Abstract
Defining the pre-psychotic state in an effort to prevent illness progression, and the development of disorders such as schizophrenia, is a rapidly growing area of psychiatry. The presentation of psychotic symptoms can be influenced by culture; however there has not been any previous assessment of psychosis-risk symptoms in the continent of Africa. Our study aimed to measure the prevalence of psychosis-risk in a community sample in Nairobi, Kenya, and to evaluate the effects of key demographic variables.
A culturally modified version of the 12-item PRIME-Screen (mPRIME) was self-administered by 2,758 youth (aged 14–29) recruited through house-to-house visits in Nairobi, Kenya. The prevalence and severity of psychosis-risk items from the mPRIME, and the effects of gender and age on symptoms were evaluated. k-Means cluster analysis was used to identify symptom groups.
Depending on the mPRIME item, 1.8–19.5% of participants reported certainty of having had a psychosis-risk symptom. Overall, 45.5% reported having had any psychosis-risk symptom. Females had a significantly higher mean severity score on items evaluating persecutory ideation and auditory hallucinations. Symptom severity on five items showed a modest (R=0.09–0.13) but significant correlation with age. Cluster analysis identified four groups of participants: normative (55%), high symptom (11%), intermediate symptom (19%), and grandiose symptom (15%).
Psychosis-risk symptoms appear to be highly prevalent in Kenyan youth. Longitudinal studies are needed to determine the correlation of identified symptoms with transition to psychotic illness, as well as the associated functionality and distress, in order to develop appropriate intervention strategies.
Keywords: Psychosis, Risk, Kenya, Africa, Youth, Prime screen
Schizophrenia and other psychotic disorders are among the most disabling psychiatric disorders, estimated to affect about 3% of the world’s population (1). Early detection of psychosis has been associated with less severe symptoms and fewer hospitalizations upon emergence of psychotic illness (2), which has profound importance when considering strategies of efficient and cost-effective health care delivery (3). Preventing the future development of a severe psychotic disorder is regarded as among the most effective ways to reduce this potentially devastating burden on the affected individual and family members (4). In sub-Saharan Africa, where financial and health care resources for managing psychotic disorders are extremely limited, the need for effective preventive strategies prior to disorder onset is therefore fundamental (5).
The ultra-high-risk (UHR) criteria, a concept of early detection of help seeking patients at short-term risk of psychosis have become an increasing focus of current research (6). Retrospective studies have confirmed an average prodromal period (i.e. period before disorder onset) of 5 to 6 years (7), and the introduction of UHR criteria has significantly advanced the possibility of indicated prevention during this period (6). The substantial body of UHR research has led to criteria for the identification of UHR individuals using structured interviews (8). These schedules generally identify three groups of UHR: the familial high risk, the attenuated positive symptoms, and the brief limited intermittent psychotic symptoms group. Studies have indicated that 16% to 54% of youth who meet current UHR criteria develop a major psychotic disorder (e.g. schizophrenia, schizoaffective disorder, and bipolar or unipolar depression with psychotic features) within 1–2.5 years (6, 9, 10).
The PRIME-Screen (11, 12) is a self-reported instrument, based on the Structured Interview for Psychosis-Risk Symptoms (SIPS)(8), and designed to enable rapid identification of those at-risk for psychotic disorders. It consists of 12 items covering positive symptoms and uses a self-rated scoring system of between 0 (definitely disagree) and 6 (definitely agree), with a score of 3 indicating “not sure”. Using limited samples of patients, a high sensitivity and perfect specificity has been reported (11), although predictive validity has not been examined. General agreement on what constitutes the UHR state using the PRIME-Screen has not been established, although a score of 6 in at least one item is considered suggestive (11, 12). A modified version of the PRIME-Screen, which considered duration of symptoms, showed a specificity and sensitivity (against SIPS as a gold standard) of 0.74 and 1.00, and a concordant validity 0f 0.43 (12). A brief self-administered screen has a potential advantage in evaluating the prevalence of psychosis-risk symptoms in large community settings, were administration of a more extensive, time-consuming semi-structured interview may not be feasible. Self-administration may also reduce inherent biases that may exist in researcher assisted interviewing, particularly in cultures where certain questions may seem unfamiliar.
There have been no previous published reports evaluating prodromal or clinically high-risk individuals in the continent of Africa (5). The limited data available from more developed countries may not representative of Africa as the presentation of schizophrenia and psychosis differs across cultures (13, 14). Epidemiologic studies in Africa suggest that there may be differences in the prevalence of psychotic illness across cultures (15), though there have been variable results across studies and surveyed populations within the continent. For example, the prevalence of schizophrenia in rural African communities has ranged between 4.3–60.0 per 1,000 (16–18), which are lower than that typically reported in western countries. However, such comparisons are limited by cultural differences in the worldview of concepts, which may influence the perception of psychotic illness (19) and thus the estimated prevalence.
Our primary aim was to evaluate the prevalence of various psychotic risk-symptoms in a large (N=2,758) community sample in Nairobi, Kenya, using a culturally modified PRIME-Screen. We explored the effect of gender on symptom manifestation, hypothesizing that symptoms will be more prevalent in males compared to females, consistent with previous studies showing higher rates of schizophrenia and psychotic experiences or an earlier age of onset in males (20). Age effects on reporting psychosis-risk were also evaluated, to gain insight into screening questions that may be more useful at various stages of development. Finally, we explored subject reports on the severity of specific psychosis-risk symptoms to identify groups of subjects, using cluster analysis.
METHODS
Recruitment
Participants were recruited between August 9th and August 26th, 2010, through house-to-house visits in Kangemi, a slum neighborhood of the city of Nairobi, Kenya, located 6 miles from the city center. Conditions in Kangemi are very poor, and many of its residents lack access to basic services, including electricity and running water, however the majority of youth attend public schools and are proficient in reading and writing in English. There were eight recruiters involved in the study. Recruiters were trained third- and fourth-year nursing students from the University of Nairobi. Written and signed consent was obtained from all participants, who were then asked to fill the questionnaire on their own, with staff available for questions if needed. 2,800 individuals were approached to participate in the study, and 2,758 agreed to participate. The study was approved by Washington University Medical School’s institutional review board (IRB), the Kenyan Medical Research Institute (KEMRI), and the Ministry of Education, Science and Technology, Kenya.
Assessment
Participants were asked to complete the 12-item PRIME-Screen, which was slightly modified to be better understood by local Kenyan youth (mPRIME). Modifications were determined following a series of discussions by Kenyan AMHF researchers and Washington University researchers. Item modifications were relatively minor, and involved minimal edits to the phrasing of some questions. In additional, item 9 of the original PRIME-Screen was deleted, since it was felt that that the question “I think I might feel like my mind is playing tricks on me”, would be difficult to understand in the local culture. We substituted this item, with another: “Has your mental state or thinking worsened in the last year”, to evaluate recent for change in subject experiences. The PRIME-Screen is structured such that each item can be answered on a severity scale: 0 - definitely disagree, 1 - somewhat disagree, 2 - slightly disagree, 3 - don’t know, 4 – slightly agree, 5 – somewhat agree, and 6 – definitely agree. For purposes of evaluating items as continuous measures, “don’t know” answers were excluded, and scales were condensed into 0-to-5 range scales.
Statistical Analysis
General statistical analyses were done using SAS 9.1 (SAS Institute, Cary, NC). Gender differences in item prevalence severity were compared using the student’s t-test (two-tailed). Age effects on severity scores were evaluated using Pearson’s correlation coefficient. Significant p-values were set at 0.004 (0.05/12), to correct for multiple comparisons.
We used k-means iterative cluster analysis (21) to identify latent subgroups of subjects with related patterns of psychosis-risk symptoms, from items on the mPRIME. Gender was also included in the analysis, as a co-variate. Only participants that completed all items on the mPRIME, did not answer “I don’t know” for any item, and indicated gender were included in the analysis (N=908). k-means iterative cluster analyses handle larger data sets more efficiently than hierarchical agglomerative methods (21). We used an algorithm in which each item is assigned to the cluster having the nearest centroid (mean). This nonhierarchical method initially takes the number of components of the population equal to the final required number of clusters. The final required number of clusters is chosen such that the points are mutually farthest apart. Next, it examines each component in the population and assigns it to one of the clusters depending on the minimum distance. The centroid’s position is recalculated every time a component is added to the cluster and this continues until all the components are grouped into the final required number of clusters. As there are no completely satisfactory methods for determining the number of population clusters (22), we ran numerous analyses with various values of k (from k=2 to k=10), with the goal of finding clusters with high concentrations of subjects. A four-cluster solution provided the most clarity with regard to the interpretability of the scores revealed on each item for the participants forming these clusters. A four-cluster solution represented a relatively large change (29.9%) in the overall R-square from a three-class solution, and the R-square further increased by less than 20% with additional numbers of clusters.
RESULTS
Demographics
In total, 2,758 individuals participated in the study. Participant ages ranged 14 and 29, with a mean age of 18.5 yrs (SD=3.4), and a median age of 18. There were 1,628 (60.5%) males and 1,064 (39.5%) females among the participants, with 66 participants not disclosing their gender.
Prevalence of Psychosis-Risk Symptoms
1,255 (45.5%) of those surveyed indicated certainty (i.e. “definitely agree” on the mPRIME) on having had any psychosis-risk symptom in their lifetime. Among the total population, the percentages stating “definitely agree” was 16.7% for 1 symptom, 11.0% for 2 symptoms, 8.2% for 3 symptoms, 5.0% for 4 symptoms, 2.4% for 5 symptoms, 1.4% for 6 symptoms, 0.5% for 7 symptoms, 0.1% for 8 symptoms, 0.1% for 9 symptoms, and 0.04% for 11 symptoms. Prevalence rates were 46.3% in males, and 44.8% in females, and differences were not significant (χ2=0.6, p=0.4). Excluding item 9 (i.e. “Worsening in Last 12 Months”) of the mPRIME did not significantly affect the prevalence of having any psychosis-risk symptom (45.2%).
Table 1 lists the prevalence of the six severities of mPRIME psychosis-risk items. Across all items, 3.7% (“Going Crazy”) to 34.7% (“Supernatural/Special”) of individuals reported having risk symptoms to any degree. However, only 1.8% to 19.5% reported the highest degree of certainty (i.e. “definitely agree”) to having experienced these symptoms. In contrast, 54.6% to 90.4% of individuals disagreed with having symptoms to any degree, and 37.7% to 83.8% “definitely disagreed” with having symptoms.
Table 1.
mPRIME-Screen Psychotic-Risk Symptom Endorsement (N=2,758)
| Experiences* | Definitely Disagree |
Somewhat Disagree |
Slightly Disagree |
TOTAL Disagree |
Definitely Agree |
Somewhat Agree |
Slightly Agree |
TOTAL Agree |
|
|---|---|---|---|---|---|---|---|---|---|
| 1. | Odd or unusual things going on I can’t explain | 1222 (45.4) | 294 (10.9) | 171 (6.4) | 1687 (62.7) | 275 (10.2) | 110 (4.1) | 264 (9.8) | 649 (24.1) |
| 2. | I may be able to predict future | 1209 (44.4) | 243 (8.9) | 202 (7.4) | 1654 (60.7) | 272 (10.0) | 128 (4.7) | 287 (10.5) | 687 (25.2) |
| 3. | Have felt something interrupting/controlling thoughts or actions | 1029 (37.7) | 346 (12.7) | 245 (9.0) | 1620 (59.3) | 292 (10.7) | 184 (6.7) | 311 (11.4) | 787 (28.8) |
| 4. | Experienced doing something differently due to superstitions | 1345 (49.2) | 242 (8.9) | 206 (7.5) | 1793 (65.6) | 267 (9.8) | 119 (4.4) | 252 (9.2) | 638 (23.3) |
| 5. | Get confused whether something is real or imaginary/dream | 1184 (43.2) | 221 (8.1) | 186 (6.8) | 1591 (58.1) | 307 (11.2) | 159 (5.8) | 309 (11.3) | 775 (28.3) |
| 6. | Might be possible others can read my mind, or I can read others’ | 1133 (41.5) | 274 (10.0) | 235 (8.6) | 1642 (60.1) | 301 (11.0) | 160 (5.9) | 306 (11.2) | 767 (28.1) |
| 7. | Wonder if people planning to hurt me | 1185 (43.6) | 249 (9.2) | 194 (7.1) | 1628 (60.0) | 254 (9.3) | 121 (4.5) | 238 (8.8) | 613 (22.5) |
| 8. | I have special or (super)natural gifts beyond my talents | 1152 (42.1) | 176 (6.4) | 164 (6.0) | 1492 (54.6) | 533 (19.5) | 160 (5.9) | 256 (9.4) | 949 (34.7) |
| 9. | My mental state has gotten worse in the last 12 monthsa | 1780 (65.2) | 220 (8.1) | 152 (5.6) | 2152 (78.8) | 102 (3.7) | 71 (2.6) | 130 (4.8) | 303 (11.1) |
| 10. | Heard sounds of people when no one is near | 1645 (60.1) | 242 (8.8) | 134 (4.9) | 2021 (73.8) | 181 (6.6) | 95 (3.5) | 199 (7.3) | 475 (17.4) |
| 11. | Hear my own thoughts out loud | 1578 (57.8) | 215 (7.9) | 163 (6.0) | 1956 (71.7) | 187 (6.9) | 109 (4.0) | 196 (7.2) | 492 (18.0) |
| 12. | Been concerned that I may be “going mad” | 2303 (83.8) | 105 (3.8) | 74 (2.7) | 2482 (90.4) | 49 (1.8) | 18 (0.7) | 34 (1.2) | 101 (3.7) |
Values are given in raw numbers (percentages). Remaining percentages of subjects either answered “I don’t know” or did not complete item.
Listed items from modified-PRIME-Screen are abbreviated to fit table
The content of this item was changed from the original PRIME-Screen, which had in its place an item evaluating experiences involving “mind tricks”.
Gender Effects
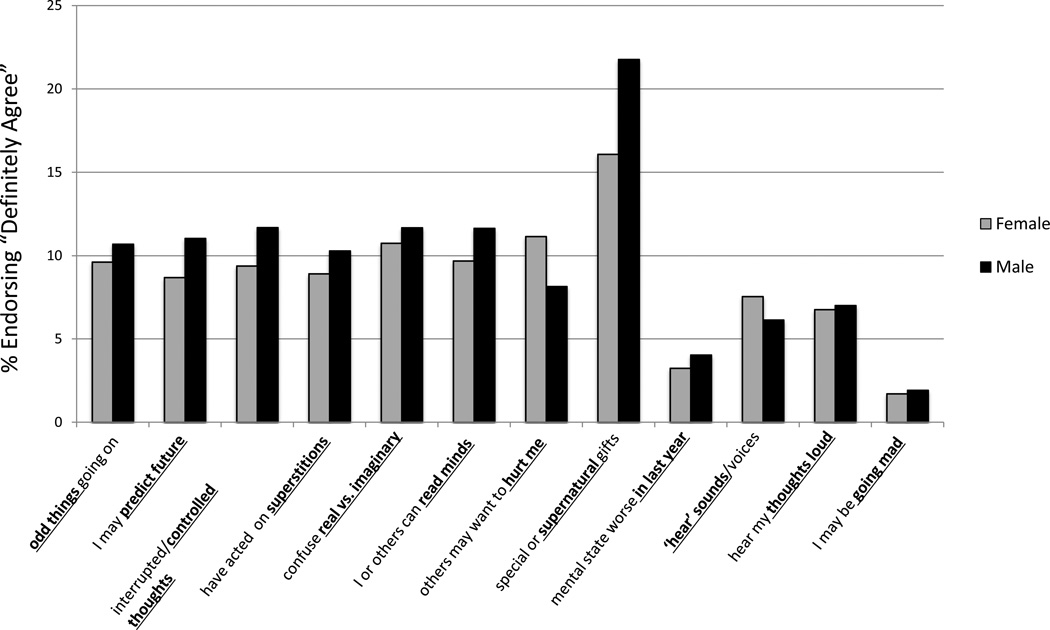
Gender differences in the prevalence of psychosis-risk symptoms are depicted in Figure 1. The mPRIME item with the largest gender prevalence difference was “(Super)natural/Special”, with males reporting “definitely agree” 35.8% more than females (f=16.1%, m=21.8%). Answering “definitely agree” was also more prevalent in males compared to females in nine other items by 8.7–27.0% (“Odd Things”: f=9.6%, m=10.7%; “Predict Future”: f=8.7%, m=11.0%; “Controlled Thoughts”: f=9.4%, m=11.7%; “Superstitions”: f=8.9%, m=10.3%, “Real vs. Imaginary”: f=10.7%, m=11.7%; “Mind Reading”: f=9.7%, m=11.6%; “Worsening in Last 12 Months”: f=3.2%, m=4.0%; “Hearing Thoughts Out Loud”: f=6.8%, m=7.0%; and “Going Mad”: f=1.7%, m=1.9%). Females had a higher prevalence than males in only two mPRIME items: “Planning to Hurt Me”, by 26.9% (f=11.2%, m=8.2%) and “Hearing Voices/Sounds”, by 18.8% (f=7.6%, m=6.1%).
FIGURE 1. Gender differences in psychosis-risk symptom prevalence in Nairobi.
The modified PRIME-Screen (mPRIME) was used to evaluate psychosis-risk symptoms, shown on the x-axis. The y-axis indicates the percentage of individuals who answered “definitely agree” on an mPRIME item. Exact values are shown in the results section.
We also evaluated difference in mean severity scores (ranging from 0–5) between genders on individual mPRIME items (Table 2). Significant mean [SD] score differences were only found for two items: “Planning to Hurt Me” (f=1.2[1.7], m=0.9[1.5]; p=0.001), and “Hearing Voices/Sounds” (f=0.8[1.5], m=0.5[1.2]; p=0.0007), both of which were higher in females compared to males.
Table 2.
Effects of Gender and Age on mPRIME-Screen Item Severity
| GENDER Mean Severity Score |
AGE Pearson’s Corr. Coeff. |
||||||
|---|---|---|---|---|---|---|---|
| Experiences | Female | Male | F | p | R | p | |
| 1. | Odd or unusual things going on I can’t explain | 0.9 (1.5) | 1.1 (1.7) | 1.7 | 0.19 | 0.07 | 0.036* |
| 2. | I may be able to predict future | 0.9 (1.5) | 1.1 (1.7) | 2.8 | 0.09 | 0.12 | 0.0002** |
| 3. | Have felt something interrupting/controlling thoughts or actions | 1.2 (1.6) | 1.3 (1.7) | 0.6 | 0.42 | 0.09 | 0.009* |
| 4. | Experienced doing something differently due to superstitions | 1.1 (1.7) | 0.9 (1.6) | 2.3 | 0.13 | 0.13 | <0.0001** |
| 5. | Get confused whether something is real or imaginary/dream | 1.2 (1.6) | 1.1 (1.7) | 0.5 | 0.48 | 0.11 | 0.0006** |
| 6. | Might be possible others can read my mind, or I can read others’ | 1.3 (1.8) | 1.2 (1.7) | 0.1 | 0.72 | 0.12 | 0.0002** |
| 7. | Wonder if people planning to hurt me | 1.2 (1.7) | 0.9 (1.5) | 10.4 | 0.001** | 0.03 | 0.39 |
| 8. | I have special or (super)natural gifts beyond my talents | 1.2 (1.8) | 1.4 (2.0) | 2.5 | 0.11 | 0.02 | 0.57 |
| 9. | My mental state has gotten worse in the last 12 months | 0.5 (1.2) | 0.4 (1.0) | 4.7 | 0.03* | 0.04 | 0.20 |
| 10. | Heard sounds of people when no one is near | 0.8 (1.5) | 0.5 (1.2) | 11.6 | 0.0007** | −0.05 | 0.16 |
| 11. | Hear my own thoughts out loud | 0.7 (1.4) | 0.6 (1.4) | 0.9 | 0.35 | 0.02 | 0.52 |
| 12. | Been concerned that I may be “going mad” | 0.2 (0.8) | 0.2 (0.8) | 0.3 | 0.56 | 0.05 | 0.16 |
Answers given for mPRIME-Screen items were numerically scored at 0–5 severity, with “0” indicating “Definitely Disagree” and “5” indicating “Definitely Agree”.
Results for gender are reported as means (standard deviation).
Results for age are reported as the Pearson’s Correlation coefficient.
p<0.05;
p<0.004 (corrected for multiple comparisons)
Age Effects
Age effects are shown on Table 2. Pearson’s correlational analysis on age against mPRIME item scores (0–5 range), showed a significant positive relationship only for five items, however R-values were very modest. R-values for these were: 0.12 for “Predict Future” (p=0.0002), 0.09 for “Controlling Thoughts” (p=0.009), 0.13 for “Superstitions” (p=<0.0001), 0.11 for “Real vs. Imaginary” (p=0.0006), and 0.12 for “Mind Reading” (p=0.0002).
Correlational analysis done separately in males also showed a significant effect for “Predict Future” (R=0.09; p=0.001), 0.09 for “Controlling Thoughts” (R=12; p=0.004), 0.13 for “Superstitions” (R=0.18; p=<0.0001), 0.11 for “Real vs. Imaginary” (R=0.15; p=0.0003), and 0.12 for “Mind Reading” (R=0.15; p=0.0002), but not other items. Age effects in females did not show a significant correlation in any mPRIME item, after controlling for multiple comparisons. However, there was a trend level effect for “Hearing Voices” in females (R= −0.13; p=0.02).
Cluster Analysis of Psychosis-Risk Symptoms
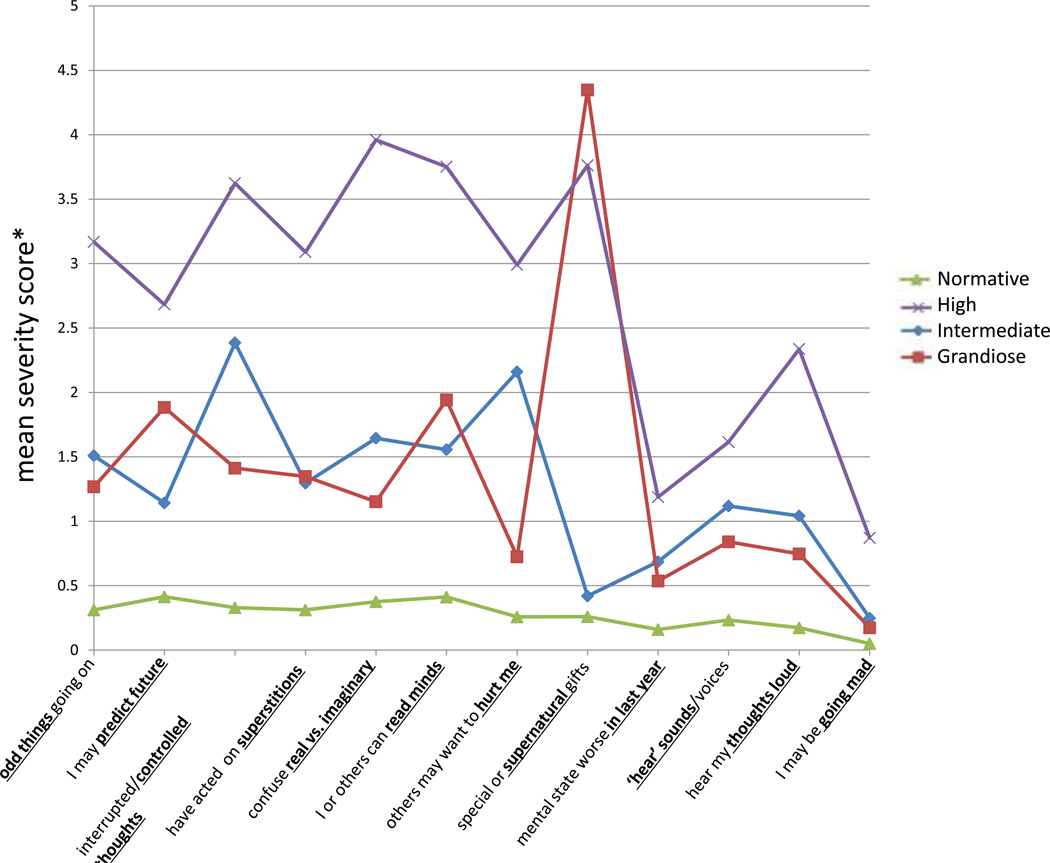
k-means cluster analyses of the 908 subjects who completed all mPRIME items, with a severity score, generated four clusters (k=4). The sample used for cluster analysis and the total sample (N=2,758) did not significantly differ in gender (χ2=1.7, p=0.2). The mean age of cluster-analyzed subjects was only minimally higher (18.8 yrs) than that of the total sample (18.5 yrs; p=0.03). The four clusters were: 1) a normative group (NG; 55%), with scores approaching 0 in all items, 2) a high symptom group (HG1; 11.1%), with mean scores >2.5 in the majority of items, 3) an intermediate symptom group (IG; 18.6%), with mean scores <2.5 in all items, and 4) a grandiose symptom group (GG; 15.2%), with intermediate mean scores <2.0 in all items except on that inquiring about being exceptionally “(Super)natural/Special” where the mean score was 4.3.
Figure 2 depicts and Table 3 lists the mean (SD) mPRIME item severity scores (ranging from 0–5) of participants in each of the four clusters. HG were on average about 2 years older (mean age=20.4 yrs) than NG, IG or GG. There was a statistically similar predominance of males in every group, which approximated the gender distribution of the original 2,758-subject sample. Participants in each group had relatively low mean scores on the “Worsening in Last 12 Months’ item (NG=0.2; HG=1.2; IG=0.7; and GG=0.5). The mean scores for endorsing feelings of “Going Mad” where very low in all groups; the highest score was in HG (mean score=1.9). Other mean scores in HG were 2.5 or higher, with the exception of the items “Hearing Voices/Sounds” and “Hearing Thoughts Loud” which were lower.
FIGURE 2. Cluster analysis of psychosis-risk questionnaire reports.
k-Means cluster analysis was used on results from the mPRIME item severity scores, with gender included in the analysis. In total, 908 participants were included in the analysis, which required completion of all items, without answering “don’t know” on any item. Severity scores on each item were renumbered such that 0=definitely disagree, 1=somewhat disagree, 2=slightly disagree, 3=slightly agree, 4=somewhat agree, and 5=definitely agree.
Table 3.
Characteristics of four groups of youth derived from cluster analysis of subject responses on mPRIME-Screen (N=908).
| Features | Normative | High Symptom |
Intermediate Symptom |
Grandiose Symptom |
F/χ2 | p |
|---|---|---|---|---|---|---|
| Number (%) | 500 (55.0) | 101 (11.1) | 169 (18.6) | 138 (15.2) | - | - |
| Age | 18.7 (3.4) | 20.4 (3.3) | 18.8 (3.4) | 18.1 (3.5) | 9.0 | <0.0001 |
| Gender – N (%) | 4.7 | 0.2 | ||||
| Female | 177 (35.4) | 36 (35.6) | 75 (44.4) | 49 (35.5) | ||
| Male | 323 (64.6) | 65 (64.4) | 94 (55.6) | 89 (64.5) | ||
| mPRIME-Screen Items: | ||||||
| 1. Odd things | 0.3 (1.8) | 3.2 (1.8) | 1.6 (1.9) | 1.3 (1.8) | 147.6 | <0.0001 |
| 2. Predict Future | 0.4 (0.9) | 2.7 (2.1) | 1.1 (1.6) | 1.9 (2.1) | 91.5 | <0.0001 |
| 3. Controlled Thoughts | 0.3 (0.6) | 3.6 (1.5) | 2.4 (1.7) | 1.4 (1.8) | 272.1 | <0.0001 |
| 4. Superstitions | 0.3 (0.8) | 3.1 (1.9) | 1.3 (1.8) | 1.3 (1.8) | 126.0 | <0.0001 |
| 5. Real vs. Imaginary | 0.4 (0.9) | 4.0 (1.4) | 1.5 (1.8) | 1.2 (1.6) | 228.8 | <0.0001 |
| 6. Mind Reading | 0.4 (0.9) | 3.8 (1.7) | 1.6 (1.6) | 1.9 (2.0) | 193.2 | <0.0001 |
| 7. Hurt Me | 0.3 (0.6) | 3.0 (2.0) | 2.2 (1.8) | 0.7 (1.3) | 197.2 | <0.0001 |
| 8. Supernatural | 0.3 (0.7) | 3.8 (1.7) | 0.4 (0.9) | 4.3 (0.9) | 1057.8 | <0.0001 |
| 9. Last 12 Months | 0.2 (0.5) | 1.2 (1.8) | 0.7 (1.4) | 0.5 (1.3) | 32.0 | <0.0001 |
| 10. Hear Sounds | 0.2 (0.7) | 1.6 (2.0) | 1.1 (1.6) | 0.8 (1.5) | 48.4 | <0.0001 |
| 11. Thoughts Loud | 0.2 (0.5) | 2.3 (2.1) | 1.0 (1.5) | 0.7 (1.6) | 97.5 | <0.0001 |
| 12. Going “Mad” | 0.1 (0.3) | 0.9 (1.6) | 0.2 (0.8) | 0.2 (0.7) | 34.5 | <0.0001 |
Values are given in means (SD) unless stated otherwise.
Chi square analysis was used to compare frequencies. ANOVA was used to compare means.
k-means cluster analysis was used to generate clusters. mPRIME item scores are derived from answer on a 0–5 severity scale, with a score of 0 being “Definitely Disagree”, and 5 being “Definitely Agree”. (Individuals that answered, “don’t know” on any item or did not indicate gender were not included in cluster analysis).
DISCUSSION
Our studies showed a relatively high (45.5%) adolescent and young adult lifetime prevalence of symptoms suggesting psychosis-risk in a region within Nairobi, Kenya. The most commonly reported symptom (34.7%) involved feelings of having special gifts beyond one’s natural ability. Other symptoms reported, of decreasing prevalence, were interrupted or controlled thinking, difficulty identifying reality, thoughts of mind reading, thoughts of predicting the future, feeling odd things are going on, superstition, persecutory ideation, and auditory hallucinations. A previous community survey of Mexican adolescents using the PRIME-Screen reported psychosis-risk rates of only 18.4%, lower than that found in our study (23). In Australia and Britain, community surveys evaluating “psychotic-like experiences” using alternative screening tools have reported prevalence estimates between 5–12% (24–27). However, higher rates of prodromal symptoms have also been reported. For example, in a survey of U.S. college students, 43% of students reported as having eight or more positive symptoms on a prodromal questionnaire (28), while 10–50% of high school students have reported prodromal symptoms by other authors (29, 30). Discrepancies across studies may reflect differences in the number and types of questions on the screening tools used, but may also indicate that psychotic symptoms vary across population groups, with the poorest socioeconomic regions in Kenya having particularly high rates. This would be consistent with the relationship of psychosis with high environmental stress. In our study, 3.7% of those surveyed stated they may be “going mad”, which may indicate that these participants that may have more severe symptoms or have an already existing psychotic disorder - considering this is only slightly above the estimated community rates of psychotic disorders (1). Thoughts of going crazy may however underestimate the severity of illness due to poor insight (31) or may indicate the presence of other underlying conditions that are associated with significant anxiety symptoms (32). We also found that 11.1% of the population stated that their symptoms might have worsened within the last year, a number that may estimate the incidence rate of psychosis-risk symptoms. However, it is unclear if worsening was due to psychosis-risk symptoms, or to other unrelated conditions, such as depression, anxiety or other environmental stressors, as these was not specifically investigated.
Females were found to be more likely to report persecutory ideation and auditory hallucinations males. This difference was observed both by measuring mean severity scores, and by measuring the prevalence rates of definitive symptoms. A higher rate of auditory and visual hallucinations in females is consistent with that previously reported by other authors (27, 33). Although these researchers found a higher prevalence of persecutory delusions in males (27), unlike our findings, an association of victimization (including for example, bullying, violence, and sexual assault) with both hallucinations (27, 34) and persecutory delusions (27) has been noted. Thus, it is plausible that higher rates hallucinations and persecutory ideation in our female participants, may reflect a greater history of physical or psychological trauma compared to males. Our studies also found that male participants were somewhat more likely to report certainty in having special or supernatural self-attributions, interrupted or controlled thinking, difficulty identifying reality, thoughts of mind reading, superstitious behaviors, thoughts of predicting the future, or feelings of oddness. However, males did not show a significant difference in mean severity scores from females, although there was a trend in that direction for some symptoms.
We found a statistically significant effect of increasing age on increased endorsement of superstitious behavior, thoughts of predicting the future, thoughts of mind reading, difficulty with identifying reality, and interrupted thinking. These correlations were however very modest, with R-values from 0.09 to 0.13. Analysis separately by gender indicated that these correlations were present in males but not in females, suggesting that the noted risk symptoms are slightly more commonly reported with increased age in males. While the reasons for this are not clear from this data, it is plausible that the noted symptoms are more likely to occur later in development. Alternatively, the possibility that certain questionnaire items are easier to comprehend at older ages due to their content or the way they are phrased cannot be overlooked, as probing for a particular symptom using differently worded text have shown differing prevalence rates (27).
We used cluster analysis to identify subject groups that have common patterns of observable symptoms based on severity scores of mPRIME items. We identified in addition to a normative group, three groups of individuals with some degree of psychosis-risk traits. The high symptom group comprised 11.1% of the analyzed population and was characterized by a relatively high severity of all psychosis-risk symptoms. Mean scores for auditory hallucinatory experiences were observably lower than other psychotic-risk symptoms. It is of interest that on average the high symptom group consisted of somewhat older participants (20.4 years) compared to other groups, which more closely reflects the 15–35 year age range associated with psychotic disorder onset (35). Considering the multiple psychotic experiences of relatively high severity in this group, it appears probable that it partly comprises of community participants with existing psychotic disorders, estimated at about 3% of the population(1). This group would most likely also be at the greatest psychosis-risk, as higher risk scores predict the development of future psychotic illness (36). An intermediate symptom group of higher prevalence was also found using cluster analysis. It is unclear whether a lower score severity may impart any risk for the future development of psychotic illness. This group could represent an unrelated normal variant, considering that psychotic experiences are often reported in healthy individuals or may be mischaracterized (37). In the grandiose symptom group, the most characteristic symptoms reported were high scores on feeling extremely special. Our study however did not evaluate whether these individuals may have an underlying pathology with grandiose symptoms, such as mania or hypomania, as bipolar spectrum disorders are relatively prevalent in the community (38). However, grandiosity and egocentrism is not uncommon in adolescence, and can be often misjudged as pathologic (39). In either case, our findings suggest questions of grandiosity may be less specific for evaluating the psychosis risk. The symptom clusters found in our study shares some similarities with that reported by Rocchi et al (40), where latent class analysis was applied to a delusions inventory. Although only delusional symptoms were analyzed, these authors identified four classes including a prominent grandiosity/hypomania class. Two other studies using other psychosis risk questionnaires have reported four alternative classes, including an intermediate, a positive psychosis, and either a paranoid or a hallucinatory class (41, 42). Together, these findings suggest that resulting clusters are dependent on the number and type of symptoms included in the analysis.
A major limitation to our prevalence rate findings from the mPRIME is that it may overestimate true risk for developing a psychotic disorder. However, establishing psychosis-risk using more extensive “gold standard” interviews are also not highly specific, with only a fraction of individuals eventually transitioning to a psychotic disorder (6, 10). Improved estimation of conversion risk would be expected to require severity evaluation using an index of functionality and symptom duration or frequency, which was not contained within the mPRIME. Including the duration criteria only during screening as other authors have done previously (12) may improve the accuracy of predicting a psychotic disorder, but could also result in the underestimation of clinically significant cases where impairment or distress is present for shorter periods. Additional limitations include that it is based on self-report, and may not precisely reflect symptoms experienced by individuals. While every effort was made to modify the questionnaire to be easily understood in the Kenyan youth culture, the possibility remains that some items were difficult to comprehend, particularly by younger individuals or those that may have a degree of cognitive impairment in the community. Questionnaire items were also not fully completed by all study participants, which may have influenced our findings by excluding individuals who are cognitively impaired or may be distractible due to psychopathology. Thus, prevalence rates reported may underestimate the actual rates in the community. As our study was cross-sectional, it is difficult to predict whether positive symptoms reported indicate true risk for a psychotic disorder, a risk for other psychiatric conditions (such as anxiety or affective disorders) or no risk at all, as follow up studies were not done. Other potential causes of reported symptoms were also not evaluated, but may include substance abuse, infective or toxic states, malnutrition, vitamin deficiency or epilepsy that are relatively common in Africa (43). Such etiologies, while significant, may not necessarily indicate a risk for primary psychotic conditions like schizophrenia or affective psychoses.
Our study provides insight into potential psychotic risk symptoms in Africa, where this has not previously been evaluated. In addition to imparting likelihood for developing more severe illness, psychosis-risk symptoms observed in our study can by themselves be disabling. Thus, our studies may imply a larger burden of disease on individuals, families and the society at large, than would be attributable to a diagnosable mental illness. Longitudinal studies are required to evaluate the rate of transition to psychotic illness over various intervals, and how this is influenced by participants’ reports on the mPRIME. This would entail assessment of psychiatric diagnoses in participants as well as other diagnostic confounders. It will also be necessary to determine the level of functionality and distress experienced by those reporting symptoms on the questionnaire, in order to develop suitable intervention strategies.
Acknowledgements
The authors would like to thank George W. Couch, III for his generous support for conducting this research project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any conflicts of interest and declare no financial interest from this study.
References
- 1.Perala J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsa E, Pirkola S, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64(1):19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Larsen TK, Melle I, Friis S, Joa I, Johannessen JO, Opjordsmoen S, et al. One-year effect of changing duration of untreated psychosis in a single catchment area. Br J Psychiatry Suppl. 2007;51:s128–s132. doi: 10.1192/bjp.191.51.s128. [DOI] [PubMed] [Google Scholar]
- 3.Stain HJ, Clark S, O'Donnell M, Schall U. Young rural people at risk for schizophrenia: time for mental health services to translate research evidence into best practice of care. Aust N Z J Psychiatry. 2010;44(10):872–882. doi: 10.3109/00048674.2010.493857. [DOI] [PubMed] [Google Scholar]
- 4.World_Health_Organization. Prevention of mental disorders: effective interventions and policy options. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 5.Ndetei DM. Early intervention in psychosis: concepts, evidence and perspectives. World Psychiatry. 2008;7(3):164–165. doi: 10.1002/j.2051-5545.2008.tb00189.x. PMCID: 2559925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67(2–3):131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 7.Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- 8.Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 9.Woods SW, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, et al. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophr Bull. 2009;35(5):894–908. doi: 10.1093/schbul/sbp027. PMCID: 2728816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruhrmann S, Schultze-Lutter F, Salokangas RK, Heinimaa M, Linszen D, Dingemans P, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241–251. doi: 10.1001/archgenpsychiatry.2009.206. [DOI] [PubMed] [Google Scholar]
- 11.Miller TJ, Cicchetti D, Markovich D, McGlashan TH, Woods SW. The SIPS Screen: a brief self-report screen to detect the schizophrenia prodrome. Schizophr Res. 2004;70(suppl1):78. [Google Scholar]
- 12.Kobayashi H, Nemoto T, Koshikawa H, Osono Y, Yamazawa R, Murakami M, et al. A self-reported instrument for prodromal symptoms of psychosis: testing the clinical validity of the PRIME Screen-Revised (PS-R) in a Japanese population. Schizophr Res. 2008;106(2–3):356–362. doi: 10.1016/j.schres.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Sartorius N, Gulbinat W, Harrison G, Laska E, C S. Long-term follow-up of schizophrenia in 16 countries. A description of the International Study of Schizophrenia by the World Health Organization. Soc Psychiatry Psychiatr Epidemiol. 1996;31:249–258. doi: 10.1007/BF00787917. [DOI] [PubMed] [Google Scholar]
- 14.Susser E, Wanderling J. Epidemiology of nonaffective acute remitting psychosis vs schizophrenia. Sex and sociocultural setting. Arch Gen Psychiatry. 1994;51(4):294–301. doi: 10.1001/archpsyc.1994.03950040038005. [DOI] [PubMed] [Google Scholar]
- 15.Torrey EF. The epidemiology of paranoid schizophrenia. Schizophr Bull. 1981;7(4):588–593. doi: 10.1093/schbul/7.4.588. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Tovim DI, Cushnie JM. The prevalence of schizophrenia in a remote area of Botswana. Br J Psychiatry. 1986;148:576–580. doi: 10.1192/bjp.148.5.576. [DOI] [PubMed] [Google Scholar]
- 17.Kebede D, Alem A, Shibre T, Negash A, Fekadu A, Fekadu D, et al. Onset and clinical course of schizophrenia in Butajira-Ethiopia--a community-based study. Soc Psychiatry Psychiatr Epidemiol. 2003;38(11):625–631. doi: 10.1007/s00127-003-0678-4. [DOI] [PubMed] [Google Scholar]
- 18.Tafari S, Aboud FE, Larson CP. Determinants of mental illness in a rural Ethiopian adult population. Soc Sci Med. 1991;32(2):197–201. doi: 10.1016/0277-9536(91)90060-p. [DOI] [PubMed] [Google Scholar]
- 19.Maslowski J, Jansen van Rensburg D, Mthoko N. A polydiagnostic approach to the differences in the symptoms of schizophrenia in different cultural and ethnic populations. Acta Psychiatr Scand. 1998;98(1):41–46. doi: 10.1111/j.1600-0447.1998.tb10040.x. [DOI] [PubMed] [Google Scholar]
- 20.Hambrecht M, Maurer K, Hafner H, Sartorius N. Transnational stability of gender differences in schizophrenia? An analysis based on the WHO study on determinants of outcome of severe mental disorders. Eur Arch Psychiatry Clin Neurosci. 1992;242(1):6–12. doi: 10.1007/BF02190336. [DOI] [PubMed] [Google Scholar]
- 21.Everitt BS. Cluster Analysis. London: Edward Arnold; 1993. [Google Scholar]
- 22.Everitt BS. Unresolved problems in cluster analysis. Biometrics. 1979;35:169–181. [Google Scholar]
- 23.Fresan A, Apiquian R, Ulloa RE, Nicolini H. Reliability study of the translation into Spanish of the PRIME Screen Questionnaire for Prodromic Symtoms. Actas Esp Psiquiatr. 2007;35(6):368–371. [PubMed] [Google Scholar]
- 24.Saha S, J GS, Varghese D, J JM. The association between general psychological distress and delusional-like experiences: A large population-based study. Schizophr Res. 2011 doi: 10.1016/j.schres.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Scott J, Chant D, Andrews G, McGrath J. Psychotic-like experiences in the general community: the correlates of CIDI psychosis screen items in an Australian sample. Psychol Med. 2006;36(2):231–238. doi: 10.1017/S0033291705006392. [DOI] [PubMed] [Google Scholar]
- 26.Scott J, Martin G, Bor W, Sawyer M, Clark J, McGrath J. The prevalence and correlates of hallucinations in Australian adolescents: results from a national survey. Schizophr Res. 2009;107(2–3):179–185. doi: 10.1016/j.schres.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Johns LC, Cannon M, Singleton N, Murray RM, Farrell M, Brugha T, et al. Prevalence and correlates of self-reported psychotic symptoms in the British population. Br J Psychiatry. 2004;185:298–305. doi: 10.1192/bjp.185.4.298. [DOI] [PubMed] [Google Scholar]
- 28.Loewy RL, Johnson JK, Cannon TD. Self-report of attenuated psychotic experiences in a college population. Schizophr Res. 2007;93(1–3):144–151. doi: 10.1016/j.schres.2007.02.010. PMCID: 2063995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGorry PD, McFarlane C, Patton GC, Bell R, Hibbert ME, Jackson HJ, et al. The prevalence of prodromal features of schizophrenia in adolescence: a preliminary survey. Acta Psychiatr Scand. 1995;92(4):241–249. doi: 10.1111/j.1600-0447.1995.tb09577.x. [DOI] [PubMed] [Google Scholar]
- 30.Nishida A, Tanii H, Nishimura Y, Kajiki N, Inoue K, Okada M, et al. Associations between psychotic-like experiences and mental health status and other psychopathologies among Japanese early teens. Schizophr Res. 2008;99(1–3):125–133. doi: 10.1016/j.schres.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 31.Drake RJ. Insight into illness: impact on diagnosis and outcome of nonaffective psychosis. Curr Psychiatry Rep. 2008;10(3):210–216. doi: 10.1007/s11920-008-0035-0. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Tsuchiya M, Kawakami N, Furukawa TA. Non-fearful vs. fearful panic attacks: a general population study from the National Comorbidity Survey. J Affect Disord. 2009;112(1– 3):273–278. doi: 10.1016/j.jad.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Tien AY. Distributions of hallucinations in the population. Soc Psychiatry Psychiatr Epidemiol. 1991;26(6):287–292. doi: 10.1007/BF00789221. [DOI] [PubMed] [Google Scholar]
- 34.Romme MA, Escher AD. Hearing voices. Schizophr Bull. 1989;15(2):209–216. doi: 10.1093/schbul/15.2.209. [DOI] [PubMed] [Google Scholar]
- 35.Hafner H, an der Heiden W. Epidemiology of schizophrenia. Can J Psychiatry. 1997;42(2):139–151. doi: 10.1177/070674379704200204. [DOI] [PubMed] [Google Scholar]
- 36.McGlashan T, Walsh B, Woods S. The psychosis-risk syndrome: handbook for diagnosis and follow-up. 1 ed. USA: Oxford University Press; 2010. [Google Scholar]
- 37.Pierre JM. Hallucinations in nonpsychotic disorders: toward a differential diagnosis of "hearing voices". Harv Rev Psychiatry. 2010;18(1):22–35. doi: 10.3109/10673220903523706. [DOI] [PubMed] [Google Scholar]
- 38.Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. PMCID: 1931566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrop C, Trower P. Why does schizophrenia develop at late adolescence? Clin Psychol Rev. 2001;21(2):241–265. doi: 10.1016/s0272-7358(99)00047-1. [DOI] [PubMed] [Google Scholar]
- 40.Rocchi MB, Sisti D, Manca S, Siddi S, Mura T, Preti A. Latent class analysis of delusion-proneness: exploring the latent structure of the Peters et al. delusions inventory. J Nerv Ment Dis. 2008;196(8):620–629. doi: 10.1097/NMD.0b013e31818132a3. [DOI] [PubMed] [Google Scholar]
- 41.Shevlin M, Murphy J, Dorahy MJ, Adamson G. The distribution of positive psychosis-like symptoms in the population: a latent class analysis of the National Comorbidity Survey. Schizophr Res. 2007;89(1–3):101–109. doi: 10.1016/j.schres.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Murphy J, Shevlin M, Adamson G. A latent class analysis of positive psychosis symptoms based on the British Psychiatric Morbidity Survey. Pers Individ Differ. 2007;42:1491–1502. [Google Scholar]
- 43.Guinness EA. Brief reactive psychosis and the major functional psychoses: descriptive case studies in Africa. Br J Psychiatry Suppl. 1992;(16):24–41. [PubMed] [Google Scholar]