Abstract
Myelination is the culmination of a complex process in which oligodendrocyte (OL) progenitors transition through defined stages in a well-coordinated differentiation program. The signaling mechanisms that regulate this progression are poorly understood. Here we investigate the role of extracellular signal-regulated-kinase-1,-2 (Erk1/2) and the mammalian target of rapamycin (mTOR), downstream effectors of the Ras/Raf/Mek/Erk and PI3K/Akt/mTOR pathways, at specific stages of OL development in vitro. Using a panel of developmental stage-specific antigenic markers and pharmacological inhibitors, we provide evidence that Erk1/2 signaling regulates transition of early progenitors to the late progenitor stage and, as a consequence, to the immature OL stage, but not the transition of immature OL to the mature OL stage. In contrast, mTOR signaling is not required for early progenitor transition to late progenitor stage. Surprisingly, it is also not required for the transition of late progenitors to terminally differentiated immature OLs, as has been reported previously, but is required for the next sequential transition of immature OLs to the mature OL stage. Furthermore, mTOR signaling regulates OL cytoskeletal organization and major myelin protein expression. These in vitro findings correlate with our in vivo data showing that inhibition of mTOR by rapamycin injection attenuated the onset of myelination in the early postnatal brain. Thus, these studies demonstrate that Erk1/2 and mTOR signaling sequentially regulates distinct stages of OL progenitor differentiation and suggest that cells in the OL-lineage require distinct signaling mechanisms to transition through specific stages of their development.
Keywords: oligodendrocyte, myelin, Erk MAPK, mTOR
INTRODUCTION
Failure of efficient remyelination in Multiple Sclerosis and other demyelinating disorders is largely due to the inability of OL progenitors to efficiently differentiate into myelinating OLs (Franklin, 2002; Fancy et al., 2011). Therefore, understanding the diverse signaling mechanisms that control the differentiation of OLs through multiple stages of the OL-lineage is essential for developing strategies to promote myelin repair.
OL progenitors progress through distinct morphological and antigenic stages of maturation prior to myelin formation. These developmental stages have been characterized extensively in vitro and are highly correlated in vivo (Pfeiffer et al., 1993; Warrington et al., 1993; Miller, 2002; Emery, 2010). Isolated bipolar early progenitors differentiate into multipolar late progenitors, which enter terminal differentiation and become postmitotic immature OLs. These cells develop complex, branched and intertwined processes before extending networks of membranes, as they become mature OLs. This program of differentiation requires multiple integrated extrinsic and intrinsic molecular signals. the intracellular signal transduction pathways that control the transition through distinct stages of the OL-lineage are poorly understood.
Several studies have implicated the Ras/Raf/Mek/Erk and P13K/Akt/mTOR pathways in the proliferation, migration or survival of OL progenitor cells, largely as a result of their activation by various growth factors (e.g Bhat, 1995; Baron et al., 2000; Flores et al., 2000; Ebner et al., 2000; Yim et al., 2001; Bansal et al., 2003; Cui and Almazan, 2007; Frederick et al., 2007; Frost et al., 2009; Van't Veer et al., 2009). However, there is limited and often contradictory information available about the role of these signaling molecules in the differentiation of OL progenitors to mature myelinating OLs. For example, using pharmacological inhibitors of Mek (PD098059, U0126) in cultures of OL progenitors, Baron et al. (2000) and Fyffe-Maricich et al. (2011) observed inhibition of differentiation, while Younes-Rapozo et al. (2009) found inhibition of process extension by OLs, but not acquisition of myelin proteins. Further, no inhibition or only a transient and partial inhibition of OL progenitor differentiation was observed in mixed primary cortical cultures of Erk1-null or Erk2 conditional single knockout mouse brain, respectively (Fyffe-Maricich et al., 2011). Using rapamycin, an mTOR inhibitor, Tyler et al. (2009) reported an inhibition of terminal differentiation of early progenitors to GalC+ immature OLs, while Baron et al. (2000), using wortmannin, a PI3K inhibitor, did not observe any inhibition at the same stage of the OL-lineage. Part of the inconsistency between these results may be attributed to differences in culture conditions including inhibitors, source of OL progenitors, culture media and timing of analysis, etc.
To unify potential discrepancies associated with previous studies and provide a more integrated understanding of how these two signaling pathways control the stage specific physiological demands of OL-lineage progression, we investigated the effect of inhibiting these pathways in parallel. We found that inhibition of Erk1/2 with U0126 inhibited the progression of isolated early progenitor to the late progenitor stage with a concomitant delay of maturation to the immature OL stage, but had no effect on the differentiation of immature OLs to the mature OL stage. In contrast, inhibition of mTOR with rapamycin did not block the transition of early or late progenitors or of late progenitors to the immature OL stage, but attenuated their progression to the mature OL stage by inhibiting the expression levels of major myelin proteins and the normal organization of the OL cytoskeleton. The effect of mTOR inhibition on MBP expression was also observed in a more complex environment of a mixed primary culture of mouse brain and correlated with the in vivo inhibition of the onset of myelination in the early postnatal brain by rapamycin injection. Together, our data demonstrates that distinct signaling pathways control distinct stages of OL development in a sequential and non-overlapping manner. Importantly, in contrast to previous in vitro studies, neither of the pathways was found to directly play an essential role in the commitment of late progenitors to enter terminal differentiation.
MATERIALS AND METHODS
Cell Culture
Enriched cultures of OL progenitors were prepared as described previously (Bansal et al., 1996). Mixed primary cultures from neonatal (P1-2) rat telencephalon were shaken overnight in an orbital shaker. Dislodged OL progenitors (and some prepogenitors) were further purified by differential adhesion and complement lysis with anti- GalC. Early progenitors were seeded on poly-L-ornithine (25 ug/ mL, Sigma) coated 4-well plates (30,000 cells/well for immunofluorescence) or 6-well plates (250,000 cells/well for immunoblotting). Following 3 hrs of cell attachment in 5% FCS/DMEM, cells were grown in serum-free, defined medium, N2 [(DMEM supplemented with human transferrin (50 μg/ml), bovine pancreatic insulin (5 μg/ml), 3,3,5-triiodo-L-thyronine (10 ng/ml), sodium selenium (30 nM), D-biotin (10 ng/ml), hydrocortisone (10 nM), sodium pyruvate (0.11 mg/ml), penicillin-streptomycin (10 IU/ml and 100 μg/ml, respectively)], and 0.1% BSA (all from Sigma) in the presence of PDGF for 1-2 days (Preprotech; 10 ng/ml). In some experiments plated cells were not exposed to PDGF at all. The onset of differentiation (day 0) was triggered by PDGF removal with a rinse in N2 and cells were maintained in N2. U0126 and rapamycin are highly selective inhibitors of Mek1/2 (upstream of Erk1/2) and mTOR (Davies et al., 2000) used to suppress the MAPK and Akt/mTOR pathways respectively (Fig.1B). Stocks of U0126 (dissolved in DMSO; Calbiochem, LaJolla, CA), rapamycin (dissolved in EtOH; Calbiochem) or dimethysulfoxide (DMSO), as a control, were pre-mixed in N2 and added to the cultures at a final concentration of 10uM and 25uM for U0126 and 22nM for rapamycin. The concentrations of U0126 were based on immunoblotting for phospho-Erk1/2 on isolated OL progenitor cultures (data not shown). Rapamycin was used at similar doses described in previous OL studies for consistency (Baron et al., 2000; Tyler et al., 2009).
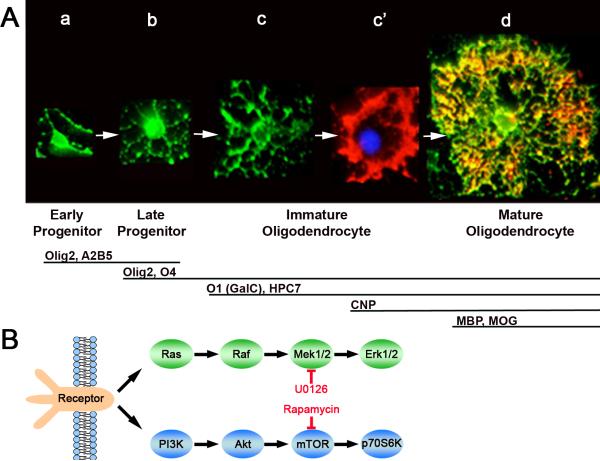
Fig. 1.
A. Immunofluorescence microscopy of enriched OL lineage cells in culture at different stages of the OL developmental pathway. Stage-specific markers and morphology of cells at each stage are shown. a. Early progenitor (Olig2+,A2B5+/O4-); b. Late progenitor (Olig2+/O4+/GalC-); c, c’ Two stages of morphological maturation of immature OL (O1(GalC)+/HPC7+/CNP+/MBP-); d. Mature OL (O1+/MBP+/MOG+). Note that early progenitors with 1-3 processes (a) transition to late progenitor with multiple thin processes (b). These late progenitors enter terminal differentiation into immature OLs by elaborating additional more branched processes (c), which subsequently intertwine into a transient ring-like structure (c’) prior to transforming into an extensive network of membranous processes of mature OLs (d). B. Simplified diagram of Ras/Raf/Mek/Erk and PI3K/Akt/mTOR signaling pathways showing targets of pharmacological inhibitors, U0126 and rapamycin.
Dissociated primary cultures of neonatal mouse telencephalon were prepared as described previously (Bansal et al., 1996) and cells plated at a density of 150,000 cells/cm2 were grown in N2 defined media following one day in 5% FCS. Rapamycin (22nM final concentration) was added with each medium change during the period indicated in the experiment.
Immunofluorescence Microscopy and quantification of cell numbers
Immunolabelling of cells was performed as described previously (Bansal et al., 1996). Primary antibodies used were anti-Olig2 (1:50, IBL), A2B5 (1:25), O4 (1:25, Bansal et al., 1992), O1 (1:25), anti-HPC7 (1:25, Baas and Barnstable, 1998), anti-CNP and anti-MBP (1:100; Sternberger Monoclonals Incorp., Lutherville, MD),. Secondary antibodies include, FITC-conjugated goat anti-mouse IgM, μ-chain specific, for O4, A2B5 and O1; Cy3-conjugated anti-mouse IgG, gama-chain specific for HCP7, CNP, MBP (Jackson Labs, Bar Harbor, Maine) or anti-rabbit IgG for Olig2, and a nuclear label, Hoechst dye (Sigma).
Three to four independent cultures, each with duplicate wells per condition, were analyzed. An average of 125 cells in ~10 fields per well were counted.
Immunoblotting
Cells were lysed in RIPA buffer (10 mM Tris-HCl, 150 mM NaCl, 0.1% SDS, 1% deoxycholate, 1% NP40 and 1% TX-100, pH 7.4) containing protease and phosphatase inhibitors (1 mM PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM orthovanadate, fluoride 50 mM, pyrophosphate 10 mM) by sonication (30 sec; 4°C). After a 10 minute incubation on ice homogenates were centrifuged (15,000 × g, 15 min, 4°C). Proteins were electrophoresed on 12% SDS polyacrylamide gels and transferred onto PVDF membranes. Membranes were blocked (Tris buffered saline, 0.2% Tween 20, and 5% non fat powdered milk or 5% BSA) prior to incubation in primary antibodies: polyclonal CNP and polyclonal MBP (each at 1:10,000, R. Bansal, Farmington, CT), monoclonal MOG (myelin oligodendrocyte glycoprotein; 1:3,000; C. Linington, Aberdeen, UK) and GAPDH (1:15,000; Biodesign International, Saco, Maine). Following several washes, the membranes were incubated for 30 min in appropriate secondary antibodies conjugated to horseradish peroxidase (1:10,000; Santa Cruz) and developed using the ECL Plus chemiluminescent kit (Amersham, Arlington Heights, IL).
Rapamycin injections and Immunohistochemistry
Starting at postnatal day (P) 6, mice were injected daily with either vehicle or rapamycin intraperitoneally at 10 mg/kg body weight (rapamycin was diluted in the vehicle solution from a stock of 20 mg/ml in 100% ethanol immediately before the injection to a final concentration of 0.8 mg/ml PBS, 5% polyethylene glycol-1000, 5% Tween 80, and 4% ethanol). On P11, brains were removed, drop fixed in 4% paraformaldehyde (overnight at 4°C), cryoprotected in 20% sucrose (overnight at 4°C), embedded in mounting media and sectioned (15 um) coronally. Sections were immersed in 100% ethanol, washed in PBS, blocked for 1hr in 10% normal goat serum, 5% bovine serum albumin, and 0.1% gelatin in PBS and incubated overnight at 4°C in polyclonal anti-rabbit MBP (1:3,000; R. Bansal, Farmington, CT). After three washes in PBS, sections were incubated for 1hr in goat anti-rabbit IgG conjugated to Alexa 488 (1:500; Molecular Probes, Eugene, OR) and Hoechst Blue 33342 (1:1,000), washed in PBS and mounted.
RESULTS
To investigate the role of Erk1/2 and mTOR in the progression of OL-lineage cells through specific stages of differentiation, we evaluated the effects of their pharmacological inhibition by U0126 (Mek inhibitor) or rapamycin (mTOR inhibitor) (Fig. 1B) on the expression of stage-specific antigenic markers and OL morphology. Normal morphology of isolated OL-lineage cells from rat forebrains and key antigenic markers used in this study are shown (Fig. 1A). Early progenitors show a simple morphology and express the transcription factor Olig2 and the lipid antigen A2B5 (Fig. 1Aa). Early progenitors transition into late progenitors that extend additional simple processes and are recognized by the O4 antibody (Fig. 1Ab, Bansal et al., 1992). Late progenitors become terminally differentiated immature OLs expressing the lipid galactocerebroside (GalC; recognized by the O1 antibody) and develop more complex branched processes (Fig. 1Ac). Immature OLs also express an unknown antigen HPC7 (Baas and Barnstable, 1998) that co-labels with O1 (data not shown) and can be used interchangeably to mark this stage. Immature OLs soon form a thick ring-like structure and begin to express the myelin protein CNP. We have classified this stage as a more advanced stage of immature OLs (Fig. 1Ac’). Finally, the immature OLs differentiate into mature OLs as they start expressing MBP and later MOG, and the ring-like structure is transformed into an extensive network of thicker processes and membranes (Fig. 1Ad). This multiprocessed morphology is characteristic of neuron-free isolated rat OLs in culture, to be distinguished from the morphology of myelinating OLs in vivo or in co-cultures with neurons, where distinct OL processes extend to and wrap the axon. Further, although “mature” OLs in isolation are able to express MBP and PLP and make “myelin-like” membranes, they are not able to make actual myelin or complete their final morphological differentiation as in vivo.
Early progenitors purified from mixed primary cultures (McCarthy and DeVellis, 1980) were plated into 4-well or 6-well dishes in defined N2 medium. Following a brief (1-2 day) expansion, PDGF was removed from the media (designated as Day 0) and cells were grown in N2 with or without U0126 or rapamycin, as indicated in each experiment. Note that no subculture of cells is involved in this culture paradigm. Purity of cultures was 99% as determined by %Olig2+ cells/total Hoescht+ cells and was ~95% as determined by %A2B5+O4 positive cells/total Hoescht+ cells after two days in N2.
Inhibition of Erk1/2 attenuates the progression of early progenitors to late progenitors and concomitantly to immature OLs
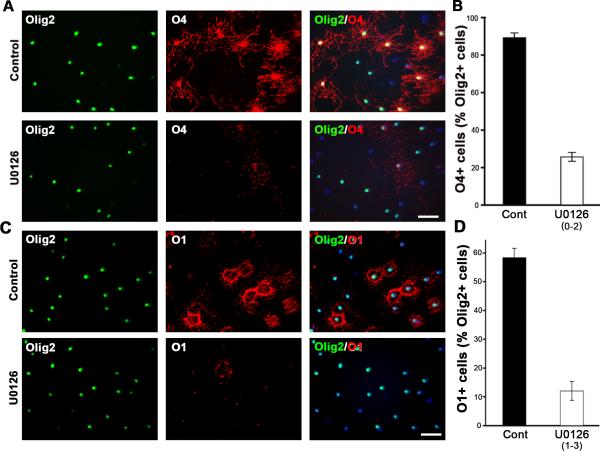
In order to examine the requirement of Erk1/2 signaling on the initial stages of the OL developmental pathway, we treated early progenitor cultures with DMSO (control) or with U0126 from 0 to 2 days and immunolabeled cells for O4 and Olig2 (Fig. 2A,B). We found that while >80% of early progenitors (Olig2+/O4-) had progressed to the late progenitor (Olig2+/O4+) stage by day 2, only 20-40% became weakly O4+ late progenitors in U0126-treated cultures (Fig. 2A,B). The effect was more pronounced at 25uM than at 10uM dose. We also investigated the consequence of Erk1/2 inhibition on further progression from the late progenitor stage to the immature OL stage. Cells were treated with U0126 on day 1 (when majority of cells are early progenitors) and analyzed on day 3 for the expression of O1 (Fig. 2C,D). Significantly reduced numbers of O1+ cells were observed in U0126-treated cultures.
Fig. 2. Inhibition of Erk1/2 attenuates the progression of early progenitors to late progenitors and concomitantly to immature OLs.
Early progenitors were grown in the absence (Cont) or presence of U0126 from 0 to 2 days (A,B) or 1 to 3 days (C,D) and analyzed by double immunolabeling for O4 and Olig2 (A,B) or Olig2 and O1 (C,D). Quantification of the percentage of late progenitors (O4+/Olig2+) and immature OLs (O1+/Olig2+) shows that inhibition of Erk1/2 signaling significantly attenuates the progression of early progenitors to late progenitors which then results in subsequent reduction in immature OLs. Error bars, SEM, N=3-4 independent cultures in duplicates. Scale bar, 50 um.
We therefore conclude that the transition from the early to the late progenitor stage requires the participation of Erk1/2 activity and that this indirectly affects the appearance of immature OLs.
Inhibition of Erk1/2 does not affect the transition of immature OLs to mature OLs
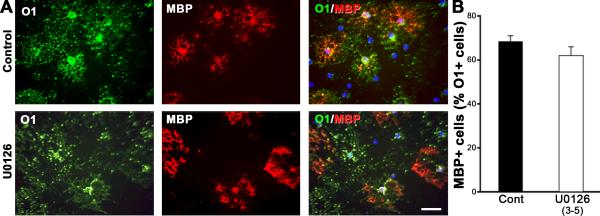
We next asked whether Erk1/2 signaling is needed for the progression of immature OL (O1+/MBP-) to the mature OL (O1+/ MBP+) stage. We treated cultures with U0126 at day 3 of differentiation (when over 50% of cells were at the immature OL stage) and analyzed cells on day 5 by double labeling with O1 and MBP (Fig. 3). We found that in both DMSO-treated control cultures and U0126-treated cultures, the majority of O1+ immature OLs acquired MBP and elaborated a network of processes characteristic of mature OLs. Therefore, in contrast to its role in early developmental transitions (above), Erk1/2 signaling is not essential for the progression of immature OL to the mature OL stage.
Fig.3. Inhibition of Erk1/2 does not affect the transition of immature OLs to mature OLs.
A. Early progenitors were first allowed to differentiate for 3 days in the culture until the majority had differentiated to the immature OL stage. They were then treated with U0126 from day 3 to 5 and analyzed by double immunolabeling for MBP and O1. B. Quantification of the percentage of MBP+ mature OLs in DMSO-treated controls (Cont) and in U0126-treated cultures shows that the progression of immature OLs (O1+/MBP-) to mature OLs (O1+/MBP+) remained unaffected by the absence of Erk1/2 signaling. Error bars, SEM, N=3 independent cultures in duplicates. Scale bar, 50 um.
Inhibition of mTOR does not block terminal differentiation of late progenitors to immature OLs
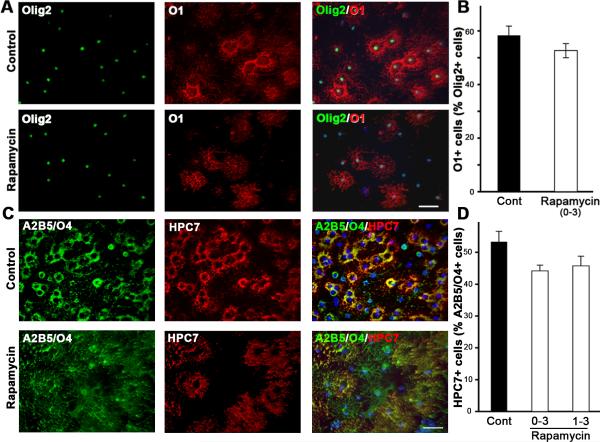
To examine the stage-specific requirements for the PI3K/Akt/mTOR pathway during OL development, we first examined the effect of rapamycin on the transition of early progenitors to late progenitors. Consistent with previous reports (Baron et al., 2000; Tyler et al., 2009), we found that rapamycin had no effect on early to late progenitor transition (data not shown). Further, rapamycin-treatment did not have any adverse effects on cell viability over long periods of time. To determine the role of mTOR signaling on the transition of late progenitor to immature OL, we treated cells with rapamycin from day 0 or 1 to day 3 and analyzed by immunolabeling the expression of immature OL markers, O1 (GalC) and HPC7 (Fig. 4). We found no difference in the percent of immature OLs (O1+, HPC7+) that appeared in control and rapamycin-treated cultures. Similarly, no difference was observed even when rapamycin treatment was initiated immediately after plating shaken OL progenitors, without pre-expansion in PDGF (data not shown). Although the numbers of immature OLs was similar, the intensity of O1 and HPC7 immunostaining was somewhat weaker in rapamycin-treated cells than controls, and these OLs did not form a dense ring-like structure like untreated immature OLs (addressed later).
Fig. 4. Inhibition of mTOR does not block terminal differentiation of late progenitors to immature OLs.
Early progenitors were grown in the absence (Cont) or presence of rapamycin from 0 to 3 days or 1 to 3 days and analyzed by double immunolabeling for the expression of Olig2 and O1 (A,B) or A2B5/O4 and HPC7 (C,D). Quantification of the numbers of O1+ or HPC7+ immature OLs, expressed as percentage of total OL-lineage cells (Olig2+ or A2B5+/O4+), shows no difference in rapamycin-treated cultures compared to untreated controls, demonstrating that late progenitors can differentiate normally to immature OLs in the absence of mTOR signaling. Error bars, SEM, N=4 independent cultures in duplicates. Scale bars, 50 um.
As determined by antigenic marker expression, we conclude that the inhibition of mTOR by rapamycin in our culture conditions does not block terminal differentiation of late progenitors to immature OLs as previously reported (Tyler et al., 2009).
Inhibition of mTOR attenuates the efficient transition of immature OLs to mature OLs
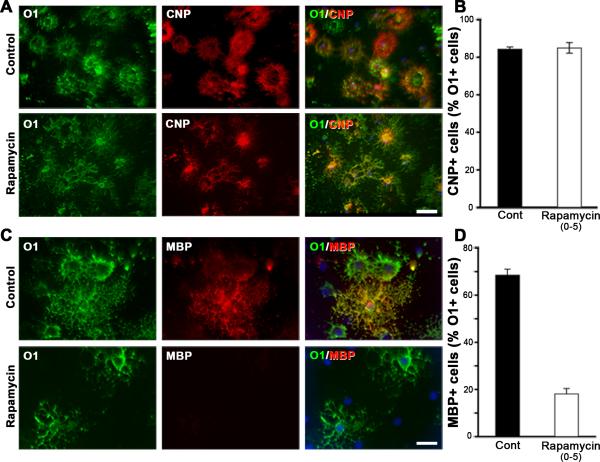
We first investigated the effect of mTOR inhibition by rapamycin on the developmental progression from the O1+ immature OL stage to a slightly later CNP+ immature OL stage as described in Fig. 1A c,c’. We found that CNP was expressed by >80% of O1 positive OLs in both the control and in cultures treated with rapamycin from 0 to 5 days (Fig. 5 A,B). This further demonstrates that mTOR inhibition does not affect differentiation of late progenitors to immature OLs, as they were able to express GalC, and CNP.
Fig. 5. Inhibition of mTOR attenuates efficient transition of immature OLs to mature OLs.
Early progenitors were grown in the absence (Cont) or presence of rapamycin from 0 to 5 days and analyzed by double immunolabeling for O1, and CNP (A,B) or O1 and MBP (C,D). Quantification of the percentage of O1+ cells that co-express CNP (B) or MBP (D) shows that while there was no effect of mTOR inhibition on the transition of O1+ immature OLs to CNP+ immature OLs, their progression to MBP+ mature OLs was significantly inhibited. Error bars, SEM, N=4 independent cultures in duplicates. Scale bars, 50 um.
We next asked if transition of immature OLs (O1+/MBP-) to mature OLs (O1+/MBP+) was affected by mTOR inhibition (Fig. 5C,D). We found that while in untreated controls approximately 70% of immature OLs expressed MBP by day 5, in the rapamycin treated cultures only about 20% progressed to the MBP+ mature OL stage. Further, the intensity of MBP immuno-reactivity in these OLs was reduced relative to untreated mature OLs.
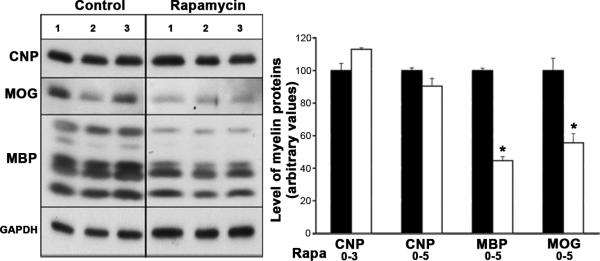
To complement immunofluorescence microscopy data, we performed immunoblot analysis on parallel cultures to evaluate the effect of rapamycin treatment on myelin protein expression (Fig. 6). Cells grown in the absence or presence of rapamycin for 0-3 and 0-5 days were analyzed for the levels of CNP, MBP and MOG. Consistent with the immunofluorescence results, the expression of CNP was unchanged in cells treated with rapamycin for 3 or 5 days, but the levels of MBP and MOG were significantly reduced. We conclude that mTOR signaling is required for the expression of normal levels of the major myelin proteins by mature OLs.
Fig. 6. Inhibition of mTOR does not affect the level of immature OL protein CNP but downregulates mature OL proteins, MBP and MOG.
Total protein lysates from cells grown in the absence (solid bars) or presence of rapamycin (open bars) from 0 to 5 days were analyzed by immunoblot analysis for the levels of CNP, MBP and MOG proteins (triplicates are shown). Quantification of the band intensity by NIH image shows that while CNP protein levels (examined also in cells treated from 0 to 3 days) remained unaffected, MBP and MOG levels were significantly reduced in the absence of mTOR signaling. GAPDH was used as a loading control. Error bars, SEM, N=3.
Inhibition of mTOR adversely affects morphological maturation of immature OLs
As described earlier, cells undergo continuous cytoskeletal alterations prior to acquiring the typical mature OL morphology (Fig. 1A). Notably, the branched processes of immature OL appear to intertwine to form a ring-like structure, which we have classified as a more advanced stage of immature OLs (Fig. 1A c,c’). We found that while in controls this ring-like morphology was evident under both phase contrast microscopy (Fig. 7a) and in O1 immunolabeled cells (Fig 7c), it was rarely seen in rapamycin-treated cultures. Instead, the immature OLs displayed a simpler multiprocess, branched morphology (Fig. 7b,d), characteristic of the early stage of immature OL (Fig. 1A c). Note that these rapamycin-treated immature OLs are antigenically and morphologically distinct from both late progenitors (that do not express O1 and have few unbranched small processes) and mature OLs (that express MBP and have a much more complex network of processes and membranes). We, therefore, conclude that the cytoskeletal organization associated with OL maturation requires the participation of mTOR signaling.
Fig. 7. Inhibition of mTOR adversely affects the morphological maturation of immature OLs.
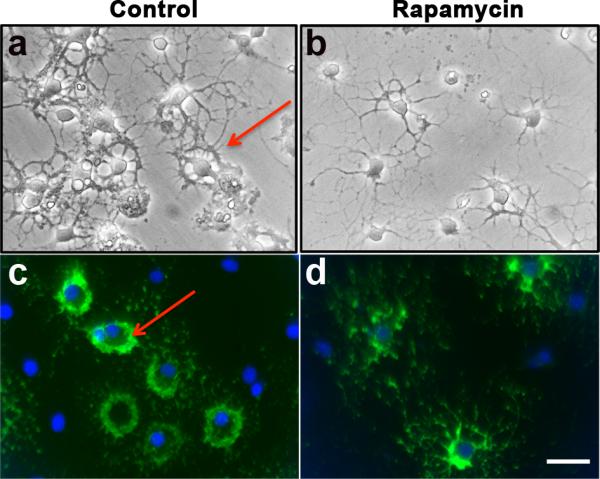
Cells were grown in the absence (Control) or presence of rapamycin from 0 to 3 days and analyzed by phase contrast microscopy (a,b) or by immunolabeling with O1 (c,d). Note that rapamycin-treated immature OLs failed to acquire the characteristic ring-like morphology with thick intertwined processes like the control immature OLs (arrows). Scale bar, 25 um.
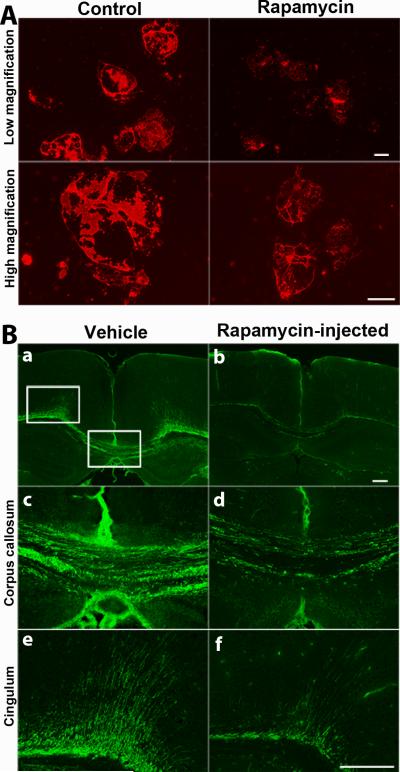
Rapamycin downregulates MBP expression in OLs in dissociated cultures of mouse brain and inhibits the onset of myelination when injected in vivo
The studies described so far were conducted on isolated OL progenitor cultures from neonatal rat brains. We next investigated the effects of mTOR inhibition by rapamycin on OLs in the context of a more complex environment of dissociated mouse brain primary cultures where OL development occurs in the presence of signals from other glial cells, mainly astrocytes. With the culture paradigm used, these cultures are virtually devoid of neurons. Cultures were treated with rapamycin from 8-17 days and immunolabeled with anti-MBP (Fig. 8A) and with O4 and HPC7 (data not shown). Consistent with our results in isolated rat OL cultures, we observed a downregulation of MBP immunoreactivity in rapamycin-treated cultures relative to untreated controls. As for isolated cultures, no inhibition of OL progenitor terminal differentiation to GalC+ stage was observed in dissociated primary cultures (data not shown).
Fig. 8. Rapamycin downregulates MBP expression in mature OLs in dissociated cultures of mouse brain and inhibits the onset of myelination when injected in vivo.
A. Dissociated primary cultures of neonatal mouse brains were treated with rapamycin from 8-17 days and immunostained with anti-MBP on day 17. The low magnification images of control and rapamycin treated cultures, taken at the same exposure shows a lower level of MBP immunoreactivity in rapamycin-treated cultures compared to controls. High magnification images of rapamycin-treated cells are shown at twice the exposure as control images to display morphology of OLs more effectively. Representative images from individual cultures of 3 pups are shown. Scale bars, 50 um. B. Mice were injected intraperitonially daily from P6 to P11 with either vehicle or rapamycin. Coronal sections from mouse brains were immunostained with anti-MBP on day 11. Representative images out of 3 rapamycin-injected and 3 vehicle-injected mouse brains shows reduced level of myelination in the rapamycin-injected (b,d,f) relative to vehicle-injected mice (a,c,e). The boxed areas of corpus callosum and cingulum are shown at higher magnification (c-f). Scale bars, 200 um.
Finally, to further explore the effect of rapamycin inhibition of mTOR on the onset of myelination in vivo, we injected mice intraperitoneally daily from P6 to P11 with either vehicle or rapamycin and immunostained brain sections with anti-MBP (Fig. 8B) on P11. Compared to vehicle-injected mice, myelination in the corpus callosum and cingulum of rapamycin-injected mice was reduced. Although this effect of rapamycin on MBP expression and myelination suggests a direct effect on OLs, since it was also seen in isolated OL cultures, the possibility exists that in vivo these effects could be secondary as a result of perturbed mTOR signaling in other cells types such as neurons, astrocytes or microglia.
We conclude that inhibition of mTOR attenuates the onset of myelination in the postnatal mouse brain, consistent with its adverse effects on the OL cytoskeleton and the expression of MBP that we observed in vitro.
DISCUSSION
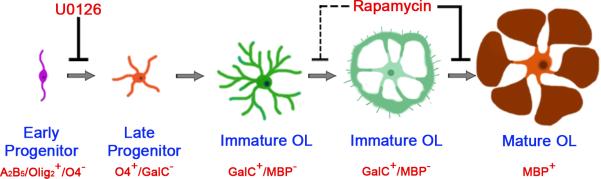
Using a panel of stage-specific antigenic markers and pharmacological inhibitors, this study demonstrates that distinct stages in the OL developmental pathway are differentially regulated by Erk1/2 and mTOR signaling in vitro (Fig. 9). Specifically, the progression of the early to late progenitor stage requires Erk1/2, but not mTOR signaling; in contrast, the immature OL to mature OL transition requires mTOR, but not Erk1/2. Interestingly, neither of these signaling pathways was found to play a direct role in the terminal differentiation of late progenitors to postmitotic immature OLs. Moreover, activation of mTOR signaling was found to be critical for OL cytoskeletal organization and the expression of normal levels of major myelin protein in vitro, and for the onset of myelination in the postnatal mouse brain.
Fig 9. Distinct stages of OL differentiation are sequentially regulated by Erk1/2 and mTOR signaling.
Inhibition of Erk1/2 by U0126 interferes with the early progenitor to late progenitor progression (solid line) and subsequently affects the appearance of immature OLs. In contrast, inhibition of mTOR by rapamycin attenuates morphological maturation of immature OLs (dashed line) and their transition to the mature OL stage (solid line). Neither Erk1/2 nor mTOR directly regulates the terminal differentiation of late progenitors to postmitotic immature OLs.
Early progenitors (O4-) and late progenitor (O4+) stages are antigenically, morphologically and functionally distinct maturation stages of OL progenitor that are often grouped as “OPCs”. Transition of early to late progenitors is a critical step in the OL-lineage when cells lose their migratory capacity and become committed to the OL-lineage (Pfeiffer et al., 1993). Our data demonstrates that Erk1/2 regulates this important transition and suggests that, as a consequence, it regulates the appearance of immature OLs. Other studies have also reported a reduction in the appearance of GalC+ immature OLs in their isolated OL progenitor culture systems using pharamacological inhibitors (Baron et al., 2000; Fyffe-Maricich et al., 2011), however the inhibition of O4 expression observed in our study was not reported previously. A possible explanation could be that in one case PD098059 instead of U0126 was used as an Erk inhibitor, and it was added to the cultures when ~50% of the OL-lineage cells were already O4+ (Baron et al., 2000), while in another, only a lower dose of U0126 was used and cells were first analyzed 3 days after treatment (Fyffe-Maricich et al., 2011). Our data showing a requirement of Erk1/2 at early stages of the OL-lineage (perhaps even at the pre-progenitor stage) correlates with in vivo findings showing that the genetic disruption of the Erk1/2 in the embryonic brain, using nestin-cre driver, resulted in attenuated OL progenitor generation in the germinal zone and cortex (Imamura et al., 2010). Further, our interpretation that Erk1/2 does not play a major role in terminal differentiation of OLs correlates with recent studies on cortical mixed cultures from Erk1-/- or Erk2-single conditional knockout mice (Fyffe-Maricich et al., 2011) and Erk1/2 double knockout mice (unpublished observations), where no reduction or only a partial (25%) transient reduction in the numbers of GalC+ cells was observed in the absence of Erk signaling. Erk1/2 signaling is also dispensable for further lineage progression since immature OLs were able to acquired MBP expression normally in isolated OL-cultures in the presence of U1026 and in the mixed primary cortical cultures of OL-lineage-specific Erk1/2 conditional knockout-mice (unpublished observations). These results together suggest that while Erk1/2 are important regulators of early progenitor and pre-progenitor stages and mediate regionally specific stimulatory effects of BDNF on myelin protein expression in the basal forebrain OLs (Du et al., 2003; Du et al., 2006), terminal differentiation and further maturation of cortical OLs are regulated by signals other than Erk1/2. However, it should be noted that these in vitro studies do not address if Erk1/2 plays a role in myelin sheath formation and wrapping per se.
Transition of late progenitors (O4+/GalC-) to immature OLs (O4+/GalC+) marks a key irreversible event in OL-lineage progression when progenitors become postmitotic and commit to terminal differentiation (Pfeiffer et al., 1993; Miller, 2002). Our present results indicate that rapamycin inhibition of mTOR did not affect this critical transition during differentiation, as assessed by the expression of stage-specific antigens. These results are at odds with a recent in vitro study that suggests that mTOR is a key regulator that drives the terminal differentiation of late progenitors to GalC+ immature OLs (Tyler et al., 2009). The main difference between the two studies is the way OL progenitors were manipulated prior to their exposure to rapamycin. Both studies employed the McCarthy and DeVellis (1980) differential shake-off procedure to obtain early progenitors from the rat brain. While we immediately plated them in defined media following the shake (or after a brief expansion of 1-2 days in PDGF, both with similar results), Tyler and co-workers first expanded the shaken-off OL progenitors in T75 flasks for 4-10 days in N2 media containing 34% B104 media and 5 ng/ml FGF-2, then passaged them before plating for rapamycin-treatment. Prolonged exposure to growth factors and undefined components of B104 and passaging of cells could alter the biology of OL progenitors, as has been observed earlier (Tang et al., 2000; Kondo and Raff, 2000), perhaps reverting them to a more immature progenitor state or slowing down their intrinsic maturation program and response to rapamycin. Thus, it is difficult at this time to definitively conclude whether mTOR regulates OL differentiation at the late progenitor to immature OL transition, as reported earlier (Tyler et al., 2009), or at the immature OL to mature OL stage, as suggested by our present study. If the former were the case (Tyler et al., 2009), one would expect to see decreased numbers of immature OLs (DM20+ premyelinating OLs) upon inhibition of mTOR by rapamycin injection in vivo. However, no such decrease in premyelinating OL number was seen in the P7 brains of rapamycin injected mice (Bercury and Macklin, Abstract # PSM01-09 ASN, 2011 and personal communications), suggesting that rapamycin does not block terminal differentiation of late progenitors in vivo, supporting the in vitro results obtained in our culture system. Despite these differences, both in vitro studies are in agreement that rapamycin inhibits the full maturation of MBP+ OLs. To definitively resolve which culture system mimics a true in vivo scenario awaits the generation of knockout mice with conditional disruption of mTOR signaling in late progenitors.
Interestingly, we have fortuitously uncovered a role for mTOR signaling in the cytoskeletal maturation of OLs. Specifically, since in our culture system rapamycin did not arrest the progression of differentiation at the early progenitor stage, we were able to see that rapamycin inhibited the characteristic cytoskeletal transformations that occur during the normal progression of the immature OL stages (Fig. 1A c,c’). Consistent with a role of mTOR in cytoskeletal maturation of OLs, recent proteomic analysis found alterations of several cytoskeletal proteins in rapamycin-treated OL cultures (Tyler et al., 2011). In addition, rapamycin treatment also inhibited the expression of major myelin proteins (MBP and MOG) and progression to mature OL stage. Since both the numbers of MBP+ mature OLs and MBP immunofluorescent-intensity per mature OL were reduced, the overall reduction in MBP protein level determined by immunoblotting could be due to a combination of the two factors. The reduced expression of major myelin proteins, accompanied by inhibition of cytoskeletal maturation, could both be mediated through mTORC1, which is the primary target of rapamycin and is known to regulate protein and lipid synthesis. However, the effect on OL morphology could, in theory, be also mediated through mTORC2 signaling, which is known to regulate cytoskeletal organization and can also be inhibited by prolonged treatment with rapamycin (Zoncu et al., 2011). Thus, mTORC1 and perhaps even mTORC2 signaling enables OLs to acquire the full antigenic and morphological complexity needed for normal myelination.
These in vitro observations correlate well with our in vivo finding where chronic inhibition of mTOR by daily injections of rapamycin in an early postnatal mouse from P 6-11 resulted in reduced myelination in the brain, demonstrating that mTOR signaling is required at the onset of myelination. The requirement of mTOR continues into the active phase of myelination since reduced myelination was also observed in the brains of mice injected with rapamycin from P21 for three weeks (Narayanan et al., 2009). Moreover, recent studies showing enhanced myelination in transgenic mice with overactive PI3K/Akt/mTOR pathway suggest a role of this pathway in myelin sheath wrapping by OLs, a process requiring major cytoskeletal alterations (Flores et al., 2008; Goebbels et al., 2010; Harrington et al., 2010). Further, rapamycin was shown to reverse this hypermyelination in transgenic mice expressing constitutively active Akt in OLs (Narayanan et al., 2009). Taken together, these findings support our in vitro observations suggesting that the PI3K/Akt/mTOR pathway plays a major role in late stages of OL differentiation, especially in the cytoskeletal organization that normally occurs during myelination.
In summary, we demonstrate that, when examined in parallel, there is a sequential and non-overlapping developmental stage-specific requirement of the Ras/Raf/Mek/Erk or the PI3K/Akt/mTOR pathways during OL-lineage progression. Specifically, transition of early progenitors to late progenitors requires Erk1/2 signaling and transition of immature OLs to mature OL requires mTOR signaling. Interestingly, neither pathway directly plays an essential role for the commitment of late progenitors to enter terminal differentiation. This study provides valuable insights about the appropriate timing and nature of the signaling pathways that could serve as useful targets to enhance OL differentiation for remyelination in neurological diseases.
Acknowledgements
We would like to thank Greg Wark for immunoblotting and for mouse brain cultures and Dr. Miki Furusho for help with the graphics. This work was supported by NIH Grant NS38878 and in part by grants from the National Multiple Sclerosis Society, RG 4087-A-3 and NIH, NS41078 to RB.
REFERENCES
- Baas D, Barnstable CJ. HPC-7: a novel oligodendrocyte lineage protein which appears prior to galactocerebroside. Glia. 1998;23:169–179. [PubMed] [Google Scholar]
- Bansal R, Stefansson K, Pfeiffer SE. Proligodendroblast antigen (POA), a developmental antigen expressed by A007/O4-positive oligodendrocyte progenitors prior to the appearance of sulfatide and galactocerebroside. J Neurochem. 1992;58:2221–2229. doi: 10.1111/j.1471-4159.1992.tb10967.x. [DOI] [PubMed] [Google Scholar]
- Bansal R, Kumar M, Murray K, Morrison RS, Pfeiffer SE. Regulation of FGF receptors in the oligodendrocyte lineage. Mol Cell Neurosci. 1996;7:263–275. doi: 10.1006/mcne.1996.0020. [DOI] [PubMed] [Google Scholar]
- Bansal R, Magge S, Winkler S. Specific inhibitor of FGF receptor signaling: FGF-2-mediated effects on proliferation, differentiation, and MAPK activation are inhibited by PD173074 in oligodendrocyte-lineage cells. J Neurosci Res. 2003;74:486–493. doi: 10.1002/jnr.10773. [DOI] [PubMed] [Google Scholar]
- Baron W, Metz B, Bansal R, Hoekstra D, de Vries H. PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol Cell Neurosci. 2000;15:314–329. doi: 10.1006/mcne.1999.0827. [DOI] [PubMed] [Google Scholar]
- Bhat NR. Signal transduction mechanisms in glial cells. Dev Neurosci. 1995;17:267–284. doi: 10.1159/000111296. [DOI] [PubMed] [Google Scholar]
- Cui QL, Almazan G. IGF-I-induced oligodendrocyte progenitor proliferation requires PI3K/Akt, MEK/ERK, and Src-like tyrosine kinases. J Neurochem. 2007;100:1480–1493. doi: 10.1111/j.1471-4159.2006.04329.x. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Fischer TZ, Lee LN, Lercher LD, Dreyfus CF. Regionally specific effects of BDNF on oligodendrocytes. Dev Neurosci. 2003;25:116–26. doi: 10.1159/000072261. [DOI] [PubMed] [Google Scholar]
- Du Y, Lercher LD, Zhou R, Dreyfus CF. Mitogen-activated protein kinase pathway mediates effects of brain-derived neurotrophic factor on differentiation of basal forebrain oligodendrocytes. J Neurosci Res. 2006;84:1692–1702. doi: 10.1002/jnr.21080. [DOI] [PubMed] [Google Scholar]
- Ebner S, Dunbar M, McKinnon RD. Distinct roles for PI3K in proliferation and survival of oligodendrocyte progenitor cells. J Neurosci Res. 2000;62:336–345. doi: 10.1002/1097-4547(20001101)62:3<336::AID-JNR3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Emery B. Transcriptional and post-transcriptional control of CNS myelination. Curr Opin Neurobiol. 2010;20:601–607. doi: 10.1016/j.conb.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Chan JR, Baranzini SE, Franklin RJ, Rowitch DH. Myelin regeneration: a recapitulation of development? Annu Rev Neurosci. 2011;34:21–43. doi: 10.1146/annurev-neuro-061010-113629. [DOI] [PubMed] [Google Scholar]
- Flores AI, Mallon BS, Matsui T, Ogawa W, Rosenzweig A, Okamoto T, Macklin WB. Akt-mediated survival of oligodendrocytes induced by neuregulins. J Neurosci. 2000;20:7622–7630. doi: 10.1523/JNEUROSCI.20-20-07622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28:7174–83. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Frederick TJ, Min J, Altieri SC, Mitchell NE, Wood TL. Synergistic induction of cyclin D1 in oligodendrocyte progenitor cells by IGF-I and FGF-2 requires differential stimulation of multiple signaling pathways. Glia. 2007;55:1011–1022. doi: 10.1002/glia.20520. [DOI] [PubMed] [Google Scholar]
- Frost EE, Zhou Z, Krasnesky K, Armstrong RC. Initiation of oligodendrocyte progenitor cell migration by a PDGF-A activated extracellular regulated kinase (ERK) signaling pathway. Neurochem Res. 2009;34:169–181. doi: 10.1007/s11064-008-9748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe-Maricich SL, Karlo JC, Landreth GE, Miller RH. The ERK2 mitogen-activated protein kinase regulates the timing of oligodendrocyte differentiation. J Neurosci. 2011;31:843–850. doi: 10.1523/JNEUROSCI.3239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Möbius W, Liu X, Lappe-Siefke C, Rossner MJ, Groszer M, Suter U, Frahm J, Boretius S, Nave KA. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J Neurosci. 2010;30:8953–64. doi: 10.1523/JNEUROSCI.0219-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington EP, Zhao C, Fancy SP, Kaing S, Franklin RJ, Rowitch DH. Oligodendrocyte PTEN is required for myelin and axonal integrity, not remyelination. Ann Neurol. 2010;68:703–16. doi: 10.1002/ana.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura O, Pages G, Pouyssegur J, Endo S, Takishima K. ERK1 and ERK2 are required for radial glial maintenance and cortical lamination. Genes Cells. 2010;15:1072–1088. doi: 10.1111/j.1365-2443.2010.01444.x. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Narayanan SP, Flores AI, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J Neurosci. 2009;29:6860–6870. doi: 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Tang DG, Tokumoto YM, Raff MC. Long-term culture of purified postnatal oligodendrocyte precursor cells. Evidence for an intrinsic maturation program that plays out over months. J Cell Biol. 2000;148:971–984. doi: 10.1083/jcb.148.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WA, Gangoli N, Gokina P, Kim HA, Covey M, Levison SW, Wood TL. Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. J Neurosci. 2009;29:6367–6378. doi: 10.1523/JNEUROSCI.0234-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WA, Jain MR, Cifelli SE, Li Q, Ku L, Feng Y, Li H, Wood TL. Proteomic identification of novel targets regulated by the mammalian target of rapamycin pathway during oligodendrocyte differentiation. Glia. 2011;59:1754–69. doi: 10.1002/glia.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van't Veer A, Du Y, Fischer TZ, Boetig DR, Wood MR, Dreyfus CF. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J Neurosci Res. 2009;87:69–78. doi: 10.1002/jnr.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington AE, Barbarese E, Pfeiffer SE. Differential myelinogenic capacity of specific developmental stages of the oligodendrocyte lineage upon transplantation into hypomyelinating hosts. J Neurosci Res. 1993;34:1–13. doi: 10.1002/jnr.490340102. [DOI] [PubMed] [Google Scholar]
- Yim SH, Hammer JA, Quarles RH. Differences in signal transduction pathways by which platelet-derived and fibroblast growth factors activate extracellular signal-regulated kinase in differentiating oligodendrocytes. J Neurochem. 2001;76:1925–1934. doi: 10.1046/j.1471-4159.2001.00199.x. [DOI] [PubMed] [Google Scholar]
- Younes-Rapozo V, Felgueiras LO, Viana NL, Fierro IM, Barja-Fidalgo C, Manhaes AC, Barradas PC. A role for the MAPK/ERK pathway in oligodendroglial differentiation in vitro: stage specific effects on cell branching. Int J Dev Neurosci. 2009;27:757–768. doi: 10.1016/j.ijdevneu.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]