Abstract
Background and Purpose
Endoplasmic reticulum stress triggers apoptotic cascades in neurons of the central nervous system after subarachnoid hemorrhage (SAH). The aim of this work was to study the mechanism of neuroprotection conferred by targeting CHOP in the acute brain injury following SAH.
Methods
A total of 172 rats were used. SAH was induced by endovascular perforation. Two small interfering RNAs for CHOP were injected 24hrs before hemorrhage induction. At 24 or 72 hours rats were neurologically evaluated and euthanized. The brains were recovered for molecular biology and histology studies.
Results
Western blot analysis revealed effective silencing of CHOP associated with suppression of Bim-Caspase-3 apoptotic pathway. Moreover, the anti apoptotic Bcl2 was found upregulated with CHOP siRNA treatment. Histological protection was reflected by a reduced number of TUNEL positive cells in the subcortex and in the hippocampus. CHOP siRNA treatment ameliorated intracranial sequelae of SAH and improved functional performance.
Conclusions
We conclude that CHOP silencing alleviates early brain injury following SAH via inhibiting apoptosis and that CHOP siRNA treatment has a clinical potential for patients with this type of hemorrhagic stroke.
Keywords: Subarachnoid hemorrhage, siRNA, CHOP, Bim, Bcl2, Apoptosis
Introduction
The extensive protein damage occurs after subarachnoid hemorrhage (SAH), which leads to overloading endoplasmic reticulum (ER) with aberrant and unfolded proteins. This phenomenon may trigger unfolded protein response as an adaptive cellular mechanism, which when overwhelmed, leads to ER stress and activation of apoptotic mechanisms. All major ER stress pathways - mediated by inositol-requiring enzyme-1 (IRE1), PKR-like ER kinase (PERK) and activating transcription factor 6 (ATF6) converge on one transcription factor named C/EBP homologous protein (CHOP alias DDIT3/GADD153). PERK phosphorylates eIF-2α, which in turn activates activating transcription factor 4 (ATF4). CHOP gene promoter contains binding site for ATF4 and ATF6 1. While PERK is required for transcriptional CHOP induction in response to ER stress 2. IRE1 may activate CHOP at the post-transcriptional level, through p38 MAPK 1. The contribution of CHOP to neuronal death via apoptosis has been evidenced in the experimental cerebral ischemia 3. In response to severe ER stress CHOP activates the expression of Bim leading to Caspase-3 cleavage and apoptosis 4. However, the contribution of this pathway to the acute brain injury after SAH remains unknown. Meanwhile, the components of extravasated blood and the impact of generalized acute brain ischemia are capable of inducing ER stress in SAH. Therefore we sought to determine whether silencing CHOP confers neuroprotection in the hemorrhagic brain. To this end we used two distinct CHOP siRNAs in the rat perforation model of subarachnoid hemorrhage.
Methods
Animal Groups and Endpoint Measures
A total of 172 male Sprague-Dawley rats were randomly assigned to the following groups: sham surgery, SAH untreated (vehicle group), and groups subjected to SAH and prior i.c.v. injection (24hrs before SAH) of either of two sequences of siRNA for CHOP or scrambled RNA. SAH was performed by endovascular perforation with 4–0 nylon monofilament suture5. At 24 and 72hrs following SAH, the rats were euthanized under ketamine (100mg/kg) and xylazine (10mg/kg) anesthesia and perfused transcardially, with ice cold PBS alone for molecular biology, followed by 10 % buffered formalin for histology. At 72hrs only brain water content and neurobehavioral tests were performed. The present study was approved by Institutional Animal Care and Use Committee at Loma Linda University.
SAH Severity and Neurological Scoring Systems
In order to confirm the equivalent level of SAH severity across groups, we applied SAH severity classification system developed by Sugawara et al. as described 6. We used modified Garcia score system for neurological testing in a blinded fashion 7. The maximum neurological score was 24 indicating a healthy rat. Seventy-two-hour mortality was calculated by dividing the number of dead animals by the number of total used animals 5.
Evans Blue Dye Extravasation
The permeability of Blood-Brain barrier (BBB) was evaluated on the basis of Evans Blue extravasation as described previously 8. The brain level of Evans Blue was determined by spectroflurophotometry at excitation wavelength 610 nm, emission wavelength of 680 nm, and a bandwidth of 10 nm 8.
Brain Water Content
Brain water content was determined at 24 and 72hrs after SAH 8 by weighing then drying brain tissues for 24hrs at 105°C in the oven. The water content was calculated according to the following formula: [(wet weight-dry weight)/wet weight]×100% 8.
CHOP Silencing
CHOP silencing experiments used intracerebroventricular (i.c.v.) infusion of two CHOP siRNAs with the following coordinates: 1.5 mm posterior, 1.0 mm lateral, and 3.2 mm below the horizontal plane of bregma 5. The sequence of the first siRNA for CHOP was: sense, 5′GGAAGAACUAGGAAACGGA; antisense, 5′UCCGUUUCCUAGUUCUUCC. The second siRNA sequence was: sense, 5′ CUGGGAAACAGCGCAUGAA; antisense, 5′ UUCAUGCGCUGUUUCCCAG. The irrelevant scrambled RNA served as a control (Dharmacon/Thermo Fisher Scientific, Lafayette, Colo). RNAs were injected in the sterile PBS at a rate of 0.5 microL/min at 24hrs prior to SAH surgery5. Sham animals received burr hole however were not subjected to i.c.v. injections.
Western Blot Analysis
Cerebral tissues facing blood clots were homogenized in the RIPA buffer containing protease/phosphatase inhibitors as described9. The aliquots of 30 micrograms of total protein were run on SDS-PAGE gels and transferred onto the nitrocellulose membranes. The membranes were probed at 4°C overnight with the following primary antibodies (Millipore; all diluted 1:500): CHOP, Bim, Bcl2, Caspase3, and β-actin, then incubated for 2hrs at room temperature (RT) with respective secondary antibodies diluted 1:2000. The chemiluminescence signal was developed with ECL kit reagents (Amersham) and detected by exposure of Kodak radiographic films. The densitometry analysis was conducted with Image J software (NIH). The results were expressed relative to β-actin bands and normalized with regard to sham.
Tripple Fluorescence Staining
Brain specimens were fixed in formalin, then processed as described to obtain 10μm frozen sections on glass slides. The antigen retrieval was done by microwaving in 0.1 M sodium citrate at pH 6 for 10min. This was followed by incubation with blocking serum for 1 hr at RT and overnight incubation with CHOP and NeuN primary antibodies (Millipore) (diluted 1:100) followed by secondary antibody (2hrs at RT) 10. TUNEL was done with a kit 11. Histological preparations were coverslipped with antifade reagent (Millipore) and looked at under OLYMPUS BX51 microscope. The photomicrographs for AMCA, Texas Red and fluorescein stains were taken with computer camera and merged with Magnafire software (Systat) 10. Negative staining procedure was conducted with the omission of primary antibodies.
Quantitative Cell Count
TUNEL positive cells were counted in a blind manner, numbers of TUNEL positive cells were calculated and subcortical regions in the hippocampus were averaged from eight photomicrographs from each animal 11. Four rats per group were used for quantitative histology analysis.
Statistical Analysis
Data are expressed as means +/− SEM. Statistical significance was verified with ANOVA followed by Tukey test for multiple comparisons. The probability level p<0.05 was considered statistically significant. The analysis of mortality was done with chi square test. Non parametric ANOVA was used for categorical variables.
Results
SAH Severity Scores
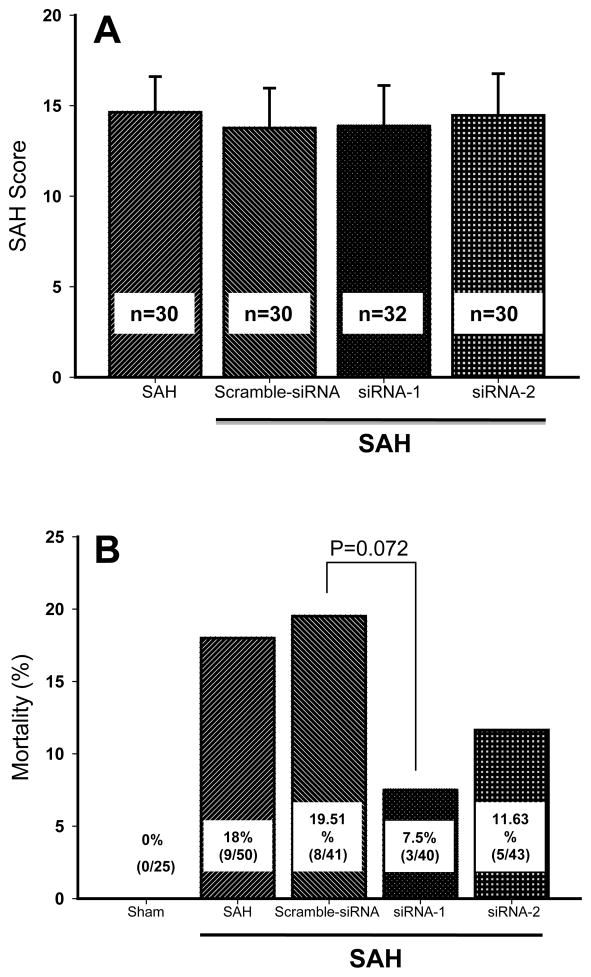
Mean SAH severity score measured 24hrs after the bleeding was within the range from 14 to 15 points (Figure 1A). There was no significant difference in the severity scores across groups (ANOVA).
Figure 1. SAH score and mortality rates in experimental groups.
(A) SAH severity scores were equivalent between untreated controls and siRNA treatment groups.
(B) The tendency towards reduced mortality was found in both groups with CHOP siRNA treatment.
Mortality Rates
No mortality was recorded in the sham operated rats (Figure 1B). Eighteen percent mortality was recorded in the vehicle group while 19.5% mortality occurred in the scramble RNA group. Treatments with siRNA1 and siRNA2 reduced mortality rates to 7% and 11.6 %, respectively. Our study however, was not powered enough to detect the statistical difference in the mortality across groups (p=0.072, Chi square).
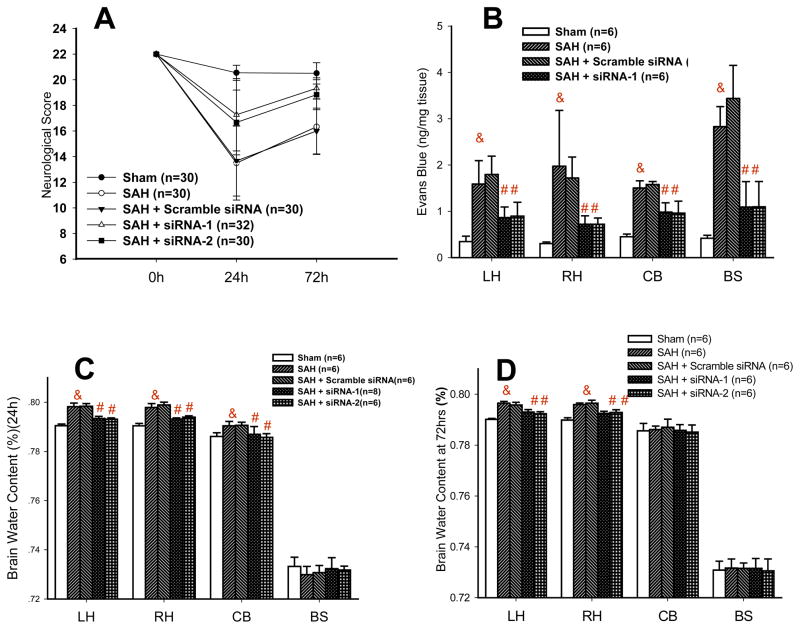
Neurological Scores
The score plunged from 22 to 13.5 points in the vehicle group and to 13.7 points in the scrambled RNA group (Figure 2A). Then there was a higher score in the CHOP siRNAs treated rats in comparison to untreated controls (17.3 points for siRNA1 and 16.7 points for siRNA2). At 72hrs the scores in the vehicle and scrambled group were 16.3 and 16 points, respectively. Small difference in neurological scores (not exceeding 1.5 point) between siRNA treated group and sham proved to be insignificant.
Figure 2. CHOP silencing improves clinically relevant outcomes after SAH.
(A) siRNA treatments brought about the amelioration of neurological scores.
(B) Reduced Evans Blue extravasation after treatment with CHOP siRNA occurred in brain hemispheres and cerebellum (& p<0.05 versus sham # p<0.05 versus SAH).
(C) CHOP siRNA reduced brain water content at 24hrs after SAH (& p<0.05 versus sham # p<0.05 versus SAH).
(D) CHOP siRNA treated groups exhibited normalized brain water level at 72hrs post SAH.
BBB Permeability
The Evans Blue level increased in cerebral tissues in the vehicle group compared to sham at 24 hours after surgery; 4.6 fold in the left hemisphere (LH), 6.51 fold in the right hemisphere (RH), 3.34 fold in the cerebellum (CB) and 6.76 fold in the brain stem (BS) (Figure 2B). The increases of similar magnitude were recorded in rats injected with the scrambled RNA. CHOP siRNA reduced brain permeability for Evans Blue in all examined brain regions, as compared to vehicle and scramble RNA treated controls. As compared to vehicle group the reduction in Evans Blue level was 46.06% and 43.60% in LH; 63.66% and 63.51% in RH, 34.84% and 36.10% in CB and 61.40% and 61.22% in BS, with siRNA1 and siRNA2 treatments, respectively (& p<0.05 versus sham # p<0.05 versus SAH).
Brain Water Content
Results are shown in the figure 2. At 24hrs after SAH the brain water content increased by 0.78% in left hemisphere (LH), 0.74% in right hemisphere (RH), 0.43% in cerebellum (CB), while no significant change was found in the brain stem (BS) (Figure 2C). At 72hrs after SAH the increase in brain water was 0.64% in LH and 0.61% in RH while CB and BS showed no increase in water content (Figure 2D). With both siRNA treatments, the brain water content was nearly normalized at 24hrs and 72hrs after SAH.
The Protein Expression of CHOP and Its Downstream Targets
CHOP silencing reduced CHOP protein level in the cerebral tissues as determined by Western Blot analysis (Figure 3A). The increase in CHOP in vehicle treated SAH was 2.74-fold (2.96-fold in the scramble RNA group) respective to sham, indicative of ER stress response activated after the hemorrhage. However in the siRNA treated rats, the increase in CHOP proved to be only 33.78% and 32.45% of the increase in the vehicle group, with siRNA1 and siRNA2 treatment, respectively. The increase in Bim level was 3.57-fold in the SAH group compared to sham (Figure 3B). Silencing CHOP with two different siRNAs reduced expression of Bim in the cerebral tissues, to 31.18% (siRNA1) and 35.22% (siRNA2) of increase noted in the vehicle-treated group. Scramble RNA had no preventive effect on the SAH-induced Bim upregulation (3.83-fold increase with regard to sham). Bcl2 level at 24hrs after SAH dropped to 27.33% of the basal level. In the scramble RNA-injected rats, bcl2 level was still depressed after SAH, at 25.11% of control. However, following CHOP siRNA treatment the levels of Bcl2 determined in the cerebral tissues were 2.27 and 2.87 times higher than those found in the vehicle group (Figure 3C). The level of cleaved Caspase-3 in the hemorrhagic brain increased in the untreated and scramble RNA treated rats, respectively, up to 365% and 347% of sham control levels (Figure 3D). However, these increases in cleaved Caspase-3 levels were reduced with the siRNA and siRNA2 treatments, by 69.85% and 72.89 %, respectively.
Figure 3. CHOP silencing attenuates ER stress apoptotic pathway and activates antiapoptotic response.
(A) Both siRNAs for CHOP downregulated CHOP protein in response to SAH at 24hrs.
(B) Downregulation of Bim after injection of siRNA found in the ipsilateral brain hemisphere.
(C) Upregulation of Bcl2 was observed in siRNA treated group
(D) Cleaved caspase-3 levels after SAH decreased with the siRNA treatment.
Apoptotic Labeling Colocalizes with Neuronal Expression of CHOP after SAH
Tripple fluorescence staining revealed increased CHOP immunoreactivity in the hippocampus and in the subcortical region and at 24hrs after SAH (Figure 4i and 4v) Merged images revealed that CHOP immunoreactivity colocalized with TUNEL in the neuronal cells (Figure 4iv and 4viii).
Figure 4.
Histological analysis of CHOP dependent apoptosis in the CA1 sector of the hippocampus and subcortical brain region reveals CHOP and TUNEL colocalization in the NeuN positive cells (AMCA: CHOP, Texas Red: NeuN, fluorescein: TUNEL; final magnification X400).
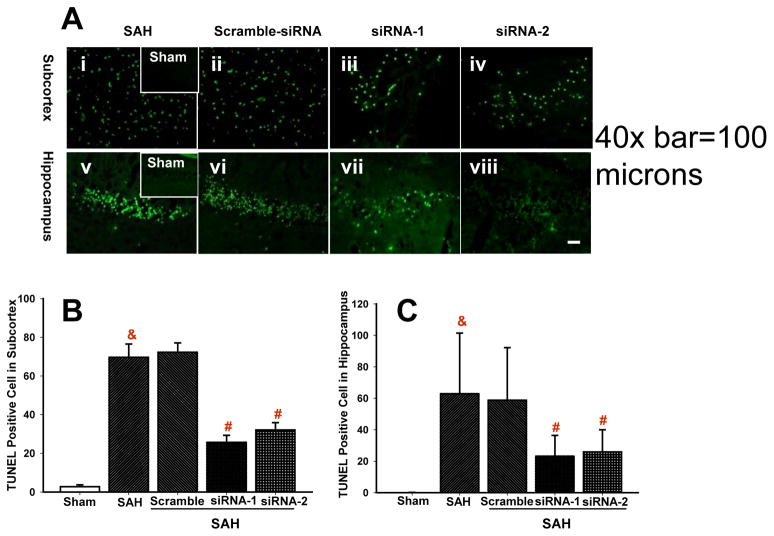
The Effect of CHOP siRNA on Apoptotic Cell Count after SAH
The quantitative analysis of cells with apoptotic labeling (TUNEL) in the posthemorrhagic brain are demonstrated in the Figure 5. Numerous TUNEL positive cells were found in the subcortical brain region after SAH treated with vehicle and scramble-RNA (Figure 5Ai and 5Aii), in contrast with weak TUNEL positivity in the sham operated animals (Figure 5Ai inset). Cell count showed 70±6 and 72±4 TUNEL positive cells per magnification field in subcortex after SAH treated with vehicle and scramble RNA, respectively (Figure 5B). Treatment with siRNA for CHOP grossly reduced numbers of TUNEL positive cells although it did not eliminate apoptotic cells completely (Figure 5Aiii and Aiv). In the subcortical region percent reduction in TUNEL positive cells was 63.2% for CHOP siRNA1 and 53.9% for siRNA2 as compared to vehicle (Figure 5B). The number of TUNEL positive cells after vehicle treated SAH amounted 62±16 in the hippocampus (Figure 5Av and 5C) while there was no hippocampal TUNEL positivity in the sham group (Figure 5Av inset). As shown by the graph (Figure 5C) the numbers of TUNEL positive hippocampal cells were significantly reduced, by 63.2% and 58.9% with siRNA1 and siRNA2 treatment, respectively.
Figure 5.
Quantitative cell count. (A) CHOP siRNA treatment reduced abundance of TUNEL positive cells in the brain. (B&C) Graphs show reduced numbers of apoptotic cells in the subcortex (B) and in the hippocampus (C).
Discussion
The results of this study indicate that CHOP siRNA treatment provides neuroprotection in the rat model of subarachnoid hemorrhage. Suppression of the major mediator of apoptotic pathway after SAH resulted in the significant upregulation of antipapoptotic protein Bcl2 while the expression of proapoptotic molecules including the executioner Caspase-3, was attenuated. Thus silencing CHOP, a major mediation of ER stress, resulted in the inhibition of apoptosis. Furthermore, the observed reduced BBB disruption brought significant reduction in the brain edema. Histological and molecular neuroprotection was accompanied by neurological improvement in the treated rats. The tendency towards reduced mortality rates with the treatment further emphasizes translational significance of the proposed siRNA-based therapy.
Our study has demonstrated that SAH induces ER stress and CHOP overexpression with activation of apoptotic cascades in the pyramidal cells of the hippocampus and subcortical neurons of the brain. This effect can be mediated by ischemic stimulation 12 but also by the action of hemoglobin degradation products including iron 13. Previous studies revealed that CHOP mediates apoptosis after cerebral ischemia 14. It has been also proven that ER stress initiates widespread pathological apoptosis, which underlies injury of the brain and other organs 4. The neurological deficits after SAH have been ascribed to this excessive apoptosis 7. Therefore, reduced apoptosis with CHOP siRNA treatment could produce the beneficial effect of CHOP inhibition on the functional performance in the treatment group. Interestingly, widespread apoptosis in this present study was detected in neurons, which is consistent with impaired neurological status after SAH. There was only little immunoreactivity of CHOP seen outside neurons. Indeed, the role of CHOP in the astrocyte death is debatable 15, 16.
Studies have documented that ER stress triggers apoptosis through activation of Bim 17. The role of Bim in early brain injury after SAH has been hypothesized by earlier authors although never thoroughly studied 18. Bim is upregulated by ER stress through direct CHOP transcriptional induction 17. Subsequent permeability transition pore activation leads to cytochrome c release, apoptosome formation and Caspase-3 activation 19. As a result of CHOP knockdown, the upregulation of Bim after SAH was prevented, which led to suppression of downstream apoptosis pathway 20. Consequently, cleaved Caspasse-3 was attenuated with the siRNA treatment.
Moreover, by blocking CHOP we were able to prevent SAH-induced Bcl2 suppression as the Bcl2 level was not significantly different from sham with either of the treatments. Bcl2 suppression via activation of transcriptional repressors is an established mechanism of CHOP-induced apoptosis 4. Thus the inhibibtion of CHOP could reduce apoptosis via prevention of Bcl2 downregulation. ER restricted Bcl2 can inhibit apoptosis induced by a variety of stimuli 20. Bcl2 can antagonize apoptotic effect via sequestrating Bim, required for mitochondrial permeabilization mediated by Bax 4. Interestingly, in primary neuronal cultures CHOP appears to protect against neuronal death 21 showing that the role of this factor varies across different experimental settings and may depend on different cell type interactions. These conflicting results further support a need to investigate the role of CHOP in distinct models of cerebrovascular diseases.
Our results, showing a robust protection of BBB with siRNA treatment may point towards inhibition of CHOP mediated apoptosis in the endothelial cell compartment. CHOP inhibition reduced vasogenic brain edema which occurs as a consequence of BBB disruption after SAH 7. The role of ER stress in the endothelial apoptosis is well documented and occurs in response to a variety of stimuli including atherosclerosis promoting factors and immunosuppressants 22, 23. Although we have shown that CHOP inhibition could reduce BBB damage further studies are warranted to gain mechanistic insight on this effect.
We used two siRNAs targeting CHOP mRNA in individual transfections rather than via pooling approach. Thereby we wanted to verify the specificity of the protective phenotype derived from gene silencing prior to SAH and to minimize the cumulative effect of off-target genes that often occurs in the pooled experiments 24. However, for the sake of clinical relevance, a rapid post SAH siRNA administration is desired. In order to work towards this requirement further investigations may employ intranasal delivery, which bypasses BBB and promotes fast onset of action for siRNA based therapeutics 25.
Summary
In summary, the results of the present study confirm the involvement of CHOP in ER stress as a major inducer of apoptosis after SAH. CHOP silencing leads to reduction of apoptosis, preservation of blood brain barrier and improved neurological function. This may suggest that antiapoptosis as therapeutic approach in the acute SAH may not be fully effective without targeting ER component. To our knowledge neuroprotective effects of targeting CHOP in SAH have been demonstrated for the first time in this present study. Our findings also reveal translational potential of siRNA based therapeutics targeting apoptotic mechanisms after SAH.
Footnotes
Conflict of Interest Disclosure
None
References
- 1.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 2.Banhegyi G, Mandl J, Csala M. Redox-based endoplasmic reticulum dysfunction in neurological diseases. J Neurochem. 2008;107:20–34. doi: 10.1111/j.1471-4159.2008.05571.x. [DOI] [PubMed] [Google Scholar]
- 3.Oida Y, Shimazawa M, Imaizumi K, Hara H. Involvement of endoplasmic reticulum stress in the neuronal death induced by transient forebrain ischemia in gerbil. Neuroscience. 2008;151:111–119. doi: 10.1016/j.neuroscience.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 4.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke. 2010;41:1783–1790. doi: 10.1161/STROKEAHA.110.586537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S, Yamaguchi M, Zhou C, Calvert JW, Tang J, Zhang JH. Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke. 2004;35:2412–2417. doi: 10.1161/01.STR.0000141162.29864.e9. [DOI] [PubMed] [Google Scholar]
- 8.Tsubokawa T, Solaroglu I, Yatsushige H, Cahill J, Yata K, Zhang JH. Cathepsin and calpain inhibitor E64d attenuates matrix metalloproteinase-9 activity after focal cerebral ischemia in rats. Stroke. 2006;37:1888–1894. doi: 10.1161/01.STR.0000227259.15506.24. [DOI] [PubMed] [Google Scholar]
- 9.Fathali N, Ostrowski RP, Lekic T, Jadhav V, Tong W, Tang, et al. Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury. Crit Care Med. 2010;38:572–578. doi: 10.1097/CCM.0b013e3181cb1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng O, Ostrowski RP, Wu B, Liu W, Chen C, Zhang JH. Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Stroke. 2011;42:484–490. doi: 10.1161/STROKEAHA.110.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matchett GA, Calinisan JB, Matchett GC, Martin RD, Zhang JH. The effect of granulocyte-colony stimulating factor in global cerebral ischemia in rats. Brain Res. 2007;1136:200–207. doi: 10.1016/j.brainres.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudo T, Kanemoto S, Hara H, Morimoto N, Morihara T, Kimura R, et al. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008;15:364–375. doi: 10.1038/sj.cdd.4402276. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira SJ, de Sousa M, Pinto JP. ER Stress and Iron Homeostasis: A New Frontier for the UPR. Biochem Res Int. 2011;2011:896474. doi: 10.1155/2011/896474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada JI, et al. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 2004;11:403–415. doi: 10.1038/sj.cdd.4401365. [DOI] [PubMed] [Google Scholar]
- 15.Benavides A, Pastor D, Santos P, Tranque P, Calvo S. CHOP plays a pivotal role in the astrocyte death induced by oxygen and glucose deprivation. Glia. 2005;52:261–275. doi: 10.1002/glia.20242. [DOI] [PubMed] [Google Scholar]
- 16.Osada N, Kosuge Y, Ishige K, Ito Y. Characterization of neuronal and astroglial responses to ER stress in the hippocampal CA1 area in mice following transient forebrain ischemia. Neurochem Int. 2010;57:1–7. doi: 10.1016/j.neuint.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 19.Cheng G, Chunlei W, Pei W, Zhen L, Xiangzhen L. Simvastatin activates Akt/glycogen synthase kinase-3beta signal and inhibits caspase-3 activation after experimental subarachnoid hemorrhage. Vascul Pharmacol. 2010;52:77–83. doi: 10.1016/j.vph.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Heath-Engel HM, Chang NC, Shore GC. The endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2 protein family. Oncogene. 2008;27:6419–6433. doi: 10.1038/onc.2008.309. [DOI] [PubMed] [Google Scholar]
- 21.Halterman MW, Gill M, DeJesus C, Ogihara M, Schor NF, Federoff HJ. The endoplasmic reticulum stress response factor CHOP-10 protects against hypoxia-induced neuronal death. J Biol Chem. 2010;285:21329–21340. doi: 10.1074/jbc.M109.095299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouvier N, Flinois JP, Gilleron J, Sauvage FL, Legendre C, Beaune P, et al. Cyclosporine triggers endoplasmic reticulum stress in endothelial cells: a role for endothelial phenotypic changes and death. Am J Physiol Renal Physiol. 2009;296:F160–F169. doi: 10.1152/ajprenal.90567.2008. [DOI] [PubMed] [Google Scholar]
- 23.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, et al. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 25.Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130:151–158. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]