Abstract
Background
Elevated serum levels of brain natriuretic peptide (BNP) have been associated with cardioembolic (CE) stroke and increased post-stroke mortality. We sought to determine whether BNP levels were associated with functional outcome after ischemic stroke.
Methods
We measured BNP in consecutive patients aged ≥18 years admitted to our Stroke Unit between 2002–2005. BNP quintiles were used for analysis. Stroke subtypes were assigned using TOAST criteria. Outcomes were measured as 6-month modified Rankin Scale score (“good outcome” = 0–2 vs. “poor”) as well as mortality. Multivariate logistic regression was used to assess association between the quintiles of BNP and outcomes. Predictive performance of BNP as compared to clinical model alone was assessed by comparing ROC curves.
Results
Of 569 ischemic stroke patients, 46% were female; mean age was 67.9 ± 15 years. In age- and gender-adjusted analysis, elevated BNP was associated with lower ejection fraction (p<0.0001) and left atrial dilatation (p<0.001). In multivariate analysis, elevated BNP decreased the odds of good functional outcome (OR 0.64, 95%CI 0.41–0.98) and increased the odds of death (OR 1.75, 95%CI 1.36–2.24) in these patients. Addition of BNP to multivariate models increased their predictive performance for functional outcome (p=0.013) and mortality (p<0.03) after CE stroke.
Conclusions
Serum BNP levels are strongly associated with CE stroke and functional outcome at 6 months after ischemic stroke. Inclusion of BNP improved prediction of mortality in patients with CE stroke.
Search Terms: Ischemic Stroke, Outcome, Biomarkers
INTRODUCTION
Long-term functional outcome after stroke is one of the most important and difficult variables to predict,1, 2 and is subject to complex interactions with multiple factors including age, gender, ethnicity, pre-existing morbidity, stroke severity, acute interventions, and post-stroke care.3–7 Utility of serum biomarkers in prediction of outcomes after acute ischemic stroke (AIS) is limited, as the data are predominantly based on analysis of short-term (up to 3 months) outcomes8 and post-stroke mortality.8–10 Furthermore, no currently validated serum biomarkers are available to assist prognostication in AIS.
Elevated serum levels of brain natriuretic peptide (BNP), a powerful predictor of outcomes in patients with cardiovascular disease,11–13 have been associated with atrial fibrillation (AF),14 cardioembolic (CE) stroke,15, 16 and higher post-stroke mortality.17, 18 However, data are controversial with regard to the potential role of BNP in prediction of long-term, functional outcomes after stroke.19, 20 We sought to determine whether admission serum BNP levels are independently associated with functional outcomes after ischemic stroke.
METHODS
Patient selection
Consecutive patients aged ≥18 years admitted to our Stroke Unit through the Emergency Department (ED) between 2002 and 2005 with diagnosis of ischemic stroke were considered for this study. The design of this ongoing single-center prospective cohort study has been described elsewhere.21 Ischemic stroke was defined as a clinical syndrome associated with a radiographically proven acute infarct consistent with a vascular pattern of involvement on brain CT or MRI. Diagnosis of ischemic stroke was confirmed for all subjects on admission for the index event. The institutional review board approved all aspects of this study, and informed consent for collection of data was obtained for all subjects or their legal guardians.
Data collection and patient follow-up
All patients were evaluated by a neurologist in the ED. Demographics and clinical characteristics including the National Institute of Health Stroke Scale (NIHSS) score, laboratory values including creatinine, past medical history, and medication use prior to admission were obtained directly during the ED evaluation or abstracted prospectively by patient or proxy interview, and/or supplemented through medical chart review. Vascular risk factors including hypertension (HTN), diabetes (DM), hyperlipidemia (HL), coronary artery disease (CAD), and AF were recorded based on existing international guidelines and as previously described.22 Cardiac measurements including left ventricular ejection fraction (LVEF) and left atrium diameter (LAD) were assessed on the echocardiogram (ECHO) completed during the admission for the index event. AIS subtypes were assigned by stroke neurologists (K.L.F.) according to TOAST criteria.23 Based on these criteria, CE stroke was defined as one presumed to be due to an embolus arising in the heart following a comprehensive evaluation for stroke etiology including laboratory testing, imaging of the cerebral and cervical vasculature, EKG, transthoracic echocardiogram, and 24-hour Holter monitoring.
Patients and their caregivers were interviewed by telephone at 3–6 months post-AIS to assess functional outcome using the modified Rankin Scale (mRS) score. Recurrent cerebrovascular events, newly diagnosed medical conditions, and medication use were specifically assessed in this interview. Good outcome was defined as mRS ≤ 2 at 6 months.
Blood Sampling and Natriuretic Hormone Assay
Serum was collected from each subject at enrollment and within 48 hours of admission. Samples were centrifuged and serum was extracted, aliquoted, and stored at −80°C until analysis. As previously described,24 serum nt-proBNP levels were determined using commercially available enzyme immunoassays without extraction (manufactured by Biomedica Gruppe, Germany). Assays were performed according to the manufacturer’s instructions and read with a Victor-X plate reader (Perkin-Elmer, CA). The immunoassay for nt-proBNP employs an immunoaffinity purified sheep antibody specific for nt-proBNP (8–29); the cross reactivity with other natriuretic peptide epitopes is < 1%. All assays were performed in duplicate and normalized to a standard curve. The intra-assay and inter-assay variances for nt-proBNP were ≤ 5%.24
Statistical analysis
All statistical analyses were performed using STATA 10.0. Continuous numerical variables were expressed as median ± inter-quartile range (IQR) with the exception of age (mean ± standard deviation (SD)). Biomarker data was log-transformed to achieve normality when used as dependent variable. When analyzed as independent variable, BNP level quintiles were used to more adequately quantify the effect size of the association between biomarker data and stroke subtypes, functional outcome, and mortality. Subjects were compared across stroke subtypes in univariate analyses using t-test, Wilcoxon rank sum, chi-square, or Fisher’s exact test as appropriate.
Multivariate logistic regression was used to assess the association between the serum BNP and functional outcome in this cohort; multivariate linear regression was used to identify baseline predictors of BNP. All variables showing a trend in association in univariate analysis (p < 0.20) were included (functional outcome model: age, gender, HTN, AF, CAD, alcohol use, antiplatelet agent use, NIHSS score, CE stroke subtype, BNP; mortality model: age, AF, BNP, alcohol use, statin use, antiplatelet or anticoagulation agent use, NIHSS score, CE stroke subtype). Final multivariable models also included LAD and LVEF, which were forced into the model in order to adequately account for possible confounding. Predictive performance of BNP for functional outcome was assessed by comparing ROC curves using multivariable models described above. Significance threshold was set at p < 0.05 (two-tailed) for all analyses.
RESULTS
Of 569 ischemic stroke patients, 187 (32.9%) had CE, 130 (22.9%) had large artery, 54 (9.5%) had small vessel, 143 (25.1%) had undetermined, and 55 (9.7%) had other stroke subtypes.(Table 1) Mean age was 67.9 ± 15 years 46% were female. BNP levels were higher among the older subjects (p<0.0001) and women (p<0.0002). When adjusted for age and gender, elevated BNP was associated with lower LVEF (p<0.0001) and greater degree of LAD (p<0.001). Furthermore, BNP was associated with AF (OR 2.0, 95%CI 1.6–2.5) and CE stroke subtype (p<0.001)(Figure 1).
Table 1.
Clinical characteristics of the ischemic stroke cohort by TOAST stroke subtype (n=569).
| Variable | CE | LA | SV | Other | Undetermined |
|---|---|---|---|---|---|
| No. Subjects | 187 | 130 | 54 | 55 | 143 |
| Age (mean, SD) | 68.8 (16.1) | 67.5 (12.5) | 67.6 (11.9) | 45.1 (13.7) | 69.5 (14.0) |
| Gender (Female), % | 50 | 45 | 40 | 44 | 51 |
| Caucasian, % | 93 | 90 | 87 | 90 | 92 |
| HTN, % | 59 | 67 | 72 | 28 | 70 |
| DM, % | 16 | 22 | 46 | 5 | 18 |
| AF, % | 46 | 7 | 1 | 0 | 10 |
| CAD, % | 25 | 18 | 26 | 8 | 21 |
| Tobacco use,* % | 55 | 78 | 57 | 67 | 65 |
| Alcohol Use,** % | 60 | 67 | 61 | 76 | 68 |
| Antiplatelet use, % | 43 | 47 | 50 | 20 | 46 |
| Warfarin use, % | 21 | 12 | 6 | 9 | 7 |
| NIHSS (median, IQR), score | 4 (1–11) | 2 (1–5) | 3 (2–4) | 2 (1–5) | 2 (1–5) |
| BNP (median, IQR), pg/ml |
273 (169–400) |
194 (139–264) |
178 (149–232) |
160 (114–210) |
208 (142–329) |
| Creatinine (mean, SD), md/dL | 1.07 (0.3) | 1.08 (0.4) | 1.03 (0.3) | 0.9 (0.1) | 1.05 (0.3) |
| LVEF (median, IQR), % | 65 (56–70) | 67 (59–72) | 65 (60–73) | 66 (61–72) | 67 (61–73) |
| LAD (median, IQR), mm | 39 (33–43) | 37 (33–40) | 37 (34–41) | 33 (31–36) | 36 (32–41) |
ever smoker
ever moderate/heavy alcohol user
Figure 1. Serum BNP levels in various stroke subtypes.
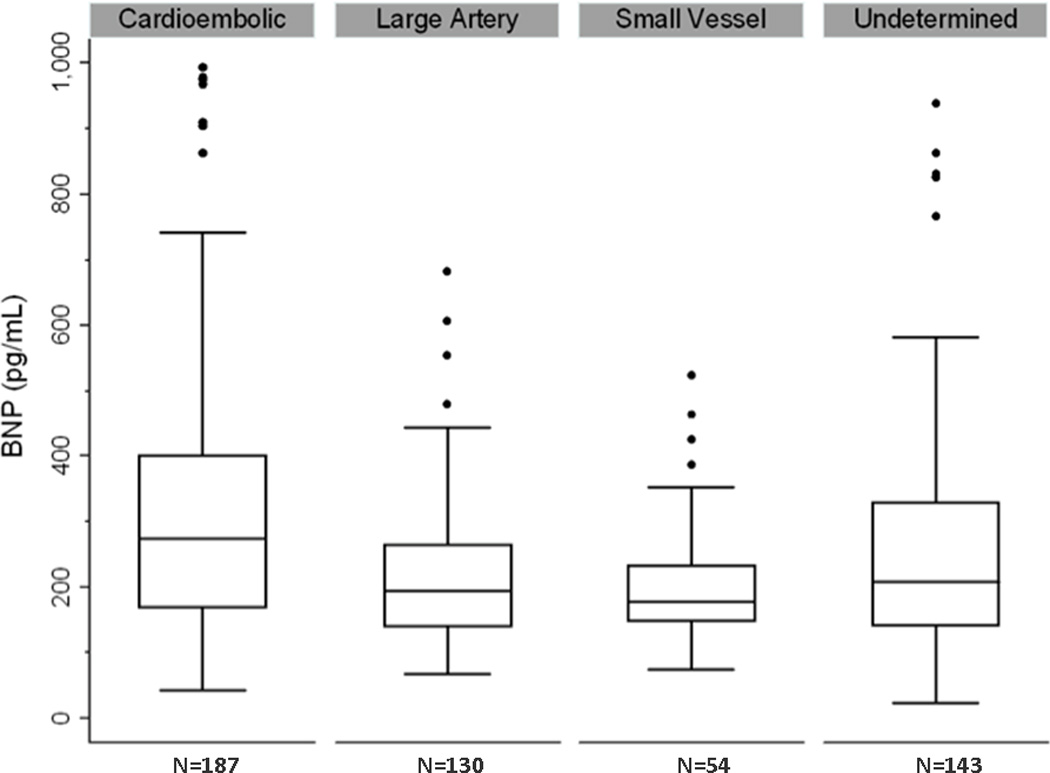
After adjustment for age and gender, serum BNP was independently associated with cardioembolic as compared to all non-cardioembolic (p<0.001) stroke subtypes; cardioembolic vs. undetermined (p<0.001) stroke subtype; and undetermined vs. small vessel or large vessel stroke subtypes (p<0.001)
In univariate analysis, age (OR 0.96, 95%CI 0.92–0.98), diagnosis of HTN (OR 0.42, 95%CI 0.3–0.7), AF (OR 0.5, 95%CI 0.3–0.75), CAD (OR 0.6, 95%CI 0.4–0.97), NIHSS score (OR 0.87, 95%CI 0.84–0.9), and BNP levels (OR 0.7, 95%CI 0.6–0.8) were associated with functional outcome. In multivariate analysis adjusted for the above variables as well as gender, alcohol use, prior antiplatelet agent use, and CE stroke subtype (all p<0.2), as well as LVEF and LAD forced into the model, only age (OR 0.97, 95%CI 0.4–0.99), NIHSS score (OR 0.86, 95%CI 0.8–0.96), and higher BNP levels (OR 0.64, 95%CI 0.41–0.98) independently predicted functional outcome. Similarly, NIHSS score (OR 1.1, 95%CI 1.01–1.19), AF (OR 3.6, 95%CI 1.2–13.2), and higher BNP levels (OR 1.75, 95%CI 1.36–2.24) were independent predictors of mortality among these subjects. (Table 2)
Table 2.
Multivariate predictors of good functional outcome (mRS ≤2) and mortality in patients with acute ischemic stroke.
| Good functional outcome* | Mortality** | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age | 0.97 | 0.4–0.99 | 1.0 | 0.97–1.04 |
| NIHSS score | 0.86 | 0.8–0.96 | 1.1 | 1.01–1.19 |
| BNP, pg/ml | 0.64 | 0.41–0.98 | 1.75 | 1.36–2.24 |
| AF | 0.81 | 0.42–1.6 | 3.6 | 1.2–13.2 |
| CE stroke subtype | 1.8 | 0.8–2.5 | 0.48 | 0.16–1.45 |
| LVEF | 1.0 | 0.97–1.02 | 1.02 | 0.99–1.07 |
| LAD | 1.01 | 0.97–1.04 | 0.95 | 0.9–1.01 |
Multiple logistic regression model including age, gender, HTN, AF, CAD, alcohol use, antiplatelet agent use, NIHSS score, CE stroke subtype, BNP level, LVEF, and LAD (all p<0.2 in univariate analysis).
Multiple logistic regression model including age, AF, BNP, alcohol use, statin use, antiplatelet or anticoagulation agent use, NIHSS score, CE stroke subtype, LVEF, and LAD (all p<0.2 in univariate analysis).
In a stroke subtype-based analysis, BNP remained independent predictor of functional outcome (OR 0.5, 95%CI 0.3 – 0.9) and mortality (OR 3.05, 95%CI 1.1 – 8.2) in CE stroke patients but not those with non-CE stroke subtype (OR 0.9, 95%CI 0.7–1.1 and OR 1.03, 95%CI 0.9–1.1 for functional outcome and mortality, respectively).
Addition of BNP to models predicting functional outcome and mortality after CE stroke increased their predictive performance (AUC estimate increase from 0.85 to 0.91, p=0.013 and 0.84 to 0.94, p<0.03, respectively).
DISCUSSION
Elevated serum BNP on hospital admission for ischemic stroke independently predicted functional outcome in the large, prospective cohort of patients at 6 months post-stroke. This was the first study to include transthoracic echocardiographic data into the analysis examining the association between serum levels of BNP and stroke outcome. These novel data further validate the importance of BNP in outcome prediction after stroke. Robust, widely-available, rapidly processed, inexpensive biomarkers such as BNP could potentially be used in the future to guide management of complex cerebrovascular patients in order to maximize their potential for recovery.
Serum BNP testing, as well as measurement of other natriuretic peptide family markers (such as mid-regional pro atrial natriuretic peptide), is widely accepted as a strategy for improving diagnostic accuracy and risk stratification in congestive heart failure and other cardiovascular conditions leading to ventricular dysfunction,11 thus allowing for earlier initiation of proper treatment and, ultimately, better patient outcomes.25, 26 In patients with cerebral ischemia, CE stroke subtype is often suspected on initial evaluation, either due to a known history of high-risk CE condition (such as AF), or due to evidence of arrhythmia on admission EKG or during first 24 hours of cardiac monitoring.27–29 In stroke patients, elevated serum BNP on admission may not only further confirm a CE etiology of stroke event, but also may signal increased risk for poor long-term outcome, including death.15, 17, 30 BNP testing has a role in risk stratification, identifying those likely to require intensive rehabilitative intervention. In addition, particularly in cases of cryptogenic stroke, the BNP level could help inform the choice of antithrombotic agent for secondary stroke prevention28. In order to improve systemic medical condition in high risk stroke patients and, as a result, their rehabilitation potential,31 BNP could also be used to determine the aggressiveness of heart failure management 32 or intensity of post-discharge monitoring.33, 34
In our study, increasing age and stroke severity also independently lowered odds of good functional outcome in patients with ischemic stroke. This is consistent with prior findings,35, 36 possibly indicating a complex interaction between the effect of survival to older age,37 increased pre-stroke morbidity,38 propensity for serious complications following their AIS,39 and less caregiver support to allow post-stroke recovery.40 The median pre-stroke mRS score of subjects enrolled in this study was 0, reflecting a population with little premorbid stroke-related disability. In this cohort, the serum BNP still independently predicted long-term mortality and poorer functional outcomes. Similarly, among the subjects with CE stroke only, increased level of BNP, but not LVEF or the degree of LAD, was independently associated with functional outcome and mortality. Conversely, despite being predictive in a combined cohort of all TOAST stroke subtypes, BNP levels played no significant role in prediction of outcomes among subjects with non-CE stroke. This finding attests to the strength of association between the BNP and outcome in the CE stroke patient subset, which provided sufficient statistical power for BNP to remain significantly correlated with outcomes in the combined cohort of CE and non-CE strokes. However, BNP levels may have limited utility in assessing outcomes following non-CE strokes based on the previously suggested pathophysiology of stroke subtypes and possible mechanisms of recovery.
In our study, serum BNP levels were measured on admission in patients with diagnosis of ischemic stroke confirmed by neuroimaging. Stroke subtype assignment was based on the TOAST criteria and assigned by the stroke neurologist (K.L.F.) blinded to BNP measurement data or patient outcomes. Despite the potential for subtype misclassification using TOAST criteria,41 BNP strongly differentiated CE stroke subtype from all non-CE subtypes, as well as CE vs. undetermined TOAST stroke subtype, which often includes mixed and possibly misclassified cases.
Limitations of this analysis are largely related to the methodological issues related to retrospective review of the otherwise prospectively collected data, including residual confounding that could not be assessed within the constraints of this study design. In particular, interaction between the timing of stroke symptom onset and BNP levels could not be evaluated. Secondly, we did not adjust for infarct volume; however, given that DWI infarct volume and admission NIHSS score are at least moderately correlated,42 we were able to partially adjust for this possible confounder. Thirdly, serum BNP levels are subject to variability as a result of physiological changes in cardiac, pulmonary, and renal function; shock and other severe systemic conditions; medication use (especially diuretics and anti-hypertensive agents); and cardiac resynchronization therapy, just to name a few.11 There is also reported biological variability in BNP levels observed in less than 50% of baseline levels,11 as well as previously reported associations of BNP with other clinical characteristics in patients with stroke,43 including possible effects of the time from symptom onset to BNP measurement. Therefore, our findings require validation. Finally, serum BNP added limited, albeit a statistically significant, advantage above and beyond the prognostic value of models built using clinical data alone. A future study designed for model validation in an independent ischemic stroke population would provide a better estimate of BNP’s predictive value.44
CONCLUSION
Serum BNP levels during stroke demonstrate a reliable association with CE stroke subtype and predict functional outcome and mortality. Further studies are warranted to establish the utility of serum BNP as a predictor of stroke outcome.
Acknowledgments
FUNDING
NIH-NINDS K23NS064052 (N.S.R.); NIH-NINDS 5P50NS051343-04 (K.L.F.); 1R01HL092577, 1R01HL104156, 5R21DA027021, 1K24HL105780 (P.T.E.)
American Heart Association/Bugher Foundation Centers for Stroke Prevention Research (0775010N), Deane Institute for Integrative Study of Atrial Fibrillation and Stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
STATEMENT OF CONTRIBUTION
Study Design: N.S.R. and K.L.F Data Acquisition: A.B., L.C., J.C., P.K., D.G., P.E., N.S.R., K.L.F. Data Analysis: A.B., N.S.R., P.E. Study Management: L.C., N.S.R., K.L.F., J.R. Manuscript Preparation: N.S.R. Manuscript Review: A.B., L.C., J.C., P.K., D.G., P.E., N.S.R., K.L.F.
DISCLOSURES
The authors have no Conflict of Interest/Disclosures
REFERENCES
- 1.Hankey G. Long-term outcome after ischaemic stroke/transient ischaemic attack. Cerebrovasc Dis. 2003;16:14–19. doi: 10.1159/000069936. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Barker-Collo S, Parag V, Senior H, Lawes CMM, Ratnasabapathy Y, et al. for the Asg. Auckland stroke outcomes study. Neurology. 2010;75:1597–1607. [Google Scholar]
- 3.Weimar C, Ziegler A, König IR, Diener H-C. Predicting functional outcome and survival after acute ischemic stroke. Journal of Neurology. 2002;249:888. doi: 10.1007/s00415-002-0755-8. [DOI] [PubMed] [Google Scholar]
- 4.Baird AE, Dambrosia J, Janket S-J, Eichbaum Q, Chaves C, Silver B, et al. A three-item scale for the early prediction of stroke recovery. The Lancet. 2001;357:2095. doi: 10.1016/s0140-6736(00)05183-7. [DOI] [PubMed] [Google Scholar]
- 5.Johnston KC, Connors AF, Jr, Wagner DP, Haley EC., Jr Predicting outcome in ischemic stroke: External validation of predictive risk models. Stroke. 2003;34:200–202. doi: 10.1161/01.str.0000047102.61863.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.German Stroke Study C. Predicting outcome after acute ischemic stroke. Neurology. 2004;62:581–585. doi: 10.1212/01.wnl.0000110309.95219.56. [DOI] [PubMed] [Google Scholar]
- 7.Roth DL, Haley WE, Clay OJ, Perkins M, Grant JS, Rhodes JD, et al. Race and gender differences in 1-year outcomes for community-dwelling stroke survivors with family caregivers. Stroke.STROKEAHA.110.595322. doi: 10.1161/STROKEAHA.110.595322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiteley W, Chong WL, Sengupta A, Sandercock P. Blood markers for the prognosis of ischemic stroke: A systematic review. Stroke. 2009;40:e380–e389. doi: 10.1161/STROKEAHA.108.528752. [DOI] [PubMed] [Google Scholar]
- 9.Elkind M, Tai W, Coates K, Paik MC, Sacco RL. High-sensitivity c-reactive protein, lipoprotein-associated phospholipase a2, and outcome after ischemic stroke. Arch Intern Med. 2006;166:2073–2080. doi: 10.1001/archinte.166.19.2073. [DOI] [PubMed] [Google Scholar]
- 10.Heuschmann PU, Wiedmann S, Wellwood I, Rudd A, Di Carlo A, Bejot Y, et al. Three-month stroke outcome. Neurology. 2011;76:159–165. doi: 10.1212/WNL.0b013e318206ca1e. [DOI] [PubMed] [Google Scholar]
- 11.Maisel A, Mueller C, Adams K, Anker SD, Aspromonte N, Cleland JGF, et al. State of the art: Using natriuretic peptide levels in clinical practice. European Journal of Heart Failure. 2008;10:824–839. doi: 10.1016/j.ejheart.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Scirica BM, Cannon CP, Sabatine MS, Jarolim P, Sloane S, Rifai N, et al. for the PITTI. Concentrations of c-reactive protein and b-type natriuretic peptide 30 days after acute coronary syndromes independently predict hospitalization for heart failure and cardiovascular death. Clin Chem. 2009;55:265–273. doi: 10.1373/clinchem.2008.117192. [DOI] [PubMed] [Google Scholar]
- 13.Pfister R, Tan D, Thekkanal J, Hellmich M, Schneider CA. Nt-pro-bnp is associated with long-term outcome in a heterogeneous sample of cardiac inpatients. European journal of internal medicine. 2007;18:215. doi: 10.1016/j.ejim.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121:200–207. doi: 10.1161/CIRCULATIONAHA.109.882241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montaner J, Perea-Gainza M, Delgado P, Ribo M, Chacon P, Rosell A, et al. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke. 2008;39:2280–2287. doi: 10.1161/STROKEAHA.107.505354. [DOI] [PubMed] [Google Scholar]
- 16.Naya T, Hosomi N, Takahashi T, Ohkita H, Mukai M, Koziol JA, et al. Brain natriuretic peptide as a surrogate marker for cardioembolic stroke with paroxysmal atrial fibrillation. Cerebrovasc Dis. 2008;26:434–440. doi: 10.1159/000155640. [DOI] [PubMed] [Google Scholar]
- 17.Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: The german stroke data bank. Stroke. 2001;32:2559–2566. doi: 10.1161/hs1101.098524. [DOI] [PubMed] [Google Scholar]
- 18.Shibazaki K, Iguchi Y, Aoki J, Sakai K, Kobayashi K. Plasma brain natriuretic peptide predicts death during hospitalization in acute ischaemic stroke and transient ischaemic attack patients with atrial fibrillation. Eur J Neurol. 2011;18:165–169. doi: 10.1111/j.1468-1331.2010.03101.x. [DOI] [PubMed] [Google Scholar]
- 19.Etgen T, Baum H, Sander K, Sander D. Cardiac troponins and n-terminal pro-brain natriuretic peptide in acute ischemic stroke do not relate to clinical prognosis. Stroke. 2005;36:270–275. doi: 10.1161/01.STR.0000151364.19066.a1. [DOI] [PubMed] [Google Scholar]
- 20.Idris I, Hill R, Ross I, Sharma JC. N-terminal probrain natriuretic peptide predicts 1-year mortality following acute stroke: Possible evidence of occult cardiac dysfunction among patients with acute stroke. Age and Ageing. 2010;39:752–755. doi: 10.1093/ageing/afq098. [DOI] [PubMed] [Google Scholar]
- 21.Kelly PJ, Kistler JP, Shih VE, Mandell R, Atassi N, Barron M, et al. Inflammation, homocysteine, and vitamin b6 status after ischemic stroke. Stroke. 2004;35:12–15. doi: 10.1161/01.STR.0000106481.59944.2F. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez-Conde J, Biffi A, Rahman R, Kanakis A, Butler C, Sonni S, et al. Hyperlipidemia and reduced white matter hyperintensity volume in patients with ischemic stroke. Stroke. 2010;41:437–442. doi: 10.1161/STROKEAHA.109.563502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 24.Ellinor PT, Low AF, Patton KK, Shea MA, MacRae CA. Discordant atrial natriuretic peptide and brain natriuretic peptide levels in lone atrial fibrillation. Journal of the American College of Cardiology. 2005;45:82. doi: 10.1016/j.jacc.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 25.Bassan R, Tura BR, Maisel AS. B-type natriuretic peptide: A strong predictor of early and late mortality in patients with acute chest pain without st-segment elevation in the emergency department. Coronary Artery Disease. 2009;20:143–149. doi: 10.1097/MCA.0b013e3283292ac6. [DOI] [PubMed] [Google Scholar]
- 26.Peacock W. Time to treatment and acute coronary syndromes: Bridging the gap in rapid decision making. Rev Cardiovasc Med. 2010;11:S45–S50. doi: 10.3909/ricm11S2S0001. [DOI] [PubMed] [Google Scholar]
- 27.Petersen P. Thromboembolic complications in atrial fibrillation. Stroke. 1990;21:4–13. doi: 10.1161/01.str.21.1.4. [DOI] [PubMed] [Google Scholar]
- 28.Adams H, Adams R, Del Zoppo G, Goldstein LB. Guidelines for the early management of patients with ischemic stroke: 2005 guidelines update a scientific statement from the stroke council of the american heart association/american stroke association. Stroke. 2005;36:916–923. doi: 10.1161/01.STR.0000163257.66207.2d. [DOI] [PubMed] [Google Scholar]
- 29.Vingerhoets F, Bogousslavsky J, Regli F, Van Melle G. Atrial fibrillation after acute stroke. Stroke. 1993;24:26–30. doi: 10.1161/01.str.24.1.26. [DOI] [PubMed] [Google Scholar]
- 30.Shibazaki K, Kimura K, Iguchi Y, Aoki J, Sakai K, Kobayashi K. Plasma brain natriuretic peptide predicts death during hospitalization in acute ischaemic stroke and transient ischaemic attack patients with atrial fibrillation. Eur J Neurol. 2011;18:165–169. doi: 10.1111/j.1468-1331.2010.03101.x. [DOI] [PubMed] [Google Scholar]
- 31.Ng YS, Stein J, Ning M, Black-Schaffer RM. Comparison of clinical characteristics and functional outcomes of ischemic stroke in different vascular territories. Stroke. 2007;38:2309–2314. doi: 10.1161/STROKEAHA.106.475483. [DOI] [PubMed] [Google Scholar]
- 32.Phua J, Lim TK, Lee KH. B-type natriuretic peptide: Issues for the intensivist and pulmonologist. Critical Care Medicine. 2005;33:2094–2013. doi: 10.1097/01.ccm.0000178351.03327.9f. [DOI] [PubMed] [Google Scholar]
- 33.Troughton RW, Frampton CM, Yandle TG, Espine EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (n-bnp) concentrations. The Lancet. 2000;355:1126. doi: 10.1016/s0140-6736(00)02060-2. [DOI] [PubMed] [Google Scholar]
- 34.Miller WL, Hartman KA, Burritt MF, Grill DE, Rodeheffer RJ, Burnett JC, Jr, et al. Serial biomarker measurements in ambulatory patients with chronic heart failure: The importance of change over time. Circulation. 2007;116:249–257. doi: 10.1161/CIRCULATIONAHA.107.694562. [DOI] [PubMed] [Google Scholar]
- 35.Hankey GJ, Spiesser J, Hakimi Z, Bego G, Carita P, Gabriel S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology. 2007;68:1583–1587. doi: 10.1212/01.wnl.0000260967.77422.97. [DOI] [PubMed] [Google Scholar]
- 36.Dhamoon MS, Moon YP, Paik MC, Boden-Albala B, Rundek T, Sacco RL, et al. Long-term functional recovery after first ischemic stroke: The northern manhattan study. Stroke. 2009;40:2805–2811. doi: 10.1161/STROKEAHA.109.549576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva G, Lima FO, Camargo E, Smith WS, Lev MH, Harris GJ, et al. Gender differences in outcomes after ischemic stroke: Role of ischemic lesion volume and intracranial large-artery occlusion. Cerebrovasc Dis. 2010;30:470–475. doi: 10.1159/000317088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wahlgren N, Ahmed N, Eriksson N, Aichner F, Bluhmki E, Davalos A, et al. for the S-MI. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe implementation of thrombolysis in stroke-monitoring study (sits-most) Stroke. 2008;39:3316–3322. doi: 10.1161/STROKEAHA.107.510768. [DOI] [PubMed] [Google Scholar]
- 39.Koton S, Green M, Bornstein N. Mortality and predictors of death 1 month and 3 years after first-ever ischemic stroke: Data from the first national acute stroke israeli survey (nasis 2004) Neuroepidemiology. 2010;34:90–96. doi: 10.1159/000264826. [DOI] [PubMed] [Google Scholar]
- 40.Clark PC, Dunbar SB, Shields CG, Viswanathan B, Aycock DM, Wolf SL. Influence of stroke survivor characteristics and family conflict surrounding recovery on caregivers' mental and physical health. Nursing Research. 2004;53:406–413. doi: 10.1097/00006199-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Ay H, Benner T, Murat Arsava E, Furie KL, Singhal AB, Jensen MB, et al. A computerized algorithm for etiologic classification of ischemic stroke: The causative classification of stroke system. Stroke. 2007;38:2979–2984. doi: 10.1161/STROKEAHA.107.490896. [DOI] [PubMed] [Google Scholar]
- 42.Menezes NM, Ay H, Wang Zhu, Lopez CJ, Singhal AB, Karonen JO, et al. The real estate factor. Stroke. 2007;38:194–197. doi: 10.1161/01.STR.0000251792.76080.45. [DOI] [PubMed] [Google Scholar]
- 43.Saritas A, Cakir Z, Emet M, Uzkeser M, Akoz A, Acemoglu H. Factors affecting the b-type natriuretic peptide levels in stroke patients. Ann Acad Med Singapore. 2010;39:385–389. [PubMed] [Google Scholar]
- 44.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MSV, et al. on behalf of the American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke C. Criteria for evaluation of novel markers of cardiovascular risk. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]


