cancer. 2006;95:1410–1414. doi: 10.1038/sj.bjc.6603444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dressing GE, Lange CA. Integrated actions of progesterone receptor and cell cycle machinery regulate breast cancer cell proliferation. Steroids. 2009;74:573–576. doi: 10.1016/j.steroids.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daniel AR, Gaviglio AL, Czaplicki LM, Hillard CJ, Housa D, Lange CA. The progesterone receptor hinge region regulates the kinetics of transcriptional responses through acetylation, phosphorylation, and nuclear retention. Mol Endocrinol. 24:2126–38. doi: 10.1210/me.2010-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Narayanan R, Adigun AA, Edwards DP, Weigel NL. Cyclin-dependent kinase activity is required for progesterone receptor function: novel role for cyclin A/Cdk2 as a progesterone receptor coactivator. Mol Cell Biol. 2005;25:264–277. doi: 10.1128/MCB.25.1.264-277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weigel NL, Moore NL. Cyclins, cyclin dependent kinases, and regulation of steroid receptor action. Mol Cell Endocrinol. 2007;265–266:157–161. doi: 10.1016/j.mce.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Beck CA, Poletti A, Edwards DP, Weigel NL. Identification of phosphorylation sites unique to the B form of human progesterone receptor. In vitro phosphorylation by casein kinase II. J Biol Chem. 1994;269:31034–31040. [PubMed] [Google Scholar]
- 53.Trembley JH, Wang G, Unger G, Slaton J, Ahmed K. Protein kinase CK2 in health and disease: CK2: a key player in cancer biology. Cell Mol Life Sci. 2009;66:1858–1867. doi: 10.1007/s00018-009-9154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 55.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 56.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, Khandekar J, Petrovitch H, McTiernan A. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. Jama. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 57.Ballare C, Uhrig M, Bechtold T, Sancho E, Di Domenico M, Migliaccio A, Auricchio F, Beato M. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol Cell Biol. 2003;23:1994–2008. doi: 10.1128/MCB.23.6.1994-2008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vicent GP, Ballare C, Nacht AS, Clausell J, Subtil-Rodriguez A, Quiles I, Jordan A, Beato M. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol Cell. 2006;24:367–381. doi: 10.1016/j.molcel.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 59.Quiles I, Millan-Arino L, Subtil-Rodriguez A, Minana B, Spinedi N, Ballare C, Beato M, Jordan A. Mutational analysis of progesterone receptor functional domains in stable cell lines delineates sets of genes regulated by different mechanisms. Mol Endocrinol. 2009;23:809–826. doi: 10.1210/me.2008-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faivre E, Skildum A, Pierson-Mullany L, Lange CA. Integration of progesterone receptor mediated rapid signaling and nuclear actions in breast cancer cell models: role of mitogen-activated protein kinases and cell cycle regulators. Steroids. 2005;70:418–426. doi: 10.1016/j.steroids.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 61.Proietti CJ, Rosemblit C, Beguelin W, Rivas MA, Diaz Flaque MC, Charreau EH, Schillaci R, Elizalde PV. Activation of Stat3 by heregulin/ErbB-2 through the co-option of progesterone receptor signaling drives breast cancer growth. Mol Cell Biol. 2009;29:1249–1265. doi: 10.1128/MCB.00853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beguelin W, Diaz Flaque MC, Proietti CJ, Cayrol F, Rivas MA, Tkach M, Rosemblit C, Tocci JM, Charreau EH, Schillaci R, Elizalde PV. Progesterone receptor induces ErbB-2 nuclear translocation to promote breast cancer growth via a novel transcriptional effect: ErbB-2 function as a coactivator of Stat3. Mol Cell Biol. 30:5456–5472. doi: 10.1128/MCB.00012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Proietti CJ, Beguelin W, Flaque MC, Cayrol F, Rivas MA, Tkach M, Charreau EH, Schillaci R, Elizalde PV. Novel role of signal transducer and activator of transcription 3 as a progesterone receptor coactivator in breast cancer. Steroids. 76:381–392. doi: 10.1016/j.steroids.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 64.Richer JK, Lange CA, Manning NG, Owen G, Powell R, Horwitz KB. Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem. 1998;273:31317–31326. doi: 10.1074/jbc.273.47.31317. [DOI] [PubMed] [Google Scholar]
- 65.Cerliani JP, Guillardoy T, Giulianelli S, Vaque JP, Gutkind JS, Vanzulli SI, Martins R, Zeitlin E, Lamb CA, Lanari C. Interaction between FGFR-2, STAT5 and progesterone receptors in breast cancer. Cancer Res. doi: 10.1158/0008-5472.CAN-10-3074. [DOI] [PubMed] [Google Scholar]
- 66.Santos SJ, Haslam SZ, Conrad SE. Signal transducer and activator of transcription 5a mediates mammary ductal branching and proliferation in the nulliparous mouse. Endocrinology. 151:2876–2885. doi: 10.1210/en.2009-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santos SJ, Haslam SZ, Conrad SE. Estrogen and progesterone are critical regulators of Stat5a expression in the mouse mammary gland. Endocrinology. 2008;149:329–338. doi: 10.1210/en.2007-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacobsen BM, Richer JK, Sartorius CA, Horwitz KB. Expression profiling of human breast cancers and gene regulation by progesterone receptors. J Mammary Gland Biol Neoplasia. 2003;8:257–268. doi: 10.1023/b:jomg.0000010028.48159.84. [DOI] [PubMed] [Google Scholar]
- 69.Chlebowski RT, Kuller LH, Prentice RL, Stefanick ML, Manson JE, Gass M, Aragaki AK, Ockene JK, Lane DS, Sarto GE, Rajkovic A, Schenken R, Hendrix SL, Ravdin PM, Rohan TE, Yasmeen S, Anderson G. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360:573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM. C/EBPbeta (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol Endocrinol. 2000;14:359–368. doi: 10.1210/mend.14.3.0434. [DOI] [PubMed] [Google Scholar]
- 71.Osborne CK, Schiff R, Arpino G, Lee AS, Hilsenbeck VG. Endocrine responsiveness: understanding how progesterone receptor can be used to select endocrine therapy. Breast. 2005;14:458–465. doi: 10.1016/j.breast.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 72.Gregory CW, Fei X, Ponguta LA, He B, Bill HM, French FS, Wilson EM. Epidermal growth factor increases coactivation of the androgen receptor in recurrent prostate cancer. J Biol Chem. 2004;279:7119–7130. doi: 10.1074/jbc.M307649200. [DOI] [PubMed] [Google Scholar]
- 73.Steeg PS, Zhou Q. Cyclins and breast cancer. Breast Cancer Res Treat. 1998;52:17–28. doi: 10.1023/a:1006102916060. [DOI] [PubMed] [Google Scholar]
- 74.Jeong Y, Xie Y, Xiao G, Behrens C, Girard L, Wistuba II, Minna JD, Mangelsdorf DJ. Nuclear receptor expression defines a set of prognostic biomarkers for lung cancer. PLoS Med. 7 doi: 10.1371/journal.pmed.1000378. e1000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 76.Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, Visvader JE, Lindeman GJ. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst. 2006;98:1011–1014. doi: 10.1093/jnci/djj267. [DOI] [PubMed] [Google Scholar]