Summary
Placental infections are major causes of maternal and fetal disease. This review introduces a new paradigm for placental infections based on current knowledge of placental defenses and how this barrier can be breached. Transmission of pathogens from mother to fetus can occur at two sites of direct contact between maternal cells and specialized fetal cells (trophoblasts) in the human placenta: (i) maternal immune and endothelial cells juxtaposed to extravillous trophoblasts in the uterine implantation site and (ii) maternal blood surrounding the syncytiotrophoblast. Recent findings suggest that the primary vulnerability is in the implantation site. We explore evidence that the placental syncytiotrophoblast evolved as a defense against pathogens, and that inflammation-mediated spontaneous abortion may benefit mother and pathogen.
Placental infections compromise the health of both mother and offspring. Besides uncalculated economic loss of livestock, they represent a major source of human morbidity and mortality. For example, 12.9 million preterm infants are born annually worldwide [1], with infection/inflammation as the leading cause [2]. Many of these infants die; others suffer long-term neurological sequelae and health problems [3]. Yet very little is known about how pathogens colonize the placenta. Infectious agents can reach the placenta either via maternal blood or by ascending the genital tract. Here, we discuss the hematogenous route, as recent work has begun to elucidate this pathway.
Relatively few pathogens are capable of placental and fetal infections in humans (Table 1) and even for these, maternal infection does not guarantee placental or fetal infection. The veterinary situation is similar, and displays much overlap in pathogenic genera [4]. Viruses, bacteria and protists, they share no universal commonalities except at least partially intracellular life cycles and hematogenous systemic dissemination. What can they tell us about placental defenses?
Table 1. Diverse pathogens are capable of transplacental infection.
Here we list the major pathogens capable of transplacental infection. Several more—e.g. Epstein-Barr Virus, Hepatitis B virus, HIV and HSV—rarely cross the placenta, more are transmitted perinatally. Others have been raised in case reports and animal studies and merit further study, e.g. Babesia spp., Coxsackie B virus, Japanese Encephalitis virus, Leptospira spp., Salmonella spp., Wuchereria bancrofti and multiple gingival bacteria including Fusobacterium nucleatum.
| Pathogen (Illness) | Type | Adult Tx | Lifestyle | Known tropisms |
|---|---|---|---|---|
| Brucella spp. (Brucellosis) a, b, c, d, e | B | Ingestion | Primarily I | Leukocytes, epithelial |
| Coxiella bumetii (Q fever) c, e, h | B | Inhalation | Obligate I | Leukocytes |
| Listeria monocytogenes (Listeriosis) a, b, c, d, g, h | B | Ingestion | I/E | Epithelial, phagocytes |
| Mycobacterium tuberculosis (TB) d, g | B | Airborne | Primarily I | Leukocytes |
| Treponema pallidum (Syphilis) b, c, f, g | B | Sexual | Primarily E* | Unknown |
| Leishmania spp. (Leishmaniasis) h | P | Vector | I/E | Leukocytes |
| Plasmodium falciparum (Malaria) b, c, e, g, h | P | Vector | Obligate I | Erythrocytes, hepatocytes |
| Toxoplasma gondii (Toxoplasmosis) a, b, d, g | P | Ingestion | Obligate I | All nucleated cells |
| Trypanosoma spp. (Chagas disease, African sleeping sickness) e, g | P | Vector | I/E | Epi/endothelial |
| Cytomegalovirus (CMV) a, b, d, g | V | Droplet | Obligate I | Leukocytes, trophoblasts |
| Lymphocytic choriomeningitis virus (LCMV) a, b, f, g | V | Ingestion, Inhalation | Obligate I | Leukocytes |
| Parvovirus B19 a, b, c, d, f | V | Droplet | Obligate I | Hematopoietic, endothelial |
| Rubella virus (German measles) g | V | Droplet | Obligate I | Many |
| Varicella zoster virus (Chicken pox) g, h | V | Airborne | Obligate I | Leukocytes, neurons, epithelial |
Recognized, common adverse pregnancy outcomes:
first trimester fetal death;
second trimester fetal death;
stillbirth;
preterm labor;
intrauterine growth restriction;
fetal hydrops;
severe neonatal infection;
increased severity of maternal disease.
Type: (B)acterial, (V)iral or (P)rotozoan. Adult Tx: most common route of transmission to adult humans. Lifestyle: I = intracellular; E = extracellular. Known tropisms = cells infected.
Treponema pallidum is generally thought to be extracellular; however, it has been documented in non-phagocytic intracellular compartments both in vivo and in vitro [56]. (Table 1 with references for each pathogen cited can be found in supplementary material.)
Placental Organization
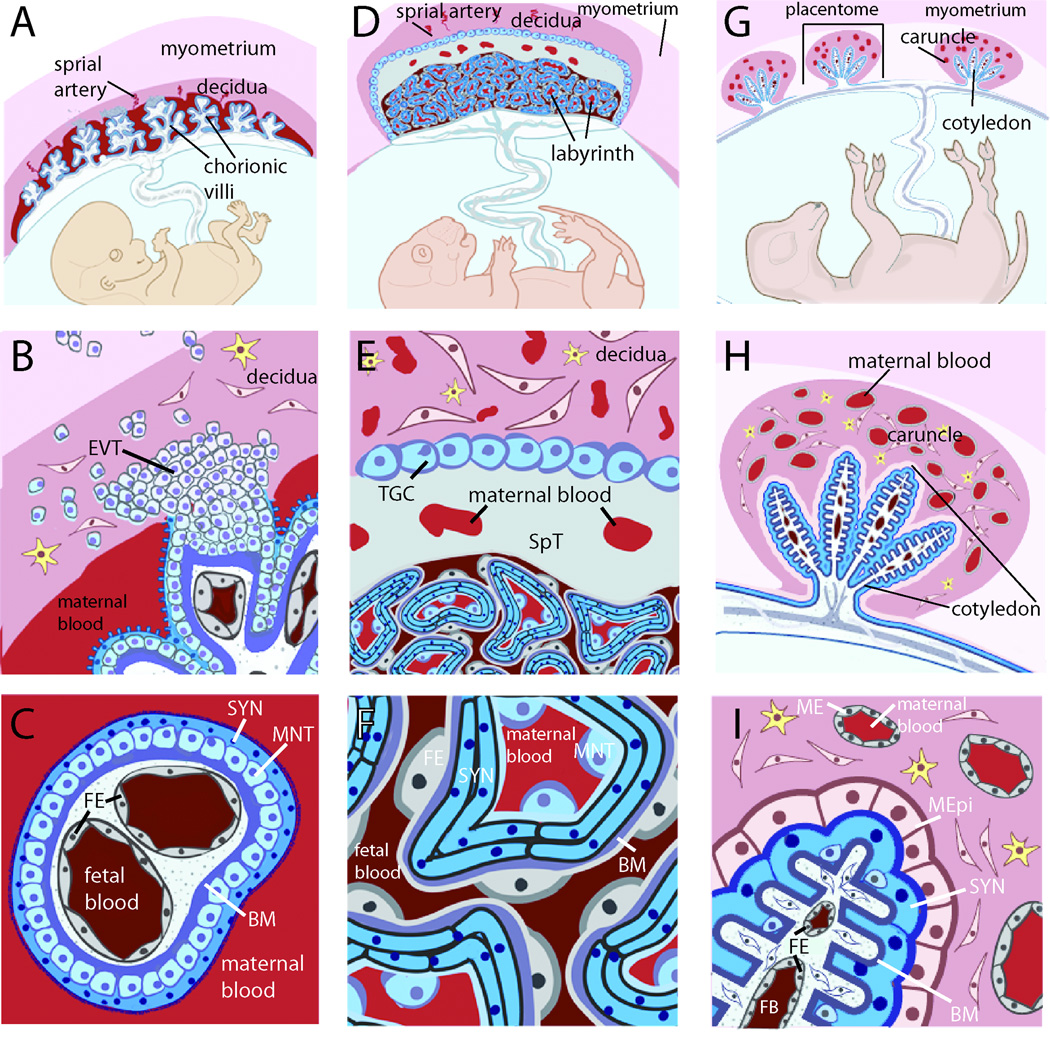
The placenta is an organ made of maternal and fetal tissues that bears two major responsibilities: to nourish and protect the fetus. The latter task includes protection from both pathogens and maternal immune rejection. Experimental investigation of placental defenses is complicated by a high inter-species variability at the maternofetal interface. A useful descriptive characteristic is the degree of uterine invasion by fetal extraembryonic epithelial cells, or trophoblasts [5]. Hemochorial placentas (Fig. 1A–F) exhibit the greatest degree of invasion, with specialized mononuclear trophoblasts invading and remodeling the uterine endometrium (decidua) and maternal spiral arteries, and even reaching the proximal myometrium in some species (e.g. humans, guinea pigs). These invasive trophoblasts anchor an extensive network of fetal capillaries. Herein lies a second interface, where maternal blood directly contacts the multinucleated trophoblast-derived syncytium that mediates molecular exchange with underlying fetal capillaries.
Fig. 1. Placental structures are diverse and do not necessarily cluster evolutionarily, complicating the selection of appropriate model systems.
A. The hemomonochorial human placenta is a villous structure bathed in maternal blood and anchored deep in the uterine decidua by extravillous trophoblasts. B. The human uterine-trophoblast interface is composed of decidual fibroblasts and maternal leukocytes juxtaposed with fetal extravillous trophoblasts (EVT), some of which migrate into the myometrium, while others remodel maternal spiral arteries (not shown). Many remain connected to a column of trophoblasts that extends into the fetal villus and stops at a basement membrane that divides trophoblasts from fetal capillaries. C. The human blood-trophoblast interface, showing villus cross-section. Maternal blood is separated from fetal vessels by a single syncytiotrophoblast (SYN), whose apical surface is covered by dense branched microvilli. Underlying that is a layer of mononuclear subsyncytial trophoblasts (MNT) that is continuous in early pregnancy but semi-discontinuous in later trimesters. Both structures are undergirded by a basement membrane. D. The hemotrichorial mouse placenta places maternal and fetal vessels in close contact within a nutrient-exchange labyrinth that is anchored in the decidua E. via a spongiotrophoblast (SpT) region and trophoblast giant cells (TGC) (analogous to EVT). The decidua is primarily composed of leukocytes and fibroblasts, as well as maternal arteries modified by trophoblast giant cells (not shown). F. Cross-section of labyrinthine region. From the maternal blood, a semi-discontinuous layer of mononuclear trophoblasts (MNT) overlays two layers of synctiotrophoblast with limited, non-overlapping cell-cell junctions. Beyond this lies fetal endothelium (FE). G. The sheep placenta is primarily epitheliochorial, and composed of multiple placentomes throughout the uterus. H. Each placentome includes a uterine caruncle rich in endometrial glands (not shown) and partially encapsulating a relatively non-invasive fetal cotyledon. I. Much of the cotyledonary surface effaces with a maternal epithelium (MEpi; which is not fully continuous and considered synepitheliochorial in regions, not shown). Maternal blood is separated from fetal blood by maternal endothelium (ME), endometrial fibroblasts, maternal epithelium, fetal syncytiotrophoblast (SYN), chorionic fibroblasts and fetal endothelium (FE). B–I. yellow cells = maternal leukocytes. A-F. Adapted from [5].
By contrast, uterine invasion by trophoblasts is diminished or absent in endotheliochorial (e.g. carnivores, tupaias) or epitheliochorial (e.g. pigs, sheep, cattle) placentas (Fig. 1G–I) [6]. Here, maternal endo- or epithelial cells are directly apposed to fetal trophoblasts in a variety of crypt-like, villous or folded arrangements. Relative to hemochorial placentas, pathogens must cross more cell layers between maternal and fetal blood (Fig. 1, compare panels C, F, I).
In addition to placental architecture, one must understand its immune components. There exists a common misconception that tolerance of the allogenic fetus requires immunosuppression; however, it is more accurate to say that maternal immunity is differentially modulated over the course of pregnancy [7]. Abundant maternal leukocytes are found in the decidua, and are required for a successful pregnancy. The emerging picture of the fetal-maternal interface is not one of an immune system blinded to “non-self” antigen, but, rather, one predisposed to tolerance in the absence of sufficient “danger” antigens.
Blood-Syncytiotrophoblast Interface
We will first consider the largest maternal-fetal interface, the site of molecular exchange with maternal blood. Almost all placentas exhibit a continuous layer of fused multinucleated trophoblasts—a syncytiotrophoblast—that in hemochorial placentas is bathed in maternal blood (Fig. 1). This structure stands in stark contrast to other mammalian epithelial barriers that serve nutritive and protective functions, such as the intestinal and alveolar epithelia, which are composed of tightly joined individual cells. A growing body of evidence suggests that the unique structure of the syncytiotrophoblast facilitates the placenta’s protective function.
The syncytiotrophoblast has been shown to resist infection by diverse pathogens (Fig. 2, pt A). In human placental organ cultures, cytomegalovirus (CMV) infects cytotrophoblasts but not syncytiotrophoblast [8], and herpes simplex virus (HSV) is only able to infect if the overlaying syncytiotrophoblast is enzymatically damaged [9]. Similarly, the syncytiotrophoblast resists colonization by the bacterial pathogen Listeria monocytogenes [10] and the protozoan parasite Toxoplasma gondii [11].
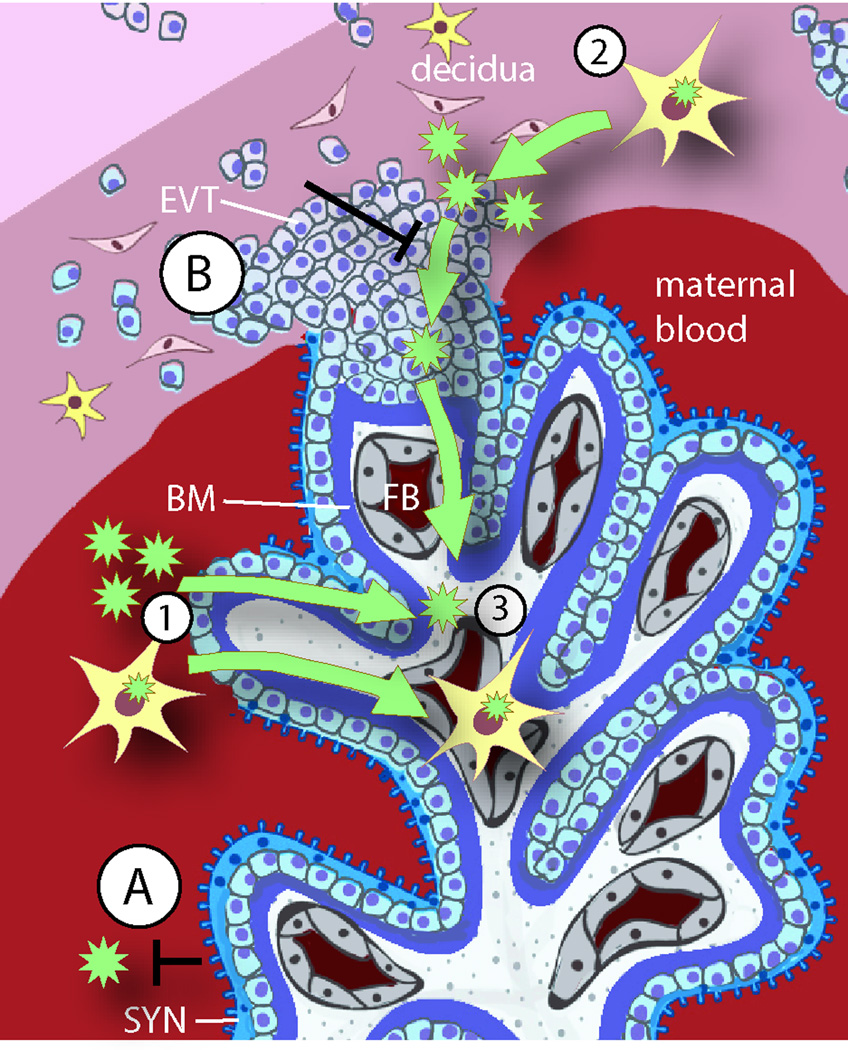
Fig. 2. Placental colonization by pathogens.
A schematic depiction of the human maternal-fetal interface is used to illustrate the barriers and possible pathways for pathogen transmission. The two sites of direct contact between maternal and fetal cells are the blood-syncytiotrophoblast (SYN) (A) and the uterus-extravillous trophoblast (EVT) (B) interfaces. Both of these trophoblast subpopulations have defense mechanisms against infection, and underneath the trophoblast barrier, the basement membrane (BM) presents an additional physical barrier. Failure of the placental barrier can occur in the presence of high pathogen titers or multiple infections. Damage of the syncytiotrophoblast enables pathogens (green stars) that are free in maternal blood or inside of maternal leukocytes (yellow cells with green stars) to cross into fetal tissues (1). However, animal and organ culture models agree that most placental infections originate in the uterine decidua (2), which is minimally accessible from the maternal blood. Pathogens can reach the decidua only by dissemination in maternal cells, most likely leukocytes. If the defense mechanisms of the EVT are overcome, the infection may spread to the fetal blood (FB) (3), act as a nidus for maternal reinfection, and/or cause trophoblast death resulting in placental insufficiency or spontaneous abortion. Finally, some pathogens may reach the fetus by traveling within maternal leukocytes on their natural way to the fetus.
What is the molecular basis of syncytial resistance to infection? E-cadherin is an important host cell receptor for L. monocytogenes binding and uptake, and is expressed on the basolateral surface of mononuclear cells that form a tight barrier for example in the intestinal epithelium [12]. Expression of E-cadherin in the human placenta is controversial. Lecuit et al. suggested that L. monocytogenes extracellular invasion of the human placenta occurs via interactions of the bacterial virulence determinant internalin A with E-cadherin on the surface of syncytiotrophoblast that is bathed in maternal blood [13]. However, other studies of the placenta have failed to find E-cadherin here [10,14–16]. All studies agree that E-cadherin is strongly expressed on subsyncytial cytotrophoblasts and proximal extravillous cytotrophoblasts, decreasing as cells migrate away from the villus tip.
Major internalization receptors for HSV [9] and CMV [17] are not significantly expressed on the syncytiotrophoblast surface that is in direct contact with maternal blood. Notably, pathogens that are able to breach the intestinal barrier typically use receptors that are important components of cell-cell junctions [12]. By eschewing most such junctions, the syncytiotrophoblast may deter multiple pathogens as well as exclude maternal leukocytes.
However, L. monocytogenes can infect cells by cell-to-cell spread as well, and the lack of intercellular junctions cannot explain syncytial resistance to this mode of entry [10], for which no host cell receptors are known. This suggests the syncytiotrophoblast can also act as a physical barrier.
Uterus-Trophoblast Interface
The second and smaller site of maternofetal contact is found where trophoblasts—termed extravillous trophoblasts (EVT) in humans or trophoblast giant cells in mice—contact and invade the uterus (Fig. 2, pt B). In the human decidua, EVT are juxtaposed to natural killer cells and macrophages—but few lymphocytes. Maternal decidual leukocytes serve to protect against pathogens, although, their activity is modulated by contact with tolerance-inducing trophoblasts [7]. EVT themselves have innate host defense mechanisms and play a significant defensive role in the presence of pathogens [7].
All ten toll-like receptors (TLRs) are expressed by trophoblasts [18], and their expression seems to be regulated in a spatial and temporal manner. For example, TLR-2 and TLR-4 are expressed by subsyncytial and extravillous trophoblasts but not by syncytiotrophoblasts in the first-trimester placenta, suggesting that innate immune responses are only elicited if the syncytial barrier is damaged or pathogens have managed to invade the implantation site [19]. Trophoblasts produce multiple anti-viral factors when TLR-3 is engaged [20]. Infection with CMV [20], T. gondii [21] and Porphyromonas gingivalis [22] all induce trophoblast apoptosis, as does exposure to viral TLR-8 agonist [7].
Our lab has provided further evidence that isolated EVT have strong innate host defense mechanisms against intracellular pathogens. EVT are bactericidal against L. monocytogenes, which normally escapes from its initial vacuole to grow in the cytosol, but this ability is severely restricted in invasive trophoblasts [23]. More recent evidence from our lab suggests that growth of some T. gondii strains in EVT is impaired as well [11], indicating a general ability of EVT to restrict growth of intracellular pathogens, an architecturally sensible defense.
Breaking Through
How might pathogens overcome the placental barriers? One possibility is syncytial damage. In hemochorial placentas, the syncytiotrophoblast is undergirded by a semi-continuous layer of mononuclear trophoblasts [5] (Fig. 1C). In a sense, removing the syncytiotrophoblast exposes a monolayer more like that of other organs’ epithelia: polarized, with stable cell-cell junctions, and supported by a basement membrane.
A useful example of how syncytial damage (Fig. 2, pt 1) might facilitate infection is presented by Plasmodium falciparum, the causative agent of malaria, which infects erythrocytes. In humans, histopathology of malarial placentas shows syncytiotrophoblast degradation [24], and P. falciparum-infected erythrocytes accumulate due to parasite-mediated erythrocyte membrane proteins that preferentially bind antigens in the intervillous space [25]. Another example is the exposure of human placental explants to high titers of Trypanosoma cruzi, which results in rapid syncytiotrophoblast detachment and apoptosis [26]. We have also observed that enzymatic syncytial damage increases underlying cytotrophoblast colonization by L. monocytogenes and T. gondii [10,11]. Even then, the basement membrane remains a potent barricade to colonization of the fetal stroma [10,11].
However, experimental infection of animals and cultured placentas strongly suggest that the more vulnerable site of entry by pathogens is at the uterine-trophoblast interface. Experimental infections of pregnant mice show that the decidua is the initial site of placental colonization for L. monocytogenes [27], T. gondii [28], Chlamydophila psittaci [29], Coxiella burnetii [30], Fusobacterium nucleatum [31] and Brucella abortus [32]. Infection of pregnant mice with Salmonella enterica results in widespread placental infection, but an attenuated mutant is found only at the decidua and adjacent trophoblasts, reconfirming the decidua as initial infection site [33]. Most CMV is found in the deciduas and EVT of naturally infected human term placentas [34]. And in human placental explants infected with L. monocytogenes and T. gondii, the EVT is the first site to be colonized [10,11]. Limited syncytial infection is possible at high listerial titers [10,13]; however, the vast majority of bacteria invade and disperse from the EVT [10].
The uterine-trophoblast interface is not directly accessible from the maternal blood. How can pathogens colonize the implantation site and reach the EVT? L. monocytogenes can infect cells either by direct invasion or by cell-to-cell spread, and mutants incapable of cell-to-cell spread are deficient in transplacental transmission in guinea pig [35] and mouse [27], while antibiotics against extracellular L. monocytogenes demonstrate that the majority of bacteria in placenta, maternal organs and blood are intracellular [36]. Maternal leukocytes are recruited to the decidua and myometrium [37], and given the propensity for intracellularity of placental pathogens (Table 1) it seems plausible that these microbes reach the decidua within leukocytes (Fig. 2, pt 2). Less commonly, they may also invade maternal endothelia [31] or possibly EVT that have invaded proximal maternal spiral arteries. On reaching the decidua, a pathogen may spread intercellularly to decidual cells or trophoblasts, or may lyse its transporting host cell and invade another resident cell. The latter may explain the fetoplacental infection defect for L. monocytogenes lacking invasion receptors [38]. Thus, the placental pathogen’s intracellular lifecycle may be explained by multiple lines of evidence that the uterine interface is the preferred portal of entry into the placenta because EVT are much more susceptible to infection than syncytiotrophoblast.
Decidual infections play an important role in the colonization of hemochorial placentas, but what about epitheliochorial placentas, with limited trophoblast invasion and uterine modification? Experimental sheep infections with Chlamydophila abortus show that the first location of C. abortus antigen is within mononuclear cells in the endometrial stroma [39]. T. gondii is also first detected in caruncular cells before spreading to trophoblasts [40].
Finally, immune cells might also allow for barrier crossing either at the decidua or at sites of syncytial damage (Fig. 2, pt 3). Human immunodeficiency virus (HIV) cannot independently cross trophoblast monolayers, but their transport can be mediated by leukocytes [41]; in like manner, maternal leukocytes do enter the fetal compartment, possibly to promote tolerance [42], and it is feasible that a pathogen could hitch a ride. For example, Parvovirus B19 has not been reported in trophoblasts, but viral replication can be detected in fetal endothelia [43].
All told, the fact that a mere fraction of the maternal pathogen load manages to colonize the placenta—and even fewer reach the fetus [36]—suggest that the placental barrier is quite strong. It is plausible that crossing is facilitated by exceptional situations such as high pathogen titers or multiple infections. The “double-hit hypothesis” postulates that the healthy body is well evolved to withstand one pathogenic insult, but a second may overcome its defenses. It is well documented that HIV increases P. falciparum placental infection specifically [44]. In human term placentas, CMV and other bacteria or HSV are often found together [34]. Pregnant mice exposed to subclinical doses of lipopolysaccharide or gammaherpesvirus alone show no pregnancy problems, but the combination initiates synergistic inflammation (i.e., cytokines produced by both trophoblasts and decidua) and can induce pre-term birth [45,46].
Crossing not Required
Once the placenta is colonized, adverse pregnancy outcomes are not limited to fetal infection. Maternal illness is another possibility. It has long been recognized that pregnant women are more susceptible to severe listeriosis, and we have shown in guinea pigs that once the placenta is colonized with L. monocytogenes it can act as a nidus of infection from which bacteria repeatedly reinfect the mother [36]. Similar results have been described for S. enterica in normally resistant pregnant mice [33]. In sheep and goats, C. abortus and C. burnetii respectively replicate abundantly in placenta even as maternal lung and liver lesions resolve [39,47], and C. burnetii disperses from there to other maternal organs [47].
Placental insufficiency due to inflammation and trophoblast apoptosis is another danger. Even pathogens not normally thought to cause transplacental fetal infections may cause placental insufficiency—Chlamydophilia pneumoniae [48] and human papilloma virus [49] in EVT of human term placentas are associated with pre-eclampsia and preterm labor respectively.
Coevolution: Pathogen and Placenta
It is not surprising that evolution has selected for strong defense of the reproductive process, despite the need for an altered immune state [7]. The unusual histology of the placenta may explain how.
The extensive conservation of syncytiotrophoblast denotes its importance. Some—but not all—animals with minimally invasive epitheliochorial placentas show only patchy or absent syncytialization (horse, pig, camelids) [6,50], though they evolved convergently from more invasive ancestors [51]. Thus, closer contact with maternal blood is coincident with robust syncytialization. But why syncytialization, when cellularized monolayers are sufficient in other epithelial barriers? To some extent, this is logically related to the need to limit traffic and stimulation of maternal and fetal leukocytes; adaptively, it also protects against blood-borne pathogens.
Ironically, a pathogen may have enabled evolution of this very tissue. Molecular evidence suggests multiple now-endogenous retroviral insertions of env-like syncytin genes were responsible for the emergence of a syncytium [52,53]. In mice, the syncytin-A knockout is embryonic lethal due to impaired syncytiotrophoblast development [54].
So, successful placental pathogens must evolve to go around this barrier, trafficking inside of maternal cells to reach the somewhat more vulnerable mononuclear trophoblasts at the uterine interface. But these have defenses of their own—among them, the ability to terminate the pregnancy. Spontaneous abortion and inflammation-mediated pre-term labor may serve an adaptive function: to preserve maternal reproductive fitness. This outcome may also favor the pathogens’ goal—transmission—since in the mammalian wild, the placenta is almost certainly consumed by the mother or a scavenger. Exposure to aerosolized conceptus is the major means of transmission for Brucella spp. both within natural hosts and to others, including humans [55]. In dense livestock conditions, this route also accounts for the majority of C. burnetii transmission in cattle and C. abortus in sheep.
Conclusion
This review summarizes recent literature that supports a new paradigm for placental infections. Although highly variable in cross-species structure, the placenta presents multiple innate defenses against pathogens: a) a syncytiotrophoblast comprising most of the maternofetal interface, whose lack of intercellular junctions protects against multiple pathogens in the blood; b) a decidual-trophoblast environment rich in innate cellular defenses; and, c) physical obstacles, including a basement membrane. Few pathogens can circumvent these barriers, and those that can do so at relatively low frequencies with tragic consequences.
Highlights.
The placenta presents multiple defenses against pathogens
The syncytiotrophoblast lacks intercellular junctions that contribute to pathogen resistance
The uterine-trophoblast environment is rich in innate cellular defenses
The few pathogens that can circumvent these barriers have intracellular life cycles
Supplementary Material
Acknowledgements
We thank Drs. Mike McCune, Guillain Mikaty and Holly Morrison for critical reading of this manuscript. We are grateful for the excellent graphic design assistance of Joseph J. Hill, and thank Dr. Kurt Benirschke for feedback on the placental drawings. We apologize to our colleagues whose work could not be directly included due to length limitations.
Abbreviations
- CMV
cytomegalovirus
- EVT
extravillous cytotrophoblasts
- HIV
human immunodeficiency virus
- HSV
herpes simplex virus
- sCTB
subsyncytial cytotrophoblasts
- SYN
syncytiotrophoblast
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88(1):31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 4.Givens MD, Marley MS. Infectious causes of embryonic and fetal mortality. Theriogenology. 2008;70(3):270–285. doi: 10.1016/j.theriogenology.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maltepe E, Bakardjiev AI, Fisher SJ. The placenta: Transcriptional, epigenetic, and physiological integration during development. J Clin Invest. 2010;120(4):1016–1025. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leiser R, Kaufmann P. Placental structure: In a comparative aspect. Exp Clin Endocrinol. 1994;102(3):122–134. doi: 10.1055/s-0029-1211275. [DOI] [PubMed] [Google Scholar]
- 7.Aldo PB, Mulla MJ, Romero R, Mor G, Abrahams VM. Viral ssrna induces first trimester trophoblast apoptosis through an inflammatory mechanism. Am J Reprod Immunol. 2010;64(1):27–37. doi: 10.1111/j.1600-0897.2010.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher S, Genbacev O, Maidji E, Pereira L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: Implications for transmission and pathogenesis. J Virol. 2000;74(15):6808–6820. doi: 10.1128/jvi.74.15.6808-6820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koi H, Zhang J, Makrigiannakis A, Getsios S, MacCalman CD, Strauss JF, 3rd, Parry S. Syncytiotrophoblast is a barrier to maternal-fetal transmission of herpes simplex virus. Biol Reprod. 2002;67(5):1572–1579. doi: 10.1095/biolreprod.102.004325. [DOI] [PubMed] [Google Scholar]
- 10.Robbins JR, Skrzypczynska KM, Zeldovich VB, Kapidzic M, Bakardjiev AI. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog. 2010;6(1):e1000732. doi: 10.1371/journal.ppat.1000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robbins JR, Zeldovich VB, Poukchanski A, Boothroyd JC, Bakardjiev AI. Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect Immun. 2012 doi: 10.1128/IAI.05899-11. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogelmann R, Amieva MR, Falkow S, Nelson WJ. Breaking into the epithelial apical-junctional complex--news from pathogen hackers. Curr Opin Cell Biol. 2004;16(1):86–93. doi: 10.1016/j.ceb.2003.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lecuit M, Nelson DM, Smith SD, Khun H, Huerre M, Vacher-Lavenu MC, Gordon JI, Cossart P. Targeting and crossing of the human maternofetal barrier by Listeria monocytogenes : Role of internalin interaction with trophoblast E-cadherin. Proc Natl Acad Sci U S A. 2004;101(16):6152–6157. doi: 10.1073/pnas.0401434101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floridon C, Nielsen O, Holund B, Sunde L, Westergaard JG, Thomsen SG, Teisner B. Localization of E-cadherin in villous, extravillous and vascular trophoblasts during intrauterine, ectopic and molar pregnancy. Mol Hum Reprod. 2000;6(10):943–950. doi: 10.1093/molehr/6.10.943. [DOI] [PubMed] [Google Scholar]
- 15.Brown LM, Lacey HA, Baker PN, Crocker IP. E-cadherin in the assessment of aberrant placental cytotrophoblast turnover in pregnancies complicated by pre-eclampsia. Histochem Cell Biol. 2005;124(6):499–506. doi: 10.1007/s00418-005-0051-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99(9):2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maidji E, Genbacev O, Chang HT, Pereira L. Developmental regulation of human cytomegalovirus receptors in cytotrophoblasts correlates with distinct replication sites in the placenta. J Virol. 2007;81(9):4701–4712. doi: 10.1128/JVI.02748-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klaffenbach D, Rascher W, Rollinghoff M, Dotsch J, Meissner U, Schnare M. Regulation and signal transduction of toll-like receptors in human chorioncarcinoma cell lines. Am J Reprod Immunol. 2005;53(2):77–84. doi: 10.1111/j.1600-0897.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 19.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173(7):4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 20.Abrahams VM, Schaefer TM, Fahey JV, Visintin I, Wright JA, Aldo PB, Romero R, Wira CR, Mor G. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, poly(i : C) Hum Reprod. 2006;21(9):2432–2439. doi: 10.1093/humrep/del178. [DOI] [PubMed] [Google Scholar]
- 21.Abbasi M, Kowalewska-Grochowska K, Bahar MA, Kilani RT, Winkler-Lowen B, Guilbert LJ. Infection of placental trophoblasts by Toxoplasma gondii. J Infect Dis. 2003;188(4):608–616. doi: 10.1086/377132. [DOI] [PubMed] [Google Scholar]
- 22.Inaba H, Kuboniwa M, Bainbridge B, Yilmaz O, Katz J, Shiverick KT, Amano A, Lamont RJ. Porphyromonas gingivalis invades human trophoblasts and inhibits proliferation by inducing g1 arrest and apoptosis. Cell Microbiol. 2009;11(10):1517–1532. doi: 10.1111/j.1462-5822.2009.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeldovich VB, Robbins JR, Kapidzic M, Lauer P, Bakardjiev AI. Invasive extravillous trophoblasts restrict intracellular growth and spread of Listeria monocytogenes. PLoS Pathog. 2011;7(3):e1002005. doi: 10.1371/journal.ppat.1002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crocker IP, Tanner OM, Myers JE, Bulmer JN, Walraven G, Baker PN. Syncytiotrophoblast degradation and the pathophysiology of the malariainfected placenta. Placenta. 2004;25(4):273–282. doi: 10.1016/j.placenta.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate a in the human placenta. Science. 1996;272(5267):1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 26.Duaso J, Rojo G, Jana F, Galanti N, Cabrera G, Bosco C, Lopez-Munoz R, Maya JD, Ferreira J, Kemmerling U. Trypanosoma cruzi induces apoptosis in ex vivo infected human chorionic villi. Placenta. 2011;32(5):356–361. doi: 10.1016/j.placenta.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Le Monnier A, Autret N, Join-Lambert OF, Jaubert F, Charbit A, Berche P, Kayal S. ActA is required for crossing of the fetoplacental barrier by Listeria monocytogenes. Infect Immun. 2007;75(2):950–957. doi: 10.1128/IAI.01570-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferro EA, Silva DA, Bevilacqua E, Mineo JR. Effect of Toxoplasma gondii infection kinetics on trophoblast cell population in calomys callosus a model of congenital toxoplasmosis. Infect Immun. 2002;70(12):7089–7094. doi: 10.1128/IAI.70.12.7089-7094.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buendia AJ, Sanchez J, Martinez MC, Camara P, Navarro JA, Rodolakis A, Salinas J. Kinetics of infection and effects on placental cell populations in a murine model of Chlamydia psittaci -induced abortion. Infect Immun. 1998;66(5):2128–2134. doi: 10.1128/iai.66.5.2128-2134.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumgartner W, Bachmann S. Histological and immunocytochemical characterization of Coxiella burnetii-associated lesions in the murine uterus and placenta. Infect Immun. 1992;60(12):5232–5241. doi: 10.1128/iai.60.12.5232-5241.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: Implication of oral bacteria in preterm birth. Infect Immun. 2004;72(4):2272–2279. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S, Lee DS, Watanabe K, Furuoka H, Suzuki H, Watarai M. Interferon-gamma promotes abortion due to Brucella infection in pregnant mice. BMC Microbiol. 2005;5(22) doi: 10.1186/1471-2180-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chattopadhyay A, Robinson N, Sandhu JK, Finlay BB, Sad S, Krishnan L. Salmonella enterica serovar typhimurium-induced placental inflammation and not bacterial burden correlates with pathology and fatal maternal disease. Infect Immun. 2010;78(5):2292–2301. doi: 10.1128/IAI.01186-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira L, Maidji E, McDonagh S, Genbacev O, Fisher S. Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J Virol. 2003;77(24):13301–13314. doi: 10.1128/JVI.77.24.13301-13314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakardjiev AI, Stacy BA, Portnoy DA. Growth of Listeria monocytogenes in the guinea pig placenta and role of cell-to-cell spread in fetal infection. J Infect Dis. 2005;191(11):1889–1897. doi: 10.1086/430090. [DOI] [PubMed] [Google Scholar]
- 36.Bakardjiev AI, Theriot JA, Portnoy DA. Listeria monocytogenes traffics from maternal organs to the placenta and back. PLoS Pathog. 2006;2(6):e66. doi: 10.1371/journal.ppat.0020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tagliani E, Shi C, Nancy P, Tay CS, Pamer EG, Erlebacher A. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J Exp Med. 2011;208(9):1901–1916. doi: 10.1084/jem.20110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Disson O, Grayo S, Huillet E, Nikitas G, Langa-Vives F, Dussurget O, Ragon M, Le Monnier A, Babinet C, Cossart P, Lecuit M. Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis. Nature. 2008;455(7216):1114–1118. doi: 10.1038/nature07303. [DOI] [PubMed] [Google Scholar]
- 39.Navarro JA, Garcia de la Fuente JN, Sanchez J, Martinez CM, Buendia AJ, Gutierrez-Martin CB, Rodriguez-Ferri EF, Ortega N, Salinas J. Kinetics of infection and effects on the placenta of Chlamydophila abortus in experimentally infected pregnant ewes. Vet Pathol. 2004;41(5):498–505. doi: 10.1354/vp.41-5-498. [DOI] [PubMed] [Google Scholar]
- 40.Buxton D, Finlayson J. Experimental infection of pregnant sheep with Toxoplasma gondii : Pathological and immunological observations on the placenta and foetus. J Comp Pathol. 1986;96(3):319–333. doi: 10.1016/0021-9975(86)90052-6. [DOI] [PubMed] [Google Scholar]
- 41.Lagaye S, Derrien M, Menu E, Coito C, Tresoldi E, Mauclere P, Scarlatti G, Chaouat G, Barre-Sinoussi F, Bomsel M. Cell-to-cell contact results in a selective translocation of maternal human immunodeficiency virus type 1 quasispecies across a trophoblastic barrier by both transcytosis and infection. J Virol. 2001;75(10):4780–4791. doi: 10.1128/JVI.75.10.4780-4791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasquinelli G, Bonvicini F, Foroni L, Salfi N, Gallinella G. Placental endothelial cells can be productively infected by Parvovirus B19. J Clin Virol. 2009;44(1):33–38. doi: 10.1016/j.jcv.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Perrault SD, Hajek J, Zhong K, Owino SO, Sichangi M, Smith G, Shi YP, Moore JM, Kain KC. Human immunodeficiency virus co-infection increases placental parasite density and transplacental malaria transmission in western Kenya. Am J Trop Med Hyg. 2009;80(1):119–125. [PMC free article] [PubMed] [Google Scholar]
- 45.Cardenas I, Mor G, Aldo P, Lang SM, Stabach P, Sharp A, Romero R, Mazaki-Tovi S, Gervasi M, Means RE. Placental viral infection sensitizes to endotoxin-induced pre-term labor: A double hit hypothesis. Am J Reprod Immunol. 2011;65(2):110–117. doi: 10.1111/j.1600-0897.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth C, Manzur A, Oyarzun E, Romero R, Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185(2):1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez J, Souriau A, Buendia AJ, Arricau-Bouvery N, Martinez CM, Salinas J, Rodolakis A, Navarro JA. Experimental Coxiella burnetii infection in pregnant goats: A histopathological and immunohistochemical study. J Comp Pathol. 2006;135(2–3):108–115. doi: 10.1016/j.jcpa.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Gomez LM, Parry S. Trophoblast infection with Chlamydia pneumoniae and adverse pregnancy outcomes associated with placental dysfunction. Am J Obstet Gynecol. 2009;200(5):526, e521–e527. doi: 10.1016/j.ajog.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez LM, Ma Y, Ho C, McGrath CM, Nelson DB, Parry S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum Reprod. 2008;23(3):709–715. doi: 10.1093/humrep/dem404. [DOI] [PubMed] [Google Scholar]
- 50.Iturrizaga DM, Verechia FT, Santos TC, Bombonato PP, Teixeira DG, Miglino MA. The materno-fetal interface in llama (lama guanicoe glama) Pesquisa Veterinaria Brasileira. 2007;27(6):8. [Google Scholar]
- 51.Vogel P. The current molecular phylogeny of eutherian mammals challenges previous interpretations of placental evolution. Placenta. 2005;26(8–9):591–596. doi: 10.1016/j.placenta.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, Mandrand B, Mallet F, Cosset FL. An envelope glycoprotein of the human endogenous retrovirus herv-w is expressed in the human placenta and fuses cells expressing the type d mammalian retrovirus receptor. J Virol. 2000;74(7):3321–3329. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blaise S, de Parseval N, Benit L, Heidmann T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2 a gene conserved on primate evolution. Proc Natl Acad Sci U S A. 2003;100(22):13013–13018. doi: 10.1073/pnas.2132646100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dupressoir A, Vernochet C, Bawa O, Harper F, Pierron G, Opolon P, Heidmann T. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc Natl Acad Sci U S A. 2009;106(29):12127–12132. doi: 10.1073/pnas.0902925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roop RM, 2nd, Gaines JM, Anderson ES, Caswell CC, Martin DW. Survival of the fittest: How Brucella strains adapt to their intracellular niche in the host. Med Microbiol Immunol. 2009;198(4):221–238. doi: 10.1007/s00430-009-0123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sykes JA, Kalan J. Intracellular Treponema pallidum in cells of a syphilitic lesion of the uterine cervix. Am J Obstet Gynecol. 1975;122(3):361–367. doi: 10.1016/0002-9378(75)90185-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.