Abstract
The central auditory system consists of the lemniscal and nonlemniscal systems. The thalamic lemniscal and non-lemniscal auditory nuclei are different from each other in response properties and neural connectivities. The cortical auditory areas receiving the projections from these thalamic nuclei interact with each other through corticocortical projections and project down to the subcortical auditory nuclei. This corticofugal (descending) system forms multiple feedback loops with the ascending system. The corticocortical and corticofugal projections modulate auditory signal processing and play an essential role in the plasticity of the auditory system. Focal electric stimulation -- comparable to repetitive tonal stimulation -- of the lemniscal system evokes three major types of changes in the physiological properties, such as the tuning to specific values of acoustic parameters of cortical and subcortical auditory neurons through different combinations of facilitation and inhibition. For such changes, a neuromodulator, acetylcholine, plays an essential role. Electric stimulation of the nonlemniscal system evokes changes in the lemniscal system that is different from those evoked by the lemniscal stimulation. Auditory signals ascending from the lemniscal and nonlemniscal thalamic nuclei to the cortical auditory areas appear to be selected or adjusted by a “differential” gating mechanism. Conditioning for associative learning and pseudo-conditioning for nonassociative learning respectively elicit tone-specific and nonspecific plastic changes. The lemniscal, corticofugal and cholinergic systems are involved in eliciting the former, but not the latter. The current article reviews the recent progress in the research of corticocortical and corticofugal modulations of the auditory system and its plasticity elicited by conditioning and pseudo-conditioning.
Keywords: Conditioning, Plasticity, Pseudo-conditioning, Reorganization, Tonotopic map
(1) Introduction
This article reviews the recent progress in the research of the modulation of the auditory system by corticocortical and corticofugal projections, conditioning and pseudo-conditioning. I will first summarize the anatomical aspects of the auditory system directly related to these physiological studies. The central auditory system is composed of the lemniscal and nonlemniscal pathways or systems. The lemniscal system consists of the central nucleus of the inferior colliculus (ICc), the ventral division of the medial geniculate body (MGBv), the primary auditory cortex (AI) and the anterior auditory field (AAF). It is tonotopically organized. The nonlemniscal system consists of the external (ICx) and dorsal (ICd) nuclei of the IC, the medial (MGBm) and the dorsal (MGBd) divisions of the MGB and the posterior intralaminar nucleus (PIN). It is poorly tonotopically organized. The ICc, ICx and ICd project to the MGBv, MGBm and MGBd, respectively (Aitkin and Webster 1972; Clarey et al. 1992 and Rouiller 1997 for reviews).
In general, MGBv neurons are sharply frequency-tuned and carry tonotopically organized auditory specific information. Their responses to sounds are consistent. On the contrary, MGBm and MGBd neurons are broadly frequency-tuned and their responses to a repeatedly delivered identical stimulus are inconsistent and quickly habituate. MGBm neurons carry poly-sensory information and are presumably involved in associative learning (Rouiller 1997, He 2003 and Hu 2003 for reviews). However, they are sensitive to changes in auditory stimuli (Kraus et al. 1994). The MGBd projects to the cortical auditory areas surrounding AI, whereas the MGBm projects to all auditory areas including AI (Imig and Morel 1983 for review; Andersen et al. 1980; Winer et al. 1977). Unlike MGBv neurons, MGBm neurons have extensive and direct connections with the amygdala, striatum and association cortex (He 2003 and Hu 2003 for reviews). Therefore, these two systems are anatomically and physiologically quite different from each other. The cortical auditory areas mutually project, so that they are not simply assigned either the lemniscal or nonlemniscal areas. However, for convenience, AI and the secondary auditory cortex (AII) are included in the lemniscal and nonlemniscal systems, respectively.
The cortical auditory areas project to the corresponding contralateral cortical areas through the corpus callosum, with certain exceptions (Imig and Brugge 1978; Liu and Suga 1997). They also project to subcortical auditory nuclei: AI and AAF project back to the MGBv, ICc and ICx; AII to the MGBd and ICd; and all auditory areas to the MGBm. The ICd receiving sparse ascending auditory projections receives a prominent descending projection from AII (Rouiller 1997 for review; Andersen et al. 1980; Weiner et al. 2001). In echolocating bats, the ICd is extremely small, whereas the ICc is very large (Pollak and Casseday 1989). Unlike the MGBd, the MGBm projects to AI and AAF, and has feedback from them. Therefore, it may be more important than the MGBd in terms of interactions between the lemniscal and nonlemniscal systems. The GABAergic thalamic reticular nucleus (TRN) receives collateral projections from the thalamocortical (Jones 1975; Crabtree 1998) and corticothalamic fibers and projects to the neighboring thalamic nuclei (He 2003 and Hu 2003 for reviews). The TRN may also play an important role in the interaction between the lemniscal and nonlemniscal systems.
The corticofugal (descending) system forms multiple feedback loops with the ascending system. The shortest feedback loop is the thalamo-cortico-thalamic loop, and the longest one is the cochlea-cortico-cochlear (via multiple auditory nuclei) loop. The colliculo-thalamo-cortico-collicular loop has an intermediate length. The changes occurring in the auditory cortex are looped back to the cortex through these multiple feedback loops.
Acoustic stimuli such as trains of 60 dB SPL tone bursts activate the auditory system and may activate the ascending reticular activating system (ARAS). For auditory fear conditioning and pseudo-conditioning, electric leg- or foot- stimulus has often been used as an unconditioned stimulus (US). The US activates not only the somatosensory system, but also the ARAS and brain aversion system (BAS). They evoke arousal and defensive behaviors, respectively. The ARAS and BAS activate the various neuromodulatory systems which broadly project to both the cerebral cortex and subcortical sensory nuclei and play an important role in their activities and organizations (Siegel 2002 and Brandao et al. 2003 for reviews).
(2) Corticocortical modulation by the lateral, contralateral, feedforward, and feedback projections
2.1. Three major types of changes evoked by focal electric stimulation of the lemniscal system
Focal repetitive stimulation with, e.g., a train of 100 nA, 0.2 ms electric pulses of AI, evokes three major types of changes in the responses and tuning curves of cortical neurons neighboring the stimulated ones. Namely, when the recorded neuron is matched in best frequency (BF) to the stimulated one, the response of the “matched” neuron is augmented at its BF and is inhibited at frequencies lower and/or higher than the BF. As a result, its frequency tuning is sharpened. When BF-unmatched, the “unmatched” neuron is inhibited at its BF and is facilitated at non-BFs so as to shift its frequency tuning (Fig. 1a and b). The frequency-tuning shift is accompanied with a shift in BF, so that such a shift has been called a “BF shift”. The BF shift develops to the peak at 0.5 h after the onset of the stimulation and disappears within 3.0 h after the peak.
Fig. 1. Tuning shifts, the auditory pathway and auditory cortex.
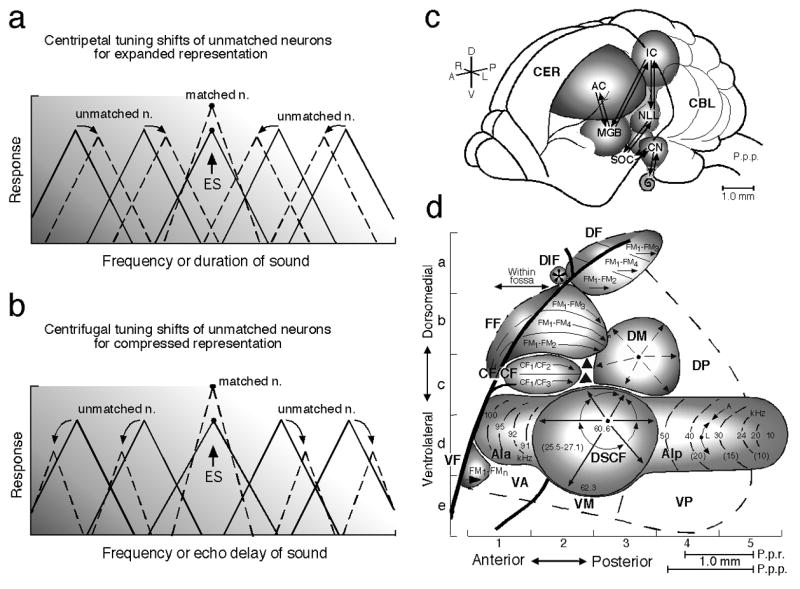
Focal electric stimulation (ES) of the lemniscal auditory system evokes facilitation, inhibition and tuning shifts in AI and subcortical auditory nuclei. There are two types of tuning shifts of “tuning-unmatched” neurons: centripetal (a) and centrifugal (b). (a) and (b) The unbroken and broken triangles represent the tuning curves in the control and shifted conditions, respectively. “Tuning-matched” neurons do not show tuning shifts, but facilitation of their auditory responses. Centripetal and centrifugal tuning shifts both have been found in the frequency and time domains. (c) The dorsolateral view of the brain of the mustached bat, Pteronotus parnellii parnellii (P.p.p.). The arrows indicate the ascending and descending (corticofugal) systems. (d) A neurophysiological map of the auditory cortex (AC) of the mustached bat. The numbers and lines in the anterior (AIa) and posterior (AIp) divisions and the Doppler-shifted constant frequency (DSCF) area in AI indicate iso-best-frequency lines. The CF/CF area sensitive to combinations of constant-frequency (CF) signals consists of two subdivisions that contain a Doppler-shift (velocity) axis. The frequency modulation-frequency modulation (*FF), dorsal fringe (DF) and ventral fringe (VF) areas are sensitive to combinations of frequency-modulated (FM) signals. Each area consists of three subdivisions. These areas contain an echo-delay (range) axis. CBL, cerebellum; CER, cerebrum; CN, cochlear nucleus; DIF, dorsal intrafossa area; DM, dorsomedial area; DP, dorsoposterior area; IC, inferior colliculus; MGB, medial geniculate body; NLL, nucleus of the lateral lemniscus; P.p.r., Pteronotus parnellii rubiginosus which is larger than P.p.p.; SOC, superior olivary complex; VA, ventroanterior area; VM, ventromedial area; VP, ventroposterior area (Suga and Ma 2003). Focal electric stimulation of the AIp, DF or VF area evokes centripetal tuning shifts, whereas that of the DSCF or FF area evokes centrifugal tuning shifts. [*The FF area had been called the FM-FM area because it consists of FM-FM combination-sensitive neurons. Both the DF and VF areas, subsequently found, also consist of FM-FM neurons. So, the FM-FM area is now called the FF area (Tang and Suga 2008).]
There are two types of BF shifts: shifts toward the BF of the stimulated neurons (“centripetal” shifts) and shifts away from it (“centrifugal” shifts) (Fig. 1a and b). The centripetal BF shift results in an increase in the number of neurons responding to the frequency equal to the stimulated cortical BF or the stimulus tone (“expanded” representation), whereas the centrifugal BF shift results in a reduced representation which is associated with the augmentation of responses and sharpening of the frequency-tuning of matched neurons (“compressed” representation). The reorganization that takes place differs depending on the cortical areas, as described in the following text (Suga et al. 2002 and Suga and Ma 2003 for reviews). The direction of the BF shifts changes from centrifugal to centripetal when cortical inhibition is removed by bicuculline methiodide, an antagonist of inhibitory GABA-A receptors (Xiao and Suga 2004, 2005; Ma and Suga 2004). The reorganization (therefore, organization) of a cochleotopic (tonotopic or frequency) map is apparently based upon the interaction between excitation and inhibition in the auditory cortex. In addition to the above three types of changes, the response latency and pattern to a sound stimulus may change (e.g., Jen et al. 1998; Fritz et al. 2007 for review). However, most of the studies on the effects of electric stimulation have been focused on the above three major changes, because an auditory neuron is tuned to at least a specific value of a parameter such as frequency, and because the tuning shift indicates the change in a neural representational map.
2.2. Modulation in the less-specialized auditory cortex
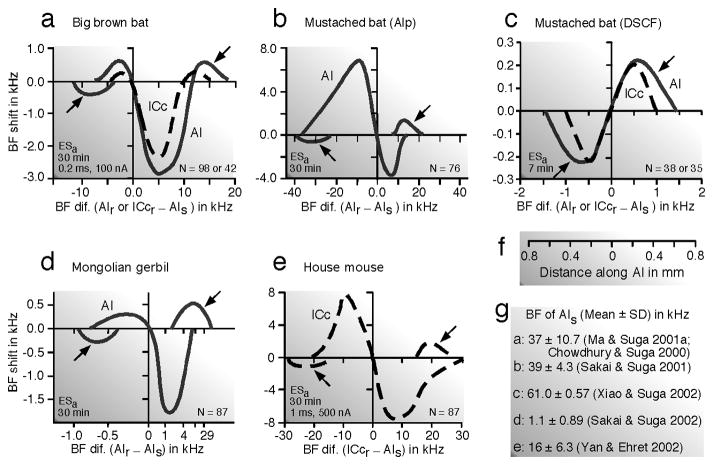
The auditory system is more or less specialized in a species-specific way. Here, “less-specialized” means that the auditory system is less specialized compared with that of the mustached bat. In the AIs of the big brown bat (Chowdhury and Suga 2000; Ma and Suga 2001a) and Mongolian gerbil (Sakai and Suga 2001, 2002), centripetal BF shifts in the ipsilateral AI through the lateral projection (horizontal connections) occur in a large area surrounding the stimulated neurons and small centrifugal BF shifts occur in a narrow zone surrounding this large area. So, the reorganization of the tonotopic map in AI is “center-surround” (Fig. 2a - c). BF shifts mean the shift of iso-BF lines. This shift is not parallel to the iso-BF line crossing the stimulated neurons, because its amount is largest around the stimulated neurons and becomes smaller away from them along the iso-BF line (Fig. 2e and f). Fig. 2d shows a scatter plot of the BF shifts of many neurons studied as the function of the difference in BF between the recorded and stimulated neurons. The curve encompassing such a plot is defined as the “BF-shift-difference” curve (Fig. 3d), which is different depending on the animal species and stimulated cortical areas. However, it always spans 0.6 ~ 0.7 mm from the stimulated neurons along the frequency axis regardless of the animal species and stimulated cortex (Fig. 3). The posterior division of AI (AIp) of the mustached bat is not particularly specialized for processing biosonar signals and is comparable to AI of the cat (Fig. 1d). The BF shift in AIp is centripetal. The amount of the BF shift is up to 6.9 kHz for the 39 kHz BF (Fig. 3b).
Fig. 2. Distribution of centripetal and centrifugal BF shifts in the primary auditory cortex (AI) evoked by focal electric stimulation of AI in the Mongolian gerbil, Meriones unguiculatus.
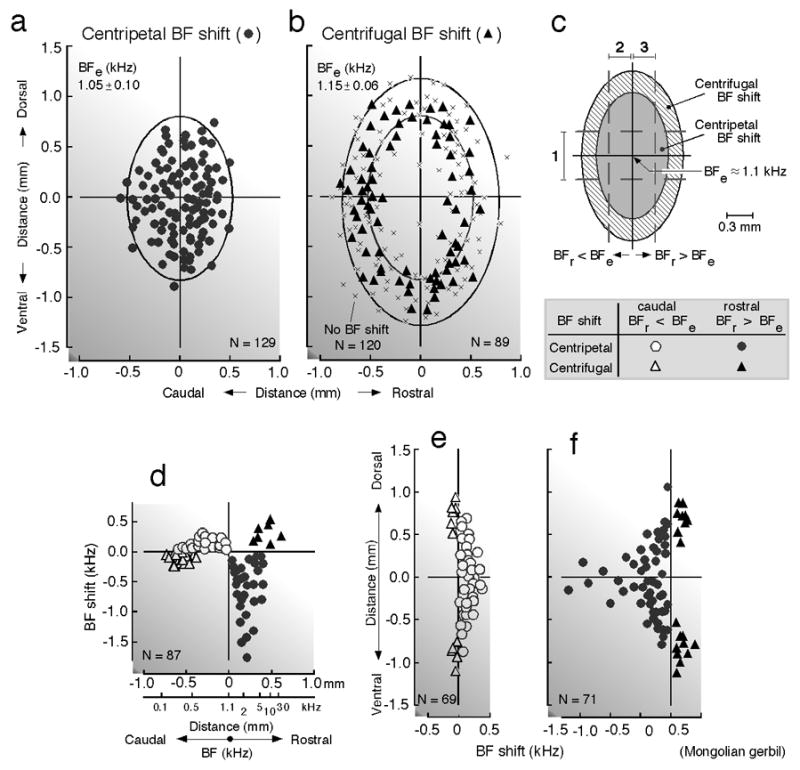
Electric stimulation of 1.1-kHz-tuned neurons in AI evokes centripetal (a, circles) and centrifugal (b, triangles) BF shifts of other AI neurons within 1.0 mm. Locations of recorded neurons along the cortical surface are plotted relative to that of the stimulated neurons at the origin of the coordinates. x- and y-axes: directions parallel and orthogonal to the cochleotopic (tonotopic) axis of AI, respectively. “x” in b indicates a neuron that showed no BF shift. Data are pooled from 16 hemispheres of 11 animals. Confidence ellipses are shown for neurons that showed centripetal (a) or centrifugal (b) BF shifts. The amounts of BF shifts were measured in a zone parallel (1) or orthogonal (2 and 3) to the cochleotopic axis (c). The directions and amounts of BF shifts of neurons in the rostro-caudal (1 in c) and dorso-ventral (2 and 3 in c) zones are respectively plotted in d – f as a function of distance along the cortical surface. BFe: BF of electrically stimulated AI neurons. BFr: BF of recorded AI neurons. See the inset at the bottom of (c) for symbols (Sakai and Suga 2002).
Fig. 3. “BF-shift-difference” curves obtained from the primary auditory cortex (AI) or the central nucleus of the inferior colliculus (ICc) of four species of mammals.
The BF shift changes as a function of the difference in BF between the recorded collicular (ICcr, dashed curves) or cortical (AIr, undashed curves) neurons and the electrically stimulated cortical neurons (AIs). Each BF-shift-difference curve encompasses a scatter plot of BF shifts of many neurons studied (N) such as in Fig. 2d. Note the differences in the curves between species and between different areas of the same species. (a, b, d and e) Centripetal BF shifts, except where indicated by arrows. A prominent centripetal BF shift occurs at ~ 5 kHz higher than the stimulated cortical BF in the big brown bat, Eptesicus fuscus (a) and at ~ 1 kHz higher than that in the Mongolian gerbil, Meriones unguiculatus (d). By contrast, the prominent centripetal BF shift occurs at ~ 10 kHz lower than the stimulated cortical BF in the posterior division of AI (AIp) of the mustached bat, Pteronotus parnellii parnellii (b). In the house mouse, Mus domesticus, prominent centripetal BF shifts occur at ~ 9 kHz higher and lower than the stimulated cortical BF (e). (c) Prominent centrifugal BF shifts occur at ~ 0.5 kHz higher and lower than the stimulated cortical BF in the Doppler-shifted constant frequency (DSCF) area of the mustached bat. The shape of these BF-shift-difference curves might change with the mean BF of stimulated cortical neurons (AIs). (f) Distance along the cochleotopic axis of AI from the stimulated cortical neurons. (g) The mean and standard deviation of the BFs of stimulated cortical neurons as well as references are shown for each figure, a – e. The characteristics of the electric stimulation (ESa) were 0.2 ms, 100 nA pulses for a - d and 1 ms, 500 nA pulses for e (Suga and Ma 2003).
In the big brown bat, BF shifts in AI evoked by focal electric stimulation of the contralateral AI are basically the same as those evoked by the stimulation of the ipsilateral AI. That is, the lateral and contralateral modulations both are predominantly centripetal BF shifts (Ma and Suga 2001a). Since centripetal tuning shifts for an expanded representation are common in the different sensory systems of various mammalian species (See 2.4. Remarks; Suga et al. 2003 for review), it may also be common that the corresponding auditory cortical areas on both sides show similar centripetal BF shifts.
2.3. Modulation in the specialized auditory cortex
Since the AIs described above are not highly specialized for processing specific auditory information, the Doppler-shifted constant frequency (DSCF) and frequency modulation-frequency modulation (FF) areas of the mustached bat were studied in comparison.
The orientation sound (hereafter, pulse) of the mustached bat consists of a long constant frequency (CF) component followed by a short frequency modulated (FM) component. Each component is composed of four harmonics. Therefore, there are eight components in each biosonar signal, CF1–4 and FM1–4. Among these eight components, the second harmonic CF component (CF2) is the most intense and plays an essential role in the Doppler shift (target relative velocity and wing motion) measurement.
2.3.1. Cortical area specialized for processing velocity information
The DSCF area consists of neurons sharply tuned to the frequencies between 60.6 and 62.3 kHz which are the frequencies of the emitted pulse and its Doppler-shifted echoes (Fig. 1d). This area occupies ~ 30% of AI and is specialized for processing the Doppler-shifted echo CF2.
Focal electric stimulation of the DSCF area evokes the centrifugal BF shifts of DSCF neurons around the stimulated ones (Fig. 4Aa). The amount of the BF shift is small, only up to 0.22 kHz for ~ 61 kHz BFs (Fig. 3c), instead of up to 6.9 kHz for ~ 39 kHz BFs in the AIp (Fig. 3b). Such a small amount of BF shift is due to the over-representation of the ~ 61 kHz sound by sharply tuned neurons in the DSCF area. The BF shift occurs within 0.6 mm from the stimulated neurons along the frequency axis in both the DSCF and AIp areas (Xiao and Suga 2002b).
Fig. 4. Best frequency (BF) shifts in the specialized auditory system of the mustached bat.
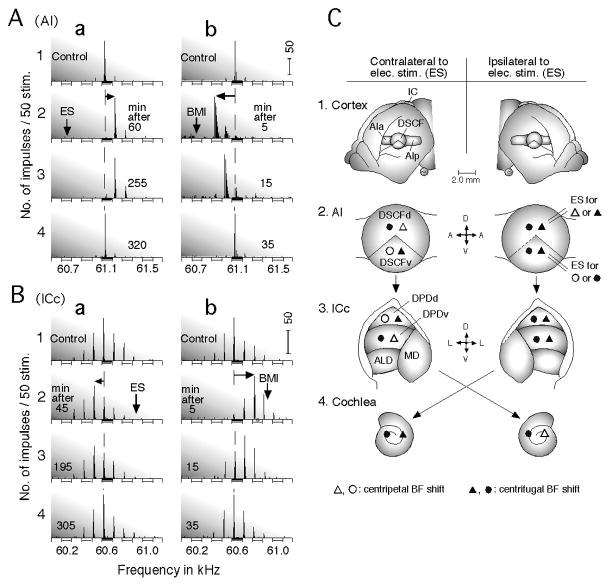
Changes in the direction of the BF shifts of a cortical (A) and a collicular (B) DSCF neuron evoked by an antagonist of inhibitory GABA-A receptors, bicuculline methiodide (BMI). A and B: Focal electric electrical stimulation (ES) of cortical DSCF neurons evokes centrifugal BF shifts (a), whereas BMI applied to the cortical DSCF neurons evokes centripetal BF shifts (b). The arrays of PST histograms display frequency-response curves of the recorded neurons. The vertical and horizontal arrows respectively indicate the BFs of cortical DSCF neurons receiving ES or BMI and centrifugal or centripetal BF shifts of the recorded neurons. 1 – 4: The PST histograms recorded before (control) and after ES or BMI applications. The amplitude of tone bursts was set at 10 dB above the minimum threshold of a given neuron. ES: 0.2-ms 100-nA electric pulses delivered at a rate of 5/s for 7.0 min; BMI: 1.0 nl of 5 mM (Xiao and Suga 2002b). The vertical scale bars indicate 50 impulses. (C) Distribution of centripetal and centrifugal BF shifts in the primary auditory cortex (AI), the central nucleus of the inferior colliculus (ICc) and the cochlea evoked by focal cortical electric stimulation (ES). (1) Dorsolateral view of the cerebral cortex. In AI, the Doppler-shifted constant frequency (DSCF) area is sandwiched between the anterior (AIa) and posterior (AIp) divisions of AI. (2) The DSCF area can be divided into dorsal and ventral divisions (DSCFd and DSCFv) in terms of the effect of cortical electric stimulation. (3) The ICc consists of the dorsoposterior (DPD), anterolateral (ALD) and medial (MD) divisions. The DPD can be divided into the dorsal (DPDd) and ventral (DPDv) portions in terms of the effect of cortical electric stimulation. (4) Cochlea where cochlear microphonic responses (CM) were recorded. Electric stimulation (ES) of DSCFd or DSCFv (2, right) evokes the changes in the BFs of DSCF and DPD neurons and CM. Centripetal and centrifugal BF shifts evoked by DSCFd stimulation are expressed by open and filled triangles, respectively, whereas those evoked by DSCFv stimulation are expressed by open and filled circles, respectively (Xiao and Suga 2005).
The contralateral modulation through the corpus collosum is unexpectedly different from the lateral one. The dorsal and ventral divisions (DSCFd and DSCFv) of the stimulated DSCF area respectively evoke the centripetal and centrifugal BF shifts of contralateral DSCFd neurons, and the centrifugal and centripetal BF shifts of contralateral DSCFv neurons (Xiao and Suga 2005). That is, the direction of BF shifts in the contralateral DSCF area shows a flip-flop, depending on the spatial relationship between the stimulated and recorded neurons (Fig. 4C). When inhibition in the stimulated DSCF area is blocked by bicuculline methiodide, all the BF shifts in the ipsilateral and contralateral DSCF areas become centripetal, regardless of the stimulated location in the DSCF area (Fig. 4Ab). Therefore, centrifugal BF shifts originate from inhibition occurring in the stimulated DSCF area (Xiao and Suga 2002b, 2005).
The DSCFd and DSCFv divisions consist of different types of binaural neurons (Manabe et al. 1978). The DSCFd is not bilaterally connected by commissural fibers, but the DSCFv is (Liu and Suga 1997). The intriguing asymmetry in corticocortical modulations described above appears to be related to binaural signal processing. In the cat’s AI, the different types of binaural neurons are separately clustered and form binaural bands (Imig and Adrian 1977). One type of band is bilaterally connected by commissural fibers, but the others are not (Imig and Brugge 1978), as those in the DSCF area. Therefore, the intriguing asymmetry in corticocortical modulations may also be found in cats.
2.3.2. Cortical areas specialized for processing distance information
The auditory cortex of the mustached bat consists of at least nine areas. Three of these are the FF, dorsal fringe (DF) and ventral fringe (VF) areas. The FF, DF and VF areas are composed of “delay-tuned” neurons sensitive to the combination of the emitted pulse FM1, and its echo FM2–4, with a specific echo delay, best delay (BDe). Each area also consists of three subdivisions in terms of the combination sensitivity of neurons, FM1-FM2, FM1- FM3 and FM1-FM4 combinations (Suga 1990, 1994 for review). The DF and VF areas are hierarchically at a higher level than the FF (Fitzpatrick et al. 1998).
Focal electric stimulation of the FF area evokes centrifugal BDe shifts in the ipsilateral and contralateral FF areas and also in the ipsilateral DF and VF areas (Fig. 5a and d). These centrifugal BDe shifts change into centripetal shifts when inhibition in the stimulated FF area is blocked by bicuculline methiodide (Xiao and Suga 2004). On the other hand, the stimulation of the DF area evokes centripetal BDe shifts in the ipsilateral and contralateral DF areas and also in the ipsilateral FF area (Fig. 5e). In other words, lateral, contralateral, feedforward and/or feedback modulations by the FF area are centrifugal but those of the DF area are centripetal (Xiao and Suga 2004; Tang et al. 2007; Tang and Suga 2008, 2009). In addition, it has also been known that feedback modulation of the VF area to the FF area is centripetal (Fig. 5b and f). The centripetal BDe shifts are ~ 2.5 times larger than the centrifugal BDe shifts. All those BDe shifts occur in BDe unmatched neurons (Fig. 5, d-f). In each of the FF, DF and VF areas, the direction of a BDe shift stays the same regardless of the combination-sensitivity of the delay-tuned neurons. In other words, iso-BDe lines shift together across the three subdivisions of each area. A flip-flop in the direction of BDe shifts does not occur. The BDe-matched neurons do not shift their BDe’s and show facilitation of their responses at their BDe’s and sharpening of their delay-tuning curves (Fig. 5c). The lateral projection within the FF area and FF-to-DF/VF feedforward projections are presumably for the finer analysis of target information at a specific distance. On the other hand, the DF-to-FF and VF-to-FF feedback projections are presumably for focusing the neural processing of target information at that specific distance represented by the FF area. These projections presumably promote finer analysis of target information at shorter distances because the target distances represented in the FF, DF and VF areas are shorter in this order (Suga 1990 for review).
Fig. 5. Changes in delay tuning evoked by electric stimulation of cortical delay-tuned neurons through the lateral, contralateral, feedforward, feedback and corticofugal projections.
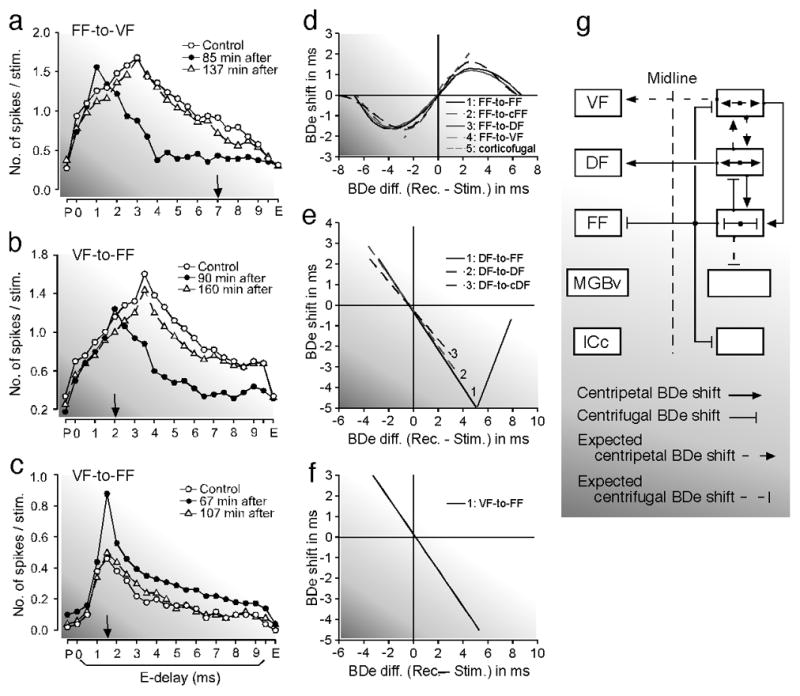
(a) The centrifugal best delay (BDe) shift of a VF neuron tuned to a 3.0 ms echo delay evoked by electric stimulation of FF neurons tuned to a 7.0 ms delay through feedforward projection. The delay-response curves of the VF neuron were obtained before (control, open circles), 85 min after (filled circles) and 137 min after (open triangles) the onset of the FF stimulation. (b) and (c) A centripetal BDe shift of a BDe-unmatched FF neuron (b) and sharpening of the tuning of a BDe-matched FF neuron (c) evoked by electric stimulation of VF neurons via the feedback projection. The BDe’s of the recorded FF and stimulated VF neurons were 3.5 and 2.0 ms, respectively in (b), but both were 1.5 ms in (c). The downward arrows indicate the BDe’s of the stimulated neurons. The BDe-shift-difference curves for the BDe shifts evoked by electric stimulation of either the FF (d), DF (e) or VF (f) neurons. (d) “1–5” respectively show the curves for the BDe shifts elicited by the lateral, contralateral, feedforward to DF or VF and corticofugal projections from (or within) the FF area. cFF, contralateral FF. (e) “1–3” respectively show the curves for the BDe shifts elicited by the feedback, lateral and contralateral projections from (or within) the DF area. cDF, contralateral DF. (f) “1” shows the curve for the BDe shifts elicited by the feedback projection from the VF area to the FF area. (g) The block diagram showing the projections evoking the centripetal (arrow) or centrifugal (line with a short bar at its end) BDe shifts. The short dashed lines indicate either centripetal or centrifugal BDe shifts that are speculated (Tang and Suga 2009).
2.4. Remarks on corticocortical modulation
The lateral projection within a specific cortical area commonly evokes the centripetal shifts of the tuning curves or receptive fields of neurons in AI’s of different mammalian species (rats, Rattus rattus, Talwar and Gerstein 2001; big brown bats, Chowdhury and Suga 2000, Ma and Suga 2001a; Mongolian gerbils and mustached bats, Sakai and Suga 2001); in the primary visual cortex (cats, Felis catus, Godde et al. 2002, Calford et al. 2003); and in the primary somatosensory cortex (rats and owl monkeys, Aotus trivirgatus boliviensis, Recanzone et al. 1992a,b). However, it evokes the centrifugal tuning shifts in the DSCF (Zhang and Suga 2000, Xiao and Suga 2005) and FF (Xiao and Suga 2004) areas. These two areas are highly specialized for processing specific biosonar information (Suga 1990 for review). The centripetal tuning shifts are apparently much more common than the centrifugal tuning shifts.
The interaction between different cortical areas has also been studied in the cat visual cortex. The deactivation of the visuoparietal cortex decreases orientation and direction sensitivities of neurons in area 18 and abolishes the global layout of the direction map in it. The higher cortical visual area significantly contributes to the creation of the basic response properties of the lower cortical visual area (Galuske et al. 2002). Corticocortical modulation between different cortical auditory areas is simple in AI of the big brown bat, but is complex in the DSCF area (a part of AI) of the mustached bat. The feedforward and feedback modulations are different between the FF, DF and VF areas. Therefore, there are apparently different types of corticocortical modulations. In macaques (Rauschecker and Tian 2000) and cats (Lomber and Malhotra 2008), the auditory cortex consists of several areas. How do the feedforward and feedback projections between these cortical areas contribute to the creation of specific response properties of neurons? Are there corticocortical modulations different from those thus far found in bats?
For a 30-min-long cortical electric stimulation, tuning shifts reach a peak 30 min after the onset of the stimulus and then gradually disappear within 3.0 hours. So, the behavioral changes which might occur have not yet been examined. The cortical tuning shifts can be elicited by repetitive acoustic stimulation or auditory fear conditioning. They represent an auditory memory trace, as described in Section 4.
(3) Corticofugal modulation: the primary auditory cortex to the subcortical auditory nuclei and to cochlear hair cells
Neural mechanisms for creating the various response properties of auditory neurons and the “computational” maps – maps different from the tonotopic map inherited from the cochlea – in the central auditory system had been explained only by neural interactions in the ascending auditory system (Suga 1990 and Covey and Casseday 1999 for reviews). However, the findings since 1995 indicate that the corticofugal system plays important roles in shaping the response properties of the neurons and in organizing the tonotopic and computational maps (Suga et al. 2002 and Suga and Ma 2003 for reviews).
Focal electric stimulation of the lemniscal system evokes basically the same plastic changes in MGBv, ICc and CN as those in AI. Namely, the stimulation evokes the augmentation of responses and sharpening of tuning of tuning-matched subcortical neurons and the tuning shifts of tuning-unmatched subcortical neurons. Almost all of the changes, if not all, first occur in AI and then are transferred down to the subcortical auditory nuclei regardless of the stimulated sites: AI (Suga and Ma 2003 and Xiong et al. 2009 for reviews; Luo et al. 2008), MGBv (Jafari et al. 2007; Wu and Yan 2007) or ICc (Zhang and Suga 2005). Since there is only one type of change in the tuning-matched neurons, but two types of changes in the tuning-unmatched neurons, the following text mainly describes centripetal and centrifugal tuning-shifts.
3.1. Modulation in the frequency domain
3.1.1. Medial geniculate body, inferior colliculus and cochlear nucleus
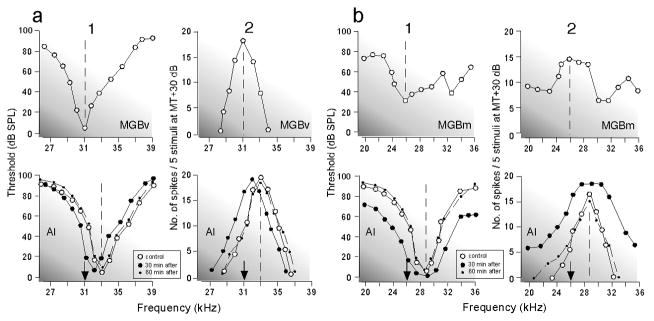
Electric stimulation of cortical DSCF neurons evokes centrifugal BF shifts of the BF-unmatched thalamic (MGBv) and collicular (ICc) DSCF neurons, and facilitation of the responses of the BF-matched thalamic and collicular neurons (Zhang and Suga 2000). Inactivation of the stimulated cortical DSCF neurons with a local anesthetic (lidocaine) changes these centrifugal BF shifts into centripetal shifts (Fig. 6). It abolishes the facilitation of the responses and reduces the responses to tone bursts of the BF-matched neurons (Zhang et al. 1997). The corticofugal system apparently improves and adjusts subcortical auditory signal processing. Inactivation of the entire DSCF area including BF-matched and – unmatched neurons by muscimol reduces the responses of the thalamic DSCF neurons by ~ 60% and those of collicular ones by ~ 24% (Zhang and Suga 1997, 2000). In the big brown bat, such inactivation of AI reduces collicular responses by ~ 38% (Gao and Suga 1998). The corticothalamic and corticocollicular feedbacks apparently amplify thalamic and collicular responses.
Fig. 6. Changes in tuning curve, best frequency and response magnitude of thalamic and collicular DSCF neurons evoked by focal activation or inactivation of cortical DSCF neurons in the mustached bat, Pteronotus parnellii parnellii.
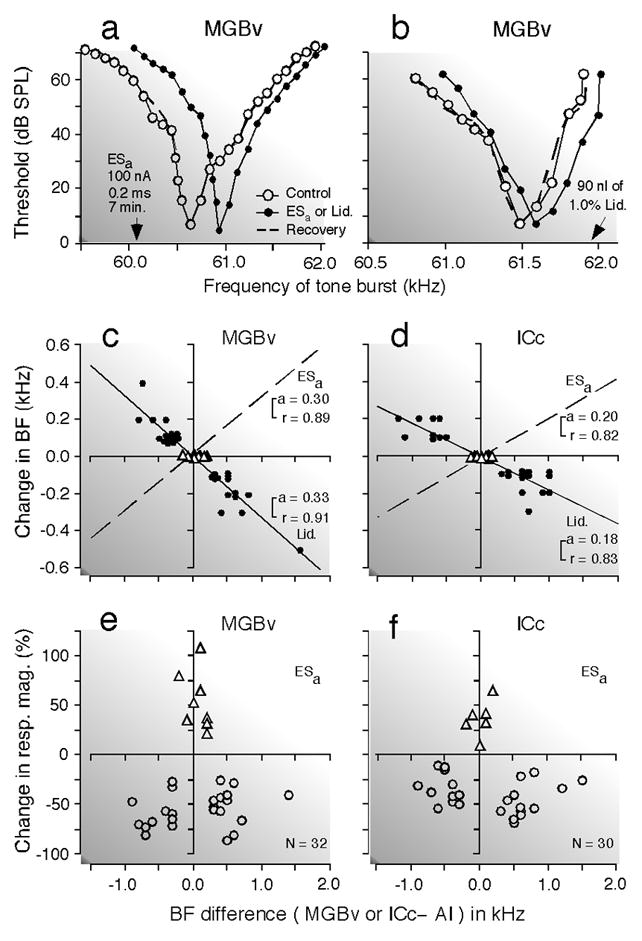
(a) and (b) Shifts in the frequency-tuning curves of two thalamic (MGBv) neurons evoked by an activation (a) or inactivation (b) of cortical neurons: activation by electric stimulation of a 0.2 ms, 100 nA electric pulse delivered at a rate of 5/s for 7 min (ESa) and inactivation by 90 nl of 1.0% lidocaine (Lid.). The best frequencies (BFs) of the activated or inactivated cortical neurons are indicated by the arrows. The curves were measured before (control, open circles), during (closed circles), and after (recovery; dashed lines) the cortical activation or inactivation. The data points for the recovery are not shown because almost all of them overlapped with those for the control. (c) and (d) The BF shifts of thalamic (c) and collicular (d) neurons evoked by a focal activation (dashed lines) or inactivation (solid lines and filled circles) of cortical neurons. The abscissae represent the differences in BF between the stimulated cortical (AI) and recorded thalamic (MGBv) or collicular (ICc) neurons in the control condition. The abscissae are the same as those in (e) and (f). The BFs of the stimulated cortical neurons were 61.2 kHz on the average. The triangles and circles represent the data obtained from matched and unmatched subcortical neurons, respectively. The regression lines, their slopes ‘a’ and correlation coefficients ‘r’ are shown in the graphs. The BF shift is centrifugal for the cortical activation, but centripetal for the cortical inactivation. (e) and (f). The ordinates represent the percent change in the response magnitude (number of pulses per tone burst) of thalamic (e) and collicular (f) neurons evoked by the cortical activation. The triangles and circles, respectively, represent percent changes in the response magnitude of matched and unmatched subcortical neurons at the BFs of individual neurons in the control condition. To measure response magnitudes, tone bursts were set at the best amplitude of each neuron in the control condition. Changes in BF (c and d) and response magnitude (e and f) both are larger in the MGBv than in the ICc (Zhang and Suga 2000).
In the MGBv (Luo et al. 2011), ICc (Yan and Ehret 2002) and cochlear nucleus (Luo et al. 2008; Liu et al. 2010) of the house mouse and the ICc of the big brown bat (Ma and Suga 2001a), electric stimulation of cortical neurons facilitates BF-matched neurons and evokes centripetal BF shifts of BF-unmatched neurons. The changes in the cochlear nucleus are smaller than those in the MGBv. As in the AI’s of the Mongolian gerbil and the big brown bat, small centrifugal BF shifts evoked by the electric stimulation are also found at the outer edge of the area where the centripetal BF shifts are evoked (Fig. 3a, d and e). Inactivation of AI by muscimol abolishes these thalamic (Zhang and Yan 2008) and collicular (Gao and Suga 1998) BF shifts.
In the big brown bat, electric stimulation of collicular neurons evokes the BF shifts of neighboring collicular neurons, which are predominantly centripetal. Inactivation of cortical neurons with lidocaine abolishes these collicular BF shifts evoked by the collicular electric stimulation (Zhang and Suga 2005). As described later, the collicular BF shifts elicited by auditory fear conditioning are abolished by muscimol inactivation of AI (Gao and Suga 1998) or atropine applied to the ICc (Ji et al. 2001). Therefore, the collicular BF shifts depend on both corticofugal feedback and acetylcholine (ACh) released into the colliculus.
ACh cortically released by the nucleus basalis (NB) of the forebrain plays an essential role in cortical plasticity (Weinberger 1998 and Suga et al. 2010 for reviews). The auditory cortex projects to the midbrain cholinergic nuclei, which in turn projects to the subcortical auditory nuclei (Schofield et al. 2010). Therefore, the auditory cortex plays an important role in the modulation of the subcortical auditory nuclei for auditory signal processing through corticofugal feedback and activation of the cholinergic nuclei.
It was also found that strong cortical stimulation in the big brown bat inhibited half of the ICc neurons and facilitated the remaining half (Jen et al. 2002 for review) and that corticofugally-inhibited neurons showed centripetal BF shifts (Jen and Zhou 2003).
3.1.2. Cochlear hair cells
In the mustached bat, the receptor potential called the cochlear microphonic response (CM) is sharply tuned to ~ 61 kHz. Electric stimulation of cortical DSCF neurons at a low rate (5/s), evokes collicular, thalamic and cortical BF shifts, but not the BF shift of the CM. However, stimulation at a high rate (33/s) evokes short-term CM changes. Namely, the BF-matched CM is increased in amplitude and is sharpened in frequency tuning, whereas the BF-unmatched CM shows the centrifugal BF shift for the contralateral cortical stimulation and the centripetal or centrifugal BF shift for the ipsilateral DSCFd or DSCFv stimulation, respectively (Fig. 4C). The BF of the CM systematically shifts as much as 0.25 kHz around 61.0 kHz, according to the BF and the location of the stimulated cortical DSCF neurons, i.e., the location of the stimulation of the cochleotopic map of the cortical DSCF area (Xiao and Suga 2002a).
Without any electric stimulation, the BF of the CM of the awake stationary mustached bat changes as much as 0.15 kHz in an unpredictable way during the emission of biosonar signals (Goldberg and Henson 1998). Such a change is presumably evoked by the corticofugal system and related to auditory attention to echoes. (The 0.25-kHz shift around 61 kHz corresponds to a Doppler shift that could be evoked by a speed of 0.71 m/s, the speed of small insects.)
Inactivation of the cortical DSCF area with muscimol reduces the BF (0.15 kHz ipsilaterally; 0.24 kHz contralaterally) and amplitude (29% ipsilaterally; 31% contralaterally) of the CM, and widens the CM frequency-tuning curve. The corticofugal system mediates the positive feedback associated with lateral inhibition even at the cochlea. Corticofugal modulation of cochlear hair cells apparently occurs in humans because otoacoustic emission decreases during cortical electric stimulation (Perrot et al. 2006) or visual selective attention (Puel et al. 1989).
3.2. Modulation in the time domain
3.2.1. Modulation of duration tuning
Duration-tuned neurons show the maximal response to a specific duration of a sound (best duration: BDu), as well as to its frequency (Pinheiro et al. 1991; Ehrlich et al. 1997; Galazyuk and Feng 1997; Ma and Suga 2001b). In the ICc of the big brown bat, the BDu changes along the tonotopic axis: the lower the BF, the shorter the BDu (Jen and Wu 2006). When cortical duration-tuned neurons are electrically stimulated, a collicular duration-tuned neuron matched (i.e., the same) in BDu and BF with the stimulated cortical neurons is augmented and its duration-tuning is sharpened (Fig. 7a), whereas an unmatched one is either shifted (Fig. 7b) or broadened in duration-tuning. The BDu shift and broadening occur toward the BDu of the stimulated cortical neurons: the larger the BDu difference, the larger the BDu shift (Fig. 7c) and the broadening (Fig. 7d). In addition to these centripetal changes in duration tuning, the duration-tuned neurons show a centripetal BF shift if their BFs are unmatched with those of the stimulated neurons. All these changes occur only when the BDu and BF differences between the recorded collicular and stimulated cortical neurons are respectively less than 4 ms and less than 6 kHz (Ma and Suga 2001b).
Fig. 7. Corticofugal modulation of collicular neurons.
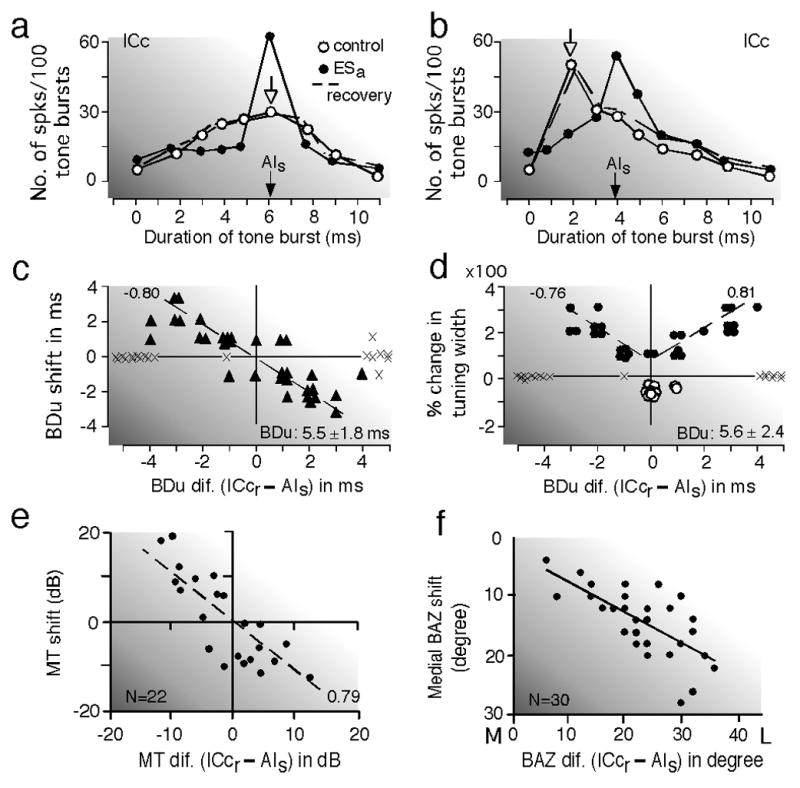
(a) and (b) Corticofugal modulation of duration-tuned collicular (ICc) neurons evoked by electrical stimulation of duration-tuned cortical (AI) neurons in the big brown bat, Eptesicus fuscus. The stimulated cortical (AIs) and recorded collicular (ICcr) neurons were matched (a) or unmatched (b) in both best frequency (BF) and best duration (BDu). The open and filled arrows indicate the BDu’s of ICcr and AIs neurons, respectively. Cortical stimulation sharpened (a) or shifted (b) duration-tuning. (c) and (d) Distributions of the BDu shifts (c) and width changes (d) in duration-tuning. The abcissae represent a BDu difference between the recorded and stimulated neurons. Each triangle in (c) represents a BDu shift. Each open or filled circle in (d) represents sharpening or broadening of a duration-tuning curve, respectively. Crosses mark neurons that showed neither BDu shift nor change in the width of a duration-tuning curve. The extent of change is linearly related to the BDu difference between ICcr and AIs neurons. The BDu’s of the stimulated neurons were 5.5 or 5.6 ms on the average (Ma and Suga 2001b). (e) Centripetal minimum-threshold (MT) shifts of BF-matched collicular neurons in the house mouse, Mus domesticus (Yan and Ehret 2002). (f) Centripetal best azimuth (BAZ) shifts of collicular neurons sensitive to the contralateral auditory fields in the big brown bat. Their BAZs shift toward the midline, i.e., toward the BAZs of the stimulated cortical neurons. L, lateral; M, medial (Zhou and Jen 2005). The MT and BAZ shifts are linearly related to the difference in MT and BAZ between the recorded collicular (ICcr) and stimulated cortical (AIs) neurons, respectively.
3.2.2. Modulation of delay tuning
As already described in the preceding text, delay-tuned neurons in the mustached bat are located in the FF, DF and VF areas of the auditory cortex. Electric stimulation of the FF area augments the response of a collicular delay-tuned neuron matched in BDe to the stimulated neurons and sharpens its delay tuning without shifting its BDe. It simultaneously suppresses the responses at the BDe’s of unmatched collicular delay-tuned neurons and shifts their BDe’s away from the BDe of the stimulated neurons. That is, cortical electric stimulation evokes centrifugal BDe shifts (Yan and Suga 1996). Thus, all the corticocortical and corticocollicular projections from the FF area evoke centrifugal BDe shifts (Fig. 5d).
Inactivation of the FF area including BDe-matched and -unmatched neurons with muscimol (an agonist of GABA-A receptors) does not cause any change in the delay-tuning curves of the thalamic and collicular delay-tuned neurons, but reduces their facilitative responses by 82% and 66%, respectively. Their facilitative responses to the combination of two FM sounds greatly depend on the corticofugal system (Yan and Suga 1999).
3.2.3. Modulation of response latencies
The response latency of auditory neurons typically shortens with an increase in stimulus intensity. However, certain cortical (AI) and collicular (ICc) neurons of the little brown (Sullivan 1982a, b; Galazyuk et al. 2005; Wang et al. 2007), big brown (Ma and Suga 2008) and Mexican free tailed (Klug et al. 2000) bats show a “paradoxical latency-shift (PLS)”: long latencies to intense sounds but short latencies to weak sounds). These neurons presumably are involved in the processing of target distance information, because they show facilitation of a response when an intense sound corresponding to an emitted pulse is followed by a weak sound corresponding to its echo (Sullivan 1982). Electric stimulation of cortical neurons evokes two types of changes in the collicular PLS neurons, depending on the relationship in BF between them. That is, the cortical stimulation does not shift the BFs of BF-matched collicular neurons and shortens their response latencies at intense sounds. Therefore, the PLS becomes smaller. However, the stimulation shifts the BFs of BF-unmatched neurons and lengthens their response latencies at intense sounds. Therefore, the PLS becomes larger. The electric stimulation also modulates the response latencies of collicular non-PLS neurons. These corticofugal modulations of the collicular responses occur only when the BF difference between the recorded and stimulated neurons is less than 6 kHz. The cortical electric stimulation produces an inhibitory frequency tuning curve or curves of a collicular neuron (Ma and Suga 2008). Bicuculline applied to a collicular PLS neuron eliminates its PLS (Galazyuk et al. 2005). Corticofugal feedback is certainly involved in shaping the temporal patterns of the responses of subcortical auditory neurons through inhibition.
3.3. Modulation in the intensity (amplitude) domain
3.3.1. Modulation of minimum thresholds
A frequency-threshold (tuning) curve is based on many thresholds measured as a function of the frequency of a tone burst stimulus. The lowest threshold -- the threshold at the BF of a neuron -- is called the “minimum” threshold (MT). In the central auditory system, a MT and a BF both differ from neuron to neuron. For focal electric stimulation of AI of the house mouse, its collicular neuron matched to the stimulated cortical neurons in BF but not in MT shows no BF shift, but a MT shift toward the MT of the stimulated neurons (Fig. 7e). On the other hand, a collicular neuron unmatched in both BF and MT increases its MT regardless of MT and BF differences (Yan and Ehret 2002). In corticofugally-inhibited collicular neurons of the big brown bat, both BF-matched and -unmatched neurons show centripetal MT shifts: the larger the MT difference, the larger the MT shift (Jen and Zhou 2003). Corticofugal modulation of MT is somewhat different between the house mouse and the big brown bat. However, this might be due to the difference in the experimental design.
In cats, electric stimulation of the medial olivo-cochlear system (MOCS) increases the threshold of peripheral neurons and shifts their intensity-response curves to tone bursts toward higher intensities without changing their dynamic range in intensity coding. When the peripheral neurons are responding to persistent noise, their dynamic range in intensity coding of tone bursts becomes very small. However, electric stimulation of the MOCS reduces the response to the noise and restores their dynamic range. Therefore, the MOCS plays a role in antimasking for detecting signals (tone bursts) in noise (Robertson and Mulders 2010). AI can control the cochlear hair cells via the olivo-cochlear neurons (Xiao and Suga 2002a), and it can be controlled by the prefrontal cortex (Fritz et al. 2007 for review, 2003). Therefore, the auditory periphery is subject to modulation by auditory attention.
3.4. Modulation in the spatial domain
The spatial tuning (i.e., directional sensitivity) of the ear varies with the frequency of a stimulus tone. In the central auditory system, binaural interactions produce neurons with spatial tunings different from those determined by the ear. In the big brown bat, focal electric stimulation of AI sharpens the spatial tuning curves of corticofugally-inhibited collicular neurons and broadens those of corticofugally-facilitated collicular neurons (Jen et al. 1998). Collicular neurons show centripetal “best azimuth (BAZ)” shifts for the electric stimulation only when the BF difference between the stimulated and recorded neurons is less than 6 kHz. The larger the difference in BAZ between them, the larger the centripetal BAZ shift (Fig. 7f; Zhou and Jen 2005).
3.5. Excitation and inhibition for tuning shifts and reorganization
Stimulated cortical AI neurons evoke both facilitation and lateral inhibition of the auditory responses of subcortical neurons as well as cortical neurons nearby. The amount of facilitation and inhibition varies with the parameter values characterizing a stimulus sound and with the relationship in tuning between the stimulated and recorded neurons. These tuning-dependent effects of the stimulated cortical neurons on the tuning-matched and -unmatched subcortical neurons improve the input to the stimulated cortical neurons and the subcortical and cortical representations of the stimulus parameters to which the stimulated cortical neurons are tuned (Zhang et al. 1997; Yan and Suga 1998; Zhang and Suga 2000; Chowdhury and Suga 2000; Ma and Suga 2001a; Sakai and Suga 2001, 2002). In other words, cortical neurons modulate the subcortical and neighboring cortical neurons to be best for their excitation. This corticofugal function has been named “egocentric selection” (Yan and Suga 1996). The changes resulting from egocentric selection are larger in thalamic neurons than in collicular ones (Zhang et al. 1997; Zhang and Suga 1997), and the changes in the collicular neurons are larger than those in the cochlear nuclear ones (Luo et al. 2008). Egocentric selection apparently takes place at different levels of the auditory system through the multiple feedback loops.
When excitation is stronger and more widespread to the neighboring unmatched neurons than is inhibition, centripetal tuning shifts in the cortical and subcortical neurons are evoked. On the contrary, when inhibition is stronger and more widespread to the neighboring unmatched neurons than is excitation, centrifugal tuning shifts are evoked (Suga et al. 2000 for review). In the highly specialized cortical auditory areas, inhibition is stronger and more widespread than excitation and evokes compressed reorganization, whereas in the less-specialized cortical auditory areas, excitation is stronger and more widespread than inhibition and evokes expanded reorganization. The removal of cortical inhibition changes the cortical and subcortical centrifugal tuning shifts into centripetal tuning shifts (Xiao and Suga 2004, 2005; Ma and Suga 2004). Therefore, the reorganization and organization of a neural map is mostly based upon the balance between excitation and inhibition occurring in the cortex.
3.6. Remarks on corticofugal modulation
Animal sounds, including human speech sounds, are characterized by multiple parameters such as frequency, intensity (amplitude), duration, time interval between sounds, etc. The central auditory system produces various types of neurons tuned to behaviorally relevant acoustic parameters, i.e., “information-bearing parameters” (Suga 1982 for review), rather than a single frequency (Suga 1973, 1990, Covey and Casseday 1999, and Rauschecker and Tian 2000 for reviews). As reviewed above, corticofugal modulation occurs for different types of subcortical neurons and is multiparametric. It occurs at multiple levels, perhaps more easily at higher levels than lower levels. Focal inactivation of AI evokes the responses and tuning shifts which are opposite to those evoked by AI stimulation (Zhang et al. 1997; Zhang and Suga 2000). So, the responses to sounds and tuning of subcortical neurons are maintained by the corticofugal system (feedback loop) as they are.
All these findings have been made only on lemniscal neurons by stimulating and recording from them. Auditory signal processing and auditory plasticity in the nonlemniscal system and interaction between the lemniscal and nonlemniscal systems remains to be studied. Based on recent findings, one may speculate that the activation of the lemniscal system suppresses the activity of the nonlemniscal system and changes the response properties of nonlemniscal neurons toward those of lemniscal neurons, that the activation of the nonlemniscal system suppresses the activity of the lemniscal system and changes the response properties of the lemniscal neurons toward those of the nonlemniscal neurons, and that ACh excites the lemniscal system and suppresses the nonlemniscal one, whereas a neuromodulator other than ACh suppresses the former and excites the latter. One may also speculate that the prefrontal cortex (which is involved in attention) may play a role in determining which system, either lemniscal or nonlemniscal, has a dominant role in auditory signal processing. These speculations remain to be explored for further understanding of the auditory system.
(4) Plasticity related to associative and non-associative learning
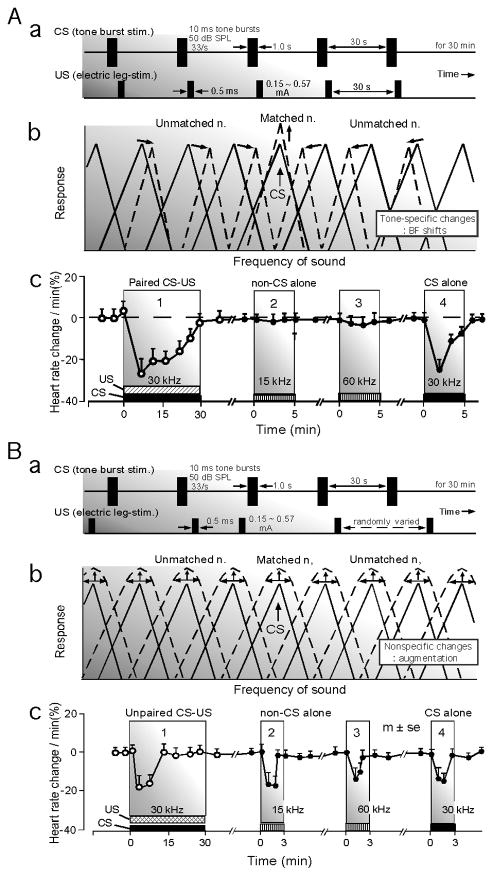
Tone burst and electric foot-stimuli have been used as conditioned (CS) and unconditioned (US) stimuli, respectively. When the CS and US are paired as CS-US for conditioning, i.e., for associative learning, an animal shows a conditioned behavioral response specific to the CS and its auditory system shows the “tone-specific” plasticity which consists of the facilitation of auditory responses and sharpening of the frequency tuning of BF-matched neurons and the BF shifts of BF-unmatched neurons (Fig. 8A). Here, “BF-matched” means that the BF of a given neuron is the same as the frequency of the tone burst used as the CS. These changes are specifically related to the tone burst, so that they are tone-specific. For simplicity, the tone-specific plasticity is represented by BF shifts. Backward conditioning (US-CS) does not evoke BF shifts.
Fig. 8. Conditioning, pseudo-conditioning, frequency-tuning changes and heart rate change.
(Aa) Paired conditioned (CS) and unconditioned (US) stimuli and the parameter values of the CS (tone bursts) and US (electric leg-shock) used for the experiments on the big brown bat. (Ab) Facilitation of the response and sharpening of the frequency-tuning of BF-matched neurons and BF shifts of BF-unmatched neurons. These changes are tone-specific changes. Here “BF-matched” means that the BF of a recorded neuron is the same as the frequency of the CS. (Ac) The bat shows a decrease in heart rate to the paired CS-US, i.e., conditioning (1). After the conditioning, the conditioned autonomic response (heart rate change) does not occur to 15-kHz (2) and 60-kHz (3) tone bursts, but to the 30-kHz tone bursts (CS) used for the conditioning (Ac by Ji and Suga 2007). When the US is unpaired with the CS by randomizing it (Ba), nonspecific augmentation (sensitization) of BF-matched and -unmatched neurons in the central auditory system is elicited (Bb). The bat shows a heart-rate decrease to the unpaired CS-US, i.e., pseudo-conditioning. After the pseudo-conditioning, it shows a heart rate decrease not only to the 30- kHz tone bursts used for the pseudo-conditioning, but also to 15-kHz and 60-kHz tone bursts (Bc by Ji and Suga 2008) (Suga et al. 2010).
On the other hand, when the CS and US are unpaired for pseudo-conditioning, i.e., for non-associative learning, the animal shows behavioral responses nonspecific to the CS and its auditory system shows the “nonspecific” plasticity which consists of the nonspecific augmentation of auditory responses, shortening of response latencies, a decrease in threshold and broadening of the tuning of BF-matched and -unmatched neurons (Fig. 8B). For simplicity, nonspecific plasticity is represented by nonspecific augmentation. Excitation and inhibition both occur in specific combinations for the tone-specific plasticity, but not for the nonspecific plasticity. The tone-specific plasticity has been well studied, but the nonspecific plasticity has not (Weinberger 1998 and Suga et al. 2002 for reviews; Ji and Suga 2008, 2009). The recent research on these two types of plasticity are reviewed below.
4.1. Tone-specific plasticity elicited by auditory fear conditioning
A train of tone bursts elicits small short-lasting cortical (AI) and collicular (ICc) BF shifts as the difference between the tone frequency and the BF of a given neuron is set at the peak of the BF-shift-difference curve (Yan and Suga 1998; Gao and Suga 1998; Chowdhury and Suga 2000; Ma and Suga 2003). When this train of tone bursts is paired as is the CS with the US for auditory fear conditioning, large long-lasting cortical and large short-lasting collicular BF shifts are elicited (Fig. 9a and b; Suga and Ma 2003 for review). The small BF shifts evoked by the train of tone bursts are apparently augmented by the conditioning activating the cholinergic system.
Fig. 9. The collicular (ICc) and cortical (AI) BF shifts elicited by auditory fear conditioning or by electric stimulation of the auditory and/or somatosensory cortices or by long repetitive acoustic stimulation (big brown bat).
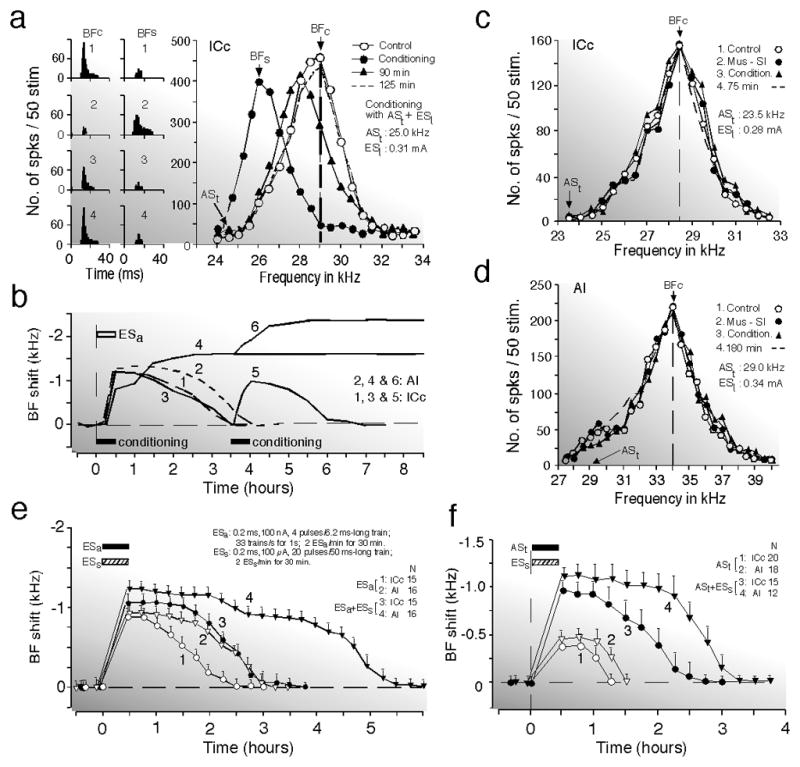
(a) Changes in the responses (left two columns) and frequency-response curves (right graph) of a collicular neuron caused by 30-min-long conditioning consisting of 60 pairs of a train of acoustic stimuli (ASt = CS) and an electric leg-stimulation (ESl = US). All of the data were obtained with tone bursts fixed at 10 dB above the minimum threshold of the neuron. The CS was 25 kHz, and the BF of the collicular neuron was 29 kHz. The data were obtained before (1, control), immediately after (2), 90 min after (3), and 125 min after the conditioning (4). BFc and BFs, BFs in the control and shifted conditions, respectively. BFs shifted back to BFc 125 min after the conditioning (Gao and Suga 1998). (b) Time courses of the BF shifts of collicular (1 and 3) and cortical (2 and 4) neurons evoked by electric stimulation of AI, ESa (1 and 2) or the conditioning (3 and 4). A second conditioning session 3.5 h after the first also evoked collicular (5) and cortical (6) BF shifts. The horizontal bars indicate the electric stimulation or conditioning of 30 min duration. Each curve is the mean of 10 – 15 curves obtained from different neurons (Ma and Suga 2001a; Gao and Suga 2000). (c) and (d) Bilateral inactivation of the somatosensory cortex (SI) with 0.4 μg of muscimol applied to its surface abolishes development of the conditioning-dependent BF shifts of a collicular (c) and a cortical (d) neuron, but does not change their responses and frequency-response curves. Frequency-response curves were obtained before the conditioning (1, control); during SI inactivation (2); immediately after the conditioning under SI inactivation (3); and 75 or 180 min after the conditioning (4). The frequencies of the CS (ASt) and the electric current of the US (ESl) are listed in each graph (Gao and Suga 2000). (e) Collicular and cortical BF shifts evoked by a short train of electric stimuli of AI (ESa) are augmented by electrical stimulation of the somatosensory cortex (ESs). ESs was delivered 1.0 s after ESa, mimicking the conditioning. Curves 1 and 2 respectively represent the time courses of collicular and cortical BF shifts evoked by ESa alone. Curves 3 and 4 respectively represent the time courses of collicular and cortical BF shifts evoked by ESa followed by ESs. These stimuli were delivered over 30 min (horizontal bars). (f) ESs following a train of acoustic stimuli (ASt) augments the collicular and cortical BF shifts evoked by ASt. Curves 1 and 2 respectively represent the time courses of the collicular and cortical BF shifts evoked by ASt. Curves 3 and 4 respectively represent the time courses of the collicular and cortical BF shifts evoked by ASt followed by ESs, mimicking the conditioning. Note that ESs has a larger and longer augmenting effect on the cortical BF shift than on the collicular BF shift. Means and standard errors (vertical bars) are based on the data obtained from the number of neurons ranging between 12 and 20, as indicated by N (Ma and Suga 2003).
The BF shifts elicited by the tone bursts alone or conditioning are basically the same as those evoked by the focal electric stimulation of AI, except for the time course of the cortical BF shifts (Fig. 9b; Gao and Suga 2000). They occur in a specific relation to the BF of the electrically stimulated neurons or the frequency of the tone burst delivered as a stimulus. The BF of the electrically stimulated neurons corresponds to the frequency of the tone burst.
The CS (weak tonal stimulation) stimulates both the lemniscal and nonlemniscal auditory systems. The US (electric leg-stimulation) is aversive to an animal and stimulates not only the somatosensory system, but also the ascending reticular activating and brain aversion systems which activate the neuromodulatory systems.
Gao and Suga (1998, 2000) found that the corticofugal auditory system and the auditory and somatosensory cortices play an essential role in eliciting the conditioning-dependent collicular (ICc) and cortical (AI) BF shifts. [Needless to say, the conditioning also elicits thalamic (MGBv) BF shifts (Edeline and Weinberger 1991).] Therefore, Suga and his collaborators (2000, 2002) proposed the so-called Gao-Suga model. This model has been developed by incorporating many important findings in bats and rodents after 1998. The following text reviews how different portions of the auditory and non-auditory systems are involved in the BF shifts elicited by conditioning and by electric stimulation of different portions of the brain.
4.1.1. The Primary auditory cortex
The direction of the cortical and collicular BF shifts changes when an antagonist of GABA-A receptors, bicuculline methiodide (Xiao and Suga 2002b; Ma and Suga 2004), or a local anesthetic, lidocaine (Zhang et al. 1997), is focally applied to AI. Inactivation of AI by muscimol abolishes the conditioning-dependent collicular BF shift without affecting the collicular frequency tuning (Gao and Suga 1998, 2000). An antagonist of muscarinic acetylcholine receptors, atropine, applied to AI completely blocks the development of the conditioning-dependent cortical BF shift and reduces the conditioning-dependent collicular BF shift by 25% without affecting the collicular auditory responses and frequency tuning (Ji et al. 2001). Therefore, the neural circuit within AI, corticofugal feedback and acetylcholine (ACh) all play an essential role in eliciting the cortical and subcortical BF shifts.
The auditory cortex projects to the nucleus basalis via multiple pathways including the amygdala or the prefrontal cortex (Zaborszky et al. 1999; Golmayo et al. 2003; Rasmusson et al. 2007). It directly projects to the ponto-mesencephalic tegmentum (PMT). The PMT projects to the multiple subcortical auditory nuclei on both sides (Schofield et al. 2011), whereas the basal nucleus broadly projects to the cortex including the auditory cortex. Thus, AI has the neural net for the adjustment (reorganization) of auditory signal processing and also the projections to the cholinergic system which augments the adjustment. The functional role of the non-AI areas in the adjustment of auditory signal processing has not yet been explored. Electric stimulation of AI mostly excites MGBv neurons and inhibits MGBm neurons through the thalamic reticular nucleus (Zhang et al. 2008).
4.1.2. The ventral and medial divisions of the medial geniculate body
As described in the introduction, MGBv neurons project specifically to AI, are sharply frequency-tuned, and habituate little. On the contrary, MGBm neurons project widely over cortical auditory areas including AI, mostly have a broad or multi-peaked frequency-tuning curve, and habituate after several stimulus presentations (Aitkin and Webster 1972; Calford 1983; Bordi and Ledoux 1994, b). MGBd neurons specifically project to AII. Their response properties are similar to those of MGBm neurons. Unlike the MGBv and MGBd, the MGBm is multisensory and projects to the amygdala as well as to the auditory cortex (He 2003 for review). The MGBm-to-amygdala projection is essential in eliciting conditioned behavioral responses (LeDoux 1993 for review; Lanuza et al. 2004).
The conditioning elicits short-lasting BF shifts in the MGBv (Edeline and Weinberger 1991b), MGBm (Edeline and Weinberger 1992) and MGBd (Edeline and Weinberger 1991a). Conditioning-elicited MGBm changes are blocked by a lesion of the amygdala (Maren et al. 2001; Poremba and Gabriel 2001; No BF shifts were examined in these studies.). Therefore, interesting questions are where the MGBm changes do originate and what kinds of cortical plastic changes are evoked by the MGBm. Focal electric stimulation of the MGBm evokes nonspecific cortical augmentation, but not the cortical BF shift (Fig. 10b; Ma and Suga 2009), whereas the stimulation of the MGBv evokes the cortical (Fig. 10a; Jafari et al. 2007; Ma and Suga 2007) and collicular (Wu and Yan 2007) BF shifts. Therefore, MGBm neurons are suited for evoking the nonspecific cortical plasticity but not the tone-specific cortical plasticity. MGBv neurons are undoubtedly implicated in evoking the cortical BF shift. It is quite possible that the BF shifts in the lemniscal system are transferred to the nonlemniscal system.
Fig. 10. Thalamo-cortical modulation.
Changes in the frequency-threshold (1) and frequency-response (2) curves of AI neurons (lower graphs) evoked by electric stimulation of a sharply-tuned MGBv neuron (a, upper graphs) or broadly tuned MGBm neurons (b, upper graphs). Electric stimulation of the MGBv neurons evokes a BF shift, i.e., a shift of the frequency-threshold and frequency-response curves of the AI neuron (a, lower graphs), whereas that of the MGBm neurons evokes broadening of those curves of the AI neuron (b, lower graphs). The open and filled circles represent the curves in the control condition and 30 min after the onset of the electric stimulation, respectively. The dashed lines with dots represent the curves obtained 60 min after the onset of the electric stimulation. The frequency-response curves were measured at 30 dB above the minimum threshold of the given neuron (Ma and Suga 2009).
As described in the preceding text, any electric stimulation of AI, MGBv or ICc, tonal stimulation, and tonal-plus-nucleus basalis stimulation can evoke the cortical BF shift without CS-US association in the MGBm and PIN. It has been shown that electrical stimulation of the PIN, not the MGBm, paired with a tone burst evokes a heart-rate change as does the conditioning (Cruikshank et al. 1992) and that a lesion of the MGBm impairs conditioning and blocks associative plasticity in the amygdala (Poremba and Gabriel 1997).
4.1.3. The central nucleus of the inferior colliculus
The ICc, ICx and ICd respectively project to the MGBv, MGBm and MGBd. The conditioning-elicited BF shift has been studied only in the ICc. It lasts up to 3.5 hours (Fig. 9b; Gao and Suga 1998, 2000; Ji et al. 2001; Ji and Suga 2003). As already described, atropine applied to AI blocks the development of the cortical BF shift and reduces the collicular one by 25%, although it does not block the corticocollicular feedback. Atropine applied to the IC blocks the collicular BF shift, reduces the cortical one by ~38%, and changes it from long-term to short-term without affecting the cortical auditory responses and frequency tuning. So, the collicular BF shift consists of the corticofugally-transferred BF shift and the intrinsic collicular BF shift based on both the corticocollicular feedback and ACh. The short-lasting collicular BF shift contributes to producing the large long-lasting cortical BF shift (Ji et al. 2001). ACh is released into the ICc by the cholinergic midbrain tegmental nuclei (Hallanger et al. 1987; Schofield et al. 2011).
In the ascending auditory system, the ICc in the midbrain is located below the MGB in the thalamus, but shows the conditioning-elicited BF shift. Therefore, the MGB is not the first place in the ascending auditory system where CS-US associated responses are found.
4.1.4. Subcollicular auditory nuclei and cochlear hair cells
It has not yet been studied whether the conditioning elicits changes in the subcollicular auditory nuclei and cochlear hair cells. These nuclei and hair cells directly or indirectly receive corticofugal and cholinergic projections. Focal electric stimulation of AI evokes the small short-lasting BF shifts of cochlear nuclear neurons (Luo et al. 2008; Liu et al. 2010) and hair cells (Xiao and Suga 2002a). The collicular BF shift evoked by focal AI stimulation is the same in amount and time course as that elicited by the conditioning (Fig. 9b). As previously stated, corticofugal modulation of cochlear hair cells apparently occurs in humans because the otoacoustic emission decreases during visual selective attention (Puel et al. 1989) or cortical electric stimulation (Perrot et al. 2006). Therefore, the conditioning may elicit the BF shifts of subcollicular neurons and hair cells as AI stimulation does.
4.1.5. The Corticofugal Auditory System and Feedback Loops
As described in the preceding text, the corticofugal auditory system and feedback loops play an essential role in eliciting the short-lasting subcortical and long-lasting cortical BF shifts. In the big brown bat, ~ 25% of the conditioning-elicited collicular BF shift is due to the cortical BF shift carried down by the corticofugal system, and the remaining ~ 75% is produced in the colliculus (ICc) by utilizing ACh and corticofugal feedback. The collicular BF shift contributes to producing the large long-lasting cortical BF shift, although the ICc itself can not produce the long-term BF shift (Ji et al. 2001). These conclusions obtained from the conditioning experiments are supported by electric stimulation experiments: (1) electric stimulation of the ICc (Zhang and Suga 2005) or the MGBv (Wu and Yan 2007) evokes the collicular BF shift via corticofugal feedback, and (2) focal electric stimulation of AI evokes the short-term collicular BF shift which is the same as that elicited by the conditioning (Ma and Suga 2001a, 2003; Gao and Suga 2000).
4.1.6. The Primary Somatosensory Cortex, SI
Bilateral inactivation of SI by muscimol does not affect the auditory responses and frequency tuning of cortical and collicular neurons and the development of nonspecific augmentation of these neurons due to pseudo-conditioning, but selectively abolishes the development of their BF shifts elicited by the conditioning (Fig. 9c and d; Gao and Suga 1998, 2000) or pseudo-conditioning (Ji and Suga 2008, 2009). Electric stimulation of SI after, but not before, focal electric stimulation of AI (Fig. 9e) or tone burst stimulation (Fig. 9f) augments the small cortical and collicular BF shifts. This augmentation particularly lengthens the duration of the cortical BF shift. It does not occur when the cholinergic nucleus basalis is lesioned (Ma and Suga 2001a, 2003). This pair of inactivation and activation experiments clearly indicates that SI plays an essential role in the development of the conditioning-dependent BF shifts through the nucleus basalis.
4.1.7. The cholinergic neuromodulator, acetylcholine
ACh applied to AI or the ICc prior to the conditioning augments both the cortical and collicular BF shifts. Atropine applied to AI or the ICc prior to the conditioning respectively blocks the cortical and collicular BF shifts (Ji et al. 2001). Focal electric stimulation of AI evokes the short-lasting cortical and collicular BF shifts. When it is accompanied with ACh applied to AI, the large long-term cortical BF shift and the large short-term collicular BF shift are evoked, as are those elicited by the conditioning (Ma and Suga 2005). Electric stimulation of the nucleus basalis augments the development of the cortical and collicular BF shifts evoked by the focal electric stimulation of AI or by tone burst stimulation (Ma and Suga 2003). It also evokes the large long-lasting cortical BF shift when it is delivered together with tone burst stimulation (in guinea pigs, Bakin and Weinberger 1996; Bjordahl et al. 1998; Kilgard and Merzenich 1998; in big brown bats, Ma and Suga 2003; in the house mouse, Yan and Zhang 2005; Zhang et al. 2005; Sarter et al. 2006: Parikh et al. 2007). ACh released in AI by the nucleus basalis apparently augments the cortical BF shift and consequently the collicular BF shift evoked by both the corticofugal feedback and ACh released in the ICc. ACh makes the cortical BF shift long-lasting. These findings confirm that the long-lasting cortical BF shift can be evoked without CS-US association in the MGBm and PIN, and that AI, corticofugal feedback loops and ACh together play a key role in evoking the long-term cortical BF shift.
Conditioning-dependent discharges occur in the nucleus basalis (Quirk et al. 1995, 1997; Maren 2000) and the lateral amygdala (Li et al. 1996; Armony et al. 1998) before AI. The development of these discharges in AI is abolished by a lesion of the amygdala (Armony et al. 1998). Inactivation of the amygdala prevents the conditioning-dependent changes in the MGBm (Poremba and Gabriel 2001; Maren et al. 2001). Therefore, the origin of these discharges in AI and the lateral amygdala is not the MGBm and PIN that project to the amygdala. It is possible that, different from the CS-dependent BF shifts, the conditioning-dependent discharges in AI are evoked via the pathway from the amygdala to the nucleus basalis and then to AI, as is the augmentation of the CS-dependent BF shifts, and that the discharges in the MGBm are evoked via the pathway from AI to the IC and then to the MGBm. It should be noted that BF shifts in the lateral amygdala have not yet been studied.
4.1.8. The amygdala
The amygdala receives the projections from the multisensory thalamic nuclei and primary sensory and association cortices, etc. (LeDoux 1993 for review; Romanski and LeDoux 1993). Both the thalamic and cortical projections to the amygdala are important for fear conditioning (Lanuza et al. 2004). The amygdala projects to the nucleus basalis which consists of cholinergic and GABAergic neurons. It shows the neural responses related to the conditioning and plays an essential role in eliciting conditioned behavioral responses (Phelps and LeDoux 2005 for review). However, activation and inactivation experiments have not yet been performed to determine whether the amygdala also plays an essential role in eliciting the cortical and collicular BF shifts.
4.1.9. The prefrontal cortex
The primary sensory cortices project to the prefrontal cortex which, in turn, projects to the nucleus basalis (Rasmusson et al. 2007). The nucleus basalis augments the cortical BF shift, as already described. The prefrontal cortex, which plays an important role in attention (Dally et al. 2004), presumably plays a role in focusing on a specific sound by augmenting tuning shifts toward the parameter values characterizing the sound.
4.1.10. A working model for tone-specific plasticity elicited by auditory fear conditioning
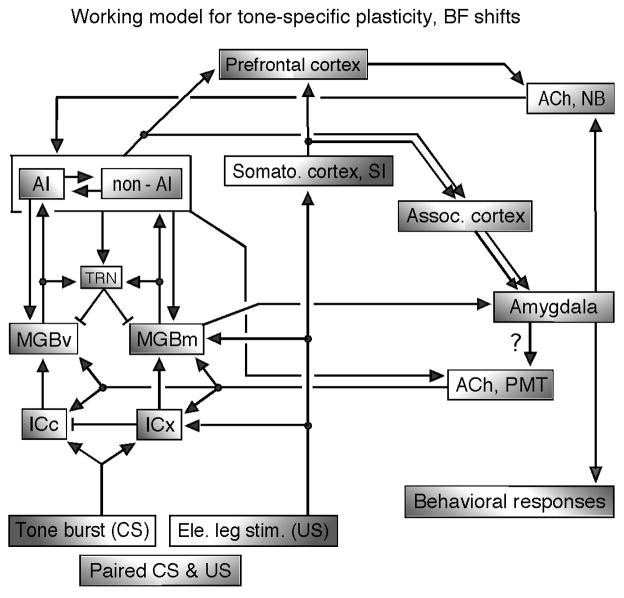
In rodents and bats, the tone-specific plasticity elicited by the conditioning is basically the same as that elicited by the focal electric stimulation of AI, other than its long-lasting cortical plasticity due to ACh. Therefore, the neural circuit explored through electric stimulation experiments can be incorporated with that for the conditioning-elicited plasticity. The Gao-Suga model proposed by Suga and his collaborators (2000, 2002) is elaborated upon in the current review article. This working model states that small (or subthreshold) short-lasting cortical, thalamic and collicular BF shifts specific to a conditioned tonal stimulus (CS) are evoked by the neural circuit within AI and corticofugal feedback loops activated by the CS through the ICc and MGBv. When the CS is paired with an unconditioned leg- or foot-stimulus (US), the auditory and somatosensory cortices respectively send the CS and US signals to the amygdala, where the CS is associated with the US. (In addition, CS-US association may also occur in the association cortex.) Then, the associated signal goes to the cholinergic basal forebrain (nucleus basalis), then the cortical acetylcholine level increases and the small cortical BF shifts evoked by the CS are augmented. AI activated by the CS projects to the cholinergic PMT as well as to the MGBv and ICc. The thalamic and collicular BF shifts are evoked by the corticofugal feedback and acetylcholine released from the PMT. Thus, the thalamic and collicular BF shifts consist of intrinsically evoked BF shifts and corticofugally transferred ones. The gain of the thalamo-cortico-thalamic feedback loop is presumably controlled by the thalamic reticular nucleus. The BF shifts that occur in the lemniscal system are presumably transferred to the nonlemniscal system in the cortical and/or subcortical levels (Fig. 11).
Fig. 11. A working model for tone-specific plasticity (BF shifts) elicited by auditory fear conditioning, paired CS – US.
ACh, acetylcholine; AI, primary auditory cortex; CS, conditioned stimulus (tone bursts); ICc and ICx, central nucleus and external cortex of the inferior colliculus; MGBv and MGBm, ventral and medial divisions of the medial geniculate body; NB, nucleus basalis in the forebrain; non-AI, auditory cortex other than AI; PMT, pontomesencephalic tegmentum; TRN, thalamic reticular nucleus; US, unconditioned stimulus (electric leg-shock). The short bar at the end of a line means a projection from inhibitory neurons. The conditioning elicits the cortical BF shift through the neural net in AI, corticofugal feedback loop and ACh from the NB, and also the collicular BF shift through the corticofugal feedback and ACh from the PMT. See the text (revised version of Suga et al. 2000).
4.2. Nonspecific plasticity elicited by pseudo-conditioning and differential gating
4.2.1. Nonspecific plasticity
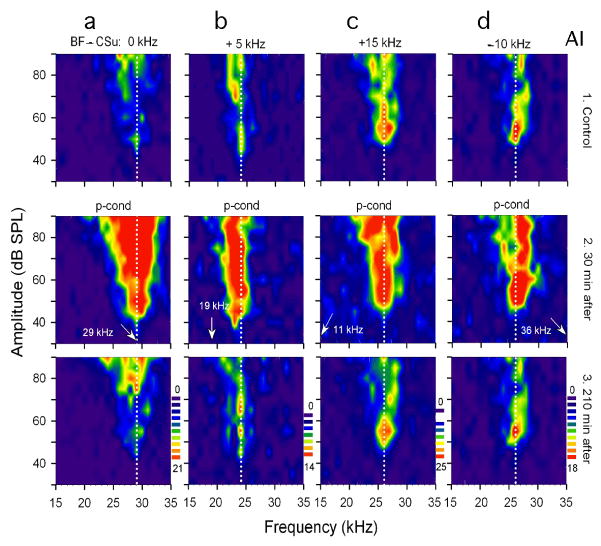
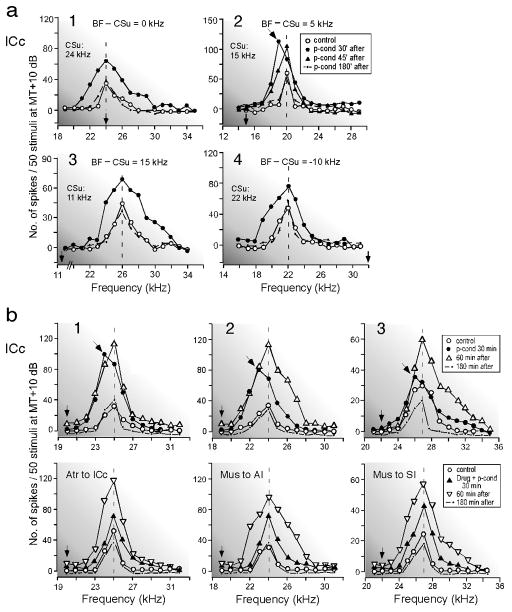
Unpaired CS and US, i.e., pseudo-conditioning, elicits nonspecific augmentation in AI, MGBv and ICc. In the big brown bat, the BF shifts are most effectively evoked when the BF of a recorded neuron is ~ 5 kHz higher than the tone burst (Fig. 3a). So, when the CS is set at 5.0 kHz lower than the BF of a given neuron and delivered with pseudo-randomized US for pseudo-conditioning (Fig. 8Ba), the neuron shows a small brief-lasting BF shift in addition to nonspecific augmentation (Figs. 12b and 13b). Then, drug effects on both the tone-specific and nonspecific plasticities can be simultaneously examined. [In the house mouse, for example, the 10-kHz difference between the CS frequency and the BF of a given neuron is perhaps appropriate in eliciting both the BF shift and nonspecific augmentation, because the peaks of the BF-shift-difference curve of the house mouse are at 10 kHz (Fig. 3e)].
Fig. 12. Nonspecific augmentation of four cortical (AI) neurons elicited by pseudo-conditioning (p-cond), unpaired CS-US.
The best frequencies (BFs) of the four collicular (ICc) neurons (a - d) were either 29, 24, 26 or 26 kHz (vertical dotted lines). The arrows along the frequency axis indicate the frequencies of the CSu (CS used for p-cond) which were either 0 (a), 5 (b), 10 (d) or 15 (c) kHz different from the BFs of the recorded neurons. In each column, 1 – 3 show the receptive fields obtained before (control), 30 and 210 min after the onset of p-cond, respectively. P-cond elicits the augmentation of responses, broadening of the receptive field, and decrease in threshold of all these neurons. For simplicity, all these changes are represented by the term “nonspecific augmentation.” The scale bars from dark blue to dark red show low to high spike counts per 10 stimuli. Note a small BF shift toward 19 kHz (i.e., tone-specific change) in b2 (Ji and Suga 2008).
Fig. 13. Changes in the frequency-response curves of single collicular (ICc) neurons elicited by pseudo-conditioning (p-cond).
(a) The BFs of four collicular neurons (1 – 4) were 24, 20, 26 and 22 kHz, respectively. The difference between the BF (vertical dashed line) and CSu (vertical arrow) frequencies is shown at the top of each graph. Note the overall augmentation of the responses 30 min after the onset of p-cond and, in addition, the 1.0 kHz BF shift (oblique arrow) only in 2. (b) The nonspecific augmentation and small BF shift elicited by p-cond were first confirmed in three collicular neurons (1–3, upper graphs). After the recovery from these changes, the drug effects on the changes elicited by the 2nd p-cond were studied in the same three neurons (1–3, lower graphs). The frequency-response curves of the same single neuron were obtained before (control), 30, 60 and 180 min after the p-cond. (b1) Atropine (Atr) was ipsilaterally applied to the ICc. (b2) Muscimol (Mus) ipsilaterally applied to AI. (b3) Muscimol bilaterally applied to SI. The BFs of these three neurons were 25, 24 or 27 kHz and were 5.0 kHz higher than the CSu frequency. Note that the drugs blocked the BF shift, but not the nonspecific augmentation. All the curves were obtained with tone bursts at 10 dB above the minimum threshold of a given neuron. The keys of the curves are shown in the inset (Ji and Suga 2009).
For the pseudo-conditioning, the big brown bat shows a nonspecific heart-rate decrease (Fig. 8Bc), and cortical (AI) and collicular (ICc) neurons show nonspecific augmentation over many different frequencies (Figs. 8Bb and 12) and, in addition, a small brief-lasting BF shift only when the CS frequency is ~ 5 kHz lower than the BF of a recorded neuron (Figs. 12b and 13a2). The time course of the development of the nonspecific augmentation is the same as that of the BF shifts elicited by the conditioning (Ji and Suga 2008, 2009).
The development of the nonspecific augmentation of cortical (Ji and Suga 2008) and collicular (Ji and Suga 2009) neurons is not affected by atropine or mecamylamine (a nicotinic cholinergic receptor antagonist) applied to AI or by muscimol applied to the somatosensory cortex. However, these drugs abolish the small brief-lasting BF shift (Fig. 13b). Therefore, different from the BF shifts, the nonspecific augmentation depends on neither the cholinergic meuromodulator nor the somatosensory cortex and the collicular nonspecific augmentation does not depend on the corticofugal feedback. The increase in the responses to tone bursts and in tuning curve width is slightly larger in AI than in the ICc, suggesting that the cortical nonspecific augmentation mostly originates from the subcortical nuclei (Ji and Suga 2009).
4.2.2. Differential gating and interaction between the lemniscal and nonlemniscal systems
The paired CS-US delivered to guinea pigs or big brown bats elicits BF shifts in their AIs, whereas the unpaired CS-US elicits the nonspecific augmentation, i.e., sensitization (Bakin and Weinberger 1992; Edeline et al. 1993; Ji et al. 2001; Ji and Suga 2008). How do the identical CS and US elicit the different types of plastic changes depending on whether they are paired or unpaired? Specifically, how can the CS and US activate neural circuits and neuromodulators in different ways depending on whether they are paired or unpaired?
The CS alone elicits the small BF shifts (Suga and Ma 2003 for review), and the randomized US alone elicits the nonspecific augmentation (Ji & Suga 2008). Therefore, the nonspecific augmentation may be decreased as the BF shift is increased by the CS paired with the US for conditioning. However, the BF shift may be reduced as the nonspecific augmentation is increased by the CS unpaired with the US for pseudo-conditioning. There appears to be a “differential” gating mechanism for such cortical plasticity and auditory signal processing. And different neuromodulators, the corticofugal system and the thalamic reticular nucleus all appear to be implicated in the differential gating mechanism (Suga 2008). Over the last 45 years or so, it has been suggested that one corticofugal function is gating or gain control (e.g., Watanabe et al. 1966; He 2003 for review).
There are findings that are favorable to the presence of the differential gating mechanism: (1) Non-focal electric stimulation of AI excites the majority of MGBv neurons and inhibits the majority of MGBm neurons (Yu et al. 2004). This inhibition is mediated by the thalamic reticular nucleus, TRN (Zhang et al. 2008). (2) Focal electric stimulation of AI evokes the centripetal BF shifts of MGBv neurons and reduces the auditory responses and broadens the frequency tuning of MGBm neurons (Tang, Yang and Suga, unpublished). (3) ACh depolarizes MGBv neurons, but hyperpolarizes MGBm neurons (Mooney et al. 2004). (4) Electric stimulation of MGBm neurons broadens the tuning curves of AI neurons, but that of MGBv neurons evokes their BF shifts (Fig. 10b; Ma and Suga 2009). (5) ICx inhibits ICc neurons (Jen et al. 2001). (6) The trains of 60 dB SPL tone bursts used as the CS activate the auditory system and may activate the ARAS. On the other hand, the electric leg-stimulus used as the US activates the somatosensory system, ARAS and BAS. The BAS and ARAS both activate the histaminergic system, which broadly releases histamine (HA) in the brain and play an essential role in eliciting defensive behaviors and arousal, respectively (Siegel 2002 for review). The HA release depends on the level of stress and arousal (Hass et al, 2008 for review). In the rat cortex, activation of postsynaptic HA3 receptors induces the release of GABA, which in turn inhibits the depolarization-induced ACh release (Giorgetti et al. 1997). It also reduces ACh release in the cortex and impairs the object recognition and passive-avoidance response by rats (Blandina et al. 1996).
Findings (1) - (3) suggest that as AI (the lemniscal system) plays a major role in fine auditory signal processing, the auditory signal processing by the nonlemniscal system is reduced and that neuromodulators for plasticity are different between the lemniscal and nonlemniscal systems. Finding (4) suggests that a high level of MGBm activity makes AI neurons somewhat similar to nonlemniscal neurons and that a high level of MGBv activity may make non-AI neurons somewhat similar to the lemniscal neurons. Finding (5) suggests that auditory signal processing in either the lemniscal or nonlemniscal system is biased at the cortical and subcortical levels according to the behavioral relevance of auditory signals. The findings in (6) suggests that HA plays an essential role in eliciting the nonspecific augmentation and in suppressing the BF shifts.
4.3. Remarks on plasticity elicited by conditioning and pseudo-conditioning
The lemniscal system including the MGBv is essential in eliciting the tone-specific plasticity, BF shifts. The nonlemniscal system including the MGBm may not be. The nonspecific plasticity already takes place in the inferior colliculus. Therefore, the MGBm may not have an essential role in eliciting it, but only a supplementary one. The lemniscal and nonlemniscal pathways form rich multiple feedback loops through the corticocortical and corticofugal projections. Therefore, plastic changes evoked by acoustic stimuli or a focal electric stimulation of a given auditory cortex spread to cortical areas and subcortical auditory nuclei. Thus far, the plastic changes in the lemniscal system elicited by either the electric stimulation, conditioning or pseudo-conditioning have been extensively studied, but not those in the nonlemniscal system. Many questions remain to be answered about the nonlemniscal system, interactions between the lemniscal and nonlemniscal systems, and differential gating.
The corticocortical and corticofugal projections sharpen and shift the tuning of cortical and subcortical neurons in the frequency, amplitude, time and spatial domains and play a key role in the adjustment of the auditory system of an adult normal animal according to an uneven distribution of neural activity over the auditory cortex which can be evoked by either acoustic stimulation, focal electric stimulation, focal drug application or focal cochlear lesion. For the tone-specific plasticity elicited by auditory fear conditioning, the auditory cortex, corticofugal feedback, somatosensory cortex, amygdala and cholinergic nuclei all play a key role. For the nonspecific plasticity elicited by pseudo-conditioning, the cortifugal feedback, somatosensory cortex and cholinergic nuclei do not play a key role. These findings are presumably applicable to many mammalian species.
The cortical BF shift evoked by repetitive acoustic stimulation may be considered as a “physiological auditory memory trace” even if it is augmented by electric stimulation of the cholinergic nucleus basalis or by the conditioning that effectively activates the nucleus basalis. It is uncertain whether the conditioning-augmented cortical BF shift is a physiological “associative” memory trace, because it has not yet been demonstrated that the BF-shifted neurons produced by the conditioning carry the associated auditory-somatosensory information (Suga 2008).
(5) Epilogue
5.1. Adjustment of the auditory system in the dynamic auditory environment
Animal sounds are dynamic, changing values of multiple parameters which characterize them. So, one may consider that the changes evoked by repetitive focal electric stimulation or conditioning using repetitive tone burst stimulation may not occur in nature. The long-lasting stimulation has been used to evoke large, long-lasting changes for the clear-cut demonstration of plasticity and drug effects on the changes. Each of the electric pulses used for the bat research was 0.2 ms, 100 nA (e.g., Ma and Suga 2001a) and that used for the house mouse research was 1.0 ms, 500 nA (e.g., Yan and Ehret 2002). The amount of the changes evoked by the focal electric stimulation greatly depends on the stimulus parameters. For example, two-minute cortical electric stimulation with short trains of 0.2 ms, 100 nA pulses evokes 0.6 kHz cortical and collicular BF shifts. Lengthing the stimulation period from 2 to 30 min increases the BF shifts 2.5 times (Ma and Suga 2001a). A cortical BF shift can be elicited by five trials of paired CS-US (Edeline et al. 1993). Therefore, the corticocortical and corticofugal modulations would occur in the natural setting. Changes in the auditory system would be fast and large if an acoustic stimulus is meaningful to an animal and the animal pays attention to it (Fritz et al. 2007 for review, 2003).
5.2. Modulation of the auditory system in different animal species by conditioning
Centripetal BF shifts elicited by conditioning have been found in the big brown bat (Gao and Suga 2000), guinea pig Cavia porcella (Bakin and Weinberger 1990), rat (Kisley and Gerstein 2001) and cat (Diamond and Weinberger 1986). An expanded tonotopic representation has been found in the rat, owl monkey (Rutkowski and Weinberger 2005) and human (Molchan et al. 1994; Morris et al. 1998). Furthermore, the corticocortical and corticofugal modulations explored by focal electric stimulation in the big brown and mustached bats are shared not only with the auditory system of non-bat species, but also with the somatosensory and visual systems, as described in Sections 2 and 3. Therefore, the bat’s auditory system is specialized for echolocation, but has the common mammalian neural mechanisms for plasticity.
On the earth, there are ~813 species of microchiropterans and ~1,687 species of rodents. Because of easy availability, the big brown bat and guinea pig or mouse have been frequently used for the research on the plasticity of the auditory system without any consideration whether they are “representative” species of the orders microchiroptera and rodentia. However, the findings thus far made in these three species of animals are basically the same (Suga and Ma 2003 for review), in spite of the comments on the bat’s auditory system and the paradigms of the conditioning used for the bat research (Weinberger 2004 for review). Therefore, there is a great possibility that the findings reviewed in the current article are shared by many different species of animals beyond these three species. It is important to identify the common and specialized mechanisms and to explore the mechanisms for specialization deriving from the common ones. Shifts in frequency and delay tuning in the mustached bat’s highly specialized auditory subsystems are centrifugal. This uniqueness derives from common neural mechanisms by strengthening inhibition in the auditory cortex. Therefore, one may conclude that even these specialized subsystems share the basic neural mechanisms with the auditory systems of non-echolocating mammals.
Highlight.
Electric stimulation and conditioning each evoke tone-specific plasticity.
Pseudo-conditioning evokes nonspecific plasticity of auditory neurons.
Neural mechanisms for the two types of plasticity are quite different.
Specialization of the auditory system for plasticity is due to enhanced inhibition.
A differential gating mechanism exists for auditory signal processing.
Acknowledgments
This work was supported by a research grant from the National Institute on Deafness and Other Communication Disorders (DC-000175). I thank Ms. Sally E. Miller for editing this current manuscript and Dr. Philip H.S. Jen for his comments on it.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen RA, Knight PL, Merzenich MM. The thalamocortical and corticothalamic connections of AI, AII, and the anterior auditory field (AAF) in the cat: evidence for two largely segregated systems of connections. J Comp Neurol. 1980;194:663–701. doi: 10.1002/cne.901940312. [DOI] [PubMed] [Google Scholar]
- Aitkin LM, Webster WR. Medial geniculate body of the cat: organization and responses to tonal stimuli of neurons in ventral division. J Neurophysiol. 1972;35:365–380. doi: 10.1152/jn.1972.35.3.365. [DOI] [PubMed] [Google Scholar]
- Armony JL, Quirk GJ, LeDoux JE. Differential effects of amygdala lesions on early and late plastic components of auditory cortex spike trains during fear conditioning. J Neuroscience. 1998;18:2592–2601. doi: 10.1523/JNEUROSCI.18-07-02592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Lepan B, Weinberger NM. Sensitization induced receptive field plasticity in the auditory cortex is independent of CS-modality. Brain Res. 1992;577:226–235. doi: 10.1016/0006-8993(92)90278-h. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjordahl TS, Dimyan MA, Weinberger NM. Induction of long-term receptive field plasticity in the auditory cortex of the waking guinea pig by stimulation of the nucleus basalis. Behav Neurosci. 1998;112:467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- Blandina P, Giorgetti M, Bartolini L, Cacchi M, Timmerman H, Leurs R, Pepeu G, Giovannini MG. Inhibition of cortical acetylcholine release and cognitive performance by histamine H3 receptor activation in rats. Br J Pharmacol. 1996;119:1656–1664. doi: 10.1111/j.1476-5381.1996.tb16086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. I. Acoustic discharge patterns and frequency receptive fields. Exp Brain Res. 1994a;98:261–274. doi: 10.1007/BF00228414. [DOI] [PubMed] [Google Scholar]
- Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. II. Cells receiving convergent auditory and somatosensory inputs and cells antidromically activated by amygdala stimulation. Exp Brain Res. 1994b;98:275–286. doi: 10.1007/BF00228415. [DOI] [PubMed] [Google Scholar]
- Brandão ML, Troncoso AC, de Souza Silva MA, Huston JP. The relevance of neuronal substrates of defense in the midbrain tectum to anxiety and stress: empirical and conceptual considerations. Eur J Pharmacol. 2003;463:225–233. doi: 10.1016/s0014-2999(03)01284-6. [DOI] [PubMed] [Google Scholar]
- Calford MB. The percellation of the medial geniculate body of the cat defined by the auditory response properties of single units. J Neurosci. 1983;3:2350–2364. doi: 10.1523/JNEUROSCI.03-11-02350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford MB, Webster WR, Semple MM. Measurement of frequency selectivity of single neurons in the central auditory pathway. Hear Res. 2003;11:395–401. doi: 10.1016/0378-5955(83)90070-9. [DOI] [PubMed] [Google Scholar]
- Calford MB, Wright LL, Metha AB, Taglianetti V. Topographic plasticity in primary visual cortex is mediated by local corticocortical connections. J Neurosci. 2003;23:6434–6442. doi: 10.1523/JNEUROSCI.23-16-06434.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SA, Suga N. Reorganization of the tonotopic map of the auditory cortex evoked by cortical electrical stimulation in the big brown bat. J Neurophysiol. 2000;83:1856–1863. doi: 10.1152/jn.2000.83.4.1856. [DOI] [PubMed] [Google Scholar]
- Clarey JC, Barone P, Imig TJ. Physiology of thalamus and cortex. In: Popper AN, Fay RR, editors. The Mammalian Auditory Pathway: Neurophysiology. Chapter 5. Springer-Verlag; New York: 1992. pp. 232–334. [Google Scholar]
- Covey E, Casseday JH. Timing in the auditory system of the bat. Ann Rev Physiol. 1999;61:457–476. doi: 10.1146/annurev.physiol.61.1.457. [DOI] [PubMed] [Google Scholar]
- Crabtree JW. Organization in the auditory sector of the cat's thalamic reticular nucleus. J Comp Neurol. 1998;390:167–182. [PubMed] [Google Scholar]
- Cruikshank SJ, Edeline JM, Weinberger NM. Stimulation at a site of auditory-somatosensory convergence in the medial geniculate nucleus is an effective unconditioned stimulus for fear conditioning. Behav Neurosci. 1992;106:471–483. doi: 10.1037//0735-7044.106.3.471. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Weinberger NM. Classical conditioning rapidly induces specific changes in frequency receptive fields of single neurons in secondary and ventral ectosylvian auditory cortical fields. Brain Res. 1986;372:357–360. doi: 10.1016/0006-8993(86)91144-3. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Pham P, Weinberger NM. Rapid development of learning-induced receptive field plasticity in the auditory cortex. Behav Neurosci. 1993;107:539–551. doi: 10.1037//0735-7044.107.4.539. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weiberger NM. Subcortical adaptive filtering in the auditory system: associative receptive field plasticity in the dorsal medial geniculate body. Behav Neurosci. 1991a;105:154–175. doi: 10.1037//0735-7044.105.1.154. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Thalamic short-term plasticity in the auditory system: associative retuning of receptive fields in the ventral medial geniculate body. Behav Neurosci. 1991b;105:618–639. doi: 10.1037//0735-7044.105.5.618. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Associative retuning in the thalamic source of input to the amygdala and auditory cortex: receptive field plasticity in the medial division of the medial geniculate body. Behav Neurosci. 1992;106:81–105. doi: 10.1037//0735-7044.106.1.81. [DOI] [PubMed] [Google Scholar]
- Ehrlich D, Casseday JH, Covey E. Neural tuning to sound duration in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Neurophysiol. 1997;77:2360–2372. doi: 10.1152/jn.1997.77.5.2360. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Olsen JF, Suga N. Connections among functional areas in the mustached bat auditory cortex. J Comp Neurol. 1998;391:366–396. [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, David SV, Shamma SA. Does attention play a role in dynamic receptive field adaptation to changing acoustic salience in A1? Hear Res. 2007;229:186–203. doi: 10.1016/j.heares.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Galazyuk AV, Feng AS. Encoding of sound duration by neurons in the auditory cortex of the little brown bat, Myotis lucifugus. J Comp Physiol [A] 1997;180:301–311. doi: 10.1007/s003590050050. [DOI] [PubMed] [Google Scholar]
- Galazyuk AV, Lin W, Llano D, Feng AS. Leading inhibition to neural oscillation is important for time-domain processing in the auditory midbrain. J Neurophysiol. 2005;94:314–326. doi: 10.1152/jn.00056.2005. [DOI] [PubMed] [Google Scholar]
- Galuske RA, Schmidt KE, Goebel R, Lomber SG, Payne BR. The role of feedback in shaping neural representations in cat visual cortex. Proc Natl Acad Sci USA. 2002;99:17083–17088. doi: 10.1073/pnas.242399199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent corticofugal adjustment of midbrain tonotopic map in bat auditory system. Proc Natl Acad Sci USA. 1998;95:12663–12670. doi: 10.1073/pnas.95.21.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent plasticity in the auditory cortex and the inferior colliculus of bats: role of the corticofugal system. Proc Natl Acad Sci USA. 2000;97:8081–8086. doi: 10.1073/pnas.97.14.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti M, Bacciottini L, Bianch L, Giovannini MG, Cecchi M, Blandina P. GABAergic mechanism in histamine H3 receptor inhibition of K+-evoked release of acetylcholine from rat cortex in vivo. Ingflamm Res. 1997;46:S3–S4. doi: 10.1007/s000110050044. [DOI] [PubMed] [Google Scholar]
- Godde B, Leonhardt R, Cords SM, Dinse HR. Plasticity of orientation preference maps in the visual cortex of adult cats. Proc Natl Acad Sci USA. 2002;99:6352–6357. doi: 10.1073/pnas.082407499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RL, Henson OW., Jr Changes in cochlear mechanics during vocalization: evidence for a phasic medial efferent effect. Hear Res. 1998;122:71–81. doi: 10.1016/s0378-5955(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Golmayo L, Nunez A, Zaborszky L. Electrophysiological evidence for the existence of a posterior cortical-prefrontal-basal forebrain circuitry in modulating sensory responses in visual and somatosensory rat cortical areas. Neuroscience. 2003;119:597–609. doi: 10.1016/s0306-4522(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol. 1987;262:105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- Hass HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- Hass H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- He J. Corticofugal modulation of the auditory thalamus. Experimental Brain Research. 2003;153:579–590. doi: 10.1007/s00221-003-1680-5. [DOI] [PubMed] [Google Scholar]
- Hu B. Functional organization of lemniscal and nonlemniscal auditory thalamus. Exp Brain Res. 2003;153:543–549. doi: 10.1007/s00221-003-1611-5. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Adrián HO. Binaural columns in the primary field (A1) of cat auditory cortex. Brain Res. 1977;138:241–257. doi: 10.1016/0006-8993(77)90743-0. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Brugge JF. Sources and terminations of callosal axons related to binaural and frequency maps in primary auditory cortex of the cat. J Comp Neurol. 1978;182:637–660. doi: 10.1002/cne.901820406. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Morel A. Organization of the thalamocortical auditory system in the cat. Annu Rev Neurosci. 1983;6:95–120. doi: 10.1146/annurev.ne.06.030183.000523. [DOI] [PubMed] [Google Scholar]
- Jafari MR, Zhang Y, Yan J. Multiparametric changes in the receptive field of cortical auditory neurons induced by thalamic activation in the mouse. Cereb Cortex. 2007;17:71–80. doi: 10.1093/cercor/bhj125. [DOI] [PubMed] [Google Scholar]
- Jen PH, Chen QC, Sun XD. Corticofugal regulation of auditory sensitivity in the bat inferior colliculus. J Comp Physiol [A] 1998;183:683–697. doi: 10.1007/s003590050291. [DOI] [PubMed] [Google Scholar]
- Jen PH, Sun X, Chen QC. An electrophysiological study of neural pathways for corticofugally inhibited neurons in the central nucleus of the inferior colliculus of the big brown bat, Eptesicus fuscus. Exp Brain Res. 2001;137:292–302. doi: 10.1007/s002210000637. [DOI] [PubMed] [Google Scholar]
- Jen PH, Wu CH. Duration selectivity organization in the inferior colliculus of the big brown bat, Eptesicus fuscus. Brain Res. 2006;1108:76–87. doi: 10.1016/j.brainres.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Jen PH, Zhang JP. Corticofugal regulation of excitatory and inhibitory frequency tuning curves of bat inferior collicular neurons. Brain Res. 1999;841:184–188. doi: 10.1016/s0006-8993(99)01786-2. [DOI] [PubMed] [Google Scholar]
- Jen PH, Zhou X. Corticofugal modulation of amplitude domain processing in the midbrain of the big brown bat, Eptesicus fuscus. Hear Res. 2003;184:91–106. doi: 10.1016/s0378-5955(03)00237-5. [DOI] [PubMed] [Google Scholar]
- Ji W, Gao E, Suga N. Effects of acetylcholine and atropine on plasticity of central auditory neurons caused by conditioning in bats. J Neurophysiol. 2001;86:211–225. doi: 10.1152/jn.2001.86.1.211. [DOI] [PubMed] [Google Scholar]
- Ji W, Suga N. Development of reorganization of the auditory cortex caused by fear conditioning: effect of atropine. J Neurophysiol. 2003;90:1904–1909. doi: 10.1152/jn.00363.2003. [DOI] [PubMed] [Google Scholar]
- Ji W, Suga N. Serotonergic modulation of plasticity of the auditory cortex elicited by fear conditioning. J Neurosci. 2007;27:4910–4918. doi: 10.1523/JNEUROSCI.5528-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Suga N. Tone-specific and nonspecific plasticity of auditory cortex elicited by pseudoconditioning: Role of acetylcholine receptors and the somatosensory cortex. J Neurophysiol. 2008;100:1384–1396. doi: 10.1152/jn.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Suga N. Tone-specific and nonspecific plasticity of inferior colliculus elicited by pseudoconditioning: Role of acetylcholine and auditory and somatosensory cortices. J Neurophysiol. 2009;102:941–952. doi: 10.1152/jn.00222.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Suga N. Histaminergic modulation of nonspecific plasticity of the auditory system and differential gating. J Neurophysiol. 2011 doi: 10.1152/jn.00930.2011. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Suga N, Gao E. Effects of agonists and antagonists of NMDA and ACh receptors on plasticity of bat auditory system elicited by fear conditioning. J Neurophysiol. 2005;94:1199–1211. doi: 10.1152/jn.00112.2005. [DOI] [PubMed] [Google Scholar]
- Jones EG. Some aspects of the organization of the thalamic reticular complex. J Comp Neurol. 1975;162:285–308. doi: 10.1002/cne.901620302. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kisley MA, Gerstein GL. Daily variation and appetitive conditioning-induced plasticity of auditory cortex receptive fields. Eur J Neurosci. 2001;13:1993–2003. doi: 10.1046/j.0953-816x.2001.01568.x. [DOI] [PubMed] [Google Scholar]
- Klug A, Khan A, Burger RM, Bauer EE, Hurley LM, Yang L, Grothe B, Halvorsen MB, Park TJ. Latency as a function of intensity in auditory neurons: influences of central processing. Hear Res. 2000;148:107–123. doi: 10.1016/s0378-5955(00)00146-5. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee T, Littman T, Nicol T, King C. Nonprimary auditory thalamic representation of acoustic change. J Neurophysiol. 1994;72:1270–1277. doi: 10.1152/jn.1994.72.3.1270. [DOI] [PubMed] [Google Scholar]
- Lanuza E, Nader K, Ledoux JE. Unconditioned stimulus pathways to the amygdala: effects of posterior thalamic and cortical lesions on fear conditioning. Neuroscience. 2004;125:305–315. doi: 10.1016/j.neuroscience.2003.12.034. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory systems in the brain. Behav Brain Res. 1993;58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- Li XF, Stutzmann GE, LeDoux JE. Convergent but temporally separated inputs to lateral amygdala neurons from the auditory thalamus and auditory cortex use different postsynaptic receptors: in vivo intracellular and extracellular recordings in fear conditioning pathways. Learning and Memory. 1996;3:229–242. doi: 10.1101/lm.3.2-3.229. [DOI] [PubMed] [Google Scholar]
- Liu X, Yan Y, Wang Y, Yan J. Corticofugal modulation of initial neural processing of sound information from the ipsilateral ear in the mouse. PLoS One. 2010;5:e14038. doi: 10.1371/journal.pone.0014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Suga N. Binaural and commissural organization of the primary auditory cortex of the mustached bat. J Comp Physiol [A] 1997;181:599–605. doi: 10.1007/s003590050143. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Malhotra S. Double dissociation of 'what' and 'where' processing in auditory cortex. Nat Neurosci. 2008;11:609–616. doi: 10.1038/nn.2108. [DOI] [PubMed] [Google Scholar]
- Luo F, Wang Q, Kashani A, Yan J. Corticofugal modulation of initial sound processing in the brain. J Neurosci. 2008;28:11615–11621. doi: 10.1523/JNEUROSCI.3972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Liu X, Wang C, Yan J. The pedunculopontine tegmental nucleus: a second cholinergic source for frequency-specific auditory plasticity. J Neurophysiol. 2011;105:107–116. doi: 10.1152/jn.00546.2010. [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Plasticity of bat's central auditory system evoked by focal electric stimulation of auditory and/or somatosensory cortices. J Neurophysiol. 2001a;85:1078–1087. doi: 10.1152/jn.2001.85.3.1078. [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Corticofugal modulation of duration-tuned neurons in the midbrain auditory nucleus in bats. Proc Natl Acad Sci USA. 2001b;98:14060–14065. doi: 10.1073/pnas.241517098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Suga N. Augmentation of plasticity of the central auditory system by the basal forebrain and/or somatosensory cortex. J Neurophysiol. 2003;89:90–103. doi: 10.1152/jn.00968.2001. [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Lateral inhibition for center-surround reorganization of the tonotopic map of bat auditory cortex. J Neurophysiol. 2004;92:3192–3199. doi: 10.1152/jn.00301.2004. [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Long-term cortical plasticity evoked by electric stimulation and acetylcholine applied to the auditory cortex. Proc Natl Acad Sci USA. 2005;102:9335–9340. doi: 10.1073/pnas.0503851102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Suga N. Multiparametric corticofugal modulation of collicular duration-tuned neurons: modulation in the amplitude domain. J Neurophysiol. 2007;97:3722–3730. doi: 10.1152/jn.01268.2006. [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Corticofugal modulation of the paradoxical latency shifts of inferior collicular neurons. J Neurophysiol. 2008;100:1127–1134. doi: 10.1152/jn.90508.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Suga N. Specific and nonspecific plasticity of the primary auditory cortex elicited by thalamic auditory neurons. J Neurosci. 2009;29:4888–4896. doi: 10.1523/JNEUROSCI.0167-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T, Suga N, Ostwald J. Aural representation in the Doppler-shifted-CF processing area of the auditory cortex of the mustache bat. Science. 1978;200:339–342. doi: 10.1126/science.635594. [DOI] [PubMed] [Google Scholar]
- Maren S. Auditory fear conditioning increases CS-elicited spike firing in lateral amygdala neurons even after extensive overtraining. European Journal of Neuroscience. 2000;12:4047–4054. doi: 10.1046/j.1460-9568.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Yap SA, Goosens KA. The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J Neurosci. 2001;21:RC135. doi: 10.1523/JNEUROSCI.21-06-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molchan SE, Sunderland T, McIntosh AR, Herscovitch P, Schreurs BG. A functional anatomical study of associative learning in humans. Proc Natl Acad Sci USA. 1994;91:8122–8126. doi: 10.1073/pnas.91.17.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney DM, Zhang L, Basile C, Senatorov VV, Ngsee J, Omar A, Hu B. Distinct forms of cholinergic modulation in parallel thalamic sensory pathways. Proc Natl Acad Sci USA. 2004;101:320–324. doi: 10.1073/pnas.0304445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Dolan RJ. Experience-dependent modulation of tonotopic neural responses in human auditory cortex. Proc Biol Sci. 1998;265:649–657. doi: 10.1098/rspb.1998.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh H, Marzullo TC, Yazdan-Shahmorad A, Gage GJ, Kipke D. Laminar characterization of spiking activity in the rat motor cortex. Conf Proc IEEE Eng Med Biol Soc. 2007:4735–4738. doi: 10.1109/IEMBS.2007.4353397. [DOI] [PubMed] [Google Scholar]
- Perrot X, Ryvlin P, Isnard J, Guénot M, Catenoix H, Fischer C, Mauguière F, Collet L. Evidence for corticofugal modulation of peripheral auditory activity in humans. Cereb Cortex. 2006;16:941–948. doi: 10.1093/cercor/bhj035. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pinheiro AD, Wu M, Jen PH. Encoding repetition rate and duration in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Comp Physiol [A] 1991;169:69–85. doi: 10.1007/BF00198174. [DOI] [PubMed] [Google Scholar]
- Pollak GD, Casseday JH. The Neural Basis of Echolocation in Bats. Springer-Verlag; Berlin: 1989. [Google Scholar]
- Poremba A, Gabriel M. Medial geniculate lesions block amygdalar and cingulothalamic learning-related neuronal activity. J Neurosci. 1997;17:8645–8655. doi: 10.1523/JNEUROSCI.17-21-08645.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Gabriel M. Amygdalar efferents initiate auditory thalamic discriminative training-induced neuronal activity. J Neurosci. 2001;21:270–278. doi: 10.1523/JNEUROSCI.21-01-00270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel JL, Rebillard G, Bonfils P, Pujol R. Effect of Visual Selective Attention on Otoacoustic Emissions. In: Wilson JP, Kemp DT, editors. Cochlear Mechanisms. Plenum Press; New York: 1989. pp. 315–321. [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Smith SA, Semba K. Inactivation of prefrontal cortex abolishes cortical acetylcholine release evoked by sensory or sensory pathway stimulation in the rat. Neuroscience. 2007;149:232–241. doi: 10.1016/j.neuroscience.2007.06.057. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of "what" and "where" in auditory cortex. Proc Natl Acad Sci USA. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Dinse HR. Expansion of the cortical representation of a specific skin field in primary somatosensory cortex by intracortical microstimulation. Cereb Cortex. 1992a;2:181–196. doi: 10.1093/cercor/2.3.181. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992b;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- Robertson D, Mulders WHAM. Central effects of efferent activation. In: Ryugo DK, Fay RR, Popper AN, editors. Auditory and Vestibular Efferents. Chapter 10. Springer; 2010. pp. 291–312. [Google Scholar]
- Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex. 1993;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- Rouiller EM. Functional organization of the auditory pathways. In: Ehret G, Ramand R, editors. The Central Auditory System. Oxford University Press; New York, New York: 1997. pp. 3–96. [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci USA. 2005;102:13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai M, Suga N. Plasticity of the cochleotopic (frequency) map in specialized and nonspecialized auditory cortices. Proc Natl Acad Sci USA. 2001;98:3507–3512. doi: 10.1073/pnas.061021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai M, Suga N. Centripetal and centrifugal reorganizations of tonotopic map of the auditory cortex in gerbils. Proc Natl Acad Sci USA. 2002;99:7108–7112. doi: 10.1073/pnas.102165399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Motts SD, Mellott JG. Cholinergic cells of the pontomesencephalic tegmentum: Connections with auditory structures from cochlear nucleus to cortex. Hear Res. 2011;30:1–11. doi: 10.1016/j.heares.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. The Neural Control of Sleep and Waking. Springer-Verlag; New York: 2002. [Google Scholar]
- Suga N. Feature Extraction in the Auditory System of Bats. In: Moller AR, editor. Basic Mechanisms in Hearing. Academic Press; New York, New York: 1973. pp. 675–742. [Google Scholar]
- Suga N. Functional organization of the auditory cortex. In: Woolsey C, editor. Cortical Sensory Organization. Humana Press; Clifton, New Jersey: 1982. pp. 157–218. [Google Scholar]
- Suga N. Cortical computational maps for auditory imaging. Neural Networks. 1990;3:3–21. [Google Scholar]
- Suga N. Role of corticofugal feedback in hearing. J Comp Physiol [A] 2008;194:169–183. doi: 10.1007/s00359-007-0274-2. [DOI] [PubMed] [Google Scholar]
- Suga N, Gao E, Zhang Y, Ma X, Olsen JF. The corticofugal system for hearing: recent progress. Proc Natl Acad Sci USA. 2000;97:11807–11814. doi: 10.1073/pnas.97.22.11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N, Ji W, Ma X, Tang J, Xiao Z, Yan J. Corticofugal modulation and beyond for auditory signal processing and plasticity. In: Ryugo DK, Fay RR, Popper AN, editors. Auditory and Vestibular Efferents. Vol. 11. Springer; 2010. pp. 313–352. [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- Suga N, Xiao Z, Ma X, Ji W. Plasticity and corticofugal modulation for hearing in adult animals. Neuron. 2002;36:9–18. doi: 10.1016/s0896-6273(02)00933-9. [DOI] [PubMed] [Google Scholar]
- Sullivan WE., 3rd Neural representation of target distance in auditory cortex of the echolocating bat, Myotis lucifugus. J Neurophysiol. 1982;48:1011–1032. doi: 10.1152/jn.1982.48.4.1011. [DOI] [PubMed] [Google Scholar]
- Tang J, Suga N. Modulation of auditory processing by cortico-cortical feed-forward and feedback projections. Proc Natl Acad Sci USA. 2008;105:7600–7605. doi: 10.1073/pnas.0802961105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Suga N. Corticocortical interactions between and within three cortical auditory areas specialized for time-domain signal processing. J Neurosci. 2009;29:7230–7237. doi: 10.1523/JNEUROSCI.0373-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Xiao Z, Suga N. Bilateral cortical interaction: modulation of delay-tuned neurons in the contralateral auditory cortex. J Neurosci. 2007;27:8405–8413. doi: 10.1523/JNEUROSCI.1257-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar SK, Gerstein GL. Reorganization in awake rat auditory cortex by local microstimulation and its effect on frequency-discrimination behavior. J Neurophysiol. 2001;86:1555–1572. doi: 10.1152/jn.2001.86.4.1555. [DOI] [PubMed] [Google Scholar]
- Wang X, Galazyuk AV, Feng AS. FM signals produce robust paradoxical latency shifts in the bat’s inferior colliculus. J Comp Physiol [A] 2007;193:13–20. doi: 10.1007/s00359-006-0167-9. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yanagisawa K, Kanzaki J, Katsuki Y. Cortical efferent flow influencing unit responses of medial geniculate body to sound stimulation. Exp Brain Res. 1966;2:302–317. doi: 10.1007/BF00234776. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Physiological Memory in Primary Auditory Cortex: Characteristics and Mechanisms. Neurobiol Learn Mem. 1998;70:226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nature Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Diamond IT, Raczkowski D. Subdivisions of the auditory cortex of the cat: the retrograde transport of horseradish peroxidase to the medial geniculate body and posterior thalamic nuclei. J Comp Neurol. 1977;176:387–417. doi: 10.1002/cne.901760307. [DOI] [PubMed] [Google Scholar]
- Winer JA, Diehl JJ, Larue DT. Projections of auditory cortex to the medial geniculate body of the cat. J Comp Neurol. 2001;430:27–55. [PubMed] [Google Scholar]
- Wu Y, Yan J. Modulation of the receptive fields of midbrain neurons elicited by thalamic electrical stimulation through corticofugal feedback. J Neurosci. 2007;27:10651–10658. doi: 10.1523/JNEUROSCI.1320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Suga N. Modulation of cochlear hair cells by the auditory cortex in the mustached bat. Nat Neurosci. 2002a;5:57–63. doi: 10.1038/nn786. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Suga N. Reorganization of the cochleotopic map in the bat's auditory system by inhibition. Proc Natl Acad Sci USA. 2002b;99:15743–15748. doi: 10.1073/pnas.242606699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Suga N. Reorganization of the auditory cortex specialized for echo-delay processing in the mustached bat. Proc Natl Acad Sci USA. 2004;101:1769–1774. doi: 10.1073/pnas.0307296101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Suga N. Asymmetry in corticofugal modulation of frequency tuning in mustached bat auditory system. Proc Natl Acad Sci USA. 2005;102:19162–19167. doi: 10.1073/pnas.0509761102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Zhang Y, Yan J. The neurobiology of sound-specific auditory plasticity: a core neural circuit. Neurosci Biobehav Rev. 2009;33:1178–1184. doi: 10.1016/j.neubiorev.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Yan J, Ehret G. Corticofugal modulation of midbrain sound processing in the house mouse. Eur J Neurosci. 2002;16:119–128. doi: 10.1046/j.1460-9568.2002.02046.x. [DOI] [PubMed] [Google Scholar]
- Yan J, Suga N. Corticofugal modulation of time-domain processing of biosonar information in bats. Science. 1996;273:1100–1103. doi: 10.1126/science.273.5278.1100. [DOI] [PubMed] [Google Scholar]
- Yan W, Suga N. Corticofugal modulation of the midbrain tonotopic map in the bat auditory system. Nat Neurosci. 1998;1:54–58. doi: 10.1038/255. [DOI] [PubMed] [Google Scholar]
- Yan J, Suga N. Corticofugal amplification of facilitative auditory responses of subcortical combination-sensitive neurons in the mustached bat. J Neurophysiol. 1999;81:817–24. doi: 10.1152/jn.1999.81.2.817. [DOI] [PubMed] [Google Scholar]
- Yu YQ, Xiong Y, Chan YS, He J. Corticofugal gating of auditory information in the thalamus: an in vivo intracellular recording study. J Neurosci. 2004;24:3060–3069. doi: 10.1523/JNEUROSCI.4897-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. The basal forebrain corticopetal system revisited. Annals of the New York Academy of Sciences. 1999;877:339–367. doi: 10.1111/j.1749-6632.1999.tb09276.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hakes JJ, Bonfield SP, Yan J. Corticofugal feedback for auditory midbrain plasticity elicited by tones and electrical stimulation of basal forebrain in mice. Eur J Neurosci. 2005;22:871–879. doi: 10.1111/j.1460-9568.2005.04276.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Suga N. Corticofugal amplification of subcortical responses to single tone stimuli in the mustached bat. J Neurophysiol. 1997;78:3489–3492. doi: 10.1152/jn.1997.78.6.3489. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Suga N. Modulation of responses and frequency tuning of thalamic and collicular neurons by cortical activation in mustached bat. J Neurophysiol. 2000;84:325–333. doi: 10.1152/jn.2000.84.1.325. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Suga N. Corticofugal feedback for collicular plasticity evoked by electric stimulation of the inferior colliculus. J Neurophysiol. 2005;94:2676–2682. doi: 10.1152/jn.00549.2005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Suga N, Yan J. Corticofugal modulation of frequency processing in bat auditory system. Nature. 1997;387:900–903. doi: 10.1038/43180. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yan J. Corticothalamic feedback for sound-specific plasticity of auditory thalamic neurons elicited by tones paired with basal forebrain stimulation. Cereb Cortex. 2008;18:1521–1528. doi: 10.1093/cercor/bhm188. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu CH, Yu YQ, Fujimoto K, Chan YS, He J. Corticofugal projection inhibits the auditory thalamus through the thalamic reticular nucleus. J Neurophysiol. 2008;99:2938–2945. doi: 10.1152/jn.00002.2008. [DOI] [PubMed] [Google Scholar]
- Zhou X, Jen PH. Corticofugal modulation of directional sensitivity in the midbrain of the big brown bat, Eptesicus fuscus. Hear Res. 2005;203:201–215. doi: 10.1016/j.heares.2004.12.008. [DOI] [PubMed] [Google Scholar]