Abstract
Background
Because exposure to UV radiation accounts for a significant portion of endogenous vitamin D production, cutaneous lupus (CLE) subjects practicing sun-protective measures are at risk for vitamin D insufficiency. Previous studies have shown light-skinned CLE subjects to have lower serum 25-hydroxy (25-OH) vitamin D levels than normal controls.
Objective
To assess the status of vitamin D insufficiency in dark-skinned CLE individuals.
Methods
We performed a cross-sectional study comparing serum 25-OH vitamin D levels in 25 CLE and 26 normal African American (AA) age-, gender-, and season-matched subjects in Dallas, Texas. A questionnaire on demographics, medical history, and lifestyle habits was administered to determine factors potentially affecting vitamin D levels. Findings were contrasted to a similar comparison in 26 CLE and 24 age-, gender-, and season-matched normal Caucasian and Hispanic (C/H) subjects.
Results
We found similar 25-OH vitamin D levels in CLE (52.0±18.5 nmol/L) and normal AA subjects (54.8±21.2 nmol/L) (p = 0.62). Almost half of AA subjects in both groups were vitamin D-insufficient. A larger gap in 25-OH vitamin D levels was found in C/H CLE (59.4±21.0 nmol/L) and normal subjects (70.5±27.4 nmol/L) (p = 0.12). Two-way ANOVA analysis demonstrated that skin color (AA vs. C/H) had a significant effect on 25-OH vitamin D levels (p = 0.008), though CLE status (CLE vs. normal) did not (p = 0.13).
Conclusions
Providers are encouraged to address vitamin D insufficiency concerns in all dark-skinned individuals. Future studies should stratify subjects by skin color in determining differences between CLE and normal controls.
Keywords: African American, cutaneous lupus, skin color, vitamin D
Introduction
Vitamin D has consistently been shown to be immune-protective and anti-inflammatory via its down-regulation of cytokine-mediated inflammatory pathways and regulation of humoral- and cell-mediated immunity and dendritic cell response to antigens.1-5 For these reasons, vitamin D insufficiency has shown an association with systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE).6-10 Because CLE is an autoimmune disease that is often exacerbated by ultraviolet light, CLE subjects are encouraged to practice sun-protective measures and are increasingly predisposed to having lower vitamin D levels10 because endogenous levels of vitamin D depend on ultraviolet exposure.11
To date, a small number of studies have shown an association between CLE subjects and low vitamin D levels.6, 8-10 In a study of 55 CLE and 37 normal control age- and sex-matched subjects in Valencia, Spain, 25-hydroxy (25-OH) vitamin D levels were lower in CLE subjects (20.0±8.9 ng/mL) than in normal subjects (23.8±7.5 ng/mL) (p = 0.03).6 In Berlin, Germany, decreased serum levels of 25-OH vitamin D were found in 41 CLE subjects year-round, with lower levels noted in wintertime than in summertime.10 Another study demonstrated that 85% and 64% of subjects with subacute cutaneous lupus erythematosus (N=13) and chronic discoid lupus erythematosus (N=14), respectively, had insufficient vitamin D levels (defined as less than 50 nmol/L).9 In another study, approximately 65% of CLE subjects (N=52) showed low vitamin D levels, especially in sun avoiders and sunscreen users.8
Of note, none of the studies investigated dark-skinned individuals, specifically those with Fitzpatrick skin types V and VI. Vitamin D levels have been shown previously to be lower in subjects with darker skin types versus those with lighter skin types because of increased melanin in dark skin. This is likely due to melanin’s ability to block ultraviolet light and decrease endogenous production of vitamin D.11 The extent of vitamin D insufficiency in dark-skinned CLE individuals is unclear. Therefore, we conducted a study comparing 25-OH vitamin D levels in African American (AA) CLE subjects and age-, gender-, and season-matched normal controls, in which all were either skin type V or VI. For comparison, we also performed a secondary comparison of 25-OH vitamin D levels in age-, gender-, and season-matched Caucasian and Hispanic (C/H) CLE subjects and normal controls, ranging from skin type I to IV. For all subjects, we attempted to identify lifestyle habit differences between the groups that could affect 25-OH vitamin D levels.
Materials and Methods
Subject Recruitment
From April 11, 2007, to March 17, 2011, a total of 25 African American (AA) CLE subjects, 26 normal AA controls, 26 Caucasian/Hispanic (C/H) CLE subjects, and 24 C/H normal controls were recruited at the outpatient clinics of University of Texas Southwestern (UTSW) Medical Center and Parkland Memorial Hospital. All were registered into the UTSW Medical Center Cutaneous Lupus Registry or the Dallas Regional Autoimmune Disease Registry. Each CLE subject was individually matched for sex, age, skin color, and season of blood draw (November-April, May-October) with a normal subject to control for these potentially confounding factors. CLE subjects were defined as those having a diagnosis of cutaneous lupus based on clinical, histopathological, and laboratory findings at the time of serum draw. CLE subjects were excluded if any of the following were met at the time of serum draw: less than 18 years of age, prednisone greater than 10 mg/day, alanine aminotransferase and/or aspartate aminotransferase levels greater than 40 IU, and glomerular filtration rate less than 30 mL/minute. The criteria for inclusion of normal control subjects included: ANA less than 20 ELISA units and negative history of autoimmune diseases. Normal controls were excluded if they were less than 18 years of age or had history of liver disease, kidney disease, short bowel syndrome, or cystic fibrosis.
The design and execution of the studies were authorized by the Internal Review Board of the UTSW Medical Center. All subjects consented by written agreement to inclusion in this study, which was approved by the UTSW Institutional Review Board.
Measurement of 25-OH vitamin D levels
Once informed consents from subjects were obtained, blood draws were performed. Sera were separated from blood samples by centrifugation and stored in aliquots at −80°C. Following the manufacturer’s directions, 25-OH vitamin D levels were measured using the IDS 25-Hydroxy Vitamin D EIA kit (Immunodiagnostic Systems, Fountain Hills, AZ), which employs a sandwich enzyme-linked immunosorbant assay method. There is a cross-reactivity of 100% with 25-hydroxyvitamin D3 and 24, 25-dihydroxyvitamin D3, 75% with 25-hydroxyvitamin D2, and less than 0.3% with cholecalciferol (D3) and ergocalciferol (D2). Normal levels, as indicated by the manufacturer, of 25-OH vitamin D are above 47.7 nmol/L. Furthermore, the Institute of Medicine recently recommended having 25-OH vitamin D levels greater than 50 nmol/L sufficient for good bone health.12
A questionnaire was administered at the time of blood draw to identify demographics and lifestyle factors contributing to the subjects’ resulting vitamin D levels. For subjects whose serum samples were drawn without administration of questionnaires, phone interviews were conducted with subjects answering questions based on the time of blood drawn. Questionnaire information included: demographics, calcium and vitamin D supplementation, sun exposure, skin type, sun protective habits, occupation, and dietary habits. A previously validated sun protective habits score (SPH) was used to quantify sun protection by assessing five variables, including sun avoidance, sunscreen use, and wearing of sleeves, sunglasses, and hat.13, 14 Responses to questions were graded on a scale of 1 to 4, where 1 represented rare compliance and 4 represented complete compliance. Scores from these five questions were averaged.13, 14 Skin disease activity was evaluated for each CLE subject using the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI).15
Statistical Analysis
A sample size of 25 per group yielded sufficient power to detect a mean difference between groups in 25-OH vitamin D levels of 16 nmol/L with a standard deviation of 20 nmol/L, based on a type I error of 0.05 and type II error of 0.20. We summarized 25-OH vitamin D levels by mean and standard deviation or median and percentiles for each group. Two-way and three-way analysis of variance (ANOVA) models were used to assess the effects of disease status, race, and season on 25-OH vitamin D levels. 25-OH vitamin D was log transformed prior to conducting parametric analyses. Comparisons of lifestyle habits were performed using Wilcoxon Rank Sum test and Fisher’s exact tests for ordinal and nominal categorical variables, respectively. Statistical significance was declared for a two-sided p-value < 0.05. All statistical analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
Results
Subject characteristics
Characteristics of CLE and normal age-, gender-, and season-matched subjects are summarized in Tables 1 (AA subjects) and 2 (C/H subjects). Of note, there was a predominant female to male ratio in all populations. All AA subjects had skin type V or VI, and all C/H subjects had skin type I-IV, with the most prevalent being skin type III.
Table 1.
Subject Characteristics (AA)
| Group | CLE (N=25) |
Normal (N=26) |
p value | |
|---|---|---|---|---|
| Gender | Females | 23 | 24 | 0.97 |
| Males | 2 | 2 | ||
| Age (years) |
Mean | 42.0 | 41.7 | 0.93 |
| SD | 11.0 | 12.2 | ||
| Season | Winter/Spring (Nov-Apr) |
12 | 12 | 0.89 |
| Summer/Fall (May-Oct) |
13 | 14 | ||
| Skin Type† |
V | 13 | 10 | 0.55 |
| VI | 12 | 13 | ||
| BMI | Mean | 29.8 | 28.9 | 0.67 |
| SD | 7.3 | 7.1 | ||
| CLE Subtypes |
ACLE | 3 | NA | NA |
| DLE | 20 | |||
| SCLE | 2 | |||
| SLE Status†† |
Positive | 11 | N/A | N/A |
| Negative | 14 |
Abbreviations: ACLE = acute cutaneous lupus erythematosus, AA = African American, DLE = discoid lupus erythematosus, SCLE = subacute cutaneous lupus erythematosus, SD = standard deviation.
Skin type was not assessed in 3 normal AA controls.
SLE status is defined by the presence of four or more American College of Rheumatology criteria for the diagnosis of SLE.17
25-OH vitamin D levels in CLE and normal African American subjects
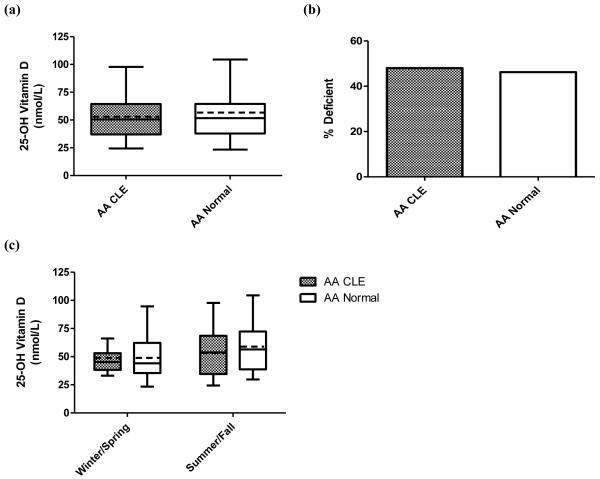
CLE and normal AA controls showed similar levels of 25-OH vitamin D. The overall mean 25-OH vitamin D levels for CLE AA subjects (N=25) and normal AA controls (N=26) were 52.0±18.5 nmol/L and 54.8±21.2 nmol/L, respectively (p = 0.62) (Fig. 1a). Vitamin D insufficiency (defined as less than 50 nmol/L)12 was present in 48.0% of CLE AA subjects and 46.2% of normal AA subjects (p = 0.89) (Fig. 1b). There were no statistically significant differences between CLE subjects and normal control AA subjects based on season of blood draw. Mean 25-OH vitamin D levels in winter/spring were 48.8±14.3 nmol/L for CLE AA subjects (N=12) and 49.6±19.6 nmol/L for normal AA controls (N=12) (p = 0.91). Likewise, summer/fall 25-OH vitamin D levels were 54.9±21.9 nmol/L and 59.2±22.2 nmol/L for CLE AA (N=13) and normal AA controls (N=14), respectively (p = 0.62) (Fig. 1c). There was no notable correlation observed between CLASI activity scores and 25-OH vitamin D levels in CLE AA subjects (data not shown).
Fig. 1.
25-OH vitamin D levels in African American (AA) CLE subjects and normal controls. (a) Mean 25-OH vitamin D levels among AA CLE (N=25) and normal controls (N=26). The boxes contain results between the 25th and 75th percentile, with the dark lines in the boxes representing the median (AA CLE=50.6 nmol/L, AA normal=51.7 nmol/L) and the dashed lines in the boxes representing the mean (AA CLE=52.0 nmol/L, AA normal=54.8 nmol/L). Traditional Tukey whiskers were used for each group. (b) Prevalence of vitamin D insufficiency in AA CLE (N=25) and normal controls (N=26). Vitamin D insufficiency is defined as less than 50 nmol/L. (c) Mean 25-OH vitamin D levels by season among AA CLE and normal controls. Winter/spring draws were obtained from CLE (N=12) and normal controls (N=12) from November to April. CLE (N=13) and normal controls (N=14) underwent summer/fall draws from May to October. The boxes contain results between the 25th and 75th percentile, with the dark lines in the boxes representing the median (AA CLE: winter/spring=45.3 nmol/L, summer/fall=53.8 nmol/L; AA normal: winter/spring=44.1 nmol/L, summer/fall=56.4 nmol/L) and the dashed lines in the boxes representing the mean (AA CLE: winter/spring=48.8 nmol/L, summer/fall=54.9 nmol/L; AA normal: winter/spring=49.6 nmol/L, summer/fall=59.2 nmol/L). Traditional Tukey whiskers were used for each group.
Lifestyle habits in CLE and normal African American subjects
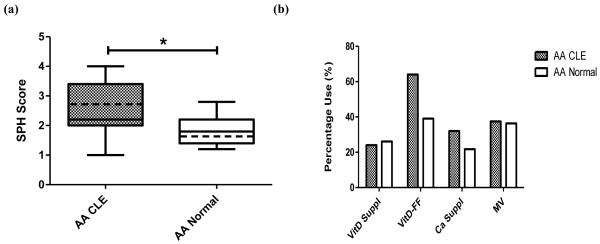
Lifestyle habits were assessed to account for their effect on 25-OH vitamin D levels. Since sun exposure and diet play integral roles in determining 25-OH vitamin D levels, information pertaining to sun protective habits, vitamin D supplementation, and inclusion of vitamin D-fortified foods in the diet were collected. CLE AA subjects (sun protective habits (SPH) average score = 2.6±0.9) were more likely to practice photoprotection than normal AA controls (SPH average = 2.0±0.6) (p = 0.003) (Fig. 2a). Little difference in vitamin D supplementation was seen between the two groups, as 24.0% of CLE AA subjects and 26.1% of normal control AA subjects practiced such behavior (p = 0.87). However, CLE AA subjects (64.0%) were more likely to include vitamin D-fortified foods in their diets as compared to normal control AA subjects (39.1%) (p = 0.08) (Fig. 2b). Other factors, including calcium and multi-vitamin supplementation (Fig. 2b), hours spent in the sun per week, and outdoor occupation (data not shown), were not found to be significantly different between groups.
Fig. 2.
Sun protective and dietary habits in AA CLE subjects and normal controls. (a) Sun protective habits (SPH) scores among AA CLE (N=25) and normal controls (N=23). SPH scores were based on averages of five categories (e.g. likelihood to use sleeves, sunscreen, hat, umbrella, and sunglasses). Each category was scored 1 to 4, where 1 = Rarely, 2 = Sometimes, 3 = Often, and 4 = Always. The boxes contain results between the 25th and 75th percentile, with the dark lines in the boxes representing the median (AA CLE=2.2, AA normal=1.8) and the dashed lines in the boxes representing the mean (AA CLE=2.6, AA normal=2.0). (b) Dietary habits among AA CLE and normal controls. The percentages of those taking vitamin D supplements (VitD Suppl), vitamin D-fortified foods (VitD-FF), calcium supplements (Ca Suppl), and multi-vitamins (MV) in the diet were calculated in AA CLE (N=25 for VitD Suppl, VitD-FF, and Ca Suppl; N=24 for MV) and normal controls (N=23 for VitD Suppl, VitD-FF, and Ca Suppl; N=22 for MV). *: p < 0.05.
25-OH vitamin D levels and lifestyle habits in CLE and normal Caucasians and Hispanic subjects
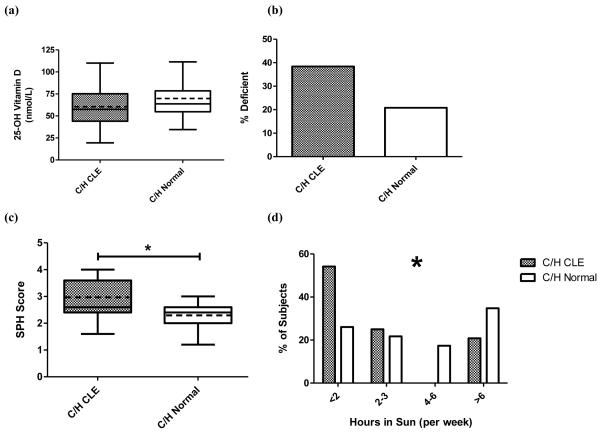
A greater difference in 25-OH vitamin D levels between CLE and normal C/H subjects was found compared to that between CLE and normal AA subjects. Overall mean 25-OH vitamin D levels were 59.4±21.0 nmol/L for CLE C/H subjects (N=26) and 70.5±27.4 nmol/L in normal C/H controls (N=24) (p = 0.12) (Fig. 3a). When looking at skin type I and II, the difference between CLE (59.3±20.0 nmol/L) and normal controls (88.2±36.9 nmol/L) was significant (p = 0.02) (Supplemental Fig. 1). Vitamin D insufficiency was present in 38.5% of CLE C/H subjects and 20.8% of normal C/H control subjects (p = 0.17) (Fig. 3b). There was no correlation between CLASI activity scores and 25-OH vitamin D levels in CLE C/H subjects (data not shown). Comparing lifestyle habits affecting vitamin D levels, CLE C/H subjects were more likely to practice photoprotection than normal C/H controls, with mean SPH averages of 3.0±0.7 and 2.3±0.5, respectively (p = 0.0005) (Fig. 3c). Normal C/H controls were found more likely to spend time in the sun than their CLE counterparts (p = 0.04) (Fig. 3d). Other factors, including vitamin D, calcium, and multi-vitamin supplementation and consumption of vitamin D-fortified foods, were not significantly different between C/H and CLE subjects and normal controls (data not shown).
Fig. 3.
25-OH vitamin D levels and sun protective habits in Caucasian and Hispanic (C/H) CLE and normal controls. (a) Mean 25-OH vitamin D levels among C/H CLE (N=26) and normal controls (N=24). The boxes contain results between the 25th and 75th percentile, with the dark lines in the boxes representing the median (C/H CLE=57.4 nmol/L, C/H normal=63.7 nmol/L) and the dashed lines in the boxes representing the mean (C/H CLE=59.4 nmol/L, C/H normal=70.5 nmol/L). (b) Prevalence of vitamin D insufficiency in C/H CLE and normal controls. Vitamin D insufficiency is defined as less than 50 nmol/L. (c) Sun protective habits (SPH) among C/H CLE (N=24) and normal controls (N=23). The boxes contain results between the 25th and 75th percentile, with the dark lines in the boxes representing the median (C/H CLE=2.6, C/H normal=2.4) and the dashed lines in the boxes representing the mean (CLE=3.0, normal=2.3). (d) Hours in sun among C/H CLE (N=24) and normal controls (N=23). Percentage of CLE and control C/H subjects spending <2, 2-3, 4-6, and >6 hours in the sun per week were calculated. *: p < 0.05.
Comparison of 25-OH vitamin D levels across skin color and disease groups
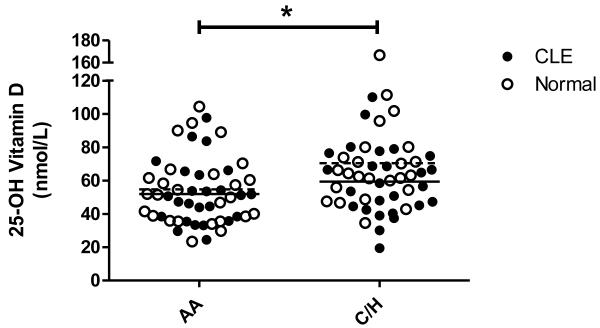
Two-way ANOVA analyses were performed to assess the effects of CLE disease status (i.e. CLE vs. normal) and skin color (i.e. AA vs. C/H) on 25-OH vitamin D levels. Controlling for CLE disease status, AAs were found to have significantly lower levels of 25-OH vitamin D than C/Hs (p = 0.008) (Fig. 4). However, when controlled for skin color, CLE subjects and normal controls were not found to have significantly different levels of 25-OH vitamin D (p = 0.13). A similar two-way ANOVA analysis was performed looking at skin type (i.e. skin type I/II vs. III/IV vs. V/VI) and CLE disease status when controlling for disease status. Skin type was found to have a significant effect on 25-OH vitamin D levels (p = 0.01). Three-way ANOVA analyses that evaluated contributions of CLE disease status, season (i.e. summer/fall vs. winter/spring), and skin color to 25-OH vitamin D levels confirmed that AAs still had significantly lower levels of 25-OH vitamin D than C/H, when controlled for CLE disease status and season (p = 0.003). CLE disease status (p = 0.12) and season (p = 0.07) did not show statistically significant effects on 25-OH vitamin D levels when controlled for other factors.
Fig. 4.
25-OH vitamin D levels in AA and C/H CLE subjects and normal controls. Levels were assessed in AA CLE (N=25) and normal subjects (N=26), and C/H CLE (N=26) and normal subjects (N=24). Mean lines are represented as solid lines for CLE subjects (AA=52.0 nmol/L, C/H=59.4 nmol/L) and dashed lines for normal subjects (AA=54.8 nmol/L, C/H=70.5 nmol/L). *: p < 0.05.
Discussion
This was a cross-sectional study comparing 25-OH vitamin D levels in AA and C/H CLE and normal control subjects, who were matched by age, gender, and season of serum draw to reduce confounding. This is the first study to date exclusively comparing 25-OH vitamin D levels in dark-skinned CLE subjects and normal controls. We found no statistically significant difference in 25-OH vitamin D levels between CLE and normal AA control subjects. Almost half of both CLE and normal AA subjects were vitamin D-insufficient. This observation is in agreement with previous reports of widespread vitamin D insufficiency in AAs.11 While we saw a greater difference in 25-OH vitamin D levels in CLE and normal C/H subjects, skin color appears to have a greater role than CLE disease status in determining 25-OH vitamin D levels in dark-skinned individuals.
Individually matched for age, gender, and season, CLE and normal AA subjects exhibited similar levels of 25-OH vitamin D. We investigated various lifestyle habits, particularly those related to photoprotection and diet, which could help explain the non-difference seen between CLE subject and normal control 25-OH vitamin D levels in AAs. As expected, CLE AA subjects displayed a significantly greater likelihood to practice photoprotection (exemplified by average SPH score) versus normal AA subjects, contributing to a decrease in CLE AA subjects’ 25-OH vitamin D levels. In contrast, the greater tendency of AA CLE subjects to include vitamin D-fortified foods in their diets may result in a relative increase in 25-OH vitamin D levels and could partially counteract the decrease caused by photoprotective methods of CLE subjects. No significant differences were seen in other forms of supplementation, including vitamin D, calcium, and multi-vitamins.
On the other hand, there was a larger difference in 25-OH vitamin D levels between C/H CLE and normal subjects. Moreover, skin type I and II CLE subjects had significantly lower 25-OH vitamin D levels than their normal counterparts. When considering the impact of these lifestyle factors in determining 25-OH vitamin D levels in C/H subjects, C/H CLE subjects were significantly more likely to practice photoprotection than their normal counterparts. This lifestyle factor was likely the most critical in causing a subsequent decrease in 25-OH vitamin D levels in CLE subjects. Dietary adjustments through vitamin D supplements, calcium supplements, multi-vitamins, and vitamin D-fortified foods were not found to be different between both groups (data not shown).
The 25-OH vitamin D and vitamin D insufficiency differences seen in CLE and normal Caucasian and Hispanic individuals reflect similar findings seen in previous studies in European populations.6, 10 This was supported by our data showing that CLE subjects were significantly more likely to practice photoprotection and spend less time outdoors than their normal counterparts. Thus, for lighter-skinned individuals, where CLE subjects had lower levels of 25-OH vitamin D than normal controls, CLE disease status plays a greater role in affecting 25-OH vitamin D levels. As such, we encourage practitioners to pay close attention to educating light-skinned CLE subjects on vitamin D supplementation in addition to their sun protective habits.
The overall lower levels of 25-OH vitamin D in both CLE and normal AA subjects compared to their C/H counterparts were noteworthy. Thus, the effects of skin color (AA vs. C/H) and CLE disease status (CLE vs. normal) on 25-OH vitamin D levels were compared in a two-way ANOVA analysis. The analysis confirmed a statistically significant effect of skin color on 25-OH vitamin D levels, but yielded a non-significant effect of CLE disease status. Thus, we postulate that skin color plays a greater role in affecting 25-OH vitamin D levels in dark-skinned individuals defined by Fitzpatrick skin type V and VI than CLE disease status. This finding suggests that the melanin content in dark skin can decrease endogenous production of vitamin D more than the photoprotective methods that CLE subjects regularly practice. Interestingly, for the CLE AA subjects, who were significantly more likely to protect themselves from the sun than their normal controls, their photoprotective practices did not further substantially decrease their 25-OH vitamin D levels. Our findings heighten awareness for providers to screen all dark-skinned individuals, with or without CLE, for vitamin D insufficiency and counsel them appropriately about vitamin D supplementation.
Only one other study has compared 25-OH vitamin D levels between lupus subjects and normal controls, who were stratified by skin color. A study of 123 SLE subjects and 240 controls, which consisted of 138 AAs and 225 Caucasians, showed that SLE Caucasians had significantly lower 25-OH vitamin D levels than normal Caucasian controls (p = 0.04), but this trend was not seen in AA SLE and normal subjects (p = 0.36).16 Compared to the difference between Caucasian SLE and control subjects, the smaller, non-significant difference in 25-OH vitamin D levels in AA SLE and controls aligns with our results.
Limitations to this study included lifestyle habits data that were gathered retrospectively. For subjects who did not fill out questionnaires when blood was drawn, phone interviews were conducted by asking them to answer questions based on recollection at the time of blood draw, thus introducing recall bias. A small amount of lifestyle habit data was not collected, which unlikely affected statistical analyses greatly. While the study had a small sample size and recruitment was limited to a tertiary care university hospital, the findings between CLE and normal subjects showed a significant effect of skin color on 25-OH vitamin D levels rather than CLE disease status. Larger multi-center studies could be pursued in the future to confirm this novel finding. Finally, dietary supplementation and intake of vitamin D-fortified foods were not quantified since most subjects could not recall definitive amounts.
In conclusion, this study showed that AA CLE and normal subjects had similar 25-OH vitamin D levels, with almost half being vitamin D-insufficient. Thus, CLE and normal dark-skinned individuals are similarly at risk for vitamin D insufficiency. In comparison, 25-OH vitamin D levels trended lower in CLE C/H subjects compared to normal counterparts, particularly in the lightest skin types. Thus, the study demonstrated a significant effect of skin color, rather than CLE disease status, on 25-OH vitamin D levels in dark-skinned individuals. We encourage practitioners to educate these subjects to supplement their diets with vitamin D through vitamin D supplements, multi-vitamins, or vitamin D-fortified foods.
Supplementary Material
Supplementary Fig. 1. 25-OH vitamin D levels in CLE subjects and normal controls, stratified by skin type. Levels were assessed in CLE (N=9) and normal subjects (N=8) with skin type I/II, CLE (N=15) and normal subjects (N=15) with skin type III/IV, and CLE (N=25) and normal subjects (N=23) with skin type V/VI. Skin types were determined clinically. The boxes contain results between the 25th and 75th percentile, with the dark lines in the boxes representing the median (Skin Type I/II: CLE=64.8 nmol/L, normal=75.7 nmol/L; Skin Type III/IV: CLE=53.5 nmol/L, normal=62.5 nmol/L; Skin Type V/VI: CLE=50.6 nmol/L, normal=52.0 nmol/L) and the dashed lines in the boxes representing the mean (Skin Type I/II: CLE=59.3 nmol/L, normal=88.2 nmol/L; Skin Type III/IV: CLE=57.6 nmol/L, normal=62.9 nmol/L; Skin Type V/VI: CLE=52.0 nmol/L, normal=55.9 nmol/L). Differences between medians and means for other skin type categories were less than 5 nmol/L between CLE subjects and normal controls. *: p < 0.05.
What’s already known about this topic?
Light-skinned CLE subjects have lower serum 25-hydroxy (25-OH) vitamin D levels and greater vitamin D insufficiency (defined as less than 50 nmol/L) prevalence versus normal control subjects.
What does this study add?
African American CLE subjects and age-, gender-, and season-matched African American normal controls have similarly low 25-OH vitamin D levels.
Skin color may play a greater role in affecting 25-OH vitamin D levels than CLE status.
Table 2.
Subject Characteristics (C/H)
| Group | CLE (N=26) |
Normal (N=24) |
p value | |
|---|---|---|---|---|
| Gender | Females | 25 | 23 | 0.95 |
| Males | 1 | 1 | ||
| Age (years) |
Mean | 48.2 | 47.7 | 0.91 |
| SD | 12.9 | 13.4 | ||
| Season | Winter/Spring (Nov-Apr) |
17 | 17 | 0.68 |
| Summer/Fall (May-Oct) |
9 | 7 | ||
| Ethnicity | Caucasian | 23 | 22 | 0.71 |
| Hispanic | 3 | 2 | ||
| Skin Type† |
I | 5 | 4 | 0.82 |
| II | 4 | 4 | ||
| III | 11 | 13 | ||
| IV | 4 | 2 | ||
| BMI | Mean | 27.8 | 27.2 | 0.67 |
| SD | 7.2 | 5.1 | ||
| CLE Subtypes |
ACLE | 3 | N/A | N/A |
| Bullous | 1 | |||
| DLE | 13 | |||
| SCLE | 8 | |||
| TLE | 1 | |||
| SLE Status†† |
Positive | 16 | N/A | N/A |
| Negative | 10 |
Abbreviations: ACLE = acute cutaneous lupus erythematosus, C/H = Caucasian/Hispanic, DLE = discoid lupus erythematosus, SCLE = subacute cutaneous lupus erythematosus, SD = standard deviation, TLE = tumid lupus erythematosus.
Skin type was not assessed in 2 C/H CLE subjects and 1 normal C/H control.
SLE status is defined by the presence of four or more American College of Rheumatology criteria for the diagnosis of SLE.17
Acknowledgements
We would like to thank David Karp, Jack Cohen, Melissa Costner, Valerie Branch, Julie Song, Jonathan Tran, and Azza Mutwally for aiding in the recruitment of the study participants.
Financial Support: This project was funded in part by NIH CTSA Grant #UL1 RR024982.
Abbreviations
- 25-OH vitamin D
25-hydroxyvitamin D
- AA
African Americans
- C/H
Caucasians and Hispanics
- CLE
cutaneous lupus erythematosus
- CLASI
Cutaneous Lupus Erythematosus Disease Area and Severity Index
- SLE
systemic lupus erythematosus
Footnotes
Conflicts of Interest: Dr. Chong is an investigator for Celgene Corporation and Amgen Incorporated.
References
- 1.Chen S, et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179(3):1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Zvi I, et al. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS One. 2010;5(2):e9193. doi: 10.1371/journal.pone.0009193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamen D, Aranow C. Vitamin D in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20(5):532–7. doi: 10.1097/BOR.0b013e32830a991b. [DOI] [PubMed] [Google Scholar]
- 4.Borba VZ, et al. Vitamin D deficiency in patients with active systemic lupus erythematosus. Osteoporos Int. 2009;20(3):427–33. doi: 10.1007/s00198-008-0676-1. [DOI] [PubMed] [Google Scholar]
- 5.Nancy AL, Yehuda S. Prediction and prevention of autoimmune skin disorders. Arch Dermatol Res. 2009;301(1):57–64. doi: 10.1007/s00403-008-0889-3. [DOI] [PubMed] [Google Scholar]
- 6.Cutillas-Marco E, et al. Serum 25-hydroxyvitamin D levels in patients with cutaneous lupus erythematosus in a Mediterranean region. Lupus. 2010;19(7):810–4. doi: 10.1177/0961203309360807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thudi A, et al. Vitamin D levels and disease status in Texas patients with systemic lupus erythematosus. Am J Med Sci. 2008;335(2):99–104. doi: 10.1097/MAJ.0b013e318134eeb6. [DOI] [PubMed] [Google Scholar]
- 8.Cusack C, et al. Photoprotective behaviour and sunscreen use: impact on vitamin D levels in cutaneous lupus erythematosus. Photodermatol Photoimmunol Photomed. 2008;24(5):260–7. doi: 10.1111/j.1600-0781.2008.00373.x. [DOI] [PubMed] [Google Scholar]
- 9.Renne J, Werfel T, Wittmann M. High frequency of vitamin D deficiency among patients with cutaneous lupus erythematosus [corrected] Br J Dermatol. 2008;159(2):485–6. doi: 10.1111/j.1365-2133.2008.08632.x. [DOI] [PubMed] [Google Scholar]
- 10.Heine G, et al. Vitamin D deficiency in patients with cutaneous lupus erythematosus is prevalent throughout the year. Br J Dermatol. 2010;163(4):863–5. doi: 10.1111/j.1365-2133.2010.09948.x. [DOI] [PubMed] [Google Scholar]
- 11.Egan KM, et al. Vitamin D insufficiency among African-Americans in the southeastern United States: implications for cancer disparities (United States) Cancer Causes Control. 2008;19(5):527–35. doi: 10.1007/s10552-008-9115-z. [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine . Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; Washington, D.C.: 2010. [PubMed] [Google Scholar]
- 13.Glanz K, et al. Skin cancer prevention in outdoor recreation settings: effects of the Hawaii SunSmart Program. Eff Clin Pract. 2000;3(2):53–61. [PubMed] [Google Scholar]
- 14.Geller AC, et al. Impact of skin cancer prevention on outdoor aquatics staff: the Pool Cool program in Hawaii and Massachusetts. Prev Med. 2001;33(3):155–61. doi: 10.1006/pmed.2001.0870. [DOI] [PubMed] [Google Scholar]
- 15.Albrecht J, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005;125(5):889–94. doi: 10.1111/j.0022-202X.2005.23889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamen DL, et al. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev. 2006;5(2):114–7. doi: 10.1016/j.autrev.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Tan EM, et al. Special Article - the 1982 Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis Rheum. 1982;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. 25-OH vitamin D levels in CLE subjects and normal controls, stratified by skin type. Levels were assessed in CLE (N=9) and normal subjects (N=8) with skin type I/II, CLE (N=15) and normal subjects (N=15) with skin type III/IV, and CLE (N=25) and normal subjects (N=23) with skin type V/VI. Skin types were determined clinically. The boxes contain results between the 25th and 75th percentile, with the dark lines in the boxes representing the median (Skin Type I/II: CLE=64.8 nmol/L, normal=75.7 nmol/L; Skin Type III/IV: CLE=53.5 nmol/L, normal=62.5 nmol/L; Skin Type V/VI: CLE=50.6 nmol/L, normal=52.0 nmol/L) and the dashed lines in the boxes representing the mean (Skin Type I/II: CLE=59.3 nmol/L, normal=88.2 nmol/L; Skin Type III/IV: CLE=57.6 nmol/L, normal=62.9 nmol/L; Skin Type V/VI: CLE=52.0 nmol/L, normal=55.9 nmol/L). Differences between medians and means for other skin type categories were less than 5 nmol/L between CLE subjects and normal controls. *: p < 0.05.