Abstract
Basic research and clinical studies have implicated a role for hyperuricemia and for xanthine oxidoreductase (XOR), the enzyme that generates uric acid (UA), in not only gout but also vascular diseases. At present, asymptomatic hyperuricemia (i.e., in the absence of gout, urate nephrolithiasis, or tumor lysis syndrome) is not an indication for therapy. With the rise over the past several decades in prevalence of both gout and hyperuricemia, clarifying the potential adverse effects of hyperuricemia (in patients with and without gout) is of public health importance. UA is not simply an inert end-product of purine metabolism in humans, but rather has potential antioxidant, pro-oxidant, and pro-inflammatory effects. However controversy remains as to which, if any, of these effects are of clinical relevance in development and complications of human vascular diseases in gout and asymptomatic hyperuricemia. Clearly, not all individuals with hyperuricemia develop gout, and studies to date have also been unable to clarify in which subjects hyperuricemia may have detrimental effects on the vasculature. Further, studies of urate-lowering therapy with XOR inhibition or uricosuric agents have not been able to definitively identify whether any such effects may be mediated by UA versus XO. Adequately sized, prospective randomized clinical trials of sufficient duration, and employing appropriate biomarkers, now appear critical to resolve the putative toxic roles of UA and XO in the human arterial circulation.
Keywords: urate, uric acid, hypertension, endothelium, smooth muscle cell, nitric oxide, myeloperoxidase, xanthine oxidase, allopurinol, atherosclerosis
Introduction
In addition to its critical role in gout, hyperuricemia is increasingly being considered a potential pathogenic factor for hypertension, metabolic syndrome and type 2 diabetes, and renal disease, as well as atherosclerosis, and several adverse consequences of vascular disease (stroke, myocardial infarction, and cardiovascular death).1, 2 Uric acid (UA) is generated via the action, in the purine degradation pathway, of both the oxidized and reduced forms of xanthine oxidoreductase (XOR) (known as xanthine oxidase (XO), and xanthine dehydrogenase (XDH), respectively) on hypoxanthine and xanthine.3-6 In addition to UA itself, increasing evidence implicates the oxidized form XO in vascular diseases.3, 7 This is noteworthy, given the role of XOR inhibition as first-line urate-lowering pharmacologic therapy in gout.8
At physiologic pH, UA exists as its ionized salt, monosodium urate. Humans and higher primates have higher serum urate levels than other mammals, due to loss in hominid evolution of expression of an active form of the hepatic peroxisomal enzyme uricase (uric acid oxidase).9 Uricase catalyzes breakdown of UA to 5-hydroxyisourate (5-HIU). It has been reported that 5-HIU is subject to hydrolysis by transthyretin-related protein10 and degradation to allantoin in humans and higher primates; this is in contrast to more rapid, hepatic intracellular enzymatic chain of UA degradation to 5-HIU by uricase, and of 5-HIU to allantoin by two other enzymes, in lower mammals.9
The limited solubility of urate predisposes to deposition of monosodium urate crystals, and thereby the development of gout. Hyperuricemia (as defined by a single reading over 7 mg/dL) was estimated to exist in ~21.4% of adults in the United States in 2007-8, with the mean serum urate in USA adults continuing to rise over the last few decades, and attaining an estimated level of close to 5.5 mg/dL.11 The duration and level of hyperuricemia directly correlate with the risk of development of gout, and begins to accelerate when serum urate rises above 8 mg/dL and rises robustly in those with serum urate greater than 9 mg/dL.12 In 2007-8, a self-reported diagnosis of gout was estimated to be present in ~8.3 million Americans (~3.9% of all adults (defined as age 20 and older), and ~5.9% of male, and ~2% of female adults).11 Importantly, gout prevalence is substantially higher in the aged population subset, a group disproportionately affected by vascular disease.13
Since a minority of individuals with sustained hyperuricemia go on to develop gout as a clinical disease, asymptomatic hyperuricemia is neither considered a disease state nor is an accepted indication for urate-lowering treatment. Reflecting this, serum urate levels are not part of the routine metabolic panel of blood tests, and asymptomatic hyperuricemia is not presently considered an issue for preventive clinical care. However, as reviewed here, asymptomatic hyperuricemia, and hyperuricemia in gout patients, from a vascular biology point of view alone, may not be benign. Further, how to identify those at risk for developing adverse sequelae of hyperuricemia, whether it is gout or vascular disease, is not yet clear.
RECENT CHANGES IN THINKING ABOUT UA AND XO ACTIONS PERTINENT TO VASCULAR DISEASE
Table 1 summarizes recent frame shifts in thinking on the roles of soluble urate and of XO in vascular disease, discussed in this review.
Table 1.
Major Hypotheses From New Knowledge of UA, Hyperuricemia, and XO to be Resolved in Human Clinical Investigation of Gout and Vascular Disease
|
Recent research advances, principally from in vitro and animal models |
Specific Hypotheses Suggested for Testing in Well-Controlled, Prospective Human Clinical Studies and Trials In Patients with and without Gout |
|---|---|
|
|
UA is not an inert end-product of purine metabolism in humans
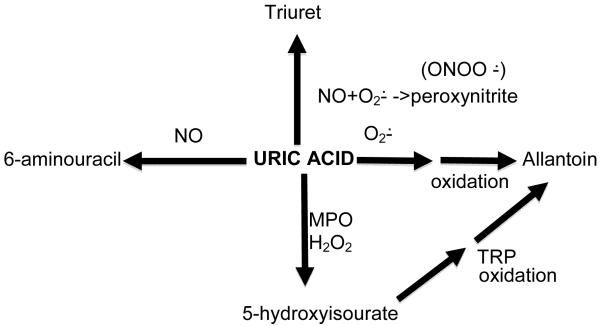
Clearly gout attacks would not occur were UA to simply be an inert endproduct of purine metabolism in humans subject to simple disposition triggered via excretion unchanged into the urine and small intestine. Of relevance for the vasculature, UA interfaces with superoxide, the major vasodilator nitric oxide (NO),14, 15 the potent long-lived NO-derived oxidant peroxynitrite,16 and with myeloperoxidase (MPO)17 (Figure 1). UA-derived endproducts of these reactions, including oxidative catabolites (6-aminouracil, allantoin, triuret), are demonstrable in human urine.18 Whether there are biologically significant activities of these catabolites in hyperuricemic humans remains unclear, but they do have the potential to be used as biomarkers for the interface of oxidative stress with UA. For example, MPO potentially links the neutrophil activation-mediated pathology of gouty arthritis, and “spillover” to systemic inflammation in gout, including leukocytosis, with vascular pathology.
Figure 1.
Uric acid (UA) is not an inert endproduct of purine catabolism, and interacts with other molecules in alternative pathways of degradation that can modulate oxidative stress, as illustrated here and discussed in the text.
Abbreviations:
ONOO− = peroxynitrite
O2− = superoxide anion
MPO = myeloperoxidase
NO = nitric oxide
H2O2 = hydrogen peroxide
TRP = transthyretin-related protein
MPO is expressed by neutrophils and monocytes in azurophil granules and lysosomes, respectively, and is principally secreted by activated neutrophils.17 MPO-catalyzed UA oxidation, in the presence of hydrogen peroxide, generates 5-hydroxyisourate, urate radical and hydroperoxide.17 UA enhances MPO-dependent consumption of NO, and in the presence of UA, MPO accelerates the oxidation of the critical intracellular antioxidant glutathione (GSH) to oxidized glutathione (GSSG).17 MPO exerts multiple pro-inflammatory effects and impairs anti-inflammatory function of HDL, and is a promoter and biomarker of atherosclerotic plaque instability and rupture.19 Neutrophil and monocyte adhesion and activation occurs at sites of hypoxic injury to arteries, and therapeutic arterial neovascularization. Hence, the potential for MPO effects on UA to affect endothelial cell homeostasis and blood pressure, as schematized in Figure 2, warrants assessment in clinical studies, and particularly in those with acute and chronic gouty arthritis.
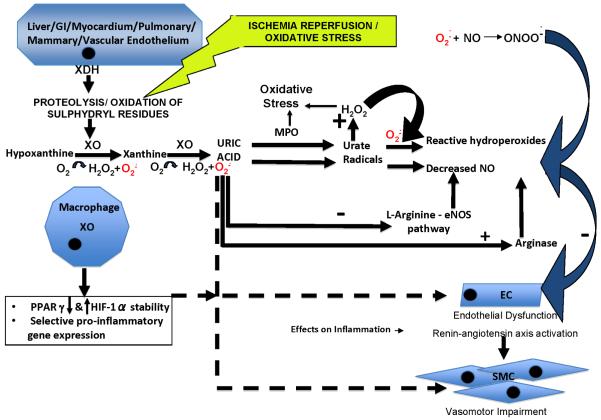
Figure 2.
Model for UA and XO interactions with vascular cells that affect oxidative stress and vascular pathophysiology (see also text and Table 1). In this model, UA turns on “inflammatory”, cytotoxic, and dysfunctional responses, including up-regulation of the renin-angiotensin system in cultured ECs, and arterial SMC proliferation and migration. These effects are mediated by UA-induced oxidative stress in ECs, scavenging of NO and induction of EC arginase that reduces production of vasodilatory NO. There are additional adverse consequences for cell redox status and NO levels of oxidative degradation of UA (in the presence of peroxide) by neutrophil-derived MPO. Soluble UA-induced promotion of NO degradation by oxidation and effects on arginase expression are illustrated, as are adverse effects of peroxynitrite whose oxidant effects are inhibited by UA. In this model, XO expression is increased in macrophages, and on EC surfaces by inflammatory conditions (e.g., gouty arthritis) and ischemia. Moreover, XO promotes oxidative stress in ECs, and impairs endothelial function independent of UA generation. XO and UA also stimulate macrophage-mediated inflammation in the artery wall. Not depicted here, but discussed in the text, are potential effects of XOR inhibition on accumulation of upstream precursors such as inosine and adenosine that have anti-inflammatory properties.
Abbreviations:
ONOO− = peroxynitrite
O2− = superoxide anion
MPO = myeloperoxidase
NO = nitric oxide
H2O2 = hydrogen peroxide
GI = gastrointestinal
XDH = xanthine dehydrogenase
XO = xanthine oxidase
PPARγ = peroxisome proliferator-activated receptor gamma
HIF-1α = hypoxia inducible factor alpha
EC = endothelial cell
SMC = smooth muscle cell
eNOS = endothelial nitric oxide synthase
UA is both an antioxidant and a pro-oxidant
UA, like other compounds with labile electrons and a redox potential, can act as pro- or anti-oxidant, dependent on its redox potential relative to the donor or acceptor substrates undergoing oxidation or reduction. Hence, the simultaneous pro- and anti-oxidant actions of UA, described in numerous and contradictory studies20-34 have not been particularly informative. For example, administration of intravenous UA systemically in small controlled studies of healthy volunteers improved the serum antioxidant capacity at rest32 and reduced exercise-induced oxidative stress.30 However, UA infusion had no acute effects, positive or negative, on endothelial function in another study.33
UA-derived free radicals include urate anion and aminocarbonyl which is formed by interaction with peroxynitrite.27 In this context, a clinical study of hyperuricemic gout patients treated with the recombinant PEGylated uricase pegloticase, which leads to marked lowering of serum urate, promoted a trend for decrease in levels of some plasma oxidative stress markers, but the effect did not reach statistical significance.35
UA indirectly and directly regulate vascular cell functions in vitro, with apparent consequences in vivo
Evidence, from in vitro and in vivo studies, performed over the last decade has presented a cohesive model on how UA can act in arterial pathophysiology.1, 2 In this paradigm, originating largely from an impressive body of in vitro and in vivo work by Richard J. Johnson and colleagues, UA turns on “inflammatory”, cytotoxic, and dysfunctional responses (including up-regulation of the angiotensin system) in cultured endothelial cells (ECs) and proliferation and migration of arterial smooth muscle cells (SMCs) (Figure 2).1, 2, 36-42 UA-induced oxidative stress in ECs, and scavenging of NO and induction of EC arginase that reduces NO production, form part of this model,14, 15 and triggering of activation of the renin-angiotensin-aldosterone axis has been implicated in some studies.1, 2, 36 Many of the in vitro findings in this model, including NO depletion43 and activation of the renin-angiotensin-aldosterone axis, are complemented by in vivo studies in rats fed with a uricase inhibitor, oxonic acid, which produces increase in the basal low level of serum urate of ~1 mg/dL to sustained levels of 2-3 mg/dL, and likely, even higher, transitory spikes in levels of serum urate.1, 2, 40
Oxonic acid-induced hyperuricemia in rats is associated with worsening of renal function triggered by a variety of insults, including cisplatin and cyclosporine administration.1, 2, 40, 44, 45 In a translational context, recent, small, randomized human clinical trials evaluating efficacy of XOR inhibition in limiting renal progression have been positive,46-48 supporting a potential role for urate-lowering as a therapeutic target in human renal disease.
A prominent feature of the oxonic acid-induced hyperuricemic rat model is hypertension, with afferent glomerular arteriolopathy, which is reversed by XOR inhibitor and uricosuric treatments that resolve the hyperuricemia.1, 2, 40 Moreover, in this rat model, inhibition of angiotensin signaling by losartan inhibits the hypertension and renal pathology, whereas diuretic therapy that resolves the hypertension, but not the elevated serum urate, does not eliminate the renal vascular pathology.40 These findings mirror findings in large-scale human randomized trials. In the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study, approximately a third of the improved cardiovascular mortality was attributed to an independent effect on serum urate levels in those who received losartan, a drug with uricosuric effects, as compared with those who received atenolol, a beta-blocker with no such effects.49 On the other hand, in the Systolic Hypertension in the Elderly (SHEP) trial, those with appropriately-controlled hypertension on a thiazide diuretic but who concomitantly also had an increase in their serum urate levels failed to demonstrate a cardiovascular benefit compared with placebo.50 Recently, a randomized placebo-controlled crossover trial of allopurinol vs. placebo was conducted among hyperuricemic adolescents with hypertension, demonstrating significant efficacy of allopurinol.51 Because these effects may be related to XOR inhibition rather than urate lowering, results from trials using uricourics are awaited. A pathogenic role of hyperuricemia in hypertension in humans, and therapeutic use of urate-lowering in hypertension, merits further investigation.
An entire field linking fructose intake and metabolism to uric acid biology has recently emerged. It has been proposed that fructose intake, linked with hepatic ATP depletion that promotes uric acid generation and the development of hyperuricemia, influences the development of various features of the metabolic syndrome. This includes nonalcoholic hepatosteatosis “fatty liver” putatively mediated in part by hepatic ATP depletion promoted by dysregulated intrahepatic fructose metabolism.52 Furthermore, the metabolic syndrome, a frequent comorbidity among persons with gout, clearly influences cardiovascular disease susceptibility and pathogenesis by multiple direct and indirect effects of insulin resistance on the vasculature. The topic of fructose metabolism, hyperuricemia and the metabolic syndrome is beyond the scope of this review, and is addressed in depth elsewhere.53
Critical appraisal of data for potential vascular toxicity of UA
One caution with interpreting the oxonic acid-induced hyperuricemia rat model is that oxonate alone can promote inflammatory differentiation of cultured macrophage lineage cells.54 Moreover, oxonate potentially modulates renal transport of UA, and thereby could affect intracellular handling and effects of UA in renal proximal tubular cells.55 Hence, some mechanisms of renal disease in the in vivo model of oxonic acid-induced hyperuricemia remain to be resolved; ideally, an alternative model of hyperuricemia would help in this task. In this context, as discussed above, intravenous infusions of urate either did not appear to impair forearm blood flow in healthy human adults, or in fact seemed to improve endothelial function.30-33 This indicates that, at least acutely in these small studies, urate does not worsen endothelial function in humans. Moreover, short duration urate lowering using a single dose of intravenous uricase was neutral on endothelial function in a small (n=10), randomized, single blind, placebo-controlled crossover study in human type II diabetics.56 Whether long-term, sustained elevations in UA result in different effects can not be discerned from these studies.
Not only cultured arterial cells, but also macrophage lineage cells,54 mesangial cells and adipocytes have also been observed to respond to soluble UA in the hyperuricemic range.27, 57 As such, the reported ability of soluble UA to induce oxidative stress, inflammatory responses such as NF-κB and mitogen-activated protein kinase activation and chemokine expression in cells has huge potential ramifications if valid. As one example, hyperuricemia in humans has been suggested to promote heightened ex vivo pro-inflammatory responses of neutrophils.58 However, the proposal that hyperuricemia predisposes to gouty arthritis by inflammatory effects beyond promotion of crystalline monosodium urate crystal formation remains provocative, and gout could primarily be a signal of heightened inflammatory responses of phagocytes.
Collectively, results of studies of high ambient UA in cell culture, and of oxonic acid treated rats, cannot be simply extrapolated to humans. Indeed, human randomized trials of therapeutic agents with uricosuric effects such as sulfinpyrazone and estrogen have been conflicting in regards to cardiovascular outcomes.59-63 Importantly, trials of the XOR inhibitor febuxostat have not demonstrated a cardiovascular benefit. In fact, an increase in cardiovascular events (though not statistically significant) in those who took febuxostat compared with allopurinol in the clinical trials program necessitated further study into febuxostat’s cardiovascular safety.64-67 Potential beneficial effects of serum urate-lowering on vascular function have the potential to be outweighed by effects of increased flares of gout, which are well-documented to occur with more intense serum urate-lowering regimens and can induce systemic inflammation, with release of pro-atherogenic cytokines (e.g., Il-1β, TNFα, IL-6, IL-8) and other mediators. Further, in the LIFE trial described above, losartan may have other beneficial effects beyond those of beta-blockers, and in the SHEP trial, mitigation of beneficial effects for stroke or “any cardiovascular event” was not demonstrated, raising concerns about a false positive result. Thus, it is not clear based on these collective human trials as to whether UA and/or arthritis-related inflammation are playing a pathogenic role, or rather that serum urate level is a biomarker or epiphenomenon.
Acceptance of the aforementioned results for soluble UA effects on vascular cells in vitro also assumes that inflammatory artifacts of submicroscopic, cell-modulated urate crystallization have been adequately excluded, particularly when assessing results using UA concentrations well above 7 mg/dL. Vascular cell uptake of UA has been reported, and linkage with intracellular UA oxidation is plausible and has translational relevance, since vascular cells can express urate anion transporters, and uricosurics inhibit the capacity of UA to activate vascular cells.38, 41 One can argue that uptake kinetics of UA by cultured ECs and SMCs in studies published to date do not appear robust,38, 39, 41 and uricosurics have nonselective effects on ion transport and cell physiology. However, extracellular (and indirect) effects of UA on NO and on oxidative stress are likely sufficient to drive certain “inflammatory” effects of high levels of UA on vascular cells.
Our appraisal is that host factors, such as intrinsic inflammation and/or oxidative stress in gout, CHF, metabolic syndrome, diabetes, and chronic kidney disease, are likely major determinants of whether high levels of soluble UA are benign or promote pathology without crystallization. One such host factor to consider in this context is activity of the XO form of XOR.
XO, Vascular Oxidative Stress, and Inflammation
Irrespective of its role as an anti-oxidant, pro-oxidant or both, the nature of localized vascular urate production (by XO vs. XDH) could be more relevant than systemic serum urate level, which reflects multiple influences on uric acid production and elimination. This may particularly be the case in atherosclerotic plaques, where both substantial concentrations of UA and allantoin have been found.68-70 This can be considered analogous to local microenvironment UA concentrations within joints affected by gout or areas of tophaceous deposits.
Local UA levels are influenced by XOR activity. XOR is widely distributed throughout various human organs including the liver, gut, lung, kidney, heart, brain,3 with highest levels in gut and the liver.5 In myocardium, it is localized to the capillary endothelial cells.7 Mammalian XOR is present in vivo as the dehydrogenase form (XDH), but is easily converted to XOR by oxidation of the sulphydryl residues or by proteolysis.3 Although XDH has a much greater affinity for NAD+ compared to oxygen (and therefore is practically incapable of directly producing ROS), both XOR and XDH can oxidize NADH, which results in ROS formation.6 Fully reduced XO contains six electrons and its re-oxidation involves electron transfer to oxygen molecules which generates two H2O2 and two O2− species71 for every fully reduced XO molecule. XDH can theoretically produce more O2− per mole of oxygen during NADH oxidization than XOR. However, studies using rat liver indicate that the rate of reaction is very slow (25% of XO Vmax).72 Unlike XDH, XOR has very little reactivity with NADH.73
Regulation and functions of XO
Variability in human XOR expression can be several fold, and on average, expression is 20% higher in men than women.74 Although basal expression of XOR is low in humans, hypoxia, ischemia-reperfusion, IL-1, IL-6, TNFα, LPS as well as corticosteroid treatment can increase XOR transcription.4 XDH conversion to XO is also accelerated in hypoxia.75 XO is significantly elevated in a variety of conditions including limb ischemia,76 major surgery,77 coronary artery disease(CAD),78 and heart failure.79 XO is also up-regulated in Chronic Obstructive Pulmonary Disease (COPD),80 and by tobacco smoke in pulmonary artery endothelial cells.81 NO is an endogenous suppressor of XO;3 therefore, reduced tonic NO suppression of XO promotes oxidative stress and endothelial dysfunction.82
Circulating XO binds to glycosaminoglycans on the surface of endothelial cells, where it can acquire modified kinetics (higher Km and Ki, oxidant producing capacity, and increased stability).83 This form of circulating and depositing XO appears to be more important in the pathogenesis of endothelial injury, compared with XO constitutively produced from endothelial cells.84
XO effects on both EC function and inflammation appear substantial. For example, when infused acutely, XO produced a decrease in cardiac contractility, cardiac index and left ventricular systolic pressure in anesthetized dogs.85 XOR and UA have both been implicated in evolution of innate immune inflammatory responses.86, 87 XOR expression is inducible by macrophage differentiation, the chemokine MCP-1, and Th1 cytokines in monocyte-macrophage lineage cells.54 XOR promotes inflammatory differentiation, caspase-1 activation, IL-1β release, and chemokine expression in these cells, partly mediated by effects on HIF-1α and on PPARγ SUMOylation.54, 88 Interestingly, forced expression of XOR, as well as exogenous UA and oxonate appear to suppress alternative, anti-inflammatory M2 macrophage differentiation.54
Allopurinol as a “probe” into noxious XO activity in arteries
Allopurinol has been demonstrated, in several studies, to improve endothelial function in humans, including two placebo-controlled crossover trials in CHF,89, 90 and a small RCT in type 2 diabetics with mild hypertension.91 In an experimental murine myocardial infarction model, in which myocardial XO increased, allopurinol significantly attenuated LV dilatation, hypertrophy, fibrosis and dysfunction.92 Allopurinol in combination with vitamins C and E appeared beneficial in post coronary artery bypass surgery, where reduced ischemic events and less ST segment depression were noted with this regimen.93 Moreover, 600 mg/day of allopurinol significantly improved endothelial function as well as indices of vascular stiffness (measured by pulse wave analysis) in optimally treated patients with coronary artery disease (CAD).94 Allopurinol 600 mg/day also significantly increased time to chest pain and ST segment changes in CAD patients undergoing exercise ECG testing compared with placebo.95
Other studies have demonstrated conflicting results. Oxypurinol administration (600mg/d) improved left ventricular ejection fraction in a post-hoc analysis of only a select subset whose baseline EF was <40% in a small RCT,96 and in another large RCT in CHF, no benefit was seen except for those whose serum urate levels were above 9.5 mg/dL.97 Direct infusion of oxypurinol improved endothelial function in persons with hypercholesterolemia, but not in those with hypertension.98 Exercise capacity has also been evaluated as endpoints in RCTs, with high-dose (600mg/d) allopurinol being associated with improved exercise capacity in unstable angina,95 while 300mg/d did not improve exercise capacity in CHF.99 Whether some of these differences are related to dose, formulation (allopurinol versus oxypurinol), or disease physiology, let alone XO or UA, is not clear.
It is also not clear if positive effects of XO inhibition on vascular function are mediated by “oxygen-sparing” effects (due to reduced consumption of molecular oxygen by XO during periods of ischemia), a purine salvage mechanism due to reduced hypoxanthine breakdown, or other mechanisms. A direct effect of lower circulating UA levels as an explanation is difficult to support, as the relationship of allopurinol to improved endothelial function has not consistently been associated with the extent of urate lowering.90 Moreover, the uricosurics probenecid90 and benzobromarone100 were neutral on endothelial function in studies of CHF. Compelling effects of XO (and allopurinol) on myocardial oxygen consumption and mechanoergetics are being actively investigated.101-108 However, certain clinical studies in CHF, that have actively lowered urate, failed to demonstrate improvements either in NYHA class, as in the Oxypurinol Therapy in Chronic Heart Failure (OPT-CHF) trial 109, 110 or in other measures of clinical improvement such as the 6-minute walk test99. In contrast to these studies of urate-lowering, direct UA infusion has also been studied as discussed above for its potential beneficial endothelial effects with conflicting results (either positive or neutral).
Currently, any evidence for beneficial effects of allopurinol in vascular disease need to be interpreted as potentially reflective of effects on XOR, UA levels, nonspecific drug effects on pyrimidine metabolism, or all these. Allopurinol also has some direct antioxidant effects,111-114 though this may only be physiologically significant at high doses.115, 116 Last, dosages of allopurinol that impact on endothelial dysfunction may not be the same as those needed to potentially modulate inflammation or simply reduce UA. In this context, a statin, but not allopurinol 300 mg daily, significantly suppressed circulating MPO in a small clinical study.117 Lastly, effects of XOR inhibition on accumulation of upstream precursors such as inosine and adenosine118-120 have also been proposed to contribute to beneficial effects of XOR inhibition in models of vascular disease and in pain. Adenosine has anti-inflammatory properties and protects endothelial cells from leuckocyte-mediated injury, and inosine suppresses certain phagocyte functions and inhibits experimental inflammation.121-124
Next steps needed in understanding the role of UA and XO in vascular disease
Despite the substantial body of evidence regarding the role of UA and XO in vascular disease summarized herein, this review emphasizes the conflicting results on effects of UA and XO in human vascular biology. We have underlined why speculation remains regarding direct roles of UA or XO, or both (or neither) human vascular pathology. In brief, numerous observational epidemiologic studies have examined the association of serum urate levels with cardiovascular endpoints and proxies for those endpoints, with conflicting results. Moreover, some studies have demonstrated neutral or even positive effects of direct UA infusion, while others support a negative effect of elevated UA and/or XO in humans.
Several methodologic challenges limit our ability to make definitive conclusions from these studies. It could be argued that there is sufficient clinical equipoise to justify a large-scale clinical trial to evaluate these effects. It would not be feasible to infuse UA in such a setting, but rather, with the bulk of the evidence supporting a potential negative effect of UA or XO, effects of lowering UA and of inhibiting XOR need to be tested. A definitive evaluation is needed to disentangle the effects of UA lowering from that of XOR inhibition. This can only be achieved by testing mechanisms of lowering UA that do not rely solely on XOR inhibition, such as with use of uricosuric agents. A few small studies have provided intriguing preliminary data to suggest that it is XOR inhibition rather than UA lowering that is conferring benefit, but this needs to be rigorously studied in large RCTs. Should high-quality RCT(s) demonstrate a beneficial effect of XOR inhibition rather than simply the lowering of UA itself, UA would still play a role in clinical management of vascular disease, as a biomarker for effective XOR inhibition. The need for such trials to definitively answer these questions needs to be balanced with the potential costs and adverse effects of these agents.
Does the lack of evidence of a protective cardiovascular effect from febuxostat trials in gout dampen the enthusiasm for testing the utility of either UA lowering or XOR inhibition in management of vascular disease? We would argue that it does not. These trials did not have sufficient power or durations needed to detect differences in cardiovascular outcomes. Additionally, it could be argued that gouty arthritis patients may not be an optimal model in which to assess these effects. The inflammatory nature of early urate-lowering therapy-induced exacerbation of gouty arthritis may outweigh any potential benefit conferred by UA lowering or XOR inhibition, and might be clouded by use of certain types of gout attack prophylaxis and treatment, including use of NSAIDs and corticosteroids. Such effects of acute gouty arthritis could be analogous to the capacity of infectious disease stimuli to trigger vascular events.125 Nonetheless, evaluation of UA lowering by XOR inhibition and uricosurics is warranted in a population of persons at risk for vascular events, which includes those with gout. With a sufficiently powered trial of long enough duration, the theoretical confounding inflammatory effects of gout (which have not yet been established as a risk factor for CV events), and effects of NSAIDs on inflammation may not be an issue.
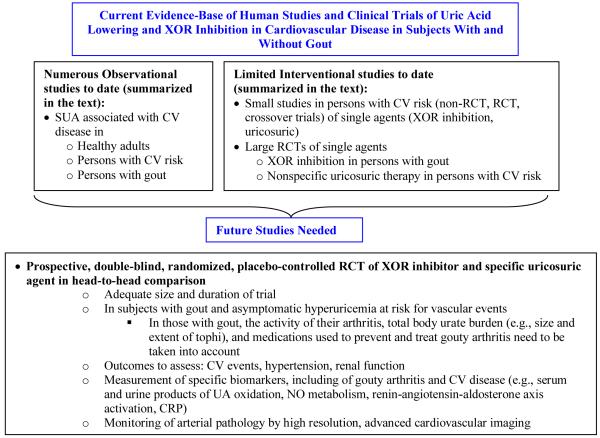
In addition to vascular outcomes, appropriately sized trials with the outcome of hypertension would also be useful. Lastly, there will need to be particular attention to assay of specific biomarkers that may help shed light on pathophysiologic mechanisms by which UA-lowering agents, with and without modulation of gout inflammation, may be exerting their effects on vascular disease, including UA oxidation and NO metabolism. Figure 3 provides a schematic of the currently available evidence-base, and a road map of the necessary components of future studies to enable more definitive evaluation of these unanswered questions in gout and asymptomatic hyperuricemia.
Figure 3.
Schematic of currently available clinical evidence-base from which inferences regarding vascular effects of UA (and XO) have been made. The schematic summarizes key needs and appropriate design considerations for future studies to enable truly definitive clinical evaluation of the role of UA and/or XO in vascular disease.
CONCLUSIONS
UA is not an inert endproduct of purine catabolism in humans, and UA can act as an antioxidant or pro-oxidant. XO, which generates UA, also induces oxidative stress, and both UA and XO may promote inflammation. Large bodies of in vitro and animal model evidence support pathogenic effects of hyperuricemia and XO that promote endothelial dysfunction and certain vascular pathologies. The bulk of human epidemiologic evidence also supports hyperuricemia to be an independent risk factor for certain vascular diseases, and complications of atherosclerosis. Host factors, such as intrinsic inflammation and/or oxidative stress in gout, CHF, metabolic syndrome, diabetes, and chronic kidney disease, are likely major determinants of whether high levels of soluble UA are benign or promote pathology without monosodium urate crystallization. One such host factor is very likely XO activity in the vasculature. Given the prevalence of both gout and asymptomatic hyperuricemia, larger, randomized, well-controlled, and prospective clinical trials, using XOR inhibition and other strategies to lower serum urate are urgently required. Future clinical trials in this area should be accompanied by appropriate monitoring of biomarkers (e.g., NO metabolism, allantoin, and renin-angiotensin axis activity) and changes in arterial pathology (e.g., atherosclerotic plaque size and stability) by sensitive, advanced imaging. Due to the huge scope of the problems of gout and hyperuricemia, such large, prospective, and well-designed clinical trials are urgently needed to resolve the putative toxic roles of hyperuricemia and XO in the human arterial circulation.
Acknowledgments
Supported by the VA Research Service, and NIH grants HL077360, HL087252 (RT), and AR055127 (TN).
References
- 1.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008;359:1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzali M, Kanbay M, Segal MS, Shafiu M, Jalal D, Feig DI, Johnson RJ. Uric acid and hypertension: cause or effect? Curr Rheumatol Rep. 2010;12:108–17. doi: 10.1007/s11926-010-0094-1. [DOI] [PubMed] [Google Scholar]
- 3.Pacher P, Nivorozhkin A, Szabo C. Therapeutic Effects of Xanthine Oxidase Inhibitors: Renaissance Half a Century after the Discovery of Allopurinol 10.1124/pr.58.1.6. Pharmacol. Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parks DA, Granger DN. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol. Scand. Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- 6.Zhang Z, Blake DR, Stevens CR, Kanczler JM, Winyard PG, Symons MC, Benboubetra M, Harrison R. A reappraisal of xanthine dehydrogenase and oxidase in hypoxic reperfusion injury: the role of NADH as an electron donor. Free Radic. Res. 1998;28:151–64. doi: 10.3109/10715769809065801. [DOI] [PubMed] [Google Scholar]
- 7.Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, Zeni P, Zardini P. Elevated serum uric acid levels are associated with diastolic dysfunction in patients with dilated cardiomyopathy. Am. Heart J. 2002;143:1107–11. doi: 10.1067/mhj.2002.122122. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, Gerster J, Jacobs J, Leeb B, Liote F, McCarthy G, Netter P, Nuki G, Perez-Ruiz F, Pignone A, Pimentao J, Punzi L, Roddy E, Uhlig T, Zimmermann-Gorska I. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann. Rheum. Dis. 2006;65:1312–24. doi: 10.1136/ard.2006.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramazzina I, Folli C, Secchi A, Berni R, Percudani R. Completing the uric acid degradation pathway through phylogenetic comparison of whole genomes. Nat Chem Biol. 2006;2:144–8. doi: 10.1038/nchembio768. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y, Park BC, Lee do H, Bae KH, Cho S, Lee CH, Lee JS, Myung PK, Park SG. Mouse transthyretin-related protein is a hydrolase which degrades 5-hydroxyisourate, the end product of the uricase reaction. Mol. Cells. 2006;22:141–5. [PubMed] [Google Scholar]
- 11.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population. Arthritis Rheum. 2011 doi: 10.1002/art.30520. Online first:DOI: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 12.Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am. J. Med. 1987;82:421–6. doi: 10.1016/0002-9343(87)90441-4. [DOI] [PubMed] [Google Scholar]
- 13.Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J. Rheumatol. 2004;31:1582–7. [PubMed] [Google Scholar]
- 14.Zharikov S, Krotova K, Hu H, Baylis C, Johnson RJ, Block ER, Patel J. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295:C1183–90. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids. 2008;27:967–78. doi: 10.1080/15257770802257952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imaram W, Gersch C, Kim KM, Johnson RJ, Henderson GN, Angerhofer A. Radicals in the reaction between peroxynitrite and uric acid identified by electron spin resonance spectroscopy and liquid chromatography mass spectrometry. Free Radic. Biol. Med. 2010;49:275–81. doi: 10.1016/j.freeradbiomed.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meotti FC, Jameson GN, Turner R, Harwood DT, Stockwell S, Rees MD, Thomas SR, Kettle AJ. Urate as a physiological substrate for myeloperoxidase: implications for hyperuricemia and inflammation. J. Biol. Chem. 2011 doi: 10.1074/jbc.M110.172460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KM, Henderson GN, Frye RF, Galloway CD, Brown NJ, Segal MS, Imaram W, Angerhofer A, Johnson RJ. Simultaneous determination of uric acid metabolites allantoin, 6-aminouracil, and triuret in human urine using liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:65–70. doi: 10.1016/j.jchromb.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao B, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein. Chem. Res. Toxicol. 2010;23:447–54. doi: 10.1021/tx9003775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc. Natl. Acad. Sci. U. S. A. 1981;78:6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker BF. Towards the physiological function of uric acid. Free Radic. Biol. Med. 1993;14:615–31. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- 22.Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem. J. 1986;235:747–54. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kutzing MK, Firestein BL. Altered uric acid levels and disease states. J. Pharmacol. Exp. Ther. 2008;324:1–7. doi: 10.1124/jpet.107.129031. [DOI] [PubMed] [Google Scholar]
- 24.Proctor P. Similar functions of uric acid and ascorbate in man? Nature. 1970;228:868. doi: 10.1038/228868a0. [DOI] [PubMed] [Google Scholar]
- 25.Radi R, Tan S, Prodanov E, Evans RA, Parks DA. Inhibition of xanthine oxidase by uric acid and its influence on superoxide radical production. Biochim. Biophys. Acta. 1992;1122:178–82. doi: 10.1016/0167-4838(92)90321-4. [DOI] [PubMed] [Google Scholar]
- 26.Regoli F, Winston GW. Quantification of total oxidant scavenging capacity of antioxidants for peroxynitrite, peroxyl radicals, and hydroxyl radicals. Toxicol. Appl. Pharmacol. 1999;156:96–105. doi: 10.1006/taap.1999.8637. [DOI] [PubMed] [Google Scholar]
- 27.Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27:608–19. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Squadrito GL, Cueto R, Splenser AE, Valavanidis A, Zhang H, Uppu RM, Pryor WA. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch. Biochem. Biophys. 2000;376:333–7. doi: 10.1006/abbi.2000.1721. [DOI] [PubMed] [Google Scholar]
- 29.Teng RJ, Ye YZ, Parks DA, Beckman JS. Urate produced during hypoxia protects heart proteins from peroxynitrite-mediated protein nitration. Free Radic. Biol. Med. 2002;33:1243–9. doi: 10.1016/s0891-5849(02)01020-1. [DOI] [PubMed] [Google Scholar]
- 30.Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ, Maxwell SR. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin Sci (Lond) 2003;105:425–30. doi: 10.1042/CS20030149. [DOI] [PubMed] [Google Scholar]
- 31.Waring WS, McKnight JA, Webb DJ, Maxwell SR. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes. 2006;55:3127–32. doi: 10.2337/db06-0283. [DOI] [PubMed] [Google Scholar]
- 32.Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J. Cardiovasc. Pharmacol. 2001;38:365–71. doi: 10.1097/00005344-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Waring WS, Adwani SH, Breukels O, Webb DJ, Maxwell SR. Hyperuricaemia does not impair cardiovascular function in healthy adults. Heart. 2004;90:155–9. doi: 10.1136/hrt.2003.016121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkinson SR, Prathalingam SR, Taylor MC, Horn D, Kelly JM. Vitamin C biosynthesis in trypanosomes: a role for the glycosome. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11645–50. doi: 10.1073/pnas.0504251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hershfield MS, Roberts LJ, 2nd, Ganson NJ, Kelly SJ, Santisteban I, Scarlett E, Jaggers D, Sundy JS. Treating gout with pegloticase, a PEGylated urate oxidase, provides insight into the importance of uric acid as an antioxidant in vivo. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14351–6. doi: 10.1073/pnas.1001072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J. Hypertens. 2008;26:269–75. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 37.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, Johnson RJ. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–93. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 38.Kang DH, Han L, Ouyang X, Kahn AM, Kanellis J, Li P, Feng L, Nakagawa T, Watanabe S, Hosoyamada M, Endou H, Lipkowitz M, Abramson R, Mu W, Johnson RJ. Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am. J. Nephrol. 2005;25:425–33. doi: 10.1159/000087713. [DOI] [PubMed] [Google Scholar]
- 39.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 2005;16:3553–62. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 40.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, Lan HY, Johnson RJ. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am. J. Physiol. Renal Physiol. 2002;282:F991–7. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 41.Price KL, Sautin YY, Long DA, Zhang L, Miyazaki H, Mu W, Endou H, Johnson RJ. Human vascular smooth muscle cells express a urate transporter. J. Am. Soc. Nephrol. 2006;17:1791–5. doi: 10.1681/ASN.2006030264. [DOI] [PubMed] [Google Scholar]
- 42.Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J. Biol. Chem. 1991;266:8604–8. [PubMed] [Google Scholar]
- 43.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–42. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 44.Roncal CA, Mu W, Croker B, Reungjui S, Ouyang X, Tabah-Fisch I, Johnson RJ, Ejaz AA. Effect of elevated serum uric acid on cisplatin-induced acute renal failure. Am. J. Physiol. Renal Physiol. 2007;292:F116–22. doi: 10.1152/ajprenal.00160.2006. [DOI] [PubMed] [Google Scholar]
- 45.Mazzali M, Kim YG, Suga S, Gordon KL, Kang DH, Jefferson JA, Hughes J, Kivlighn SD, Lan HY, Johnson RJ. Hyperuricemia exacerbates chronic cyclosporine nephropathy. Transplantation. 2001;71:900–5. doi: 10.1097/00007890-200104150-00014. [DOI] [PubMed] [Google Scholar]
- 46.Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincon A, Arroyo D, Luno J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388–93. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Momeni A, Shahidi S, Seirafian S, Taheri S, Kheiri S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010;4:128–32. [PubMed] [Google Scholar]
- 48.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am. J. Kidney Dis. 2006;47:51–9. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Hoieggen A, Alderman MH, Kjeldsen SE, Julius S, Devereux RB, De Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H, Chen C, Dahlof B. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int. 2004;65:1041–9. doi: 10.1111/j.1523-1755.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 50.Franse LV, Pahor M, Di Bari M, Shorr RI, Wan JY, Somes GW, Applegate WB. Serum uric acid, diuretic treatment and risk of cardiovascular events in the Systolic Hypertension in the Elderly Program (SHEP) J. Hypertens. 2000;18:1149–54. doi: 10.1097/00004872-200018080-00021. [DOI] [PubMed] [Google Scholar]
- 51.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–32. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, Patel JM, Johnson RJ. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Renal Physiol. 2006;290:F625–31. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 53.Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol. 2005;1:80–6. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 54.Gibbings S, Elkins ND, Fitzgerald H, Tiao J, Weyman ME, Shibao G, Fini MA, Wright RM. Xanthine Oxidoreductase Promotes the Inflammatory State of Mononuclear Phagocytes through Effects on Chemokine Expression, Peroxisome Proliferator-activated Receptor-{gamma} Sumoylation, and HIF-1{alpha} J. Biol. Chem. 2011;286:961–75. doi: 10.1074/jbc.M110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leal-Pinto E, Cohen BE, Abramson RG. Functional analysis and molecular modeling of a cloned urate transporter/channel. J. Membr. Biol. 1999;169:13–27. doi: 10.1007/pl00005897. [DOI] [PubMed] [Google Scholar]
- 56.Waring WS, McKnight JA, Webb DJ, Maxwell SR. Lowering serum urate does not improve endothelial function in patients with type 2 diabetes. Diabetologia. 2007;50:2572–9. doi: 10.1007/s00125-007-0817-7. [DOI] [PubMed] [Google Scholar]
- 57.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:C584–96. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 58.Martin WJ, Grainger R, Harrison A, Harper JL. Differences in MSU-induced superoxide responses by neutrophils from gout subjects compared to healthy controls and a role for environmental inflammatory cytokines and hyperuricemia in neutrophil function and survival. J. Rheumatol. 2010;37:1228–35. doi: 10.3899/jrheum.091080. [DOI] [PubMed] [Google Scholar]
- 59.Sulfinpyrazone in the prevention of cardiac death after myocardial infarction. The Anturane Reinfarction Trial. N. Engl. J. Med. 1978;298:289–95. doi: 10.1056/NEJM197802092980601. [DOI] [PubMed] [Google Scholar]
- 60.The Anturane Reinfarction Trial Research Group Sulfinpyrazone in the prevention of sudden death after myocardial infarction. N. Engl. J. Med. 1980;302:250–6. doi: 10.1056/NEJM198001313020502. [DOI] [PubMed] [Google Scholar]
- 61.Sulphinpyrazone in post-myocardial infarction. Report from the Anturan Reinfarction Italian Study. Lancet. 1982;1:237–42. [PubMed] [Google Scholar]
- 62.Cairns JA, Gent M, Singer J, Finnie KJ, Froggatt GM, Holder DA, Jablonsky G, Kostuk WJ, Melendez LJ, Myers MG, et al. Aspirin, sulfinpyrazone, or both in unstable angina. Results of a Canadian multicenter trial. N. Engl. J. Med. 1985;313:1369–75. doi: 10.1056/NEJM198511283132201. [DOI] [PubMed] [Google Scholar]
- 63.Simon JA, Lin F, Vittinghoff E, Bittner V. The relation of postmenopausal hormone therapy to serum uric acid and the risk of coronary heart disease events: the Heart and Estrogen-Progestin Replacement Study (HERS) Ann. Epidemiol. 2006;16:138–45. doi: 10.1016/j.annepidem.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, Lademacher C. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12:R63. doi: 10.1186/ar2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Becker MA, Schumacher HR, Jr., Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N. Engl. J. Med. 2005;353:2450–61. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 66.Schumacher HR, Jr., Becker MA, Lloyd E, MacDonald PA, Lademacher C. Febuxostat in the treatment of gout: 5-yr findings of the FOCUS efficacy and safety study. Rheumatology (Oxford) 2009;48:188–94. doi: 10.1093/rheumatology/ken457. [DOI] [PubMed] [Google Scholar]
- 67.Schumacher HR, Jr., Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, Lademacher C, Joseph-Ridge N. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540–8. doi: 10.1002/art.24209. [DOI] [PubMed] [Google Scholar]
- 68.Patetsios P, Song M, Shutze WP, Pappas C, Rodino W, Ramirez JA, Panetta TF. Identification of uric acid and xanthine oxidase in atherosclerotic plaque. Am. J. Cardiol. 2001;88:188–91. A6. doi: 10.1016/s0002-9149(01)01621-6. [DOI] [PubMed] [Google Scholar]
- 69.Ciari I, Terzuoli L, Porcelli B, Coppola MG, Marinello E. Antioxidant status and purine bases in carotid artery plaque. Nucleosides Nucleotides Nucleic Acids. 2008;27:624–7. doi: 10.1080/15257770802138608. [DOI] [PubMed] [Google Scholar]
- 70.Terzuoli L, Marinello E, Felici C, Frosi B, Setacci C, Giubbolini M, Porcelli B. Purine bases and atheromatous plaque. Int J Immunopathol Pharmacol. 2004;17:31–3. [PubMed] [Google Scholar]
- 71.Hille R, Massey V. Studies on the oxidative half-reaction of xanthine oxidase. J. Biol. Chem. 1981;256:9090–5. [PubMed] [Google Scholar]
- 72.Saito T, Nishino T. Differences in redox and kinetic properties between NAD-dependent and O2-dependent types of rat liver xanthine dehydrogenase. J. Biol. Chem. 1989;264:10015–22. [PubMed] [Google Scholar]
- 73.Waud WR, Rajagopalan KV. The mechanism of conversion of rat liver xanthine dehydrogenase from an NAD+-dependent form (type D) to an O2-dependent form (type O) Arch. Biochem. Biophys. 1976;172:365–79. doi: 10.1016/0003-9861(76)90088-6. [DOI] [PubMed] [Google Scholar]
- 74.Guerciolini R, Szumlanski C, Weinshilboum RM. Human liver xanthine oxidase: nature and extent of individual variation. Clin. Pharmacol. Ther. 1991;50:663–72. doi: 10.1038/clpt.1991.205. [DOI] [PubMed] [Google Scholar]
- 75.Doehner W, Anker SD. Uric acid in chronic heart failure. Semin. Nephrol. 2005;25:61–6. doi: 10.1016/j.semnephrol.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 76.Tan S, Gelman S, Wheat JK, Parks DA. Circulating xanthine oxidase in human ischemia reperfusion. South. Med. J. 1995;88:479–82. doi: 10.1097/00007611-199504000-00021. [DOI] [PubMed] [Google Scholar]
- 77.Pesonen EJ, Linder N, Raivio KO, Sarnesto A, Lapatto R, Hockerstedt K, Makisalo H, Andersson S. Circulating xanthine oxidase and neutrophil activation during human liver transplantation. Gastroenterology. 1998;114:1009–15. doi: 10.1016/s0016-5085(98)70321-x. [DOI] [PubMed] [Google Scholar]
- 78.Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, Reepschlager N, Hornig B, Drexler H, Harrison DG. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2003;107:1383–9. doi: 10.1161/01.cir.0000056762.69302.46. [DOI] [PubMed] [Google Scholar]
- 79.de Jong JW, Schoemaker RG, de Jonge R, Bernocchi P, Keijzer E, Harrison R, Sharma HS, Ceconi C. Enhanced expression and activity of xanthine oxidoreductase in the failing heart. J. Mol. Cell. Cardiol. 2000;32:2083–9. doi: 10.1006/jmcc.2000.1240. [DOI] [PubMed] [Google Scholar]
- 80.Komaki Y, Sugiura H, Koarai A, Tomaki M, Ogawa H, Akita T, Hattori T, Ichinose M. Cytokine-mediated xanthine oxidase upregulation in chronic obstructive pulmonary disease’s airways. Pulm. Pharmacol. Ther. 2005;18:297–302. doi: 10.1016/j.pupt.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 81.Kayyali US, Budhiraja R, Pennella CM, Cooray S, Lanzillo JJ, Chalkley R, Hassoun PM. Upregulation of xanthine oxidase by tobacco smoke condensate in pulmonary endothelial cells. Toxicol. Appl. Pharmacol. 2003;188:59–68. doi: 10.1016/s0041-008x(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 82.Terada LS, Radisavljevic Z, Mahr NN, Jacobson ED. Xanthine oxidase decreases production of gut wall nitric oxide. Proc. Soc. Exp. Biol. Med. 1997;216:410–3. doi: 10.3181/00379727-216-44190. [DOI] [PubMed] [Google Scholar]
- 83.Radi R, Rubbo H, Bush K, Freeman BA. Xanthine Oxidase Binding to Glycosaminoglycans: Kinetics and Superoxide Dismutase Interactions of Immobilized Xanthine Oxidase-Heparin Complexes. Arch. Biochem. Biophys. 1997;339:125–35. doi: 10.1006/abbi.1996.9844. [DOI] [PubMed] [Google Scholar]
- 84.Panus PC, Wright SA, Chumley PH, Radi R, Freeman BA. The contribution of vascular endothelial xanthine dehydrogenase/oxidase to oxygen-mediated cell injury. Arch. Biochem. Biophys. 1992;294:695–702. doi: 10.1016/0003-9861(92)90743-g. [DOI] [PubMed] [Google Scholar]
- 85.Prasad K, Kalra J, Bharadwaj L. Cardiac depressant effects of oxygen free radicals. Angiology. 1993;44:257–70. doi: 10.1177/000331979304400401. [DOI] [PubMed] [Google Scholar]
- 86.Vorbach C, Harrison R, Capecchi MR. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol. 2003;24:512–7. doi: 10.1016/s1471-4906(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 87.Kono H, Chen CJ, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J. Clin. Invest. 2010;120:1939–49. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nicholas SA, Bubnov VV, Yasinska IM, Sumbayev VV. Involvement of xanthine oxidase and hypoxia-inducible factor 1 in Toll-like receptor 7/8-mediated activation of caspase 1 and interleukin-1beta. Cell. Mol. Life Sci. 2011;68:151–8. doi: 10.1007/s00018-010-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–6. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 90.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–16. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 91.Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–51. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 92.Engberding N, Spiekermann S, Schaefer A, Heineke A, Wiencke A, Muller M, Fuchs M, Hilfiker-Kleiner D, Hornig B, Drexler H, Landmesser U. Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? Circulation. 2004;110:2175–9. doi: 10.1161/01.CIR.0000144303.24894.1C. [DOI] [PubMed] [Google Scholar]
- 93.Sisto T, Paajanen H, Metsa-Ketela T, Harmoinen A, Nordback I, Tarkka M. Pretreatment with antioxidants and allopurinol diminishes cardiac onset events in coronary artery bypass grafting. Ann. Thorac. Surg. 1995;59:1519–23. doi: 10.1016/0003-4975(95)00197-s. [DOI] [PubMed] [Google Scholar]
- 94.Rajendra NS, Ireland SE, George J, Belch JJF, Lang CC, Struthers AD. Mechanistic insights into the therapeutics use of Allopurinol in Angina Pectoris. Journal of the American College of Cardiology (JACC) 2011 doi: 10.1016/j.jacc.2010.12.052. in press. [DOI] [PubMed] [Google Scholar]
- 95.Noman A, Ang DS, Ogston S, Lang CC, Struthers AD. Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomised, placebo controlled crossover trial. Lancet. 2010;375:2161–7. doi: 10.1016/S0140-6736(10)60391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cingolani HE, Plastino JA, Escudero EM, Mangal B, Brown J, Perez NG. The effect of xanthine oxidase inhibition upon ejection fraction in heart failure patients: La Plata Study. J. Card. Fail. 2006;12:491–8. doi: 10.1016/j.cardfail.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 97.Hare JM, Mangal B, Brown J, Fisher C, Jr., Freudenberger R, Colucci WS, Mann DL, Liu P, Givertz MM, Schwarz RP. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J. Am. Coll. Cardiol. 2008;51:2301–9. doi: 10.1016/j.jacc.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 98.Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Quyyumi AA, Panza JA. Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients. Hypertension. 1997;30:57–63. doi: 10.1161/01.hyp.30.1.57. [DOI] [PubMed] [Google Scholar]
- 99.Gavin AD, Struthers AD. Allopurinol reduces B-type natriuretic peptide concentrations and haemoglobin but does not alter exercise capacity in chronic heart failure. Heart. 2005;91:749–53. doi: 10.1136/hrt.2004.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ogino K, Kato M, Furuse Y, Kinugasa Y, Ishida K, Osaki S, Kinugawa T, Igawa O, Hisatome I, Shigemasa C, Anker SD, Doehner W. Uric Acid Lowering Treatment with Benzbromarone in Patients with Heart Failure: a Double-blind Placebo-controlled Crossover Preliminary Study. Circ Heart Fail. 2009 doi: 10.1161/CIRCHEARTFAILURE.109.868604. [DOI] [PubMed] [Google Scholar]
- 101.Amado LC, Saliaris AP, Raju SV, Lehrke S, St John M, Xie J, Stewart G, Fitton T, Minhas KM, Brawn J, Hare JM. Xanthine oxidase inhibition ameliorates cardiovascular dysfunction in dogs with pacing-induced heart failure. J. Mol. Cell. Cardiol. 2005;39:531–6. doi: 10.1016/j.yjmcc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 102.Ekelund UE, Harrison RW, Shokek O, Thakkar RN, Tunin RS, Senzaki H, Kass DA, Marban E, Hare JM. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure. Circ. Res. 1999;85:437–45. doi: 10.1161/01.res.85.5.437. [DOI] [PubMed] [Google Scholar]
- 103.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J. Biol. Chem. 2010;285:28938–45. doi: 10.1074/jbc.M110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, Li D, Berkowitz DE, Hare JM. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15944–8. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Naumova AV, Chacko VP, Ouwerkerk R, Stull L, Marban E, Weiss RG. Xanthine oxidase inhibitors improve energetics and function after infarction in failing mouse hearts. Am J Physiol Heart Circ Physiol. 2006;290:H837–43. doi: 10.1152/ajpheart.00831.2005. [DOI] [PubMed] [Google Scholar]
- 106.Perez NG, Gao WD, Marban E. Novel myofilament Ca2+-sensitizing property of xanthine oxidase inhibitors. Circ. Res. 1998;83:423–30. doi: 10.1161/01.res.83.4.423. [DOI] [PubMed] [Google Scholar]
- 107.Saavedra WF, Paolocci N, St John ME, Skaf MW, Stewart GC, Xie JS, Harrison RW, Zeichner J, Mudrick D, Marban E, Kass DA, Hare JM. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ. Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- 108.Stull LB, Leppo MK, Szweda L, Gao WD, Marban E. Chronic treatment with allopurinol boosts survival and cardiac contractility in murine postischemic cardiomyopathy. Circ. Res. 2004;95:1005–11. doi: 10.1161/01.RES.0000148635.73331.c5. [DOI] [PubMed] [Google Scholar]
- 109.Baldus S, Koster R, Chumley P, Heitzer T, Rudolph V, Ostad MA, Warnholtz A, Staude H-J, Thuneke F, Koss K. Oxypurinol improves coronary and peripheral endothelial function in patients with coronary artery disease. Free Radical Biology and Medicine. 2005;39:1184–90. doi: 10.1016/j.freeradbiomed.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cleland JGF, Coletta AP, Clark AL. Clinical trials update from the Heart Failure Society of America meeting: FIX-CHF-4, selective cardiac myosin activator and OPT-CHF. European Journal of Heart Failure. 2006;8:764–6. doi: 10.1016/j.ejheart.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 111.Das DK, Engelman RM, Clement R, Otani H, Prasad MR, Rao PS. Role of xanthine oxidase inhibitor as free radical scavenger: a novel mechanism of action of allopurinol and oxypurinol in myocardial salvage. Biochem. Biophys. Res. Commun. 1987;148:314–9. doi: 10.1016/0006-291x(87)91112-0. [DOI] [PubMed] [Google Scholar]
- 112.Hoey BM, Butler J, Halliwell B. On the specificity of allopurinol and oxypurinol as inhibitors of xanthine oxidase. A pulse radiolysis determination of rate constants for reaction of allopurinol and oxypurinol with hydroxyl radicals. Free Radic. Res. Commun. 1988;4:259–63. doi: 10.3109/10715768809055151. [DOI] [PubMed] [Google Scholar]
- 113.Keshavarzian A, Morgan G, Sedghi S, Gordon JH, Doria M. Role of reactive oxygen metabolites in experimental colitis. Gut. 1990;31:786–90. doi: 10.1136/gut.31.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ricardo SD, Bertram JF, Ryan GB. Podocyte architecture in puromycin aminonucleoside-treated rats administered tungsten or allopurinol. Exp. Nephrol. 1995;3:270–9. [PubMed] [Google Scholar]
- 115.Augustin AJ, Boker T, Blumenroder SH, Lutz J, Spitznas M. Free radical scavenging and antioxidant activity of allopurinol and oxypurinol in experimental lens-induced uveitis. Invest. Ophthalmol. Vis. Sci. 1994;35:3897–904. [PubMed] [Google Scholar]
- 116.Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and Hepatocellular Peroxynitrite Formation during Acetaminophen Toxicity: Role of Mitochondrial Oxidant Stress 10.1093/toxsci/62.2.212. Toxicol. Sci. 2001;62:212–20. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- 117.Andreou I, Tousoulis D, Miliou A, Tentolouris C, Zisimos K, Gounari P, Siasos G, Papageorgiou N, Papadimitriou CA, Dimopoulos MA, Stefanadis C. Effects of rosuvastatin on myeloperoxidase levels in patients with chronic heart failure: a randomized placebo-controlled study. Atherosclerosis. 2010;210:194–8. doi: 10.1016/j.atherosclerosis.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 118.Connor M. Allopurinol for pain relief: more than just crystal clearance? Br. J. Pharmacol. 2009;156:4–6. doi: 10.1111/j.1476-5381.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marro PJ, Mishra OP, Delivoria-Papadopoulos M. Effect of allopurinol on brain adenosine levels during hypoxia in newborn piglets. Brain Res. 2006;1073-1074:444–50. doi: 10.1016/j.brainres.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 120.Schmidt AP, Bohmer AE, Antunes C, Schallenberger C, Porciuncula LO, Elisabetsky E, Lara DR, Souza DO. Anti-nociceptive properties of the xanthine oxidase inhibitor allopurinol in mice: role of A1 adenosine receptors. Br. J. Pharmacol. 2009;156:163–72. doi: 10.1111/j.1476-5381.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Conta AC, Stelzner DJ. Immunomodulatory effect of the purine nucleoside inosine following spinal cord contusion injury in rat. Spinal Cord. 2008;46:39–44. doi: 10.1038/sj.sc.3102057. [DOI] [PubMed] [Google Scholar]
- 122.Hasko G, Kuhel DG, Nemeth ZH, Mabley JG, Stachlewitz RF, Virag L, Lohinai Z, Southan GJ, Salzman AL, Szabo C. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J. Immunol. 2000;164:1013–9. doi: 10.4049/jimmunol.164.2.1013. [DOI] [PubMed] [Google Scholar]
- 123.Liaudet L, Mabley JG, Pacher P, Virag L, Soriano FG, Marton A, Hasko G, Deitch EA, Szabo C. Inosine exerts a broad range of antiinflammatory effects in a murine model of acute lung injury. Ann. Surg. 2002;235:568–78. doi: 10.1097/00000658-200204000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marton A, Pacher P, Murthy KG, Nemeth ZH, Hasko G, Szabo C. Anti-inflammatory effects of inosine in human monocytes, neutrophils and epithelial cells in vitro. Int. J. Mol. Med. 2001;8:617–21. [PubMed] [Google Scholar]
- 125.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N. Engl. J. Med. 2004;351:2611–8. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]