Abstract
Objective
This research examined why smokers receiving combination medication for smoking cessation are more likely to quit smoking than are those who receive either single agent (monotherapy) or placebo.
Method
Data were collected from 1504 current smokers (58.2% women, 83.9% White, mean age 44.67 years, SD=11.08) participating in a cessation clinical trial who were randomized to one of six cessation pharmacotherapy conditions (placebo, nicotine patch, nicotine lozenge, bupropion, nicotine patch + nicotine lozenge, and bupropion + nicotine lozenge). Participants completed Ecological Momentary Assessments four times a day, concerning five hypothesized mediators (negative affect, positive affect, craving, smoking expectations, and withdrawal) of pharmacotherapy effects. Medications were provided for 8 to 12 weeks post quit along with 6 individual counseling sessions. Mediational paths were estimated via a novel Bayesian approach with estimation of multiple mediator models.
Results
Biochemically confirmed 8-week abstinence was the outcome variable, with the monotherapy and combination pharmacotherapy composites producing 45% (n = 689) and 54% (n = 478) abstinence rates, respectively. The univariate models suggested that the combination treatments produced higher abstinence rates than the monotherapies because of greater suppression of withdrawal, craving, and smoking expectations. However, multiple mediator models showed that the suppression of craving on the quit day produced the strongest mediational effects and could account for the mediational effects of other tested variables.
Conclusion
Suppression of craving on the quit day significantly mediates the clinical effects of mono- and combination smoking pharmacotherapies and the higher abstinence rates for combination therapy versus monotherapies appears primarily due to greater craving suppression.
Keywords: Smoking cessation, combination pharmacotherapy, mediation analyses, craving
Smoking cessation intervention is a key means of reducing the human and economic costs of tobacco use. Mounting evidence suggests that combining smoking cessation pharmacotherapies (i.e., using combination pharmacotherapy) improves cessation rates over those achieved by use of individual smoking cessation medications (i.e., monotherapy). For instance, both the 2008 PHS Guideline: Treating Tobacco Use and Dependence (Fiore et al., 2008) and a Cochrane report (Stead, Perera, Bullen, Mant, & Lancaster, 2008) presented meta-analyses showing that combinations of nicotine replacement therapies (NRT’s) produce higher long-term abstinence rates than do single NRT’s (also see Shah, Wilken, Winkler, & Lin, 2008). In addition, two recent, large comparative effectiveness trials demonstrated that combination pharmacotherapy interventions tended to produce higher success rates than did monotherapies (Piper et al., 2009; Smith et al., 2009; also see Blondal, Gudmundsson, Olafsdottir, Gustavsson, & Westin, 1999; Cooney et al., 2009; Kornitzer, Boutsen, Dramaix, Thijs, & Gustavsson, 1995; Puska et al., 1995; Sweeney, Fant, Fagerstrom, McGovern, & Henningfield, 2001); although cf. (Ingersoll & Cohen, 2005).
There is some evidence that the type of medication involved in the combination treatment makes a difference. Specifically, evidence suggests that combinations of NRT’s (e.g., the nicotine patch + nicotine gum or lozenge) increase cessation rates beyond combinations comprising a non-NRT medication (e.g., NRT + bupropion). In analyses reported in the 2008 PHS Guideline (see Fiore, et al., 2008); also cf. (Jorenby et al., 1999) only the combination of NRT agents, and not NRT+bupropion, produced significantly higher success rates than did the nicotine patch by itself. However, there is evidence that the combination of NRT + bupropion is also efficacious relative to monotherapy. For instance, in one of the large, recent comparative effectiveness trials (Smith, et al., 2009) a combination of bupropion + nicotine lozenge produced significantly higher 6-month abstinence rates (29.9%) than did any of the tested monotherapies (the nicotine patch, nicotine lozenge, bupropion: 16.8 – 19.9%). The abstinence rate of the bupropion + NRT combination was also modestly higher than the combination of the nicotine patch + nicotine lozenge in that study (29.9% vs. 26.9%) but not significantly so. In the second major, recent comparative effectiveness trial, both the bupropion + nicotine lozenge and the nicotine patch + nicotine lozenge combinations produced significantly higher abstinence rates at end-of-treatment than did the monotherapies (Piper, et al., 2009). In sum, there is evidence that both combination NRT and the NRT+bupropion combination produce greater success than monotherapies, although the evidence is somewhat stronger with regard to the former.
It is unknown why combination pharmacotherapies produce greater benefit than monotherapies (i.e., what therapeutic mechanisms account for their superior effects on abstinence). This issue can be addressed through formal mediation analysis. Such analyses can reveal whether the relation between a treatment (an independent variable) and a clinically important outcome (the dependent variable), is partly or wholly due to treatment effects on potentially mediating variables. Such information can shed light on the determinants of success and failure, reveal what treatments do and do not do, and may be used for purposes such as the development of treatment algorithms and the determination of treatment “dosing” (ascertaining when a person has had a sufficient dose of treatment, based on mediator status (McCarthy, Bolt, & Baker, 2007).
The study of mediation demands that investigators hypothesize a causal path leading from treatment to a clinically important outcome, identifying variables that should index intermediate change in that path. Such variables, or mediators, should be substantively and/or empirically linked with inferred causal processes. Very little research exists on the mediation of smoking cessation pharmacotherapy, and virtually all that does exist concerns monotherapies (i.e., single medications1). The extant research reveals a relatively small group of variables that has been implicated, albeit inconsistently, in mediating pharmacotherapy effects on long-term abstinence. McCarthy et al., (McCarthy et al., 2008) reported that bupropion’s impact on abstinence was partially mediated by its effects on craving and positive affect, but not by its effects on overall withdrawal2, negative affect, or effects associated with smoking a lapse cigarette. McCarthy et al., also found that some of the effects of pharmacotherapy may be related to effects on self-efficacy and motivation, which themselves could reflect changes in multiple individual symptoms and diverse appraisal processes (McCarthy, et al., 2008). Another study using bupropion implicated negative affect as a mediator, but not withdrawal or positive affect (Lerman et al., 2002), and a third study reported bupropion mediation via withdrawal and craving suppression, but not via effects on negative or positive affect (Piper, Federman, et al., 2008).
Only two studies have addressed NRT mediation. One study (Ferguson, Shiffman, & Gwaltney, 2006) reported that the increased time to first lapse caused by NRT was mediated by reductions in withdrawal and craving, especially the latter. While NRT produced other effects, such as reducing negative affect and attention disturbance and increasing positive affect, these did not mediate treatment effects on lapse latency. A second study with smokers with HIV/AIDS (Stanton, Lloyd-Richardson, Papandonatos, de Dios, & Niaura, 2009) reported that self-efficacy and decisional balance (a motivational measure), significantly mediated NRT effects on cessation outcomes. In sum, research has most consistently implicated craving as mediating the clinical effects of single agents (monotherapy); it less consistently implicates other variables such as positive affect, negative affect, withdrawal and motivation. However, it is important to note that this characterization is based on only a few studies, these studies used different methods (e.g., different outcomes, different dosings, different analytic strategies), and in all cases the mediator accounted for only a portion of the agent’s therapeutic effects – often a modest portion.
The current study sought to yield additional insight into the mediation of smoking cessation pharmacotherapy effects by identifying the proximal actions of combination therapy that account for its superior clinical outcomes. The mediators examined in this research were craving, withdrawal, negative affect, positive affect, and expectation of smoking reward. The first four variables were chosen because: (1) there is some prior evidence that these mediated the effects of monotherapies, and (2) empirical evidence and theory suggest that they should be affected by nicotine abstinence and should affect the likelihood of remaining abstinent (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; D’Souza & Markou, 2010; Hughes, 2007; McCarthy, et al., 2008). The fifth potential mediator, expectancy of smoking reward, was selected for analysis because it has been shown to be related to relapse and smoking motivation (Gwaltney, Shiffman, Balabanis, & Paty, 2005; Herd & Borland, 2009; Kirchner & Sayette, 2007), and because prior research (McCarthy, et al., 2008; Stanton, et al., 2009) suggested that such motivational factors might mediate pharmacotherapy effects.
This study used data generated by one of the large recent comparative effectiveness studies cited earlier (Piper, et al., 2009); the other comparative effectiveness study by Smith et al., (Smith, et al., 2009) did not comprise measures of potential mediators. The advantages of the former study are that it had a large sample size, involved several types of pharmacotherapy, including two different types of combination pharmacotherapy, and offered measures of diverse potential mediators assessed in real time.
The current work uses a Bayesian approach to mediation analysis (Yuan & MacKinnon, 2009) that has not previously been used to characterize the effects of smoking cessation interventions. The complexity of mediational modeling, especially in the use of repeated measures, can make model estimation a challenge. Fortunately such complexity can be handled in a straightforward fashion through the use of Bayesian estimation techniques. A Bayesian approach to mediation has many advantages, including the ability to incorporate prior information into the analysis, the capacity to construct credible confidence intervals for mediation effects, as well as the potential to accommodate multilevel data structures (Yuan & MacKinnon, 2009). The latter two advantages are of particular relevance in the current analysis. Further, the use of a Bayesian approach facilitated our testing multiple mediator models, which allowed the estimation of the magnitude of orthogonal mediational paths.
In sum, this research uses real-time measures of multiple potential mediators, which were selected on theoretical and empirical grounds, and which were modeled as latent variables in a discontinuous piecewise model that allowed for estimation of quit day increases as well as post-quit symptom trajectories. These data were analyzed using a novel, Bayesian mediation approach, which permitted the estimation of multiple mediator models. This research was intended to provide insight into why combination therapy results in superior cessation outcome relative to monotherapy; insight that can be used to develop new treatments or use existing treatments more efficiently.
Methods
Recruitment and Inclusion/Exclusion Criteria
Participants were recruited via TV, radio and newspaper advertisements, community flyers, and earned media (e.g., radio and TV interviews, press releases) in the greater Madison and Milwaukee, WI, areas. Primary inclusion criteria included: smoking at least 10 cigarettes per day for the past 6 months and being motivated to quit smoking. Exclusion criteria included: certain medications (including MAO inhibitors, bupropion, lithium, anticonvulsants, and antipsychotics); any history of psychosis, bipolar disorder, or an eating disorder; consuming six or more alcoholic beverages daily 6 or 7 days a week; pregnancy or breast-feeding; and a serious health condition that might prevent study completion. This study was approved by the University of Wisconsin Health Sciences Institutional Review Board.
Procedure
Participants who passed a phone screen, where they were told about the study and asked about the all of the inclusion/exclusion criteria, were invited to an Information Session where a study description was provided and written informed consent was obtained. Next, participants completed multiple baseline screenings, including a medical history screening, vital signs measurements and a carbon monoxide (CO) breath test. Participants also completed demographic, smoking history and tobacco dependence questionnaires.
Eligible participants were randomized to one of six treatment conditions: Bupropion SR (n=264); Nicotine lozenge (n=260); Nicotine patch (n=262); Nicotine patch + Nicotine lozenge (n=267); Bupropion SR + Nicotine lozenge (n=262) or Placebo (five placebo conditions that matched the five active conditions; n=189). All medications were provided for 8 weeks post-quit except the nicotine lozenge which was provided for 12 weeks post-quit (consistent with prescribing instructions and the 2008 PHS Guideline; (Fiore, et al., 2008). Randomization was conducted in a double-blind fashion using a blocked randomization scheme blocking on gender and race (White vs. non-White). All participants received six individual counseling sessions (each lasting 10–20 minutes), designed to provide social support and training in problem-solving and coping skills. Bachelor-level case managers provided counseling based on the study protocol and were supervised by a licensed clinical psychologist.
Measures
Baseline Assessments
Participants completed questionnaires that assessed characteristics including gender, ethnicity, age, marital status, education level, employment, and smoking history features such as number of cigarettes smoked per day, age at smoking initiation, and number of prior quit attempts. They also completed the Fagerström Test of Nicotine Dependence (FTND; α=.61) (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) and the Wisconsin Inventory of Smoking Dependence Motives (WISDM) (Piper et al., 2004) to assess tobacco dependence.
Ecological Momentary Assessment (EMA) Reports
Ecological momentary assessment allows researchers to capture, in real time, how participants are feeling and thinking, more accurately than through daily diaries (see Ferguson & Shiffman, 2011; Shiffman, Kirchner, Ferguson, & Scharf, 2009; Trull & Ebner-Priemer, 2009). Participants completed EMA reports four times a day (just after waking, prior to going to bed and 2 randomly timed prompts) for 1 week pre-quit and 2 weeks post-quit. EMA reports asked participants to rate how they felt within the last 15 minutes in terms of withdrawal symptoms (craving, hunger, and difficulty concentrating) using items from the Wisconsin Smoking Withdrawal Scale (WSWS) (Welsch et al., 1999). Other items included an urge item from an adapted Questionnaire of Smoking Urges (Sweeney, Pillitteri, & Kozlowski, 1996), and items assessing self-efficacy, motivation and cessation fatigue (i.e., “I’m tired of trying to quit smoking”). The EMA reports also assessed the number of alcoholic drinks consumed that day and number of cigarettes smoked, stress and temptation events since the last prompt. Subjects were given training on how to interpret and respond appropriately to EMA items. Withdrawal dimensions and expectancies were measured with a 10-point response scale while affect was measured with a 5-point scale (Table 1; McCarthy, Piasecki, Fiore, & Baker, 2006).
Table 1.
Descriptive Statistics for Mediator Measures Across Pre-and Post-Quit Intervals.
| Variable | Observations | Minimum | Maximum | Mean | Standard Deviation |
|---|---|---|---|---|---|
| Withdrawal | 71786 | 0 | 10 | 1.97 | 1.52 |
| Craving | 71786 | 0 | 10 | 4.23 | 3.13 |
| Negative Affect | 71788 | 1 | 5 | 1.34 | 0.69 |
| Expectations | 71781 | 0 | 10 | 3.65 | 2.92 |
| Positive Affect | 71788 | 1 | 5 | 1.98 | 1.00 |
Cessation Outcomes
The cessation outcomes were: initial cessation (defined as 24 hours of abstinence in the first week of the quit attempt), and CO-confirmed 7-day point-prevalence abstinence at 8 weeks and 6 months post-quit. Data at the 8-week mark constituted the outcome measure in mediational models as this was the end of treatment, and therefore, likely to capture net treatment effects. Alveolar CO was assessed using a Bedfont Smokerlyzer and smokers with a CO < 10 ppm were considered abstinent. Smokers who could not be reached for follow-up were considered to be smokers, using the intent-to-treat principle.
Analytic Plan
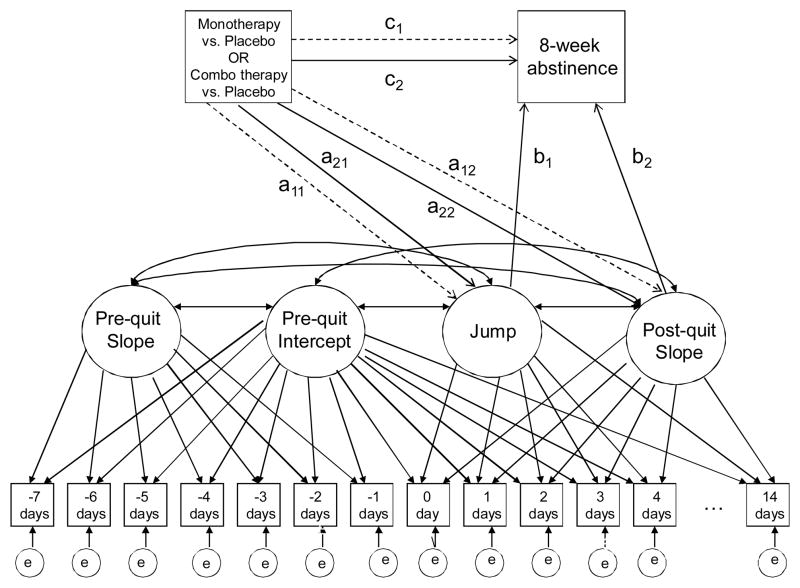
Our mediational models were similar in structure to those presented in Piper, Federman, et al. (2008). Multiple daily measures of our hypothesized mediators from 7 days prequit to up to 14 days postquit allow for growth trajectory modeling of each mediator (positive affect, negative affect, craving, withdrawal, and expectancies). In this paper we consider both univariate (single mediator variable) and multivariate (multiple mediator variables) approaches. A graphical illustration of a univariate mediational analysis is shown in Figure 1. Of particular interest are the effects of treatment on smoker experiences post-quit and the subsequent effects of those experiences on end-of-treatment abstinence. Our growth model examines post-quit experiences through two latent person variables. The first, a post-quit “jump” variable, quantifies how the studied mediator changed on the quit day (e.g., a 3-point quit-day increase in craving). The second, referred to as a post-quit slope variable, quantifies the average per-day rate of change in the mediator over the 14 days postquit (e.g., a 0.2-points per day decrease in craving). A primary question to be answered in this context is whether these mediator variables demonstrated differences in relation to treatment, and whether these differences, in turn, are related to end-of-treatment abstinence.
Figure 1.
Illustration of Latent Variable Model Used for Univariate Mediational Analysis.
Note. Boxes represent observed (measured) variables, ovals represent latent variables. Coefficients for all paths relevant to mediational effects are denoted by (a)-(c). Dashed arrows represent effects unique to the monotherapy versus placebo condition.
While the analyses above allow for evaluation of differential effects of treatments on each individual mediator, they do not clarify whether the effects represent one or more phenomena, as the mediators tend to be meaningfully correlated. The approach above can be generalized so as to study more than one mediator simultaneously. Similar to generalizations of simple regression to multiple regression, a multiple mediator model allows for evaluation of whether the potential effects observed for each mediator are statistically distinguishable or are tapping effectively the same phenomenon.
In Figure 1, pathways relevant to the evaluation of mediational effects are identified by letters (a)–(c). The figure provides only a conceptual illustration of the model, as in actuality multiple measures are often collected in the same day. Note that the effects of monotherapy and combination therapy, relative to placebo, on both the mediators and outcome are independently estimated, with the dotted arrows corresponding to unique monotherapy effects, and solid arrows to unique combination therapy effects. For example, the dashed arrow from the initial treatment variable to the Jump variable (a11) represents the effect of monotherapy relative to placebo on the jump in the mediator variable (e.g., withdrawal) on the quit day. Indirect effects are calculated as the product of the relevant pathway from treatment to mediator and the pathway from mediator to outcome. For example, the indirect effect of monotherapy on abstinence via the Jump variable would be a11b1. Because two distinct parameters (Jump and Slope) represent possible mediators, the total indirect effect of each treatment type on outcome can be calculated as the simple sum of indirect effects. Therefore, the combined indirect effect of monotherapy on EOT abstinence is a11b1+a12b2, while that for combination therapy is a21b1+a22b2.
Our decision to collapse across the monotherapies, and combination therapies was motivated by preliminary analyses that revealed no significant differences amongst the individual treatments within these categories on end-of-treatment abstinence. We also conducted a preliminary study of hypothesized mediators in relation to general treatment effects. These analyses compared a pooled active treatment condition comprising all monotherapies and combination therapies against a placebo condition with respect to the jump and slope of each mediator. These analyses allowed for a preliminary reduction in the number of potential mediators worthy of consideration for distinguishing mono- and combination therapy conditions
As our focus is on distinguishing the effects of monotherapy and combination therapy conditions, we subsequently fit models that further evaluated whether the combination therapies demonstrated a disproportionate mediational effect to monotherapies for any of the significant mediators. The model also controlled for the effects of smoking on the days on which mediational data were gathered via a binary variable coding for occurrence of smoking vs. not smoking on that day (not depicted in Fig. 1). Both single and multiple mediator models were estimated.
Markov Chain Monte Carlo Estimation
As illustrated in Yuan & MacKinnon (2009), Bayesian estimation of mediational models can be implemented through Markov chain Monte Carlo (MCMC) techniques. Unlike more traditional estimation methods such as maximum likelihood or least squares methods, for example, MCMC methods rely on sampling techniques to estimate model parameters and resulting mediation effects (i.e., iterative sampling from the parameter distributions is used to estimate credible intervals, similar to confidence intervals, to identify significant effects). An appealing feature of the methodology is its relative ease of implementation, particularly for complex statistical models. As in Yuan & MacKinnon (2009), we implemented MCMC using WinBUGS 1.4 (Spiegelhalter, 2008).
In most respects our approach follows the general strategy detailed in Yuan & MacKinnon (2009), with a couple of exceptions. The first relates to the nature of the mediational models being fit. Unlike the multilevel mediation model illustrated in Yuan & MacKinnon (2009), our models are upper-level mediation models, meaning mediation is studied with respect to subject-level variables. This approach is natural in our application given that the relevant mediational variables (e.g., treatment, jump, slope, and abstinence outcome) are modeled as single-occasion variables for each studied participant (even though the variables are estimated with multiple waves of data). One practical implication of this difference is that the mediational effect is viewed as a fixed rather than random effect across participants.
The second difference relates to the prior distributions used for the model parameters. “Priors” of model parameters can be used to incorporate known information about the model from sources other than the data being analyzed. The selection of priors can also influence the efficiency of the sampling process. To allow the data to speak most directly to the final estimates, it is appropriate to specify non-informative priors (Yuan & MacKinnon, 2009). Because the current analysis is a complex latent variable model, non-informative priors were not possible; however the priors chosen were weak, and centered at 0 to avoid any bias toward detecting intervention effects. For example, we assumed normal priors (having means of 0 and variances of 1) for each of the a–c parameters. WINBUGS code for the models fit in this paper can be provided on request to the first author.
It is important to note that while the models fit in this paper are complex, only a small number of effects are relevant to quantifying mediation. In MCMC analyses, these effects can be traced in the sampling process and evaluated for statistical significance (i.e., significant differences from 0) by inspecting a 95% credible interval (CI), analogous to a confidence interval (see Yuan & MacKinnon, 2009 for further description). As is often done in MCMC analyses, we report the mean of the sampled values as a point estimate of each parameter (Kim & Bolt, 2007). The endpoints of 95% CIs are derived from the sampled distribution of values with cut-points determined by the lowest and highest 2.5% of observations. One advantage of the MCMC approach is that inferences can also be derived with respect to functions of model parameters, such as the combined indirect effects as described above (a11b1+a12b2, a21b1+a22b2), as well as the differences in effects of monotherapies and the combination therapies on the jump (a21-a11) and slope (a22-a12) variables. We focus on both the individual trajectory parameter estimates (e.g., the quantifications of the jump and slope for the studied mediators) per se, as well as estimates of these functions of the trajectory parameter coefficients relevant to interpreting meditational effects. Consistent with a traditional meditational model, the effects of both the jump and slope variables on abstinence (c1, c2) are assumed to be equivalent for the monotherapy and combination therapy conditions. In other words, while therapy condition was allowed to have a direct effect on abstinence, it was assumed that therapy condition did not moderate relations in the pathways from jump to abstinence or from slope to abstinence.
Results
Biochemically confirmed 8-week abstinence rates for the six treatment conditions were: placebo = 33% (n = 160), patch = 47% (n = 232), lozenge = 44% (n = 229), bupropion = 42% (n = 228), patch + lozenge = 56% (n = 242), and bupropion + lozenge = 52% (n = 236)3. The 8-week abstinence rates for the monotherapy and combination pharmacotherapy composites were 45% (n = 689) and 54% (n = 478), respectively. Preliminary analyses using logistic regression were conducted to evaluate treatment effects on end-of-treatment abstinence. Treatment variables were entered as dummy-coded predictors using the placebo as a reference condition. These analyses confirmed a relation between treatment and end-of-treatment abstinence for general active treatment against placebo (b=.65, 95% CI=.22, 1.04, exp(b)=1.92), as well as when monotherapy and combination therapy conditions were evaluated independently against placebo (b=.46, 95% CI=.09,.80, exp(b)=1.58 & b=.84, 95% CI=.48,1.30, exp(b)=2.32, for monotherapy and combination therapy, respectively). Beyond being statistically significant, these treatment effects are also clinically significant, with exp(b) indicating the proportional change in the odds of abstinence when moving from placebo to treatment (e.g., a nearly two-times greater likelihood of abstinence in active treatment compared to placebo). As noted earlier, a preliminary mediational analysis using the model of Figure 1 was conducted in which treatment was coded as a binary variable distinguishing active (pooled mono- or combination therapies) from placebo. Table 1 displays descriptive statistics for each of the five potential mediating variables calculated across both persons and repeated measurements. Of the five mediators studied (negative affect, positive affect, craving, expectations, withdrawal), only positive affect failed to show a significant indirect effect as a mediator of the effects of active treatment, and therefore was dropped in subsequent analyses comparing mono- versus combo-treatment.
For each of the four remaining mediators, models conforming to Figure 1 were fit, now distinguishing placebo, mono- and combo-therapies as distinct treatment conditions. In order to check the samples simulated by the MCMC algorithm, various convergence indices are typically inspected to determine whether the sample is suitable for the construction of credible intervals for the model parameters (i.e., whether iterative draws of data from the sample yields acceptably convergent estimates of model parameters: Yuan & MacKinnon, 2009). For the current analyses, all but one of the simulated samples for the univariate mediational analyses suggested convergence within for the parameters of interest within an initial sample of 15,000 states. The one exception was negative affect, which failed the Raftery & Lewis criterion (Raftery & Lewis, 1992). Consequently, an additional 30,000 iterations were simulated for negative affect, which returned a result consistent with convergence.
Results for the univariate mediational analyses distinguishing monotherapy versus combination therapy conditions are shown in Table 2. The top portion of the table (a paths) summarize results for the effects of the treatment type on the jump and slope parameters for each mediator. In each case, the intercept (“Int”) represents the baseline effect for the placebo condition. As these effects are linear, the coefficient estimates can be interpreted in direct relation to the metric of the mediating variable. For example, the intercept estimate of .60 for the jump parameter of withdrawal implies that in the placebo condition, the mean increase in withdrawal at the quit day is approximately .60 units. A 95% CI that fails to include 0 can be taken to represent a statistically significant effect. Consequently, the intercept estimate for withdrawal in the placebo condition is statistically different from 0. The magnitude of this jump may be best understood in relation to the overall distribution of the withdrawal measure as seen in Table 1. For example, a .60 unit increase in withdrawal corresponds to approximately .60/1.52 = .4 standard deviations change in withdrawal (or alternatively, .60 units on the 0–10 scale for withdrawal). The a11 and a12 effects related to the jump parameter reflect the differential jump for the monotherapy and combination therapy effects, respectively, relative to the placebo condition. For example, the estimates of −.13 and −.32 on withdrawal imply a lower jump for each of the monotherapy and combination therapy effects, and therefore a net jump of .47 units and .28 units for the monotherapy and combination therapy conditions, respectively. Although both of these effects are in the expected direction, only the effect of the combination therapy is statistically significant, given that the credibility interval for a11 includes 0. Analogous interpretations are given to the slope estimates (i.e., the coefficients for a12 & a22), where the coefficients now reflect the per-day rate of change in the mediator over the first two weeks post-quit. Across mediators, it can be seen that the more substantial effects of treatment consistently occur with respect to the jump rather than the post-quit slope. The treatment effect on jump appears most sizable for expectations (even adjusting for metric differences). In addition, the effects of combination therapy (a21) appear to be nearly double those of monotherapy (a11) on the jump consistently across mediators, possibly even higher for withdrawal and craving.
Table 2.
Results from Univariate Mediation Analyses.
| apaths | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jump Parameter | Post-quit Slope | |||||||||||
| Int | a11 | a21 | Int | a12 | a22 | |||||||
| Mediator | Est | 95%CI | Est | 95%CI | Est | 95%CI | Est | 95%CI | Est | 95%CI | Est. | 95%CI |
|
| ||||||||||||
| Withdrawal | .60* | (.44,.76) | −.13 | (−.31,.05) | −.32* | (−.51, −.14) | −.02 | (−.04,.01) | −.02 | (−.05,.01) | −.02 | (−.05,.01) |
|
| ||||||||||||
| Craving | 1.06* | (.70,1.41) | −.31 | (−.71,.09) | −.69* | (−1.12, −.27) | −.09* | (−.14, −.05) | −.03 | (−.08,.02) | −.03 | (−.08,.02) |
|
| ||||||||||||
| Negative Affect | .16* | (.08,.23) | −.05 | (−.14,.03) | −.09* | (−.18, −.00) | −.00 | (−.02,.02) | −.01 | (−.02,.01) | −.00 | (−.02,.02) |
|
| ||||||||||||
| Expectations | 1.07* | (.71,1.40) | −.48* | (−.84, −.09) | −.86* | (−1.26, −.45) | −.09* | (−.13, −.05) | .01 | (−.03,.06) | .01 | (−.03,.06) |
| b and cpaths | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Int | b1 | b2 | c1 | c2 | ||||||
| Mediator | Est | 95%CI | Est | 95%CI | Est | 95%CI | Est. | 95% CI | Est. | 95%CI |
| Withdrawal | −.20 | (−.59,.17) | −.38* | (−.54, −.22) | −2.14* | (−3.24, −1.06) | .41* | (.06,.77) | .73* | (.36,1.11) |
| Craving | −.06 | (−.49,.36) | −.21* | (−.28, −.14) | −1.88* | (−2.56, −1.22) | .40* | (.04,.76) | .72* | (.34,1.10) |
| Negative Affect |
−.32 | (−.76,.13) | −.61* | (−1.05, −.19) | −1.15 | (−2.71,.38) | .45* | (.11,.80) | .81* | (.45,1.17) |
| Expectations | −.12 | (−.54,.30) | −.16* | (−.23, −.08) | −.81* | (−1.57, −.05) | .42* | (.07,.78) | .73* | (.36,1.11) |
| Combined Indirect Effects | TheRelative Effectsof Combo Versus Monotherapyon Mediator | |||||||
|---|---|---|---|---|---|---|---|---|
| Monotherapy (a11b1+a12b2) | Combo therapy (a21b1+a22b2) | Jump Effect (a21-a11) | Slope Effect (a22-a12) | |||||
| Mediator | Est | 95%CI | Est | 95%CI | Est. | 95%CI | Est. | 95%CI |
| Withdrawal | .09* | (.02,.19) | .16* | (.06,.27) | .19* | (.06,.32) | −.00 | (−.02,.02) |
| Craving | .14* | (.02,.27) | .20* | (.07,.34) | .38* | (.08,.68) | −.01 | (−.04,.03) |
| Negative Affect |
.04 | (−.01,11) | .06* | (+.00,.14) | .04 | (−.02,.10) | −.00 | (−.02,.01) |
| Expectations | .07* | (.12,.67) | .13* | (.05,.23) | .38* | (.11,.67) | −.00 | (−.03,.03) |
Note. “Int” reflects the intercept or baseline effects for the placebo condition. The letters (a, b, c etc.) designate estimated causal paths in the meditational models and are defined in Figure 1.
indicates statistical significance, two-tailed alpha=.05
The effects of the jump and post-quit slope variables on end-of-treatment abstinence (b1 and b2, respectively) and the residual effects of monotreatment and combotreatment (c1 and c2, respectively, which reflect treatment effects not related to change in the mediator) also demonstrate similar patterns of effects across mediators. As these effects relate to a binary outcome, both the b and c coefficients are interpreted on a logit metric. For example, for the mediator of “withdrawal,” a one unit increase in the jump reduces the likelihood of abstinence by .38 logits. As in the evaluation of treatment effects above, the b and c coefficients are often transformed to exp(b) and exp(c) to represent the proportional change in the odds of abstinence for each unit increase in the predictor. Consequently, the .38 logit decrease associated with a one unit increase in withdrawal can also be interpreted as an exp(.38) = 1.46 times lower likelihood of abstinence. All b1 and b2 estimates are in the expected direction and statistically significant with the exception of the b2 estimate for negative affect. The statistical significance of all c1 and c2 estimates implies that both monotherapies and combination therapies demonstrate significant effects on abstinence beyond those captured by any single mediator. While a comparison of b1 and b2 estimates across mediators would appear to implicate factors such as the jump in negative affect (b1 = −.61) as substantial, it is important to note both that corresponding treatment effects (a11, a21) as well as the metric of the mediator (negative affect has low variability, even adjusting for metric differences across mediators) ultimately contribute to determining the importance of mediator in understanding treatment effects.
To better quantify the strength of mediation, the bottom portion of Table 2 reports point estimates for the monotherapy (a11b1+a12b2) and combination therapy (a21b1+a22b2) total indirect effects (i.e., the effects across both components of the meditational path). As for the a coefficients reported earlier, these total indirect effects should each be interpreted as the effects of the respective treatments in comparison to the placebo condition. In addition, these estimates should also be interpreted on a logit metric, with positive coefficients implying a higher likelihood of abstinence due to treatment effects on the studied mediator. All studied mediators were found to be significant with the exception of negative affect for the monotherapy condition. Importantly, these indirect effects are comparable across mediators (despite metric differences across mediators) as each indirect effect can be interpreted as a treatment effect on the likelihood of abstinence (via the studied mediator). In this instance, craving emerges as the most important of the meditational variables as it shows the largest indirect effects for both monotherapy and combination therapy treatments. For monotherapy, exp(a11b1+a12b2) = exp(.14) = 1.15, implying a 1.15 times greater likelihood of abstinence due to the effects of monotherapy on craving, while exp(a21b1+a22b2) = exp(.20) = 1.22, implying a 1.22 times greater likelihood of abstinence due to the effects of combination therapy on craving.
The magnitude of the indirect effects is perhaps best understood in comparing the indirect effect coefficient estimates to the corresponding c (residual) estimate (the estimate of treatment effects on 8-week abstinence not accounted for by the mediational path in question;Yuan & MacKinnon, 2009). Thus, the magnitude of the mediational effects can be evaluated by comparing the corresponding indirect effect estimates (c1, c2) against the residual direct effect estimate (a11b1+a12b2, a21b1+a22b2) for each mediator to quantify how much of the treatment effect can be attributed to the studied mediator. For monotherapy effects, the ratios, i.e., (a11b1+a12b2)/c1, are estimated as .23 (95% CI =.01, 1.5) for withdrawal, .31 (95%CI = −.02, 1.92) for craving, .08 (95%CI = −.04,.46) for negative affect, and .14 (95% CI = −.02, .78) for expectations. For combination therapy effects, the ratios, i.e., (a21b1+a22b2)/c2, are estimated as .22 (95% CI =.09, .58) for withdrawal, .27 (95% CI = .09, .71) for craving, .07 (95%CI =+.00,.22) for negative affect, and .16 (95% CI = .05, .43) for expectations In this way it can be also seen that of the four studied mediators, craving appears to have the largest indirect effects and thus would appear to be the most important of the studied mediators in understanding the effects of monotherapy as well as combination therapy. Finally, by examining the difference between pathways for monotherapy and combination therapy to a common mediator (jump or slope; i.e., a21-a11 and a22-a12), we can evaluate differential effects of the two forms of therapy on the mediator. From the bottom of Table 2, it can be seen that for all mediators except negative affect, there is a statistically significant difference in the effects of combination therapy versus monotherapy on the jump in mediator at quit day, with the effects of the combination therapy being significantly stronger (as indicated by confidence intervals that do not include “0” for the “The Relative Effects of Combo versus Monotherapy on Mediator” columns in Table 2).
As the craving mediator yielded the strongest indirect effects, we next examined multivariate mediational models adding to the univariate craving mediation model the repeated measures and associated growth trajectory variables of negative affect or expectations as additional indirect effects.4 Table 3 reports results when negative affect is jointly studied as a mediator with craving. For both monotherapy and combination therapy effects, the indirect effects of negative affect not only become nonsignificant, but are also nearly 0. Thus, it would appear that whatever effect either form of treatment has on abstinence through negative affect is being simultaneously accounted for through the effects of the treatments on craving. Table 4 reports similar findings when the expectations variable is jointly studied with craving. Despite the more sizeable effects observed for expectations in the univariate analysis, statistical significance is lost in the joint analysis, and the estimated indirect effects related to expectations again fall nearly to 0. It would thus appear likely that the effects seen in the univariate analyses for both negative affect and expectations may well be a consequence of the effects of treatments on craving.
Table 3.
Results from Multivariate Mediation Analysis, Craving and Negative Affect
| Combined Indirect Effects | The Relative Effects of Combo Versus Monotherapy on Mediator | |||||
|---|---|---|---|---|---|---|
| Monotherapy | Combo therapy | Jump Effect | Slope Effect | |||
| Mediator | Est | 95%CI | Est | 95%CI | 95%CI | 95%CI |
| Craving | .13* | (.02,.26) | .19* | (.07,.34) | (.07,.67) | (−.04,.02) |
| Negative Affect |
.01 | (−.03,.06) | .01 | (−.04,.08) | (−.02,.10) | (−.02,.01) |
| Residual Direct Effects | |||
|---|---|---|---|
| Monotherapy | Combo therapy | ||
| Est | 95%CI | Est | 95%CI |
| .41* | (.05,.78) | .73* | (.35,1.11) |
indicates statistical significance, two-tailed alpha=.05
Table 4.
Results from Multivariate Mediation Analysis, Craving and Expectations
| Combined Indirect Effects | The Relative Effects of Combo Versus Monotherapy on Mediator | |||||
|---|---|---|---|---|---|---|
| Monotherapy | Combo therapy | Jump Effect | Slope Effect | |||
| Mediator | Est | 95% CI | Est | 95% CI | 95% CI | 95% CI |
| Craving | .15* | (+.00,.32) | .22* | (.05,.42) | (.10,.64) | (−.04,.03) |
| Expectations | .01 | (−.09,.12) | .00 | (−.15,.17) | (.10,.69) | (−.04,.03) |
| Residual Direct Effects | |||
|---|---|---|---|
| Monotherapy | Combo therapy | ||
| Est | 95% CI | Est | 95% CI |
| .37 | (−.00,.75) | .71* | (.33,1.11) |
indicates statistical significance, two-tailed alpha=.05
Discussion
Using a novel but powerful analytic approach, Bayesian mediation analysis, we examined why smokers who received combination medication were more likely to quit smoking than were those who received either single agent therapy (monotherapy) or placebo. A principal finding of this research is that, amongst all variables tested for mediational effects, craving suppression appeared to be the factor that best accounted for the superiority of combination pharmacotherapy.
Three types of treatment effects were obtained and for which mediational models were evaluated: at the end of treatment (1) the monotherapies were superior to the placebo condition, (2) the combination therapies were superior to the placebo condition, and (3) the combination therapies were superior to the monotherapies. Positive affect was the only candidate mediator that did not yield any evidence of treatment mediation when both monotherapies and combination therapies were combined and compared with placebo. Therefore, it was not tested further. When the group of monotherapy treatments (i.e., the nicotine patch, the nicotine lozenge, and bupropion) was compared with placebo, single-mediator (univariate) tests showed that craving, smoking expectancies, and withdrawal, but not negative affect, yielded significant mediational paths. In comparisons of the combination pharmacotherapies vs. placebo, univariate tests showed that craving, smoking expectancies, withdrawal, and negative affect all supported significant mediational (indirect) paths. Craving tended to produce stronger mediational effects than did the other potential mediators in tests of both the monotherapies and combination therapies. Finally, when the combination pharmacotherapies were compared with the monotherapies, univariate analyses showed significantly greater effects of combination therapy compared to monotherapy on the post-quit jump variable for all candidate mediators except negative affect.
Since craving seemed to yield the most powerful mediational effects of the tested mediators in univariate analyses, the multivariate models were structured to determine whether either of the other specific mediators (negative affect, expectations) would account for a significant amount of variance in the models once craving was included. These analyses showed that the growth trajectory parameters of negative affect and expectations contributed no meaningful effect when craving was in the model. This suggests that the effects of negative affect and expectancies in the univariate models were due to their association with craving.
The results of this research accord with other recent research (Ferguson, et al., 2006; McCarthy, et al., 2008; Piper, Federmen, et al., 2008) that shows that craving reduction is an important mechanism through which pharmacotherapies exert their effects. The results show that smoking cessation pharmacotherapies significantly suppress craving early in the course of a quit attempt, and the extent to which they do so, predicts the likelihood that smokers will be abstinent at follow-up time points. The fact that the jump in craving seen on the quit day proved to be more important than trajectory of craving over the two-week post-quit period, accords with other evidence that craving very early in the quit attempt is a critical determinant of long-term outcomes (e.g., McCarthy, et al., 2006). In addition, the current research adds to earlier evidence implicating craving as a mediator because it tests multiple pharmacotherapies, tests both combination pharmacotherapies and monotherapies, and uses multimediator models to help clarify the relative contributions of the potential mediators. While earlier research suggested that craving was an important mediator of the tested treatments, a lack of multimediator models held open the possibility that other variables might also contribute to mediation or account for the apparent mediation by craving. In addition, it was unknown whether combination therapies produced superior effects to monotherapies because they enhanced actions in the same mediational paths as were activated by monotherapies or because they activated additional or different mediational paths. The results suggest that craving may represent something of a “final common pathway” of pharmacotherapy-induced clinical benefit whether it be induced by combination pharmacotherapy or monotherapy; i.e., it may reflect the net effects of diverse beneficial actions of pharmacotherapy. Of course, this hypothesis must be viewed as only a tentative, working hypothesis. The results show that craving accounts for only a portion of the effects of treatment, which suggests either that other exogenous variables play important mediational roles, or that craving was inaccurately measured, thereby underestimating the extent of its effects.
It is unclear from this research exactly how the combination therapy provides additional craving suppression. It is unknown whether these effects are due to greater medication dose per se vs. due to two different forms of therapy (e.g., the nicotine patch provides a steady state of nicotine while the ad libitum nicotine lozenge allows the smoker to dose emergent cravings acutely). Addressing combination NRT specifically, the evidence is somewhat weak that increasing the nicotine patch dose beyond the standard dose (e.g., 21 mg) increases cessation rates (Fiore, et al., 2008; Killen, Fortmann, Davis, Strausberg, & Varady, 1999; Stead, et al., 2008), yet there is substantial evidence that multiple forms of NRT (the patch plus an acute dosing form) do indeed boost cessation rates (Fiore, et al., 2008; Stead, et al., 2008). There is also prior evidence that the combination of two forms of NRT produces greater craving suppression than a single form (Ferguson & Shiffman, 2009; Schneider, Cortner, Gould, Koury, & Olmstead, 2008; Sweeney, et al., 2001). Thus, with regards to NRT, the evidence suggests that the conjoint use of different types of medication is more important than received dose per se (albeit, there certainly may be strong dose effects at lower dose levels [< 21 mg]; Shiffman & Ferguson, 2008). Of course, the issue of type of medication vs. dose effects is somewhat moot with regard to the nicotine patch + bupropion combination. The two medications are obviously different types of agents neuropharmacologically and have different delivery systems, but neither permits acute dosing. Thus, the extent that this combination produces greater benefit than its constituent monotherapies (Jorenby, et al., 1999; Smith, et al., 2009) must be due to complementary neuropharmacologic actions.
These results have potential clinical relevance. First, they suggest that craving may constitute a useful early or surrogate measure of treatment success. That is, that craving early in the quit attempt can be used in addition to other variables such as early lapsing (see Baker et al., 2010; Perkins, Stitzer, & Lerman, 2006), to identify smokers who may need additional or different treatment in order to attain long-term success. Such a strategy could be used with a Sequential Multiple Assignment Randomized Trial design (Collins, Murphy, & Strecher, 2007) to evaluate how treatment should be modified based upon measures of early response.
The results could also inform treatment development. For instance, one inference that might be drawn from the results is that craving suppression is the most likely way for treatment to improve outcomes. Treatment development efforts, therefore, might focus on treatments that address the modifiable causes of craving. For example, it appears that craving is increased by smoking cues (triggers), distress, falling blood levels of nicotine, the absence of a highly mapped nonsmoking behavioral response to smoking triggers, perceptions of smoking availability or likelihood, and so on (Curtin, McCarthy, Piper, & Baker, 2006; Gloria et al., 2009; Hendricks, Ditre, Drobes, & Brandon, 2006; Juliano & Brandon, 1998; Sayette, Martin, Hull, Wertz, & Perrott, 2003; Sayette et al., 2003). Nonpharmacologic treatments that address these roots of craving might be tried as adjuvants to pharmacotherapy so as to achieve additional craving suppression. Such treatment approaches might include extensive practice of alternative behaviors in the context of smoking triggers, or treatments that systematically expose smokers to withdrawal prior to the quit day (McCarthy, Curtin, Piper, & Baker, 2009). Conversely, these results might encourage researchers to pursue adjuvant treatments that affect mediators other than craving suppression (and, therefore, add clinical benefit along a different mediational path). Treatments that might exert such complementary effects include counseling designed to enhance social support, intrinsic motivation, or self-efficacy (Hendricks, Delucchi, & Hall, 2010; McCarthy et al., 2010). The key is that intended or hypothesized impact on mediators could serve as a guide or touchstone for treatment development.
In addition to its substantive relevance, this research supports the use of a Bayesian approach to mediation analysis. The Bayesian approach recommended by Yuan & MacKinnon (2009) permitted the estimation of complex multivariate models comprising latent variable mediators and binary outcomes. The approach yielded evidence of convergence across multiple indices, and effects that were consistent with prior mediational research. Future research should explore the value of this approach in addressing additional complex questions: e.g., analyses of moderated mediation.
This research highlights questions to be addressed in future research. As noted above, researchers might systematically examine how combinations of counseling and pharmacotherapy treatments affect mediators in order to identify overlapping and distinct mediational paths. In addition, moderated mediational analyses could reveal if subgroups of subjects show different mediational paths. Further, researchers may wish to employ more penetrating mediational measures that yield more specific information on change mechanisms. For instance, craving self-report might reflect density of smoking cues, an inability to cope with craving, or discouragement or low self-efficacy. Also, researchers might want to examine multiple waves of mediators in order to identify change sequences in mediational paths. Thus, we now know that combination pharmacotherapy results in greater craving suppression than does monotherapy, and that this boosts abstinence rates. We do not, though, understand why combination NRT produces greater craving suppression; more molecular change measures and sequential mediator paths might elucidate this and suggest treatment improvements.
One limitation of this research is that a relatively small number of mediators was selected for analysis. This was done to limit the threat of experimentwise error, and to focus on mediators that had the strongest prior evidence of impact. Another limitation is that subjects in this research were participating in an intensive efficacy study and the results might not reflect what occurs in more real-world use of the tested medications. Further, it is almost certainly the case that our measures, especially our measures of the candidate mediators, were affected by considerable error, despite our using real-time data acquisition methods and latent variable modeling. For instance, subjects may have differed in their understanding of terms such as “craving”; they may have used the rating scales in idiosyncratic manners; the sampling time frame may have been nonoptimal; and the effects of smoking during the intratreatment period may not have been optimally statistically controlled. In addition, the mediational modeling approach we use is likely most sensitive to mediators demonstrating tonic changes following a quit attempt. It may well be that mediators such as negative affect demonstrate more phasic effects that are not well captured by the type of linear model used in this analysis to study changes post-quit. This possibility is also reinforced by the distribution observed for the negative affect variable as seen in Table 1, which reflects the rather substantial positive skew typically observed both pre- and post-quit for this variable5.
Conclusion
Prior research shows that combination pharmacotherapy for smoking cessation is more effective than single agent pharmacotherapy. Prior research also indicates that the clinical benefit of single agents is mediated by their suppression of craving. The current findings not only show that craving suppression mediates the clinical effects of single agents, but that it also accounts for the added benefit of combination pharmacotherapy. In fact, while several variables showed evidence that they mediated the additional benefit of combination pharmacotherapy, multiple mediator analyses showed that craving suppression that occurred in the first 24 hours of the quit attempt accounted for all of the obtained mediational effects. Moreover, this research constitutes a successful demonstration of a Bayesian approach to mediation analysis, which should permit the efficient use of complex mediational models.
Footnotes
One study of mediation (Piper et al., 2008) did use a combination of bupropion + nicotine gum in addition to bupropion alone, but that study combined these two conditions in analyses and so yielded no information on combination pharmacotherapy per se.
With withdrawal reflecting multiple symptoms such as craving, irritability, anxiety, sadness, inability to concentrate, and hunger.
These n’s differ from the numbers actually randomly assigned to groups reported by Piper et al. (2009) due to the omission of a small number of participants that provided insufficient EMA data.
We did not use the withdrawal mediator in these analyses since it comprises some of the other mediators (e.g., negative affect, craving) and therefore would have been less useful than the other mediators for highlighting specific mechanisms involved in mediation.
We report analyses using the PANAS negative affect measure as opposed to the negative affect scale of the Wisconsin Smoking Withdrawal Scale (Welsh et al., 1999) due to the PANAS’ display of slightly less positive skew, and thus potentially more tonic change.
Contributor Information
Daniel M. Bolt, Department of Educational Psychology, University of Wisconsin-Madison; Wisconsin
Megan E. Piper, Department of Medicine, Center for Tobacco Research and Intervention, University of Wisconsin School of Medicine and Public Health, Wisconsin
Wendy E. Theobald, Department of Medicine, Center for Tobacco Research and Intervention, University of Wisconsin School of Medicine and Public Health, Wisconsin
Timothy B. Baker, Department of Medicine, Center for Tobacco Research and Intervention, University of Wisconsin School of Medicine and Public Health, Wisconsin
References
- Baker TB, Mermelstein R, Collins LM, Piper ME, Jorenby DE, Smith SS, Fiore MC. New methods for tobacco dependence treatment research. Annals of Behavioral Medicine. 2010 doi: 10.1007/s12160-010-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Blondal T, Gudmundsson LJ, Olafsdottir I, Gustavsson G, Westin A. Nicotine nasal spray with nicotine patch for smoking cessation: randomised trial with six year follow up. British Medical Journal. 1999;318(7179):285–288. doi: 10.1136/bmj.318.7179.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Murphy SA, Strecher V. The Multiphase Optimization Strategy (MOST) and the Sequential Multiple Assignment Randomized Trial (SMART): New methods for more potent eHealth interventions. American Journal of Preventive Medicine. 2007;32(5 Suppl):S112–118. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Cooney JL, Perry BL, Carbone M, Cohen EH, Steinberg HR, Litt MD. Smoking cessation during alcohol treatment: a randomized trial of combination nicotine patch plus nicotine gum. Addiction. 2009;104(9):1588–1596. doi: 10.1111/j.1360-0443.2009.02624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JJ, McCarthy DE, Piper ME, Baker TB. Implicit and explicit drug motivational processes: A model of boundary conditions. In: Weirs RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Thousand Oaks, CA: Sage; 2006. pp. 233–250. [Google Scholar]
- D’Souza MS, Markou A. Neural substrates of psychostimulant withdrawal-induced anhedonia. In: Self DW, Staley JK, editors. Behavioral neuroscience of drug addiction. New York: Springer Publishing Co; 2010. pp. 119–178. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. Journal of Substance Abuse Treatment. 2009;36(3):235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. Using the methods of ecological momentary assessment in substance dependence research--smoking cessation as a case study. Substance Use & Misuse. 2011;46(1):87–95. doi: 10.3109/10826084.2011.521399. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. Journal of Consulting and Clinical Psychology. 2006;74(6):1153–1161. doi: 10.1037/0022-006X.74.6.1153. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, Wewers ME. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008. [Google Scholar]
- Gloria R, Angelos L, Schaefer HS, Davis JM, Majeskie M, Richmond BS, Baker TB. An fMRI investigation of the impact of withdrawal on regional brain activity during nicotine anticipation. Psychophysiology. 2009;46:681–693. doi: 10.1111/j.1469-8986.2009.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, Balabanis MH, Paty JA. Dynamic self-efficacy and outcome expectancies: prediction of smoking lapse and relapse. Journal of Abnormal Psychology. 2005;114(4):661–675. doi: 10.1037/0021-843X.114.4.661. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Delucchi KL, Hall SM. Mechanisms of change in extended cognitive behavioral treatment for tobacco dependence. Drug and Alcohol Dependence. 2010;109(1–3):114–119. doi: 10.1016/j.drugalcdep.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology (Berl) 2006;187(3):385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Herd N, Borland R. The natural history of quitting smoking: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction. 2009;104(12):2075–2087. doi: 10.1111/j.1360-0443.2009.02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine and Tobacco Research. 2007;9(3):315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Ingersoll KS, Cohen J. Combination treatment for nicotine dependence: state of the science. Substance Use and Misuse. 2005;40(13–14):1923–1943. 2043–1928. doi: 10.1080/10826080500294817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine. 1999;340(9):685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: evidence with urge and reaction time. Experimental and Clinical Psychopharmacology. 1998;6(1):45–53. doi: 10.1037//1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Davis L, Strausberg L, Varady A. Do heavy smokers benefit from higher dose nicotine patch therapy? Experimental and Clinical Psychopharmacology. 1999;7(3):226–233. doi: 10.1037//1064-1297.7.3.226. [DOI] [PubMed] [Google Scholar]
- Kim JS, Bolt DM. Estimating item response theory models using Markov chain Monte Carlo methods. Educational Measures. 2007;26(4):38–51. [Google Scholar]
- Kirchner TR, Sayette MA. Effects of smoking abstinence and alcohol consumption on smoking-related outcome expectancies in heavy smokers and tobacco chippers. Nicotine & tobacco research. 2007;9(3):365–376. doi: 10.1080/14622200701188893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornitzer M, Boutsen M, Dramaix M, Thijs J, Gustavsson G. Combined use of nicotine patch and gum in smoking cessation: a placebo-controlled clinical trial. Preventive Medicine. 1995;24(1):41–47. doi: 10.1006/pmed.1995.1006. [DOI] [PubMed] [Google Scholar]
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, Epstein L. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug and Alcohol Dependence. 2002;67(2):219–223. doi: 10.1016/s0376-8716(02)00067-4. [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Bolt DM, Baker TB. The importance of how: A call for mechanistic research in tobacco dependence treatment studies. In: Treat T, Bootzin RI, Baker TB, editors. Psychological clinical science: recent advances in theory and practice. Integrative perspectives in honor of Richard M. McFall. New York: Lawrence Erlbaum Associates; 2007. pp. 133–163. [Google Scholar]
- McCarthy DE, Curtin JJ, Piper ME, Baker TB. Negative reinforcement: Possible clinical implications of an integrative model. Invited chapter. In: Kassel J, editor. Substance Abuse and Emotion. Washington, DC: American Psychological Association; 2009. pp. 15–42. [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. Journal of Abnormal Psychology. 2006;115(3):454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Jorenby DE, Lawrence DL, Shiffman S, Baker TB. A multi-level analysis of non-significant counseling effects in a randomized smoking cessation trial. Addiction. 2010;105(12):2195–2208. doi: 10.1111/j.1360-0443.2010.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Baker TB. Psychological mediators of bupropion sustained-release treatment for smoking cessation. Addiction. 2008;103(9):1521–1533. doi: 10.1111/j.1360-0443.2008.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology (Berl) 2006;184(3–4):628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Piper ME, Federmen EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, Baker TB. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. Journal of Abnormal Psychology. 2008;117(1):94–105. doi: 10.1037/0021-843X.117.1.94. [DOI] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72(2):139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, Baker TB. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Archives of General Psychiatry. 2009;66(11):1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puska P, Korhonen HJ, Vartiainen E, Urjanheimo EL, Gustavsson G, Westin A. Combined use of nicotine patch and gum compared with gum alone in smoking cessation - a clinical trial in North Karelia. Tobacco Control. 1995;4:231–235. [Google Scholar]
- Raftery AE, Lewis SM. How many iterations in the Gibbs sampler? In: Bernardo JM, Berger JO, Dawid AP, Smith AF, editors. Bayesian statistics. Vol. 4. Oxford, UK: Oxford University Press; 1992. pp. 765–776. [Google Scholar]
- Sayette MA, Martin CS, Hull JG, Wertz JM, Perrott MA. Effects of nicotine deprivation on craving response covariation in smokers. Journal of Abnormal Psychology. 2003;112(1):110–118. [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Wertz JM, Martin CS, Cohn JF, Perrott MA, Hobel J. Effects of smoking opportunity on cue-elicited urge: a facial coding analysis. Experimental and Clinical Psychopharmacology. 2003;11(3):218–227. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider NG, Cortner C, Gould JL, Koury MA, Olmstead RE. Comparison of craving and withdrawal among four combination nicotine treatments. Human Psychopharmacology. 2008;23(6):513–517. doi: 10.1002/hup.947. [DOI] [PubMed] [Google Scholar]
- Shah SD, Wilken LA, Winkler SR, Lin SJ. Systematic review and meta-analysis of combination therapy for smoking cessation. Journal of the American Pharmacists Association. 2008;48(5):659–665. doi: 10.1331/JAPhA.2008.07063. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG. The effect of a nicotine patch on cigarette craving over the course of the day: results from two randomized clinical trials. Current Medical Research and Opinion. 2008;24(10):2795–2804. doi: 10.1185/03007990802380341. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kirchner TR, Ferguson SG, Scharf DM. Patterns of intermittent smoking: An analysis using Ecological Momentary Assessment. Addictive Behaviors. 2009;34(6–7):514–519. doi: 10.1016/j.addbeh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, McCarthy DE, Japuntich SJ, Christiansen B, Piper ME, Jorenby DE, Jackson TC. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Archives of Internal Medicine. 2009;169(22):2148–2155. doi: 10.1001/archinternmed.2009.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalter DJ. Understanding uncertainty. Annals of Family Medicine. 2008;6(3):196–197. doi: 10.1370/afm.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton CA, Lloyd-Richardson EE, Papandonatos GD, de Dios MA, Niaura R. Mediators of the relationship between nicotine replacement therapy and smoking abstinence among people living with HIV/AIDS. AIDS Education and Prevention. 2009;21(3 Suppl):65–80. doi: 10.1521/aeap.2009.21.3_supp.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2008;(1):CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- Sweeney CT, Fant RV, Fagerstrom KO, McGovern JF, Henningfield JE. Combination nicotine replacement therapy for smoking cessation: Rationale, efficacy and tolerability. CNS Drugs. 2001;15(6):453–467. doi: 10.2165/00023210-200115060-00004. [DOI] [PubMed] [Google Scholar]
- Sweeney CT, Pillitteri JL, Kozlowski LT. Measuring drug urges by questionnaire: do not balance scales. Addictive Behaviors. 1996;21(2):199–204. doi: 10.1016/0306-4603(95)00044-5. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Ebner-Priemer UW. Using experience sampling methods/ecological momentary assessment (ESM/EMA) in clinical assessment and clinical research: introduction to the special section. Psychological Assessment. 2009;21(4):457–462. doi: 10.1037/a0017653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Yuan Y, MacKinnon DP. Bayesian mediation analysis. Psychological Methods. 2009;14(4):301–322. doi: 10.1037/a0016972. [DOI] [PMC free article] [PubMed] [Google Scholar]