Abstract
Background and Purpose
Better understanding of fall risk post-stroke is required for developing screening and prevention programs. This study characterizes falls in the Locomotor Experience Applied Post Stroke (LEAPS) randomized clinical trial, describes the impact of two walking recovery interventions on falls, and examines the value of clinical assessments for predicting falls.
Methods
Community-dwelling ambulatory stroke survivors enrolled in LEAPS were assessed 2-months post-stroke. Falls were monitored until 12-months post-stroke and participants were characterized as multiple or injurious (M/I); single, non-injurious (S/NI); or non-fallers. Incidence and time to M/I falls was compared across interventions [home exercise (HEP) and locomotor training initiated 2-months (early-LTP) or 6-months (late-LTP) post-stroke]. Predictive value of 2-month clinical assessments for falls outcome was assessed.
Results
Among the 408 participants, 36.0% were M/I, 21.6% S/NI, and 42.4% non-fallers. Most falls occurred at home in the first three months after assessment. Falls incidence was highest for those with severe walking impairment who received early-LTP.(p=0.025). Berg Balance Scale (BBS) score ≤42/56 was the single best predictor of M/I falls.
Conclusions
As individuals with stroke improve walking capacity, risk for M/I falls remains high. Individuals walking <0.4 m/s are at higher risk for M/I falls if they receive early-LTP training. BBS, at 2-months post-stroke, is useful for informing falls risk but cannot account for the multifactorial nature of the problem. Falls prevention in stroke will require multifactorial risk assessment and management provided concomitantly with exercise interventions to improve mobility.
Keywords: stroke, falls, fall risk, falls incidence
Introduction
Despite recent advances, stroke remains the most common disabling neurological condition among American adults.1 Falls are a common complication after stroke.2, 3 Between 40 and 70 percent of individuals fall within 12 months post-stroke3, 4 and their hip fracture risk is doubled.5 Individuals with stroke are more likely to become repeat fallers than the general elderly population3 with incidence of multiple (>1) falls between 42 and 57 percent in the first year.3, 6, 7 Recurrent fallers with stroke have greater deficits in mobility and ADL function than single fallers.8 Although multiple fallers are not at higher risk for injury for any given fall, cumulative injury risk increases with each fall.9 Falls prediction and management for individuals post-stroke should thus focus on multiple falls.4, 7, 8
A primary goal of stroke rehabilitation is to improve individuals’ mobility in the presence of motor, balance, and visual-spatial deficits. Yet, increasing mobility and physical activity increases exposure to fall risks.3 A systematic review of exercise in older people suggests that strength and balance exercises reduce falls, whereas walking training alone may increase them.10 The authors speculate that this may be associated with either increased risk during walking practice or that time spent walking reduces time available for balance training. Few studies comparing interventions to improve walking recovery post-stroke have reported falls.11-14
Most studies of falls in community-dwelling stroke survivors are relatively small and do not consider the concurrent effects of physical therapy programs or increased mobility on falls risk. In contrast, the Locomotor Experience Applied Post-stroke (LEAPS) phase 3 multisite randomized clinical trial (RCT) that compared rehabilitation programs to promote walking recovery after disabling stroke, provides a unique opportunity to prospectively characterize and examine risk for falls in the context of two walking rehabilitation programs in a relatively large cohort. The purposes of this study were to characterize the incidence and consequences of falls among participants in the LEAPS RCT, examine the impact of two interventions to improve walking recovery on falls, and assess the value of clinical assessments at 2-months for predicting falls outcome at 12 months post-stroke.
Methods
Participants
Four hundred eight individuals enrolled in the LEAPS multi-site RCT were included in this study.15 Inclusion criteria included stroke in the last 45 days, residual paresis, ability to walk 10 feet with no more than 1-person assistance, ability to follow a 3-step command, and self-selected walking speed less than 0.8 m/s.15, 16 Ethics review boards at all participating centers approved the trial protocol and all participants provided written informed consent.
2-Month Assessment
Participants were assessed by trained, blinded assessors at 2-months post-stroke using standardized protocols reported previously.16 From this assessment, we selected 41 variables deemed potentially relevant to 12-month falls outcome, based on previous studies and clinical judgment, to include in this analysis (full list available online).
Physical Therapy Interventions
At 2-months post-stroke, participants were randomly assigned to one of three treatment groups: a specialized locomotor training program (LTP) that included stepping on a treadmill with body weight support followed by walking practice overground delivered early (early-LTP, 2-months post-stroke) or late (late-LTP, 6-months post-stroke) or a progressive strength and balance exercise program provided by a physical therapist in the home (HEP) initiated 2-months post-stroke.15, 16 Each intervention was provided for 30-36 sessions over 12-16 weeks by trained physical therapists.16 Participants were stratified by moderate (0.4-<0.8 m/s) or severe (<0.4 m/s) walking speed impairment. The late-LTP group received only usual care physical therapy based on current practice between 2 and 6 months post-stroke and crossed over to LTP at 6-months.
Falls Assessment
Falls incidence was monitored between 2 and 12 months post-stroke. We used international standards for defining and reporting falls17 including the following definition for a fall, “A person has a fall if they end up on the ground or floor when they did not expect to. Most often a fall starts while a person is on their feet, but a fall could also start from a chair or bed. If a person ends up on the ground, either on their knees, their belly, their side, their bottom, or their back, they have had a fall.” This explanation was provided to participants and caregivers and printed on monthly calendars issued at randomization. Participants and/or caregivers placed an “X” on the corresponding date if a fall occurred and mailed calendars to their study site each month (even if no falls occurred). Study personnel provided reminders as needed.
Participants were contacted by phone to follow-up on reported falls using a standard questionnaire. Information collected for each fall included presence and nature of any injury, location of the fall, and ability to get up independently after the fall. Three categories were used to characterize falls outcome at 12-months post-stroke: multiple and/or injurious (M/I); single fallers non-injurious (S/NI); and non-fallers. Injurious falls were those resulting in serious injury: fracture, loss of consciousness, or hospital admission.
Statistical Analysis
Conventional statistics were conducted using SAS® 9.2. Chi-square tests and analysis of variance were used to assess univariate associations between clinical assessments at 2-months and fall category; alpha value was 0.05. Chi-square tests were used to examine associations between intervention groups and faller categories. A log-rank test was used to compare the probability of M/I fall onset over time across intervention groups. The Classification and Regression Tree method (CART® Salford-System, V6) was used to establish a prediction model for 2-month clinical assessment variables and faller category at 12-months.18 Because we had three faller categories, the Twoing splitting rule was used for CART analysis. This approach reduces bias towards the largest outcome strategy. Ten-fold cross validation was used to reduce the complicity of the prediction model (pruning). This procedure can prevent over-fitted prediction models, thereby preserving generalizability.18 Logistic regression was used to evaluate the potential confounding and modification effects of treatment group assignment on the prediction model.
Results
Participants
Four hundred eight participants, 62.0±12.7 years old, were assessed at 63.8±8.5 days post-stroke and monitored for falls incidence for 10.3±2.1 months. The average number of monthly reports per participant was 9.6±2.4; there was no significant difference in reporting across the three intervention groups (p=0.80). All participants had severe (<0.4 m/s, n=218, 53.4%) or moderate (0.4m/s≤ and <0.8 m/s, n=190, 46.6%) walking speed deficits and most had moderate to moderately-severe disability (modified Rankin Scale19: 0-1, 0.5%; 2, 13.2%; 3, 42.2%; 4, 44.1%).
Incidence of falls
Among all participants, 147 (36.0%) were M/I, 88 (21.6%) S/NI, and 173 (42.4%) non-fallers. The majority of all fallers (n=235) experienced multiple falls (n=147, 62.6%). Twenty-four fallers (10.2%) experienced fall-related serious injury; 8 had one and 16 had >1 fall (p=0.43). For all falls (n=612), 55.4% occurred in the first three months monitored (3-5 months post-stroke), 86.8% occurred at home (indoors=70.8% [bathroom=10.0%, bedroom=23.9%, other room=36.9%], outdoors=16.0%), and 13.1% in the community. Of individuals who fell, 74% had at least one fall from which they could not get up independently. Fall rate per person year was 1.76 overall, 1.33 for moderately impaired walkers, and 2.13 for severely impaired walkers (p<0.001).
Characteristics of fallers
Table 1 is an abbreviated summary of the associations between clinical presentation at 2-months and faller category at 12-months post-stroke (full table online). Multiple or injurious fallers were older than S/NI fallers and non-fallers (p=0.02); S/NI and M/I fallers had worse upper (p=0.02) and lower (p=0.05) extremity motor control (Fugl-Meyer Upper and Lower Extremity Motor Scores20, respectively) compared to non-fallers; and the M/I faller group had the lowest comfortable and fast walking speeds, 6-minute walk distance, and BBS score, the greatest use of assistive devices, and the lowest balance confidence (p<0.01). The Stroke Impact Scale Participation subscale score was lowest (worst function) among S/NI and M/I fallers (p=0.01). Similarly, M/I fallers had the highest overall disability (lowest modified Rankin Scale scores), followed by S/NI fallers and non-fallers (p<0.01).
Table 1.
Characteristics of participants by faller category at 2 month post stroke
| Item | All Participants (n=408) |
Non-fallers (n=173) |
Single, Non- injurious fallers (n=88) |
Multiple or injurious fallers (n=147) |
p- value |
|---|---|---|---|---|---|
| PERSONAL FACTORS | |||||
| Demographics | |||||
| Male | 224 (54.9%) | 99(57.2%) | 47 (53.4%) | 78(53.1%) | 0.72 |
| Female | 184 (45.1%) | 74 (42.8%) | 41 (46.6%) | 69 (46.9%) | |
| Age at stroke onset | 62.0±12.7 | 60.8±13.2 | 60.5±12.9 | 64.4±11.9 | 0.02 |
| Co-morbidities | |||||
| Depression score (PHQ-9) | 4.68±5.22 | 4.36±5.2 | 4.8±5.5 | 5.0±5.1 | 0.55 |
| Atrial fibrillation | 31 (7.6%) | 14 (8.1%) | 7 (8%) | 10 (6.8%) | 0.90 |
| Coronary Artery Disease | 56 (13.8%) | 20 (11.6%) | 13 (14.8%) | 23 (15.8%) | 0.53 |
| On Beta-blocker | 163 (40%) | 69 (39.9%) | 39 (44.3%) | 55 (37.4%) | 0.58 |
| Diabetes Mellitus | 140 (34.4%) | 57 (33.1%) | 30 (34.1%) | 53 (36.1%) | 0.86 |
| History of alcohol abuse | 15 (3.7%) | 5 (2.9) | 0 (0%) | 10 (6.8%) | 0.02 |
| BODY FUNCTION AND STRUCTURE | |||||
| Stroke Characteristics | |||||
| Location | |||||
| Left | 164 (40.2%) | 71 (41%) | 31 (35.2%) | 62 (42.2%) | 0.53 |
| Right | 199 (48.8%) | 85 (49.1%) | 43 (48.9%) | 71 (48.3%) | |
| Undefined | 45 (11%) | 17 (9.8%) | 14 (15.9%) | 14 (9.5%) | |
| Stroke Type | |||||
| Ischemic | 290 (71.1%) | 121 (69.9%) | 65 (73.9%) | 104 (70.7%) | 0.48 |
| Hemorrhagic | 70 (17.2%) | 34 (19.7%) | 10 (11.4%) | 26 (17.7%) | |
| Uncertain | 48 (11.8%) | 18 (10.4%) | 13 (14.8%) | 17 (11.6%) | |
| Cognition | |||||
| MMSE | 26.1±3.5 (N=368) |
26.1±3.4 (N=151) |
26.2±3.5 (N=82) |
26.0±3.6 (N=135) |
0.91 |
| Trail Making (B - A in seconds) |
134.1±88.5 (N=375) |
127.8±86.6 (N=157) |
134.8±82.3 (N=83) |
141.1±94.1 (N=135) |
0.44 |
| WAIS Digit Symbol Subscale |
31.02±16.98 (N=385) |
32.3±17.8 (N=160) |
31.1±14.8 (N=86) |
29.5±17.3 (N=139) |
0.37 |
| Sensory-Motor | |||||
| FM Total Motor Score | 58.3±25.3 | 62.5±25.0 | 55.0±25.4 | 55.4±25.0 | 0.02 |
| FM LE Motor Score | 24.4±6.4 | 25.3±6.23 | 24.06±6.9 | 23.6±6.3 | 0.05 |
| FM UE Motor Score | 34.0±20.68 (N=407) |
37.4±20.7 (N=172) |
31.0±20.9 (N=88) |
31.8±20.2 (N=147) |
0.02 |
| FM Sensation Score | 19.5±5.9 (N=400) |
20.1±5.4 (N=170) |
19.1±6.0 (N=87) |
19.0±6.4 (N=143) |
0.19 |
| Paresis in dominant hand | 171 | 77 (45.03%) | 33 (19.3%) | 61 (35.67%) | 0.55 |
| ACTIVITY LIMITATIONS | |||||
| Comfortable walking Speed (m/s) |
0.38±0.22 | .42±0.22 | 0.37±0.23 | 0.34±0.21 | <0.01 |
| 6-minute walk distance (m) | 125±78 | 141±77 | 118±82 | 112±75 | <0.01 |
| Berg Balance Score | 35.8±14.0 | 38.9±13.1 | 36.4±14.5 | 31.7±13.8 | <0.01 |
| Use of Assistive Device | 359 (88%) | 144 (83.2%) | 79 (89.8%) | 136 (92.5%) | 0.03 |
| ABC Scale | 45.1±23.9 (N=406) |
49.8±23.8 (N=172) |
44.2±24.9 (N=87) |
40.0±22.5 (N=147) |
<0.01 |
| PARTICIPATION | |||||
| Steps/day | 2552±2570 (N=384) |
2893±2676 (N=163) |
2308±2343 (N=81) |
2295±2540 (N=140) |
0.08 |
| Stroke Impact Scale (Participation Subscale) |
45.51±23.25 | 49.54±23.31 (N=173) |
42.14±21.16 (N=88) |
42.78±23.81 (N=147) |
0.01 |
| Modified Rankin Scale | |||||
| Rankin 0-2 | 56 (13.7%) | 32 (18.5%) | 11 (12.5%) | 13 (8.8%) | <0.01 |
| Rankin 3-4 | 352 (86.3%) | 141 (81.5%) | 77 (87.5%) | 134 (91.2%) | |
| SF-36 Physical Function | 27.8±9.6 (N=406) |
28.4±9.35 (N=171) |
28.1±9.4 (N=88) |
26.8±9.9 (N=147) |
0.33 |
Categorical variables are N(%), continuous variables are mean ± standard deviation.
N=408 unless otherwise specified.
A full table of all items assessed can be accessed online.
PHQ-9=Personal Health Questionnaire Depression Scale; MMSE=Mini Mental State Exam; WAIS=Wechsler Adult Intelligence Scale; FM=Fugl-Meyer; LE=Lower Extremity; UE=Upper Extremity; ABC=Activities Balance Confidence; SF-36 PF=Short-Form-36 Physical Function subsection;
Impact of RCT Physical Therapy Interventions
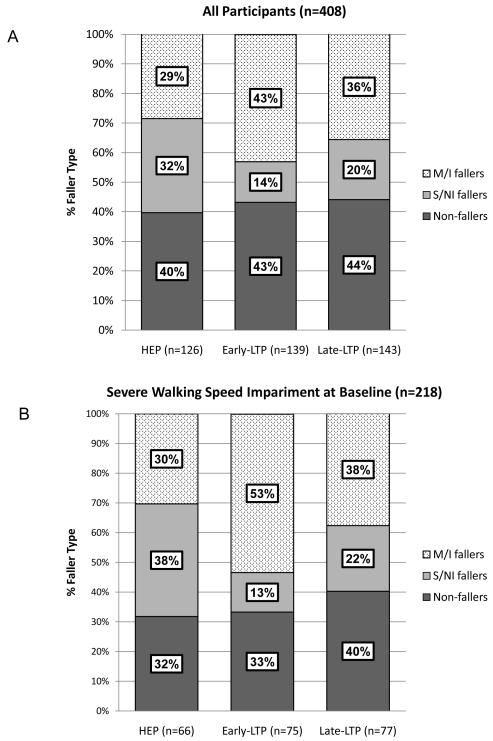
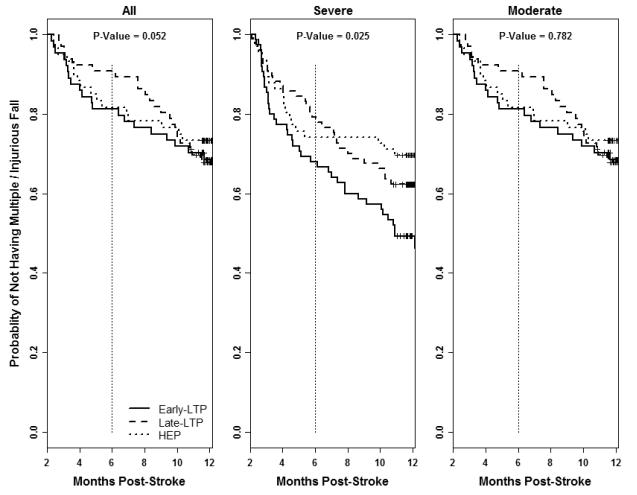
There was no difference across intervention groups (HEP, early-LTP and late-LTP) in overall fall incidence between 2 and 12 months post-stroke. However, significantly more individuals who received early-LTP experienced M/I falls than individuals who received late-LTP or HEP (p=0.047, Figure 1a). The difference in M/I falls across intervention groups is attributable to those with severe walking impairment (<0.4 m/s) in the early-LTP group (Figure 1b). Figure 2 provides log rank test results and Kaplan-Meier estimates for probability of not having M/I falls from randomization to 12-months post-stroke by intervention group and initial walking speed impairment.
Figure 1.
Distribution of participants’ fall outcome category at 12-months post-stroke by RCT intervention arm. Panel A: All participants; Panel B: Participants with severe walking speed impairment at baseline (<0.4 m/s).
Figure 2.
Kaplan-Meier Curves for probability of not having a second or injurious fall between 2-12 months post-stroke by intervention group and initial walking speed impairment.
A secondary analysis at 6-months post-stroke demonstrated that early-LTP and HEP groups’ M/I falls rates were 25.2% and 22.2% respectively compared to 14.0% for the late-LTP group which had received only usual care (p=0.05). However, as reported in a previous analysis15, at 6-months, the late-LTP group was also less mobile (SIS mobility: early-LTP =15.3±21.4, HEP= 14.9±20.0, late-LTP=7.0±15.7) and took almost half as many steps in comparison with early-LTP and HEP (Steps per day: early-LTP=1017, HEP=1357, late-LTP =566).
Predicting Faller Category
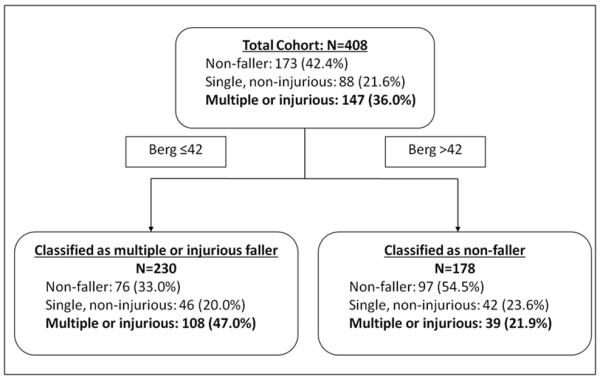
Analysis using the CART method revealed that a BBS score ≤42 at 2-months post-stroke was the single best predictor of M/I falls (Figure 3). Sensitivity and specificity were 73% and 53%, respectively, for the LEAPS cohort, and 78% and 39% with cross validation. Adding variables to the model enhanced prediction accuracy within the LEAPS cohort but had poor generalizability with cross-validation. The association between BBS and fall category was neither confounded nor modified by intervention group assignment.
Figure 3.
Illustration of the Classification and Regression Tree result. The top box shows distribution of the population by fall outcome category. The bottom boxes show distribution by fall outcome when participants are split by Berg Balance Scale score of 42/56.
Discussion
Incidence and Consequences of Falls
The LEAPS study provided a valuable opportunity to evaluate falls in a cohort of individuals receiving interventions to improve walking. The successful prospective capture of falls in this study reveals that even for individuals who are improving mobility, balance, and walking post-stroke15, incidence of falls is high. Well over half of participants fell between 2 and 12 months post-stroke. This high rate of falls is consistent with previous reports.4, 6, 7, 21, 22 Our data also support previous findings that individuals are most likely to fall at home8, 23, 24, and that a large number experience falls from which they are unable to get up independently.4, 23
Impact of RCT Physical Therapy Interventions
The LEAPS RCT assessed the impact of two physical therapy interventions on walking speed. Both LTP and HEP interventions were associated with clinically relevant improvements in walking speed, endurance, functional status, and quality of life in the severity groups categorized with either moderate or severe walking speed impairment.15 Between 2 and 6-months post-stroke, both groups receiving early intervention had a higher fall rate than individuals in the late-LTP group (which had received only usual care at 6 months)—this despite the fact that the early groups had experienced almost twice the improvement in walking and mobility as the late-LTP group.15The process of gaining mobility post-stroke appears to be associated with higher risk for falls. Between 2 and 12-months post-stroke, individuals with severe walking speed impairment in the early-LTP group experienced a significantly higher incidence of M/I falls than those in the HEP group. We are uncertain about the causes of this difference. For those with severe walking speed impairment, locomotor training may have resulted in over-confidence in walking ability and/or perhaps HEP resulted in improved strength or balance.
Characteristics of Fallers and Predicting Faller Category
Our results corroborate others’ findings that fall risk post-stroke is associated with a wide range of characteristics including older age, greater disability, more prevalent use of an assistive device, and reduced balance, motor function, and walking speed.4, 6, 8, 21-25 Unlike some reports24, 26, we did not find an association between falls and cognition or depression. This was probably attributable to the relatively high cognitive function (mean Mini Mental State Exam Score = 26.1±3.5 ) and low incidence of depression (16.4% with PHQ-9 scores >9) in the cohort.15
The BBS emerged as the most robust predictor of M/I falls. Persson and colleagues22 identified the same BBS cutoff score for predicting fallers versus non-fallers in the first year post-stroke. This convergence of findings suggests that the cutoff score of 42 may be a useful tool for identification of both single and M/I falls risk. Post-test probability for M/I falls was 25% for BBS >42 and 42% for BBS ≤42 . The low sensitivity and specificity for predicting M/I falls reflect the multi-factorial causes of falls and suggest that a measure of balance, while useful, cannot independently account for fall risk.3 Additionally, there may be items in the BBS that fail to distinguish between faller groups and detract from its predictive value. Identifying item subsets that are more robust for falls prediction may be useful.
The clinician and patients with stroke face a conundrum: walking may increase risk for M/I falls while not walking will lead to a known plethora of deficits associated with inactivity including recurrent stroke.25 Clearly, there is a need for efficacious interventions that provide concomitant mobility training and fall prevention. For example, multi-risk factor falls prevention programs should include Center for Disease Control recommended home patient and caregiver education, progressive exercise program, medication review and management, vision exam and improvement, and home safety assessment and modification.27 Attention to patient-specific deficits identified by the BBS may also be beneficial.
A strength of this study is that it was prospective and longitudinal with excellent falls capture rate in a well-defined cohort. The methods follow international standards for fall injury prevention trials.17 Additionally, we were able to capture fall rates in association with interventions designed to improve mobility. The primary limitation of this study is the selective nature of the population. The population in this study was highly screened and living in the community; hence, results are not generalizable to a larger stroke population. For example, our cohort did not include non-ambulatory individuals nor individuals walking at speeds associated with community mobility (>0.8 m/s). Likewise, participants had no previous history of stroke or significant cardiac or neurologic co-morbidities and had relatively high cognitive function. Our analysis of risk factors was also limited to those collected for the RCT. Some risk factors (e.g. history of falls and urinary incontinence) identified by others were not collected.
Conclusion
In a highly screened and selective population, individuals with stroke may improve walking and mobility but they remain at high risk for falls. Among those with severe impairment, participation in early-LTP appeared to increase M/I falls risk. The BBS is useful for informing fall risk but has limitations related to the multifactorial nature of the problem. Fall prevention should focus on risk at home in the early months post-stroke and should include multifactorial risk assessment and management concomitant with exercise interventions to improve walking and mobility.
Supplementary Material
Acknowledgements
Sources of Funding This work was supported by funding from National Institute of Neurological Disorders and Stroke and the National Center for Medical Rehabilitation Research (RO1 NS050506). Trial registration: NCT0024391.
Footnotes
Clinical Trial Registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00243919
LEAPS Investigators include: Duke University Administrative Coordinating Center: Pamela Duncan, PT, PhD, FAPTA, FAHA; Sarah Hayden; Mysha Sissine; Quishi Feng, PhD; Brooks Rehabilitation Hospital, Jacksonville, FL: Deborah Stewart, MD; Trevor Paris, MD; Joann Gllichio, PT, DSc; Florida Hospital, Orlando, FL: Mitchell Freed, MD; Michelle Dolske, PhD; Craig Moore, PT; Bettina Brutsch, PT; Long Beach Memorial Hospital, Long Beach, CA; H. Richard Adams, MD; Diehma Hoang, MD; Anita Correa, PT; Sharp Rehabilitation Center, San Diego, CA; Jerome Stenehjem, MD; Roxanne Hon, MD; Molly McLeod, PT; University of Southern California: David Alexander, MD, UCLA Medical Center; Julie Hershberg, DPT; Samneang Ith-Chang, DPT; Official Coordinating Center - University of Florida: Andrea L. Behrman, PT, PhD, FAPTA; Dorian K. Rose, PT, PhD; Clinical Coordinating Center – University of Southern California: Julie K. Tilson, DPT, MS; Data management and Analysis Center - University of Southern California: Steven Cen, PhD; Chris Han, MS; James Gardner; University of Florida, Gainesville, FL: Yunfeng Dai, MS; Xiaomin Lu, PhD; Consultants: Anatole D. Martin, PhD, University of Florida; Richard Schofield, MD, University of Florida; Steering Committee: Pamela Duncan, PT, PhD, FAPTA, FAHA; Andrea L. Behrman, PT, PhD, FAPTA; Stanley P. Azen, PhD, University of Southern California; Samuel S Wu, PhD, University of Southern California; Bruce H. Dobkin, MD, University of California, Los Angeles; Stephen Nadeau, MD, University of Florida; Sarah K. Hayden, Duke University.
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: A report from the american heart association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davenport RJ, Dennis MS, Wellwood I, Warlow CP. Complications after acute stroke. Stroke. 1996;27:415–420. doi: 10.1161/01.str.27.3.415. [DOI] [PubMed] [Google Scholar]
- 3.Weerdesteyn V, de Niet M, van Duijnhoven HJ, Geurts AC. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45:1195–1213. [PubMed] [Google Scholar]
- 4.Forster A, Young J. Incidence and consequences of falls due to stroke: A systematic inquiry. BMJ. 1995;311:83–86. doi: 10.1136/bmj.311.6997.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pouwels S, Lalmohamed A, Leufkens B, de Boer A, Cooper C, van Staa T, et al. Risk of hip/femur fracture after stroke: A population-based case-control study. Stroke. 2009;40:3281–3285. doi: 10.1161/STROKEAHA.109.554055. [DOI] [PubMed] [Google Scholar]
- 6.Andersson AG, Kamwendo K, Seiger A, Appelros P. How to identify potential fallers in a stroke unit: Validity indexes of 4 test methods. J Rehabil Med. 2006;38:186–191. doi: 10.1080/16501970500478023. [DOI] [PubMed] [Google Scholar]
- 7.Ashburn A, Hyndman D, Pickering R, Yardley L, Harris S. Predicting people with stroke at risk of falls. Age Ageing. 2008;37:270–276. doi: 10.1093/ageing/afn066. [DOI] [PubMed] [Google Scholar]
- 8.Hyndman D, Ashburn A, Stack E. Fall events among people with stroke living in the community: Circumstances of falls and characteristics of fallers. Arch Phys Med Rehabil. 2002;83:165–170. doi: 10.1053/apmr.2002.28030. [DOI] [PubMed] [Google Scholar]
- 9.Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: A prospective study. J Gerontol. 1991;46:M164–170. doi: 10.1093/geronj/46.5.m164. [DOI] [PubMed] [Google Scholar]
- 10.Sherrington C, Whitney JC, Lord SR, Herbert RD, Cumming RG, Close JC. Effective exercise for the prevention of falls: A systematic review and meta-analysis. J Am Geriatr Soc. 2008;56:2234–2243. doi: 10.1111/j.1532-5415.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 11.Ada L, Dean CM, Morris ME, Simpson JM, Katrak P. Randomized trial of treadmill walking with body weight support to establish walking in subacute stroke: The mobilise trial. Stroke. 2010;41:1237–1242. doi: 10.1161/STROKEAHA.109.569483. [DOI] [PubMed] [Google Scholar]
- 12.Bernhardt J, Dewey H, Thrift A, Collier J, Donnan G. A very early rehabilitation trial for stroke (avert): Phase ii safety and feasibility. Stroke. 2008;39:390–396. doi: 10.1161/STROKEAHA.107.492363. [DOI] [PubMed] [Google Scholar]
- 13.Boysen G, Krarup LH, Zeng X, Oskedra A, Kõrv J, Andersen G, et al. Exstroke pilot trial of the effect of repeated instructions to improve physical activity after ischaemic stroke: A multinational randomised controlled clinical trial. BMJ. 2009;339:b2810. doi: 10.1136/bmj.b2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang MY, Eng JJ, Dawson AS, McKay HA, Harris JE. A community-based fitness and mobility exercise program for older adults with chronic stroke: A randomized, controlled trial. J Am Geriatr Soc. 2005;53:1667–1674. doi: 10.1111/j.1532-5415.2005.53521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364:2026–2036. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Protocol for the locomotor experience applied post-stroke (LEAPS) trial: A randomized controlled trial. BMC Neurol. 2007;7:39. doi: 10.1186/1471-2377-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamb SE, Jørstad-Stein EC, Hauer K, Becker C, Group PoFNEaOC Development of a common outcome data set for fall injury prevention trials: The prevention of falls network europe consensus. J Am Geriatr Soc. 2005;53:1618–1622. doi: 10.1111/j.1532-5415.2005.53455.x. [DOI] [PubMed] [Google Scholar]
- 18.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees (The Wadsworth Statistics/Probability Series) Chapman and Hall; New York, NY: 1984. pp. 1–358. [Google Scholar]
- 19.Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke. 1988;19:1497–1500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]
- 20.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 21.Mackintosh SF, Hill KD, Dodd KJ, Goldie PA, Culham EG. Balance score and a history of falls in hospital predict recurrent falls in the 6 months following stroke rehabilitation. Arch Phys Med Rehabil. 2006;87:1583–1589. doi: 10.1016/j.apmr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Persson CU, Hansson PO, Sunnerhagen KS. Clinical tests performed in acute stroke identify the risk of falling during the first year: Postural stroke study in gothenburg (postgot) J Rehabil Med. 2011;43:348–353. doi: 10.2340/16501977-0677. [DOI] [PubMed] [Google Scholar]
- 23.Yates JS, Lai SM, Duncan PW, Studenski S. Falls in community-dwelling stroke survivors: An accumulated impairments model. J Rehabil Res Dev. 2002;39:385–394. [PubMed] [Google Scholar]
- 24.Kerse N, Parag V, Feigin VL, McNaughton H, Hackett ML, Bennett DA, et al. Falls after stroke: Results from the auckland regional community stroke (arcos) study, 2002 to 2003. Stroke. 2008;39:1890–1893. doi: 10.1161/STROKEAHA.107.509885. [DOI] [PubMed] [Google Scholar]
- 25.Divani AA, Vazquez G, Barrett AM, Asadollahi M, Luft AR. Risk factors associated with injury attributable to falling among elderly population with history of stroke. Stroke. 2009;40:3286–3292. doi: 10.1161/STROKEAHA.109.559195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jørgensen L, Engstad T, Jacobsen BK. Higher incidence of falls in long-term stroke survivors than in population controls: Depressive symptoms predict falls after stroke. Stroke. 2002;33:542–547. doi: 10.1161/hs0202.102375. [DOI] [PubMed] [Google Scholar]
- 27.National Center for Injury Prevention and Control . Preventing Falls: How to Develop Community-based Fall Prevention Programs for Older Adults. Centers for Disease Control and Prevention; Atlanta, GA: 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.