Summary
Bacterial growth in the host is required for pathogenesis. To successfully grow in vivo, pathogens have adapted their metabolism to replicate in specific host microenvironments. These adaptations reflect the nutritional composition of their host niches, inter-bacterial competition for carbon and energy sources, and survival in the face of bactericidal defense mechanisms. A sub-group of Escherichia coli, which cause urinary tract infection, bacteremia, sepsis, and meningitis, have adapted to grow as a harmless commensal in the nutrient-replete, carbon-rich human intestine but rapidly transition to pathogenic lifestyle in the nutritionally poorer, nitrogen-rich urinary tract. We discuss bacterial adaptations that allow extraintestinal pathogenic E. coli to establish both commensal associations and virulence as the bacterium transits between disparate microenvironments within the same individual.
Keywords: uropathogenic, extraintestinal, ExPEC, UTI, UPEC
Introduction
Escherichia coli, one of the most important model organisms in the laboratory, is the best studied microorganism. The primary niche occupied by E. coli is the lower intestinal tract of mammals, where it resides as a beneficial component of the commensal microbiota. Although it is well-known that E. coli resides in the human intestine as a harmless commensal, specific strains or pathotypes have the potential to cause a wide spectrum of intestinal and diarrheal diseases. For example, at least six pathotypes have been described: enterohemorragic, enteropathogenic, enterotoxigenic, enteroaggregative, diffusely-adherent, and enteroinvasive E. coli (respectively, EHEC, EPEC, ETEC, EAEC, DAEC, and EIEC). On the other hand, extra-intestinal diseases that include urinary tract infection (UTI), bacteremia, septicemia, and meningitis can be caused by additional pathotypes known as extraintestinal pathogenic E. coli (ExPEC) [1]. The loss or gain of mobile genetic elements is responsible for the ability of E. coli to cause a broad range of human diseases. The core genome shared by all E. coli strains represents approximately 2,000 genes, while the pan genome that represents the collective gene content for all sequenced E. coli strains exceeds 10,000 genes [2•]. Thus, for each E. coli strain, which contains 4,800 genes on average, it is the specific composition of horizontally acquired genetic material that determines its ability to cause a certain disease and be defined as a specific pathotype [3].
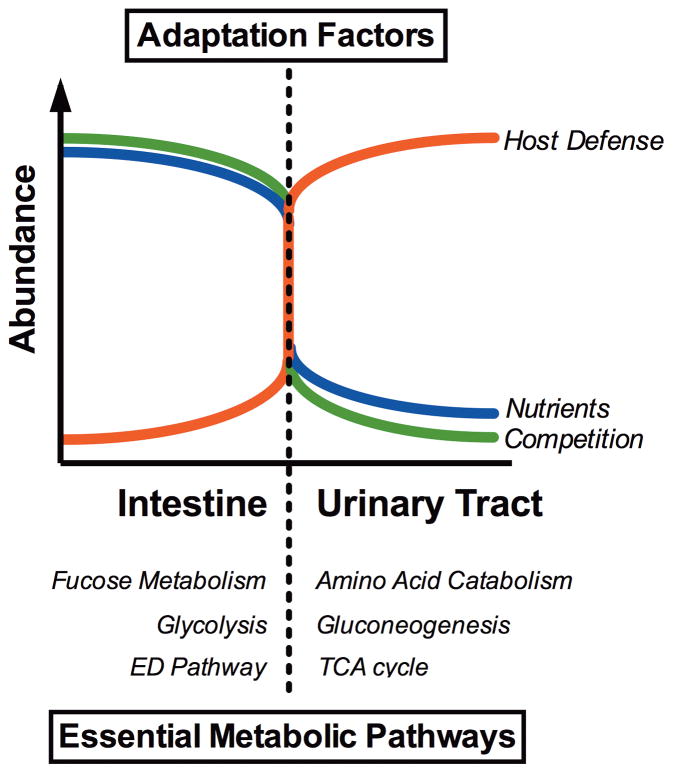
Intestinal E. coli pathotypes, like the infamous EHEC O157:H7 serotype, resides in the bovine intestine as a commensal bacterium and cause severe diarrheal disease only when accidentally introduced into the human intestinal tract. In contrast, extraintestinal E. coli pathotypes reside harmlessly in the human intestinal microenvironment but, upon access to sites outside of the intestine, become a major cause of human morbidity and mortality as a consequence of invasive UTI (pyelonephritis, bacteremia, or septicemia) [4,5]. Thus, extraintestinal pathotypes like uropathogenic E. coli (UPEC) possess an enhanced ability to cause infection outside of the intestinal tract and colonize the urinary tract, the bloodstream, or cerebrospinal fluid of human hosts [5,6]. It follows that extraintestinal pathogenic E. coli possess the unique ability to shift its behavior between harmless colonizer of the nutrient-rich human intestine and virulent pathogen of the nutritionally limited bladder (Fig. 1) [7–9]. Here, we discuss the current understanding of the role for uropathogenic E. coli metabolism and physiology in adapting to these diverse host microenvironments.
Figure 1. Adaptation of metabolism and basic physiology allows E. coli to replicate in diverse host microenvironments.
Extraintestinal pathogenic E. coli that cause urinary tract infection, bacteremia, sepsis, and meningitis, have adapted to grow as a harmless commensal in the nutrient-replete, carbon-rich human intestine but rapidly transition to pathogenic lifestyle in the nutritionally poor, nitrogen-rich urinary tract. In order to establish a commensal association within the human intestine, adaptive factors such as metabolic flexibility allow E. coli to successfully compete for carbon and energy sources with a large and diverse bacterial population. E. coli acquires nutrients from the intestinal mucus, including N-acetylglucosamine, sialic acid, glucosamine, gluconate, arabinose, fucose and simple sugars released upon breakdown of complex polysaccharides by anaerobic gut residents. When UPEC transition to the urinary tract, the bacteria encounter a drastic reduction in the abundance of nutrients and bacterial competition. Consequently, to replicate in a new host microenvironment, UPEC utilization of metabolic pathways required for growth in the dilute mixture of amino acids and peptides in the bladder signals the bacterium to elaborate virulence properties to successfully cause invasive disease and survive the onslaught of bactericidal host defenses. These adaptations are a unique and essential characteristic of ExPEC that enable a successful transition between disparate microenvironments within the same individual.
Traditional extraintestinal E. coli virulence factors
Traditional studies of bacterial pathogenesis have focused on pathogen-specific virulence properties including toxins, adhesins, secretion, and iron acquisition systems, and mechanisms to avoid the innate and adaptive immune response. Epidemiological studies have identified a number of specific virulence factors or genetic determinants associated with extraintestinal pathogenic E. coli (ExPEC) isolates. Extraintestinal virulence genes like those encoding P-fimbriae or hemolysin are frequently clustered in genomic islands known as pathogenecity-associated islands (PAIs) [10] and encode a variety of fimbrial and non-fimbrial adhesins, toxins, and iron acquisition systems [4,11]. Of E. coli pathotypes, ExPEC isolates generally have the largest number of PAIs and horizontally acquired genes; specifically, the prototype pyelonephritis UPEC strain CFT073 has 13 PAIs and is the largest genome (5,388 predicted genes) of sequenced E. coli strains [2•]. One outstanding question, however, is whether these horizontally acquired determinants are maintained in UPEC because they confer an advantage during intestinal colonization or are selected for increased extraintestinal fitness [12,13••].
An intriguing aspect of the pathogenesis of E. coli UTI is the lack of a dominant virulence factor or common set of virulence determinants shared by all UPEC strains but absent from commensal E. coli or intestinal pathogens. Bacterial metabolism during infection has only been recently appreciated to contribute to persistence as much as their virulence properties. Due to the requirement for these E. coli to replicate in and colonize both the intestine and extraintestinal environments, we posit that physiology and metabolism of ExPEC strains is paramount. Indeed, we propose that the ability to survive in the urinary tract depends as much on bacterial physiology and metabolism as it does on the well-considered virulence determinants [14].
Life as an intestinal commensal: nutrient-rich with diverse bacterial competitors
E. coli strains reside and grow in the nutrient-rich mucus lining of the mammalian intestine [15]. Important early colonization studies show the Entner Duodoroff pathway, and gluconate or other sugar acids, are required for intestinal growth of commensal E. coli [16]. E. coli acquires nutrients from the intestinal mucus, a complex mixture of glycoconjugates, and up-regulates genes that encode enzymes involved in the catabolism of N-acetylglucosamine, sialic acid, glucosamine, gluconate, arabinose and fucose [16,17]. EHEC O157:H7 requires the same central carbon pathways as do commensal strains, and mutations in pathways that utilize galactose, hexuronates, mannose, and ribose resulted in colonization defects [17]. Further, multiple mutations in a single strain had an additive effect on colonization levels suggesting that some E. coli strains depend on the simultaneous metabolism of up to six sugars to support colonization of the intestine [17]. These findings suggest that E. coli uses multiple limiting sugars for growth in the intestine and supports the assertion that E. coli grows in the intestine using simple sugars released upon breakdown of complex polysaccharides by anaerobic gut residents [18,19].
Synthesis and degradation of glycogen, an endogenous glucose polymer, plays an important role for E. coli and Salmonella during colonization of the mouse intestine by functioning as an internal carbon source for the bacterium during nutrient limitation [20,21]. When faced with limiting sugars due to consumption by other colonizing bacteria, E. coli can also switch from glycolytic to gluconeogenic substrates to sustain growth in the intestine [22]. For example, EHEC utilizes glycolytic substrates and switches to gluconeogenic substrates when present in the intestine with commensal E. coli that are utilizing glycolytic pathways for in vivo growth [22]. This finding, that competition in vivo can alter preferred routes of carbon flux through the central pathways, introduces the notion that metabolic flexibility and an increased capacity to utilize diverse carbon sources are likely important for successful long-term intestinal colonization by extraintestinal E. coli.
Commensal E. coli that are resident in the intestine are three times more likely to belong to the phylogenetic group B2 (ExPEC primarily belong in B2) than transient intestinal colonizers [23,24]. If ExPEC strains are superior colonizers of the human intestine then it is expected that horizontally acquired genomic islands and PAIs must be contributing to enhanced persistence in the intestine. Recent studies have shown that acquisition and regulation of genes that encode proteins to transport and catabolize prebiotic fructooligosaccharides provides a fitness advantage in the intestine for ExPEC strains [25,26]. The gene cluster required for ExPEC growth on fructooligosaccharides encodes two glycoside hydrolases that belong to family 32 in the carbohydrate active enzyme database (CAZY) and a predicted cytoplasmic fructokinase [25]. The latter is important because fructose must be phosphorylated to be catabolized by E. coli. Another horizontally acquired gene cluster proposed to provide a fitness advantage in the intestine has been discovered in ExPEC, the frz operon, which also encodes a sugar kinase in addition to two aldolases, and IIA, IIB, and IIC fructose family PTS transporter subunits [27]. These findings suggest that ExPEC genomic islands and PAIs may indeed provide added metabolic flexibility and promote persistence in the competitive intestinal microenvironment.
Rapid in-host transition from intestinal colonizer to extraintestinal pathogenic lifestyle
Many studies, primarily from the Conway and Cohen laboratories, have contributed much to the understanding of the in vivo metabolic requirements of E. coli gastrointestinal colonization [16,17,21,22,28]. These groups have also found that mutants that accumulate L-fucose-1-phosphate stimulate catabolism of ribose in vivo and thus fucose metabolites play a role in regulating E. coli carbon utilization in the intestine [29]. This finding that intracellular levels of one metabolite control the expression of genes encoding enzymes for utilization of an unrelated metabolite implies that nutritional sensing may be important for competing with other bacteria for limiting nutrients in the intestine [18] or could represent a mechanism signaling the arrival into a different host microenvironment such as the urinary tract. Signal transduction using two component regulatory systems, which contain a transmembrane sensor kinase and a cognate response regulator transcription factor, are the best described mechanism used by bacteria to coordinate gene expression in response to specific external stimuli. In UPEC, disruption of the QseBC two component regulatory system attenuates virulence in E. coli within the urinary tract by altering carbon flux through central pathways, which negatively affects the expression of multiple adhesins that are involved in bladder colonization [30]. The horizontally acquired frz operon has also been shown to link the metabolic capacity of ExPEC with expression of genes required for adherence to the bladder epithelium; the presence of the frz operon favors the ON orientation of the invertible type 1 fimbriae promoter [27]. UPEC catabolism of D-serine in the urinary tract is also an important signaling mechanism to trigger virulence gene expression [31–33]. These studies suggest that movement of ExPEC from a commensal-like lifestyle in the nutrient- and carbon-rich intestine to the nutritionally poor, nitrogen-rich lower urinary tract would be quickly sensed by changes in metabolism, which would trigger expression of genes required for pathogen colonization of an extraintestinal microenvironment (Fig. 1).
Life as an uropathogen: nutrient-poor with decreased competition and increased host defense
In contrast to the nutritionally diverse intestine, urine in the bladder is a high-osmolarity, moderately oxygenated, iron-limited environment that contains mostly amino acids and small peptides [34–37]. It is therefore not surprising that defects in the both branches of the pentose phosphate pathway, the Entner-Doudoroff pathway, and glycolysis have limited or no impact on E. coli fitness in the bladder and kidney microenvironments [14]. Studies on UPEC metabolism during UTI have revealed that the ability to catabolize the amino acid D-serine in urine, which is both a nutrient and a signaling mechanism to trigger virulence, supports UPEC growth in the nitrogen-rich urinary tract [33]. The utilization of short peptides and amino acids as a carbon source during bacterial infection of the bladder and kidneys is also supported by the observation that UPEC mutants defective in peptide import have reduced fitness during UTI while auxotrophic strains do not [14]. Metabolism of nucleobases is also required for E. coli colonization of the bladder. Signature-tagged mutagenesis screening identified a mutant in the dihydroorotate dehydrogenase gene pyrD [38] and in a separate transposon screen, a gene involved in guanine biosynthesis, guaA, was also identified; a guaA mutant was found to be attenuated in vivo during UTI [39]. Both are supported by the recent finding that E. coli are rapidly replicating in the bladder [40•].
UPEC growing in human urine induces expression of multiple isoforms of both dipeptide- and oligopeptide-binding proteins, both of which were found to be required for UPEC to effectively colonize the bladder and kidneys [14]. Since the nutrient content in the kidney is expected to be very different from urine in the bladder, it is surprising that mutants lacking the ability to produce peptide-transport proteins were attenuated in both the kidneys and bladder because growth in urine mainly mimics only the bladder microenvironment. The host renal physiology might be expected to provide UPEC with several metabolizible carbon sources during reabsorption of the kidney glomerular filtrate in the tubules; however the ischemic damage caused by UPEC during pyelonephritis could alter nutrient availability [41]. Lack of a fitness defect for UPEC amino acid auxotrophs during bladder and kidney colonization and impaired colonization of bladder and kidneys for peptide-transport deficient mutants indicates that these bacteria actively import short peptides found in urine and suggests that peptides or amino acids represent the primary carbon source for E. coli during UTI [14]. In fact, dissimilatory acetate metabolism coupled to the degradation of amino acids during E. coli colonization of the bladder and kidneys shows that ExPEC are adapted to acetogenic growth rather than acetate assimilation [42].
Further, prolonged asymptomatic carriage of E. coli in the bladder selects for mutations that increase expression of D-serine deaminase and peptide/amino acid transport in E. coli [43••]. Gluconeogenic amino acids, like D- and L-serine, can be degraded to oxaloacetate or to pyruvate that can enter the TCA cycle, which is necessary to provide substrates for gluconeogenesis when E. coli use amino acids as a carbon source. Consistent with peptides and amino acids being important carbon sources during UTI, only bacteria with defects in peptide transport, gluconeogenesis, or the TCA cycle demonstrate a fitness defect during colonization of the host urinary tract [14].
Energy metabolism and surviving bactericidal activities of the host
The TCA cycle, in E. coli and in nearly all living cells, is an amphibolic pathway because TCA intermediates are important for anabolic processes in addition to catabolism of acetogenic and gluconeogenic carbon sources like amino acids. Both the complete aerobic TCA cycle and the incomplete reductive pathway are intimately linked to energy metabolism and respiration; pyruvate from glycolysis is oxidized to CO2 concomitant with production of NADH and FADH2, both of which must be reoxidized because all pathways would cease in that absence of NAD+. During respiration, the reoxidation of NADH is coupled to the generation of a proton gradient (ΔpH) that is used, among many processes, to drive ATP production via proton influx through the F1F0 ATPase stator. Much like the flexibility of E. coli carbon metabolism in the intestine, the modular respiratory chains of E. coli can be assembled in a variety of configurations depending on the available terminal electron acceptor and the energy needs of the bacterium [44]. In the intestine, the ability to respire aerobically and anaerobically provides a substantial fitness advantage for E. coli [28]. In the extraintestinal environment, loss of the TCA cycle enzyme succinate dehydrogenase, SdhB, results in a UPEC strain that has reduced fitness in vivo [14], suggesting that a complete TCA cycle and aerobic respiration are important in the urinary tract. At first approximation, aerobic respiration and acetogenic growth (fermentation) during UTI appear contradictory; however, during rapid growth in the host urinary tract it is likely that acetate metabolism reflects a certain degree of reductive overflow, or an electron sink, to relieve carbon flux through the TCA cycle when the respiratory capacity has been reached [40•].
The flexibility of energy metabolism and the modular respiratory chains for E. coli is also critical for adapting to respiratory stress [45•]. Utilization of alternative respiratory chains not only confers flexibility dependent upon the available energy source or terminal electron acceptor [44] but also allows for modulation of the proton motive force (μH+), the gradient of charge and protons across the cytoplasmic membrane [46••]. By reducing membrane potential (Δψ), E. coli can limit or prevent uptake of antibiotics and exist as a bacterial “persister” that can only be eradicated by antibiotics when specific metabolites are present to stimulate respiratory generation of μH+ [47•]. This strategy is also utilized by E. coli to prevent killing by the bactericidal activities of mammalian phagocytes. In the urinary tract, extraintestinal E. coli infection elicits a massive inflammatory response characterized by neutrophil influx [48,49] and host production of the antimicrobial peptide LL-37 cathelicidin [50]. In response to antimicrobial peptides and acidic pH generated by neutrophils, the PhoPQ regulatory system becomes activated and up-regulates genes that encode electroneutral respiratory chain components, cation import systems, and anion efflux channels to limit respiratory generation of μH+ and reverse membrane polarity from inside-negative to inside-positive, respectively [51••]. This control of membrane potential promotes E. coli resistance to cationic antimicrobial peptides and acidic pH, which potentiates bacterial survival against bactericidal activities of neutrophils during UTI. Indeed, UPEC lacking PhoP are exquisitely sensitive to polymyxin and acidic pH, and are severly attenuated during acute infection of the bladder [51••].
Conclusions
In the future, studies using deep sequencing and expression technologies, like RNAseq will provide a clearer understanding of how the transcriptional architecture of E. coli changes between intestinal and extraintestinal environment. This will be important because it is not only the presence or absence of genes that confers virulence, but rather how pathogenic E. coli coordinate expression of genes and regulons, shared by all E. coli, to silently enhance commensal associations in the intestine and rapidly respond to environmental cues and unveil pathogenicity in the urinary tract. It will also be important to understand how the presence or acquisition of genomic islands and PAIs affects the metabolome and metabolic capacity of E. coli. Investigating changes in carbon flux through central metabolism caused by transcriptional regulators, nutrient-uptake systems, and carbohydrate utilization systems encoded on PAIs will help to identify traits that confer a fitness advantage for UPEC when faced with bacterial competition for nutrients in the human intestine. For E. coli transitioning from the carbon-rich intestine to the nitrogen-rich urinary tract, it is likely that the ratio of carbon:nitrogen, or fluctuations in the intracellular C:N levels plays an important role controlling pathogenicity. Nitrogen metabolism and ammonia generated by catabolism of amino acids can act as a form of long-range interbacterial communication to induce oxidative stress responses and increase resistance to antibiotics [52••].
Basic principles of physiology, shared by nearly all living cells, are beginning to be appreciated as playing a key role in processes that are essential for pathogenesis. Bacterial respiration coordinates type three secretion system (TTSS) assembly with movement of Shigella from the intestinal lumen to the mucosa [53]. E. coli modulate their respiratory activity and limit or reverse membrane potential as a protective counter-measure against mammalian host defenses during UTI [51••]. These studies demonstrate the important link between fundamental bioenergetics and pathogenesis and suggest that energy metabolism might be an important signal used by bacterial pathogens to identify specific host microenvironments. By studying carbon metabolism, key differences between E. coli growing in the intestine and colonizing the urinary tract have been identified. Commensal E. coli require the Entner-Doudoroff pathway and glycolysis for intestinal colonization; while the TCA cycle, pentose phosphate pathway, and gluconeogenesis are dispensable [16]. In contrast, for E. coli infecting the urinary tract, the pathways required for commensal colonization are dispensable while the TCA cycle and gluconeogenesis are required [14]. Other aspects of UPEC physiology such as determining if the numerous well-considered or presently uncharacterized fimbriae that predominate in ExPEC strains [54] function as adhesive organelles to promote bacterial adherence in the intestine or in the urinary tract will be beneficial to understand the selective pressures that actively shape and maintain the UPEC pan-genome. Once we better understand how ExPEC are able to transition and adapt to both the intestinal and extraintestinal host microenvironments within the same individual it will be feasible to develop antimicrobials that target pathogenic strains and avoid eradicating beneficial commensal bacteria.
Highlights.
Uropathogenic E. coli are uniquely adapted to colonize disparate host niches in humans.
Flexible carbon and energy metabolism give UPEC a fitness advantage in the intestine and urinary tract.
The ability to survive in the urinary tract depends as much on bacterial physiology as it does on the well-considered virulence determinants.
Acknowledgments
The authors thank Ariel Brumbaugh for assistance with graphics. This work was supported in part by Public Health Service grants AI43363, AI59722, and DK94777 from the National Institutes of Health.
Glossary
- Pathotype
sub-groups of E. coli defined by type of disease caused in humans
- Pan genome
the total gene pool derived from all E. coli genomic sequences
- Virulence factor
usually a gene or collection of related genes that confer a pathogenic trait, e.g. invasion or iron acquisition
- TCA cycle
tricarboxylic acid cycle; also known as the citric acid cycle, or Krebs cycle
- Membrane potential (Δψ)
the electrochemical gradient of charge across the cytoplasmic membrane one component of proton motive force (μH+)
- Persister
dormant bacteria that are refractory to treatment with antibiotics
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Recommended papers, published within the past 2 years, have been annotated as being of special interest (•) or outstanding interest (••).
- 1.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 2•.Touchon M, Hoede C, Tenaillon O, Barbe V, Baeriswyl S, Bidet P, Bingen E, Bonacorsi S, Bouchier C, Bouvet O, et al. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 2009;5:e1000344. doi: 10.1371/journal.pgen.1000344. This study reannotated the genomes of 20 commensal and pathogenic E. coli strains to define the E. coli pan genome and identified an adaptive role for metabolic diversity among ExPEC strains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol. 2010;8:26–38. doi: 10.1038/nrmicro2265. [DOI] [PubMed] [Google Scholar]
- 4.Johnson JR, Kuskowski MA, Gajewski A, Soto S, Horcajada JP, Jimenez de Anta MT, Vila J. Extended virulence genotypes and phylogenetic background of Escherichia coli isolates from patients with cystitis, pyelonephritis, or prostatitis. J Infect Dis. 2005;191:46–50. doi: 10.1086/426450. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JR, Russo TA. Molecular epidemiology of extraintestinal pathogenic (uropathogenic) Escherichia coli. Int J Med Microbiol. 2005;295:383–404. doi: 10.1016/j.ijmm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Russo TA, Johnson JR. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 2003;5:449–456. doi: 10.1016/s1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 7.Chen SL, Hung CS, Pinkner JS, Walker JN, Cusumano CK, Li Z, Bouckaert J, Gordon JI, Hultgren SJ. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc Natl Acad Sci U S A. 2009;106:22439–22444. doi: 10.1073/pnas.0902179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hommais F, Gouriou S, Amorin C, Bui H, Rahimy MC, Picard B, Denamur E. The FimH A27V mutation is pathoadaptive for urovirulence in Escherichia coli B2 phylogenetic group isolates. Infect Immun. 2003;71:3619–3622. doi: 10.1128/IAI.71.6.3619-3622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson JR, Russo TA. Extraintestinal pathogenic Escherichia coli: “the other bad E coli”. J Lab Clin Med. 2002;139:155–162. doi: 10.1067/mlc.2002.121550. [DOI] [PubMed] [Google Scholar]
- 10.Hacker J, Blum-Oehler G, Mühldorfer I, Tschape H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd AL, Rasko DA, Mobley HL. Defining genomic islands and uropathogen-specific genes in uropathogenic Escherichia coli. J Bacteriol. 2007;189:3532–3546. doi: 10.1128/JB.01744-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Gall T, Clermont O, Gouriou S, Picard B, Nassif X, Denamur E, Tenaillon O. Extraintestinal virulence is a coincidental by-product of commensalism in B2 phylogenetic group Escherichia coli strains. Mol Biol Evol. 2007;24:2373–2384. doi: 10.1093/molbev/msm172. [DOI] [PubMed] [Google Scholar]
- 13••.Diard M, Garry L, Selva M, Mosser T, Denamur E, Matic I. Pathogenicity-associated islands in extraintestinal pathogenic Escherichia coli are fitness elements involved in intestinal colonization. J Bacteriol. 2010;192:4885–4893. doi: 10.1128/JB.00804-10. This study showed that deletion of all seven PAIs in UPEC strain 536 caused a drastic reduction in fitness during intestinal colonization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alteri CJ, Smith SN, Mobley HL. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog. 2009;5:e1000448. doi: 10.1371/journal.ppat.1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moller AK, Leatham MP, Conway T, Nuijten PJ, de Haan LA, Krogfelt KA, Cohen PS. An Escherichia coli MG1655 lipopolysaccharide deep-rough core mutant grows and survives in mouse cecal mucus but fails to colonize the mouse large intestine. Infect Immun. 2003;71:2142–2152. doi: 10.1128/IAI.71.4.2142-2152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang DE, Smalley DJ, Tucker DL, Leatham MP, Norris WE, Stevenson SJ, Anderson AB, Grissom JE, Laux DC, Cohen PS, et al. Carbon nutrition of Escherichia coli in the mouse intestine. Proc Natl Acad Sci U S A. 2004;101:7427–7432. doi: 10.1073/pnas.0307888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabich AJ, Jones SA, Chowdhury FZ, Cernosek A, Anderson A, Smalley D, McHargue JW, Hightower GA, Smith JT, Autieri SM, et al. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect Immun. 2008;76:1143–1152. doi: 10.1128/IAI.01386-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martens EC, Roth R, Heuser JE, Gordon JI. Coordinate regulation of glycan degradation and polysaccharide capsule biosynthesis by a prominent human gut symbiont. J Biol Chem. 2009;284:18445–18457. doi: 10.1074/jbc.M109.008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peekhaus N, Conway T. What's for dinner?: Entner-Doudoroff metabolism in Escherichia coli. J Bacteriol. 1998;180:3495–3502. doi: 10.1128/jb.180.14.3495-3502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonafonte MA, Solano C, Sesma B, Alvarez M, Montuenga L, Garcia-Ros D, Gamazo C. The relationship between glycogen synthesis, biofilm formation and virulence in Salmonella enteritidis. FEMS Microbiol Lett. 2000;191:31–36. doi: 10.1111/j.1574-6968.2000.tb09315.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones SA, Jorgensen M, Chowdhury FZ, Rodgers R, Hartline J, Leatham MP, Struve C, Krogfelt KA, Cohen PS, Conway T. Glycogen and maltose utilization by Escherichia coli O157:H7 in the mouse intestine. Infect Immun. 2008;76:2531–2540. doi: 10.1128/IAI.00096-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miranda RL, Conway T, Leatham MP, Chang DE, Norris WE, Allen JH, Stevenson SJ, Laux DC, Cohen PS. Glycolytic and gluconeogenic growth of Escherichia coli O157:H7 (EDL933) and E. coli K-12 (MG1655) in the mouse intestine. Infect Immun. 2004;72:1666–1676. doi: 10.1128/IAI.72.3.1666-1676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowrouzian FL, Wold AE, Adlerberth I. Escherichia coli strains belonging to phylogenetic group B2 have superior capacity to persist in the intestinal microflora of infants. J Infect Dis. 2005;191:1078–1083. doi: 10.1086/427996. [DOI] [PubMed] [Google Scholar]
- 24.Nowrouzian FL, Adlerberth I, Wold AE. Enhanced persistence in the colonic microbiota of Escherichia coli strains belonging to phylogenetic group B2: role of virulence factors and adherence to colonic cells. Microbes Infect. 2006 doi: 10.1016/j.micinf.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Schouler C, Taki A, Chouikha I, Moulin-Schouleur M, Gilot P. A genomic island of an extraintestinal pathogenic Escherichia coli Strain enables the metabolism of fructooligosaccharides, which improves intestinal colonization. J Bacteriol. 2009;191:388–393. doi: 10.1128/JB.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porcheron G, Kut E, Canepa S, Maurel MC, Schouler C. Regulation of fructooligosaccharide metabolism in an extra-intestinal pathogenic Escherichia coli strain. Mol Microbiol. 2011;81:717–733. doi: 10.1111/j.1365-2958.2011.07725.x. [DOI] [PubMed] [Google Scholar]
- 27.Rouquet G, Porcheron G, Barra C, Reperant M, Chanteloup NK, Schouler C, Gilot P. A metabolic operon in extraintestinal pathogenic Escherichia coli promotes fitness under stressful conditions and invasion of eukaryotic cells. J Bacteriol. 2009;191:4427–4440. doi: 10.1128/JB.00103-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones SA, Chowdhury FZ, Fabich AJ, Anderson A, Schreiner DM, House AL, Autieri SM, Leatham MP, Lins JJ, Jorgensen M, et al. Respiration of Escherichia coli in the mouse intestine. Infect Immun. 2007;75:4891–4899. doi: 10.1128/IAI.00484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Autieri SM, Lins JJ, Leatham MP, Laux DC, Conway T, Cohen PS. L-fucose stimulates utilization of D-ribose by Escherichia coli MG1655 ΔfucAO and E. coli Nissle 1917 ΔfucAO mutants in the mouse intestine and in M9 minimal medium. Infect Immun. 2007;75:5465–5475. doi: 10.1128/IAI.00822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hadjifrangiskou M, Kostakioti M, Chen SL, Henderson JP, Greene SE, Hultgren SJ. A central metabolic circuit controlled by QseC in pathogenic Escherichia coli. Mol Microbiol. 2011;80:1516–1529. doi: 10.1111/j.1365-2958.2011.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anfora AT, Haugen BJ, Roesch P, Redford P, Welch RA. Roles of serine accumulation and catabolism in the colonization of the murine urinary tract by Escherichia coli CFT073. Infect Immun. 2007;75:5298–5304. doi: 10.1128/IAI.00652-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haugen BJ, Pellett S, Redford P, Hamilton HL, Roesch PL, Welch RA. In vivo gene expression analysis identifies genes required for enhanced colonization of the mouse urinary tract by uropathogenic Escherichia coli strain CFT073 dsdA. Infect Immun. 2007;75:278–289. doi: 10.1128/IAI.01319-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roesch PL, Redford P, Batchelet S, Moritz RL, Pellett S, Haugen BJ, Blattner FR, Welch RA. Uropathogenic Escherichia coli use d-serine deaminase to modulate infection of the murine urinary tract. Mol Microbiol. 2003;49:55–67. doi: 10.1046/j.1365-2958.2003.03543.x. [DOI] [PubMed] [Google Scholar]
- 34.Alteri CJ, Hagan EC, Sivick KE, Smith SN, Mobley HL. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 2009;5:e1000586. doi: 10.1371/journal.ppat.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alteri CJ, Mobley HL. Quantitative profile of the uropathogenic Escherichia coli outer membrane proteome during growth in human urine. Infect Immun. 2007;75:2679–2688. doi: 10.1128/IAI.00076-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brooks T, Keevil CW. A simple artificial urine for the growth of urinary pathogens. Lett Appl Microbiol. 1997;24:203–206. doi: 10.1046/j.1472-765x.1997.00378.x. [DOI] [PubMed] [Google Scholar]
- 37.Snyder JA, Haugen BJ, Buckles EL, Lockatell CV, Johnson DE, Donnenberg MS, Welch RA, Mobley HL. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect Immun. 2004;72:6373–6381. doi: 10.1128/IAI.72.11.6373-6381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahrani-Mougeot FK, Buckles EL, Lockatell CV, Hebel JR, Johnson DE, Tang CM, Donnenberg MS. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol Microbiol. 2002;45:1079–1093. doi: 10.1046/j.1365-2958.2002.03078.x. [DOI] [PubMed] [Google Scholar]
- 39.Russo TA, Jodush ST, Brown JJ, Johnson JR. Identification of two previously unrecognized genes (guaA and argC) important for uropathogenesis. Mol Microbiol. 1996;22:217–229. doi: 10.1046/j.1365-2958.1996.00096.x. [DOI] [PubMed] [Google Scholar]
- 40•.Hagan EC, Lloyd AL, Rasko DA, Faerber GJ, Mobley HL. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog. 2010;6:e1001187. doi: 10.1371/journal.ppat.1001187. This study provides insight into the physiology of UPEC during clinical UTI and represents the first transcriptome analysis for any pathogenic E. coli during a naturally occurring human infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melican K, Sandoval RM, Kader A, Josefsson L, Tanner GA, Molitoris BA, Richter-Dahlfors A. Uropathogenic Escherichia coli P and Type 1 fimbriae act in synergy in a living host to facilitate renal colonization leading to nephron obstruction. PLoS Pathog. 2011;7:e1001298. doi: 10.1371/journal.ppat.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anfora AT, Halladin DK, Haugen BJ, Welch RA. Uropathogenic Escherichia coli CFT073 is adapted to acetatogenic growth but does not require acetate during murine urinary tract infection. Infect Immun. 2008;76:5760–5767. doi: 10.1128/IAI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zdziarski J, Brzuszkiewicz E, Wullt B, Liesegang H, Biran D, Voigt B, Gronberg-Hernandez J, Ragnarsdottir B, Hecker M, Ron EZ, et al. Host imprints on bacterial genomes--rapid, divergent evolution in individual patients. PLoS Pathog. 2010;6:e1001078. doi: 10.1371/journal.ppat.1001078. In this work the genome of an asymptomatic bacteriuria E. coli strain was sequenced before and after long-term therapeutic bladder colonization of patients and it was found that permanent and unique adaptive changes had occurred in response to individual host environments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unden G, Bongaerts J. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta. 1997;1320:217–234. doi: 10.1016/s0005-2728(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 45•.Shepherd M, Sanguinetti G, Cook GM, Poole RK. Compensations for diminished terminal oxidase activity in Escherichia coli: cytochrome bd-II-mediated respiration and glutamate metabolism. J Biol Chem. 2010;285:18464–18472. doi: 10.1074/jbc.M110.118448. This study provides an insightful and detailed look into the metabolic flexibility of E. coli with particular emphasis on energetics and adaptation to respiratory stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Kralj JM, Hochbaum DR, Douglass AD, Cohen AE. Electrical spiking in Escherichia coli probed with a fluorescent voltage-indicating protein. Science. 2011;333:345–348. doi: 10.1126/science.1204763. This group probed E. coli membrane potential at the level of single cells and found that E. coli can control membrane polarity in response to specific external stimuli. [DOI] [PubMed] [Google Scholar]
- 47•.Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473:216–220. doi: 10.1038/nature10069. This work demonstrated that dormant bacteria can be made susceptible to aminoglycosides by stimulating respiratory generation of proton motive force. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergsten G, Samuelsson M, Wullt B, Leijonhufvud I, Fischer H, Svanborg C. PapG-dependent adherence breaks mucosal inertia and triggers the innate host response. J Infect Dis. 2004;189:1734–1742. doi: 10.1086/383278. [DOI] [PubMed] [Google Scholar]
- 49.Godaly G, Bergsten G, Hang L, Fischer H, Frendeus B, Lundstedt AC, Samuelsson M, Samuelsson P, Svanborg C. Neutrophil recruitment, chemokine receptors, and resistance to mucosal infection. J Leukoc Biol. 2001;69:899–906. [PubMed] [Google Scholar]
- 50.Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson GH, Gallo RL, Agerberth B, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 51••.Alteri CJ, Lindner JR, Reiss DJ, Smith SN, Mobley HL. The broadly conserved regulator PhoP links pathogen virulence and membrane potential in Escherichia coli. Mol Microbiol. 2011;82:145–163. doi: 10.1111/j.1365-2958.2011.07804.x. The findings presented in this work demonstrate that E. coli energy metabolism and control of membrane potential is a key protective counter-measure against mammalian host defenses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52••.Bernier SP, Letoffe S, Delepierre M, Ghigo JM. Biogenic ammonia modifies antibiotic resistance at a distance in physically separated bacteria. Mol Microbiol. 2011;81:705–716. doi: 10.1111/j.1365-2958.2011.07724.x. This nice study showed that nitrogen metabolism and ammonia production is used by bacteria as a long-range signal to influence antibiotic resistance and oxidative stress responses. [DOI] [PubMed] [Google Scholar]
- 53.Marteyn B, West NP, Browning DF, Cole JA, Shaw JG, Palm F, Mounier J, Prevost MC, Sansonetti P, Tang CM. Modulation of Shigella virulence in response to available oxygen in vivo. Nature. 2010;465:355–358. doi: 10.1038/nature08970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spurbeck RR, Stapleton AE, Johnson JR, Walk ST, Hooton TM, Mobley HL. Fimbrial Profiles Predict Virulence of Uropathogenic Escherichia coli Strains: Contribution of Ygi and Yad Fimbriae. Infect Immun. 2011;79:4753–4763. doi: 10.1128/IAI.05621-11. [DOI] [PMC free article] [PubMed] [Google Scholar]