Abstract
Enhanced sensitivity to noxious stimuli and the perception of non-noxious stimuli as painful are hallmark sensory perturbations associated with chronic pain. It is now appreciated that ATP, through its actions as an excitatory neurotransmitter, plays a prominent role in the initiation and maintenance of chronic pain states. Mechanistically, the ability of ATP to drive nociceptive sensitivity is mediated through direct interactions at neuronal P2X3 and P2X2/3 receptors. Extracellular ATP also activates P2X4, P2X7, and several P2Y receptors on glial cells within the spinal cord, which leads to a heightened state of neural-glial cell interaction in ongoing pain states. Following the molecular identification of the P2 receptor superfamilies, selective small molecule antagonists for several P2 receptor subtypes were identified, which have been useful for investigating the role of specific P2X receptors in preclinical chronic pain models. More recently, several P2X receptor antagonists have advanced into clinical trials for inflammation and pain. The development of orally bioavailable blockers for ion channels, including the P2X receptors, has been traditionally difficult due to the necessity of combining requirements for target potency and selectivity with suitable absorption distribution, metabolism, and elimination properties. Recent studies on the physicochemical properties of marketed orally bioavailable drugs, have identified several parameters that appear critical for increasing the probability of achieving suitable bioavailability, central nervous system exposure, and acceptable safety necessary for clinical efficacy. This review provides an overview of the antinociceptive pharmacology of P2X receptor antagonists and the chemical diversity and drug-like properties for emerging antagonists of P2X3, P2X2/3, P2X4, and P2X7 receptors.
Keywords: Physicochemical parameters, P2X4, P2X7, P2X3, Pain
Introduction
The ability to detect and react to noxious environmental stimuli is a highly adaptive physiological response that is mediated through the coordinated processing of sensory information by the peripheral and central nervous systems [1–4]. Chronic pain in the presence or absence of tissue trauma is characterized by increased sensitivity to painful stimuli (i.e., hyperalgesia) and the perception of pain in response to normally innocuous stimuli (i.e., allodynia) [4]. Based on many studies of the neurobiology of pain over the last 20 years, it is now appreciated that both peripheral and central neural pathways become “sensitized” in response to ongoing nociceptor stimulation [3]. This sensitized state of sensory function is mediated, at least in part, by neurochemical and phenotypic changes within sensory neurons as well as changes in neural-glial cell interactions at multiple sites including peripheral nerves, spinal cord and brain [3, 4].
A wide variety of pronociceptive neurotransmitters and neuromodulators including glutamate, substance P, CGRP, pro-inflammatory cytokines (e.g., interleukin-1β and tumor necrosis factor-α), and neurotrophic factors (e.g., nerve growth factor) contribute to initiation and maintenance of heightened states of sensory neuron excitability associated with chronic pain [4]. Emerging data also support a key role for ATP, acting as an excitatory neurotransmitter, in both acute and persistent nociception. It had been appreciated for many years that extracellular ATP can excite nerves [5, 6]. ATP administered intradermally elicits pain in humans under normal conditions and enhances inflammatory-mediated pain [6, 7]. Similar effects have been produced in experimental pain models [8–11] using ATP and structurally related agonists such as α,β-methylene ATP [1]. A mechanistically specific role for ATP as a pain neurotransmitter was provided by demonstrations that distinct ATP-gated channels are expressed on sensory neurons and that genetic and/or pharmacological inhibition of channel function led to decreased pain sensitivity in experimental models [5–7, 11].
The ability of ATP to modulate neuronal excitability associated with nociception is mediated by at least two mechanistically distinct pathways. ATP can directly activate homomeric and heteromeric P2X3 receptors on central and distal processes of peripheral nerves which results in the sensation of acute pain. ATP can also indirectly modulate chronic nociception by activating both P2X and P2Y receptors on non-neural-glial cells (astrocytes and microglia) in the periphery and spinal cord [11].
Based on the emerging appreciation for the role of P2X receptors in mediating nociceptive neurotransmission, the development of P2X receptor-selective antagonists as research tools and, in the context of pain management, as potential therapeutics has received much interest. This effort has incorporated a wide variety of medicinal chemistry research strategies including iterative structure activity relationship studies based on the prototypical purinergic antagonists suramin and pyridoxalphosphate-6-azophenyl-2′,5′-disulfonic acid (PPADS), as well as the application of high throughput screening of large chemical libraries [10, 11]. To date, structurally diverse receptor-selective antagonists for P2X3 and P2X7 receptors have been identified. Additionally, three dimensional crystal structures of P2X2 [12] and P2X4 [13] receptors have been identified which may aid the discovery of potent antagonists for these P2X receptor subunits.
Physicochemical properties of orally bioavailable drugs Over the last decade, the number of drugs reaching the market has continued to decrease with the rate of attrition rising to as much as 98% [14–20]. One noticeable trend during this timeframe is that the molecular weight and lipophilicity of molecules entering clinical studies has continued to increase [15]. In this context, the physicochemical properties of successful drugs and drug candidates can be used as key parameters for in silico predictions of in vitro absorption, distribution, metabolism, and elimination (ADME), oral bioavailability, and off-target toxicity, in attempts to improve the probabilities of success throughout the drug discovery process (Table 1). Lipinski et al. [14] first articulated a series of physicochemical parameters for drug solubility and permeability that may improve the probability of identifying compounds with acceptable (>30%) oral bioavailability. These parameters have subsequently become known as the rule of (RO)-5 because they are based on 5 s or multiples of 5 s and include the following parameter thresholds: MW < 500, ClogP < 5, HBA < 10, and HBD < 5. Lipinski found that 90% of the drugs from the World Drug Index violated not more than one of these rules [14]. Since then, a number of investigators have generated more quantitatively correlated subsets of molecular physicochemical properties related to the absorption, solubility, permeability, oral bioavailability, target specificity, and toxicity of drugs and clinically evaluated molecules [15–23]. These parameters have been used to assess the relative probability of success of clinical lead optimization research for specific drug-like compounds that have demonstrated proof of concept in subsequent clinical testing [15–23].Recently, Wager et al. [24] analyzed currently marketed drugs for a variety of central nervous system (CNS) disorders and developed a multi-parameter optimization (MPO) score, which is calculated from six in silico physicochemical properties (ClogP, ClogD, TPSA, MW, HBD, and pKa). This score is expressed as a single number ranging from 0 to 6. The reader is referred to this review and the references therein for full details [24]. In this study, the authors found that 74% of currently marketed CNS drugs have an MPO score greater than 4, which defines the desirable drug-like space. They also demonstrate that CNS drugs and clinical candidates with higher MPO scores also have a higher probability of achieving ADME and safety criteria typically necessary for successful advancement into clinical trials. Based on this analysis, novel lead molecules for CNS disorders with MPO scores greater than 4 should also have a higher probability of having acceptable oral bioavailability.The intrinsic molecular efficiency for which novel molecules bind their targets is also another critical parameter for achieving an acceptable efficacy and side-effect profile for drug candidates. Abad-Zapatero and Metz [25] illustrated the importance of binding efficiency (BEI) for a target as defined by (pIC50)/(molecular weight (MW)/1,000)), which is a measure of target potency relative to MW. This parameter is based on the hypothesis that increasing the potency of compounds within a chemical series solely by increasing the MW is less likely to improve the drug-like properties of the series relative to increasing the intrinsic binding efficiency [23]. For many CNS drugs (e.g., G-protein-coupled receptor antagonists), it can be assumed that a lead-like molecules would have an IC50 value less than or equal to 100 nM and a MW threshold of 500, thus producing a BEI greater than or equal to 14. For successful marketed oral drugs, the majority have a BEI greater than 19 (Cox, unpublished analysis). For the purposes of this review, P2X antagonists with BEI greater than 14 are characterized in lead-like chemical space and those with BEI greater than 19 are considered in drug-like space. Table 1 summarizes physicochemical properties, MPO score and BEI parameters that define the characteristics of the majority of marketed orally bioavailable drugs and a subset of orally bioavailable CNS drugs.
Table 1.
Physicochemical properties of orally bioavailable drugs
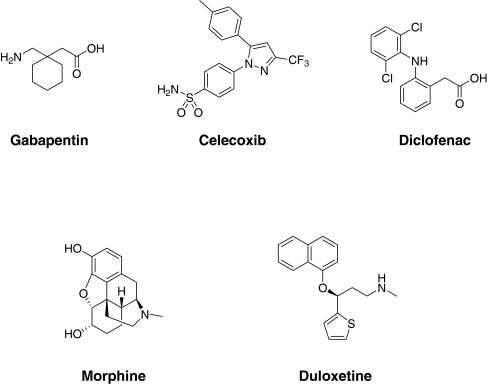
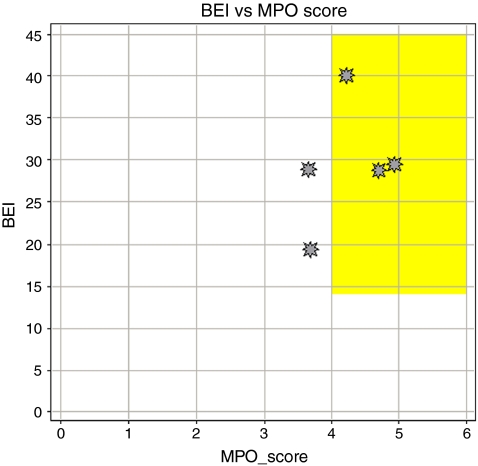
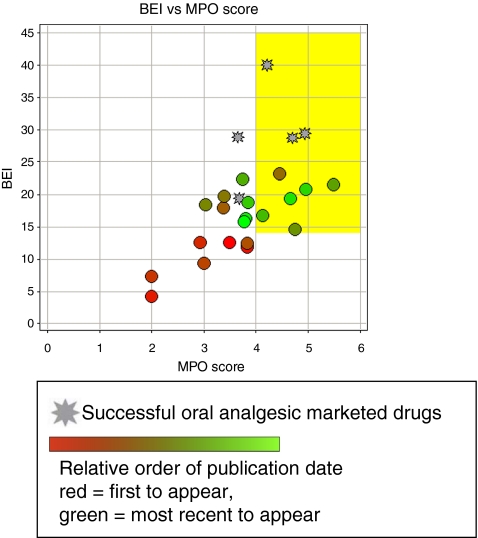
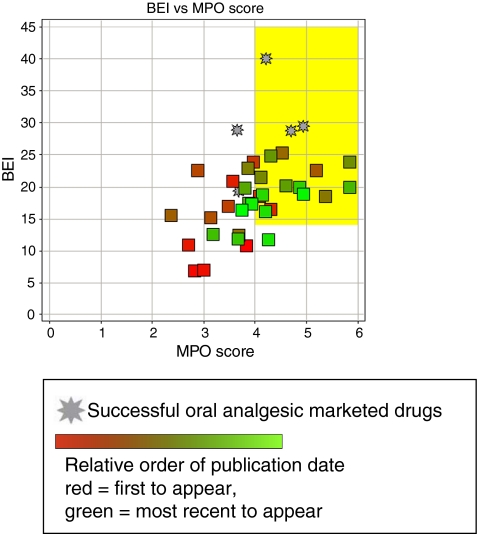
Drug-like properties of current analgesics Figure 1 and Table 2 show five marketed, orally bioavailable analgesic drugs from different chemical classes along with their putative mechanism(s) of action. To test how these oral analgesic drugs (Fig. 1) compare with respect to these properties, we have plotted the BEI against the MPO score for the five drugs (Fig. 2). This plot demonstrates how the five analgesic drugs fit into or close to BEI/MPO drug space (i.e., the yellow box represents the optimal lead-like chemical space based on the analysis described above). Three out of five of the analgesics meet the MPO criteria for the more desirable drug space while they all meet the proposed drug space in terms of binding efficiency index. Celecoxib and duloxetine are the two drugs just under the desirable CNS MPO score of >4. Although this is a relatively small sample size, this MPO analysis is fairly consistent with the observed data from the original publication where 26% of CNS drugs have MPO scores of <4 [24]. This analysis can be readily applied to the evaluation of novel ligands with the expectation that compounds falling within the yellow box will generally have more drug-like characteristics compared to compounds falling outside of the yellow box. This prediction may be even more apparent for compounds that fail to meet both criteria for lead-like chemical space (i.e., BEI, <14 and MPO, <4) relative to compounds that fulfill both criteria. In this review, we have used this analysis to evaluate the drug-like properties of antagonists for the P2X receptors involved in persistent nociception.
Fig. 1.
Chemical structures of representative orally bioavailable analgesic drugs
Table 2.
In vitro potency and physicochemical summary of selected analgesic drugs
| Name | MOA | IC50 (nM) | BEI | MPO score | MW | CLogP | PSA | HBA | HBD | LOGD | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gabapentin | Voltage-gated calcium channel 2 subunit ligand | 140 | 40.03 | 4.2 | 171.3 | −0.7 | 63.32 | 3 | 2 | −1.6 | [77] |
| Celecoxib | COX2 inhibitor | 40 | 19.4 | 3.7 | 381.4 | 4.4 | 77.98 | 3 | 1 | 3.4 | [78] |
| Diclofenac | NSAID | 3 | 28.78 | 4.7 | 296.2 | 4.7 | 49.33 | 3 | 2 | 1.4 | [79] |
| Morphine | μ opioid receptor agonist | 4 | 29.43 | 4.9 | 285.4 | 0.6 | 52.93 | 4 | 2 | 0.1 | [80] |
| Duloxetine | SNRI | 2.6 | 28.87 | 3.7 | 297.4 | 4.3 | 21.26 | 2 | 1 | 2.2 | [81] |
COX-2 cyclooxygenase-2, NSAID non-steroidal anti-inflammatory drug, SNRI serotonin norepinepherine reuptake inhibitor
Fig. 2.
Comparison of binding efficiency and multi-parameter analysis (MPO) for the orally bioavailable drugs shown in Fig. 1
Analgesic pharmacology and drug-like properties of P2X receptor antagonists
P2X3 receptors
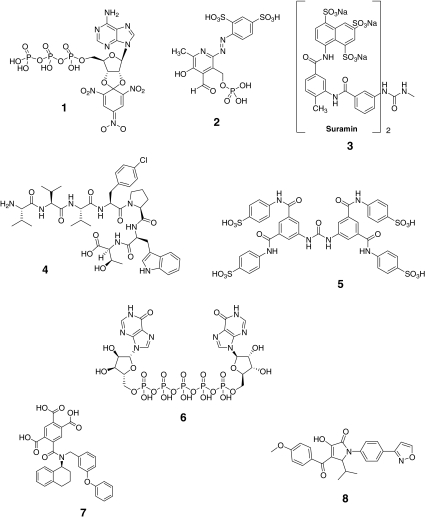
Table 3 and Fig. 3 show summary data and chemical structures, respectively, for known P2X3/P2X2/3 receptor antagonists. PPADS (compound 2) and Suramin (compound 3) are two nonselective P2X receptor antagonists that have been studied in a wide variety of animal pain models [8, 26–31]. The utility of these antagonists for delineating mechanistically specific contributions of individual P2X receptors to pain is limited by their nonselective pharmacology and generally weak potency [10]. The poly-pharmacological activities of early P2X receptor antagonists have also generated conflicting reports of both pronociceptive and antinociceptive effects following P2X receptor blockade [26].
Table 3.
In vitro potency and physicochemical summary of antagonists for P2X3 receptors
| Compound no. | Name | P2X3 IC50 (nM) | P2X2/3 IC50 (nM) | BEI P2X3 | MPO score | MW | CLogP | PSA | HBA | HBD | LOGD | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | TNP-ATP | 1 | 7 | 12.6 | 3.5 | 714 | −6.4 | 398 | 23 | 5 | −1.7 | [82] |
| 2 | PPADS | 1,000 | 11.8 | 3.8 | 507 | −9.5 | 262 | 15 | 5 | −2.6 | [82] | |
| 3 | Suramin | 3,000 | 4.3 | 2.0 | 1,291 | −27.4 | 501 | 23 | 12 | −2.5 | [82] | |
| 4 | Spinorphin | 0.008 | >10,000 | 12.6 | 2.9 | 877 | 1.0 | 285 | 11 | 10 | 0.4 | [82] |
| 5 | NF-110 | 36 | 7.4 | 2.0 | 1,005 | −17.9 | 386 | 17 | 10 | −2.1 | [83] | |
| 6 | IP5I | 3 | 2,800 | 9.4 | 3.0 | 913 | −8.1 | 483 | 28 | 11 | −8.6 | [82] |
| 7 | A-317491 | 100 | 100 | 12.4 | 3.8 | 564 | −0.9 | 147 | 8 | 3 | 0.7 | [82] |
| 8 | 10 | 17.9 | 3.4 | 447 | 6.0 | 93 | 5 | 1 | 2.3 | [84] | ||
| 9 | RO-3 | 100 | 1,000 | 23.2 | 4.5 | 302 | 2.7 | 96 | 6 | 2 | 2.3 | [36] |
| 10 | RO-4 | 13 | 25 | 19.7 | 3.4 | 400 | 3.9 | 96 | 6 | 2 | 3.3 | [36] |
| 11 | RO-51 | 2 | 5 | 18.4 | 3.0 | 474 | 3.6 | 123 | 8 | 4 | 2.5 | [85] |
| 12 | RO-85 | 398 | >5,000 | 14.6 | 4.8 | 440 | 3.3 | 70 | 4 | 1 | 2.7 | [86] |
| 13 | 2.8 | 10 | 21.5 | 5.5 | 399 | 2.4 | 86 | 5 | 1 | 2.0 | [84] | |
| 14 | 2 | 10 | 22.4 | 3.7 | 394 | 4.0 | 93 | 5 | 2 | 2.3 | [84] | |
| 15 | 11 | 11 | 16.8 | 4.1 | 475 | 2.9 | 86 | 5 | 1 | 3.6 | [84] | |
| 16 | 8 | 18.8 | 3.9 | 430 | 4.0 | 68 | 4 | 1 | 4.0 | [87] | ||
| 17 | 9 | 27 | 20.8 | 5.0 | 387 | 3.1 | 87 | 7 | 1 | 3.6 | [84] | |
| 18 | 7 | 9 | 19.4 | 4.7 | 420 | 3.1 | 92 | 5 | 1 | 2.9 | [88] | |
| 19 | AZ-2 | 13 | >3,900 | 16.3 | 3.8 | 485 | 3.8 | 82 | 6 | 1 | 3.5 | [38] |
| 20 | MK-3901 | 24 | 15.8 | 3.8 | 482 | 3.4 | 89 | 6 | 1 | 4.4 | [37] |
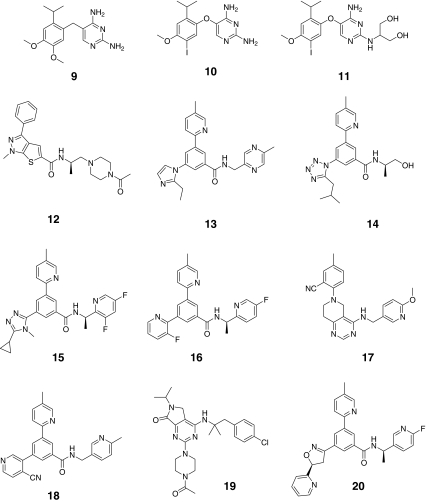
Fig. 3.
Chemical structures of antagonists for P2X3 receptors
2′(3′)-O-(2,4,6-Trinitrophenyl) ATP (TNP-ATP; compound 1) is a nonselective but highly potent antagonist of P2X1 receptors and P2X3 receptors [9, 29]. The ability to use this antagonist for preclinical pain studies in rodents is limited by its poor metabolic stability in plasma [30]. However, direct administration of TNP-ATP into relevant sites has been shown to block the pronociceptive effects of P2 receptor agonists [9, 31].
A-317491 (compound 7) has nanomolar affinity for blocking both P2X3 and P2X2/3 receptors and is a competitive antagonist [32]. Peripheral and spinal administration of A-317491 attenuates complete Freund’s adjuvant (CFA)-induced inflammatory hyperalgesia [33]. A-317491 has limited CNS penetration following systemic administration. However, systemic administration of high doses or intrathecal administration of this antagonist effectively attenuates tactile allodynia caused by peripheral nerve injury [32, 33]. Consistent with these data, ATP-evoked activation of capsaicin-insensitive spinal P2X2/3 receptors underlies an N-methyl-d-aspartate (NMDA)-dependent long lasting allodynic sensitivity in rodents [34]. Another structurally different and potent P2X2/3 and P2X3 antagonist, RO-4 (compound 4), has been reported to reverse both inflammatory and bone cancer pain in experimental models [35, 36]. Following peripheral administration, RO-4 is effective in nerve injury induced pain models, presumably resulting from its ability to readily cross the blood–brain barrier [36]. Scientists at Merck have also recently disclosed a novel P2X3 antagonist, MK-3901 (compound 20), that effectively attenuates chronic inflammatory and neuropathic pain in experimental models [37]. Interestingly, AZ-2 (compound 19) represents another novel antagonist that has been reported to have greater than 300-fold selectivity for homomeric P2X3 receptors over heteromeric P2X2/3 receptors [38]. AZ-2 effectively reversed CFA-induced mechanical allodynia following systemic and intraplantar dosing but was ineffective when dosed intrathecally [38]. These data indicate that peripheral homomeric P2X3 receptors may play a key role in inflammatory pain. Taking all the available data into account, it appears that the heteromeric P2X2/3 receptor at key synapses in the spinal cord are essential for the modulation of nociceptive input from the periphery.
Figure 4 shows the BEI/MPO analysis for existing P2X3 receptor antagonists. Early P2X3 antagonists including compounds 1–6 in Table 3 (colored red in Fig. 4) are high molecular weight antagonists with multiple phosphonate and sulfonate groups, and as expected, do not fit well into lead-like chemical space. A-317491(compound 7) was the first selective small molecule compound for P2X3 and P2X2/3. Subsequently, other potent P2X3 antagonists were reported including RO-3 (compound 9), and RO-4 (compound 10) that are at least 100-fold less active across a wide range of kinases, receptors, and ion channels [35, 36, 39]. These compounds have improved drug-like properties relative to earlier P2X3 antagonists (e.g., compounds 1–6) (Fig. 4). Comparing potent and selective P2X3 receptor antagonists, RO-3 and RO-4 have significantly improved BEI and MPO scores that correlate with enhanced oral bioavailability, lower plasma protein binding, and good CNS penetration relative to A-317491 [35, 36].
Fig. 4.
Analysis of P2X3 antagonist efficiency and physical chemical properties. Early identified antagonists lacked acceptable lead-like characteristics and are associated with low oral bioavailability and generally poor drug-like properties. In contrast, recently disclosed P2X3 antagonists have improved physicochemical properties that are consistent with orally bioavailable leads and drugs. These compounds demonstrate the significant improvement in the quality of P2X3 antagonist chemical matter over time. The newer compounds have helped to solidify the preclinical validation of the role of P2X3 in pain (see text), as well as demonstrate the feasibility of successfully advancing lead molecules into clinical trials
More recently, other potent P2X3 antagonists that are much more drug like have been disclosed (colored green in Fig. 4). Many of these newer molecules fit into lead-like space. RO-85 (compound 12), a potent and selective P2X3 antagonist with a high MPO score of 4.75, is reported to have high oral bioavailability (%F = 89) in rats [36]. MK-3901 has an MPO score of 3.8 and has good (%F = 60) oral bioavailabilty [37]. Another potent P2X3 antagonist, AF-219 (structure undisclosed), is reported to have advanced into clinical studies [39].
P2X4 receptors
A role for P2X4 receptors in the pathophysiology of chronic pain has largely been delineated based on receptor expression and genetic disruption studies. Within the CNS, cell surface expression of P2X4 receptors on microglial cells follows trauma or exposure to pro-inflammatory agents (e.g., bacterial lipopolysaccharides) [40]. This increased expression appears to be specific since P2X4 receptor protein expression is not increased in neurons or astrocytes following trauma or injury [27, 41]. Several intracellular signaling pathways have been implicated in the upregulation of P2X4 receptor expression including the PI3K/AKT pathway [42], and lyn kinase via an associated fibronectin/integrin mechanism [43–49].
P2X4 (−/−) mice show normal sensory function but diminished nociceptive sensitivity following nerve injury [45]. Additionally, intrathecal administration of a specific P2X4 receptor antisense oligodeoxynucleotide decreases P2X4 receptor expression and suppressed tactile allodynia following peripheral nerve injury [27]. Intrathecal infusion of ATP-stimulated microglia cells that express P2X4 receptors also produces allodynia in normal rats [27]. This action appears to be mediated by increased intracellular Cl− concentrations in lamina I neurons that is dependent on brain-derived neurotrophic factor (BDNF) and neurotropin (TrkB) receptor signaling pathways [45, 47]. P2X4 receptor stimulation results in down-stream signaling involving PIP2, PIP3, and p38 MAPK and subsequent release of BDNF [48, 49]. Microglial P2X4 receptor stimulation also leads to phosphorylation of the NMDA receptor NR1 on subunit spinal neurons [45] that is likely mediated by P2X7-dependent release of interleukin-1β (IL-1β). These events lead to increased synthesis of microglial BDNF that contributes to a slow and prolonged release of this trophic factor [49].
Pharmacological validation of the role of P2X4 receptors in chronic pain states is limited due primarily to the lack of receptor-selective antagonists. TNP-ATP has also been used as a putative antagonist of P2X4 receptors [41]). While TNP-ATP (compound 1) does block P2X4 receptor activation (IC50 = 15 μM), it is more than 1,000-fold more potent in blocking P2X1 and P2X3 receptors [9, 50]. Since presynaptic P2X3 and P2X2/3 receptors are also expressed on the superficial lamina of the dorsal horn of the spinal cord, the relative contributions of blocking P2X4, in addition to P2X3, receptors in chronic pain states has not been delineated. P2X4 receptor-mediated currents are insensitive to block by the nonselective antagonists suramin or PPADS and can be potentiated by ivermectin (IC50 > 500 μM) which has been used as a pharmacological indicator of P2X4 activity [51].
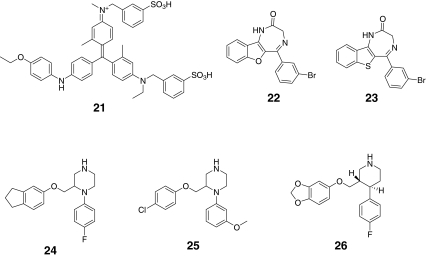
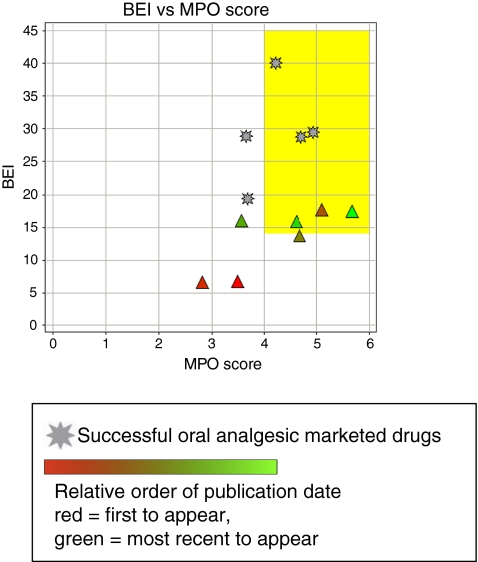
While the genetic and neurochemistry data described above provides significant insights into the potential role of P2X4 receptors in the maintenance of chronic pain states, very few P2X4 antagonists have been reported in the literature, which may reflect the difficulty of identifying potent leads or lead-like chemical matter for this P2X receptor. A summary of the available P2X4 antagonists described to date is provided in Table 4 with corresponding chemical structures shown in Fig. 5. It should be noted that compounds 22 and 23 represent a single chemical class and compounds 24–26 represent another distinct chemical series, but this is the only other small molecule chemical class known to interact with the P2X4 receptor. Importantly, compounds 24–26 belong to a class of potent serotonin reuptake inhibitors [52]. For example, compound 26, paroxetine, is a clinically useful antidepressant. Compound 22 is reported to have an IC50 value of 500 nM and has appropriate physicochemical properties to place it within lead-like chemical space (Fig. 6). However, the relative selectivity of this compound for other targets including other P2X receptors has not been reported. Although compounds 25 and 26 also fall within lead-like space (Fig. 6), they are weak antagonists and meet the BEI and MPO lead-like criteria only as a result of their low molecular weight and superior physicochemical properties. The recent elucidation of the closed state crystal structure of the P2X4 receptor [13] offers hope that potent antagonists can now be rationally designed.
Table 4.
In vitro potency and physicochemical summary of antagonists for P2X4 receptors
| Compound no. | Name | P2X4 IC50 (nM) | BEI | MPO score | MW | CLogP | PSA | HBA | HBD | LogD | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | TNP-ATP | 15,000 | 6.8 | 3.5 | 714.3 | −6.4 | 398 | 23 | 5 | −1.7 | [82] |
| 21 | Brilliant Blue G | 3,160 | 6.6 | 2.8 | 831 | −3.7 | 142 | 9 | 3 | 1.0 | [63] |
| 22 | 500 | 17.7 | 5.1 | 355.2 | 4.2 | 41 | 2 | 1 | 3.2 | [89] | |
| 23 | ~8,000 | 13.7 | 4.7 | 371.3 | 4.8 | 25 | 2 | 1 | 3.9 | [84] | |
| 24 | 6,100 | 16.0 | 3.6 | 326.5 | 4.3 | 34 | 3 | 1 | 3.2 | [84] | |
| 25 | 5,300 | 15.9 | 4.6 | 332.9 | 4.3 | 34 | 4 | 1 | 2.8 | [84] | |
| 26 | Paroxetine | 1,870 | 17.4 | 5.7 | 329 | 3.3 | 40 | 4 | 1 | 0.9 | [52] |
Fig. 5.
Chemical structures of antagonists for P2X4 receptors
Fig. 6.
Analysis of P2X4 antagonist efficiency and physical chemical properties. Early identified P2X4 antagonists suffer from low affinity and selectivity for blocking P2X4 receptors. Additionally, these antagonists also lacked lead-like characteristics. While some recently disclosed P2X4 antagonists have improved drug-like properties, potency and selectivity for specifically blocking only P2X4 receptors remains a challenge. These factors have hampered the pharmacological validation of P2X4 for pain indications
P2X7 receptors
P2X7 receptors are expressed on quiescent and activated microglia in the CNS and on macrophages in the periphery [5, 30, 40, 53]. Agonist stimulation of P2X7 receptors opens a nonselective cation channel and also leads to the rapid maturation and release of IL-1β [53–55]. Prolonged (>60 s) receptor activation leads to the formation of large cytolytic pores in the cell membrane that are produced, at least in part, by receptor-mediated down-stream signaling events linked to the recruitment of hemichannels to the cell surface [5, 48–57].
P2X7(−/−) mice exhibit disrupted cytokine signaling cascades and attenuated ATP-induced processing of pro-IL-1β [58]. In a collagen monoclonal antibody model of arthritis, P2X7(−/−) mice also show a decreased incidence and severity of arthritis as compared to wild-type control mice [58]. P2X7(−/−) mice also show reduced nociceptive sensitivity in inflammatory and neuropathic pain states compared to wild-type controls and this phenotype is consistent with the antinociceptive phenotype of mice lacking both isoforms of IL-1 (IL-1α and IL-1β) [59–61].
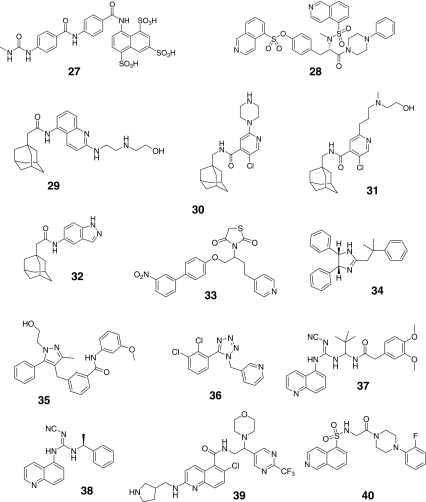
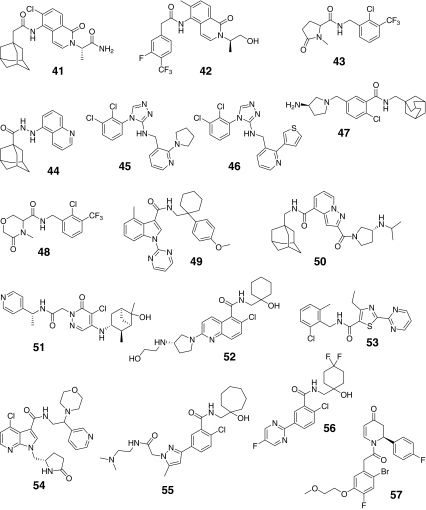
Given the demonstrated role of P2X7 in both inflammatory and pronociceptive signaling, the development of receptor-selective antagonists has received much attention. Multiple chemically distinct selective P2X7 receptor antagonists have been recently described (Table 5; Fig. 7) [62]. Many of the new antagonists are systemically bioavailable. One limitation for some P2X7 antagonists is their differential activity at rodent and human receptor isoforms [63, 64]. Examples include compounds like KN-62 (compound 28) and Brilliant Blue G (BBG; compound 21) that show preferential activity at human or rat P2X7 receptors, respectively [63]. Additionally, many of the adamantane-based antagonists (e.g., compounds 30–32) also show less activity at rat versus human P2X7 receptors [64]. Early putative P2X7 receptor antagonists also have significant ancillary pharmacological actions. Examples include oxidized-ATP which is a weak (IC50 > 1 mM) antagonist that has anti-inflammatory actions independent of its affinity for P2X7 receptors [5, 10, 65]. BBG (compound 21) has recently been shown to potently block sodium channels and P2X4 receptors [66]. NF-279 (compound 27) also is active against P2X1 [64]). KN-62 (compound 28) was the first potent P2X7 antagonist identified [10]. None of these four early compounds (shaded red) fall into lead-like space as exemplified by the yellow box in Fig. 8. However, nearly half of the more recently identified P2X7 antagonists are within lead or drug-like space, potentially indicating P2X7 is a highly druggable target (shaded green in Fig. 8).
Table 5.
In vitro potency and physicochemical summary of antagonists for P2X7 receptors
| Compound # | Name | P2X7 IC50 (nM) | P2X7 IC50 (rat, nM) | BEI | MPO score | MW | CLogP | PSA | HBA | HBD | LOGD | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | PPADS | 3,500 | 10.8 | 3.8 | 507 | −9.5 | 262 | 15 | 5 | −2.6 | [63, 64] | |
| 21 | Brilliant Blue G | 1,950 | 603 | 6.9 | 2.8 | 831 | −3.7 | 142 | 9 | 3 | 1.0 | [63, 64, 90] |
| 27 | NF-279 | 20,000 | 7.0 | 3.0 | 676 | −13.9 | 271 | 12 | 7 | −1.7 | [82] | |
| 28 | KN-62 | 14 | 10.9 | 2.7 | 722 | 5.4 | 130 | 9 | 0 | 5.1 | [89] | |
| 29 | 1 | 19 | 21.0 | 3.6 | 423 | 5.6 | 86 | 5 | 4 | 2.7 | [10] | |
| 30 | 398 | 16.5 | 4.3 | 389 | 4.4 | 57 | 4 | 2 | 2.5 | [89] | ||
| 31 | 16 | 18.6 | 4.1 | 420 | 4.3 | 65 | 4 | 2 | 2.6 | [89] | ||
| 32 | 40 | 23.9 | 4.0 | 309 | 5.5 | 58 | 2 | 2 | 3.7 | [62] | ||
| 33 | 14 | >10,000 | 17.0 | 3.5 | 464 | 3.6 | 103 | 7 | 0 | 3.7 | [63, 64] | |
| 34 | 10 | 22.6 | 2.9 | 354 | 6.8 | 24 | 2 | 1 | 4.0 | [89] | ||
| 35 | <200 | 15.2 | 3.1 | 442 | 4.2 | 76 | 4 | 2 | 3.9 | [89] | ||
| 36 | A-438079 | 126 | 316 | 22.5 | 5.2 | 306 | 3.2 | 56 | 4 | 0 | 2.7 | [63, 64] |
| 37 | A-740003 | 44 | 18 | 15.5 | 2.4 | 475 | 3.2 | 121 | 8 | 3 | 3.4 | [63, 64] |
| 38 | A-804598 | 11 | 10 | 25.2 | 4.5 | 315 | 3.2 | 73 | 5 | 2 | 3.0 | [84] |
| 39 | 100 | 12.4 | 3.7 | 564 | 1.8 | 104 | 8 | 3 | 1.9 | [91, 92] | ||
| 40 | 12 | 18.5 | 5.4 | 428 | 3.4 | 83 | 5 | 1 | 1.8 | [62] | ||
| 41 | 0.079 | 22.9 | 3.9 | 442 | 4.3 | 94 | 3 | 2 | 4.2 | [62] | ||
| 42 | 0.398 | 21.5 | 4.1 | 436 | 2.9 | 71 | 3 | 2 | 3.6 | [62] | ||
| 43 | 10 | 23.9 | 5.8 | 335 | 2.3 | 49 | 2 | 1 | 2.4 | [62] | ||
| 44 | 10 | 45 | 24.9 | 4.3 | 321 | 3.5 | 54 | 3 | 2 | 3.2 | [62] | |
| 45 | 15 | 12 | 20.1 | 4.6 | 389 | 4.0 | 59 | 5 | 1 | 3.6 | [62] | |
| 46 | 11 | 40 | 19.8 | 3.8 | 402 | 4.6 | 56 | 4 | 1 | 3.9 | [62] | |
| 47 | GSK314181A, AACBA | 18 | 29 | 17.4 | 3.9 | 402 | 4.6 | 58 | 3 | 2 | 2.9 | [70] |
| 48 | <100 | 20.0 | 5.8 | 351 | 2.7 | 59 | 3 | 1 | 1.8 | [62, 84] | ||
| 49 | <2,000 | 12.5 | 3.2 | 455 | 6.1 | 69 | 4 | 1 | 5.4 | [84] | ||
| 50 | <2,000 | 11.9 | 3.7 | 464 | 5.0 | 79 | 4 | 2 | 3.6 | [84] | ||
| 51 | 1 | 19.9 | 4.9 | 444 | 2.9 | 89 | 5 | 2 | 3.3 | [84] | ||
| 52 | 4 | 18.8 | 4.1 | 447 | 2.1 | 98 | 6 | 4 | 1.7 | [84] | ||
| 53 | <1,000 | 16.1 | 4.2 | 373 | 3.7 | 68 | 4 | 1 | 3.5 | [93] | ||
| 54 | <2,000 | 11.8 | 4.3 | 483 | 0.5 | 101 | 6 | 2 | 1.0 | [84] | ||
| 55 | 3 | 17.3 | 3.9 | 490 | 3.2 | 99 | 5 | 3 | 2.3 | [62] | ||
| 56 | 27 | 18.9 | 4.9 | 100 | 1.7 | 75 | 4 | 2 | 2.6 | [94] | ||
| 57 | 13 | 13 | 16.4 | 3.7 | 480 | 3.5 | 56 | 4 | 0 | 3.5 | [95] |
Fig. 7.
Chemical structures of antagonists for P2X7 receptors
Fig. 8.
Analysis of P2X7 antagonist efficiency and physical chemical properties Early identified antagonists lacked acceptable lead-like characteristics and are associated with low oral bioavailability and generally poor drug-like properties. Further, many of these antagonists also had generally low potency for blocking P2X7 receptors and showed significant species differences. In contrast, recently disclosed P2X7 antagonists have improved potency, selectivity, and physicochemical properties that are consistent with orally bioavailable leads and drugs. These compounds demonstrate the significant improvement in the quality of P2X7 antagonist chemical matter over time. The newer compounds have helped to solidify the preclinical validation of the role of P2X7 in inflammatory and chronic pain states (see text). Compared with the available chemical matter for antagonists of P2X3 and P2X4 receptors, the diversity of chemical matter for P2X7 antagonists suggests it may be a highly druggable target of pain and inflammation
The discovery of selective P2X7 receptor antagonists has helped clarify the role of this receptor in states of ongoing pain and inflammation [64, 67–69]. For example, AACBA (compound 47) has been reported to attenuate collagen-induced arthritis following prophylactic dosing in rats [70]. An analog from a series of adamantine P2X7 receptor-selective antagonists from AstraZeneca has provided signals of clinical efficacy in reducing the symptoms of rheumatoid arthritis [71].
In experimental models of chronic nociception, A-740003 (compound 37) and A-438079 (compound 36), represent selective and competitive P2X7 antagonists that reduce inflammatory hyperalgesia in rodents [72, 73]. These effects do not appear to be secondary to P2X7-mediated anti-inflammatory effects in models of acute inflammation that are largely driven to prostaglandin E2 [72, 73]. P2X7 receptor-selective antagonists also reduce nociception in some, but not all, experimental models of neuropathic pain [67–69]). These effects have been reported to be mediated by spinal and/or supra-spinal sites of action based on the of ability A-438079 (compound 36) to reduce the firing of nociceptive specific and wide dynamic range spinal neurons in neuropathic rats [69]. Taken together, these data support a role for P2X7 receptors on microglial cells in mediating pronociceptive cytokine production (e.g., IL-β) in states of persistent pain. Thus, the contribution of the P2X7 receptor to spinal nociceptive processing is enhanced following a neuropathic injury and appears to modulate a diverse spectrum of inputs affecting spinal neuronal excitability. However, this action may not be mechanistically relevant in all neuropathic pain conditions. Recent data from Heegaard and colleagues have demonstrated the lack of microglial cell involvement and P2X7 antagonist mediated antinociception in two murine models of cancer pain [73].
Conclusions
The molecular cloning and characterization of the P2 receptor superfamilies has greatly contributed to the development of P2 receptor-selective antagonists. In the context of pain research, these advances have enabled initial investigations into the individual contributions of specific P2 receptors that are involved in the initiation and maintenance of chronic pain. Data based on the use of receptor-selective antagonists has clearly demonstrated mechanistically specific roles for P2X3 and P2X7 receptors in mediating nociceptive sensitivity. In contrast, definitive pharmacological validation for P2X4 receptors in modulating microglial cells during ongoing pain remains to be demonstrated. From a ligand recognition perspective, it is surprising that there is a diversity of pharmacophores that have potent and selective antagonist actions at P2X7 receptors. Such diversity is much more constrained for antagonists of P2X3 receptors and essentially absent for P2X4 receptor antagonists. The present analysis also shows that progress has been made in the generation of useful pharmacological tools for P2X3 and P2X7 receptors that have drug-like characteristics. Further, P2X3 and P2X7 receptor leads have advanced into early clinical evaluation.
Acknowledgments
The authors thank Jennifer van Camp and Phil Cox for their input on the physicochemical analysis presented in this manuscript and their critical comments on earlier versions of the manuscript.
Glossary
- clogP
Calculated partition coefficient (octanol/water) of a unionized organic compound—ionization not considered and pH independent. A measure of lipophilicity
- ClogD
Calculated distribution coefficient (octanol/buffer) of a compound in all forms (ionized and unionized)—pH dependent. A pH dependent measure of lipophilicity
- PSA
Polar surface area—surface sum over all polar atoms in a molecule (generally N and O) units in Angstrom squared. A measure of polarity
- HBD
Hydrogen bond donors
- HBA
Hydrogen bond acceptors
- MW
Molecular weight
- BEI
Binding efficiency index
- MPO
Multi-parameter optimization
- ADME
Absorption, distribution, metabolism, and elimination
- CFA
Complete Freund’s adjuvant
References
- 1.Honore P, Jarvis MF. Acute and chronic pain. In: Triggle DJ, Taylor JB, editors. Comprehensive medicinal chemistry II, vol. 6. Oxford: Elsevier; 2006. pp. 327–349. [Google Scholar]
- 2.Perl ER. Ideas about pain, a historical view. Nat Rev Neurosci. 2007;8:71–80. doi: 10.1038/nrn2042. [DOI] [PubMed] [Google Scholar]
- 3.Woolf CJ. Central sensitization:implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 6.Bleehen T, Keele CA. Observation on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton SG, Warburton J, Bhattacharjee A, Ward J, McMahon SB. ATP in human skin elicits a dose-related pain response under conditions of hyperalgesia. Brain. 2000;123:1238–1246. doi: 10.1093/brain/123.6.1238. [DOI] [PubMed] [Google Scholar]
- 8.Sawynok J. Adenosine and ATP receptors. Handb Exp Pharmacol. 2007;177:309–328. doi: 10.1007/978-3-540-33823-9_11. [DOI] [PubMed] [Google Scholar]
- 9.Jarvis MF, Wismer CT, Schweitzer E, Yu H, Biesen T, Lynch KJ, Burgard EC, Kowaluk EA. Modulation of BzATP and formalin induced nociception: attenuation by the P2X receptor antagonist, TNP-ATP and enhancement by the P2X3 allosteric modulator, cibacron blue. Br J Pharmacol. 2001;132:259–269. doi: 10.1038/sj.bjp.0703793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson KA, Jarvis MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarvis MF. The neural-glial purinergic receptor ensemble in chronic pain states. Trends Neurosci. 2010;33:48–57. doi: 10.1016/j.tins.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Mio K, Ogura T, Yamamoto T, Hiroaki Y, Fujiyoshi Y, Kubo Y, Sato C. Reconstruction of the P2X(2) receptor reveals a vase-shaped structure with lateral tunnels above the membrane. Structure. 2009;17(2):266–275. doi: 10.1016/j.str.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability of drug discovery and development settings. Adv Drug Delivery Rev. 2001;46:3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 15.Leeson PD, Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat Rev/Drug Disc. 2007;6:881–889. doi: 10.1038/nrd2445. [DOI] [PubMed] [Google Scholar]
- 16.Muresan S, Sadowski J. Properties guiding drug- and lead-likeness. In: Mannhold R, editor. Molecular drug properties, measurement and prediction (Ch. 17) Weinheim: Wiley; 2008. pp. 441–461. [Google Scholar]
- 17.Paolini GV, Shapland RHB, Hoorn WP, Mason JS, Hopkins AL. Global mapping of pharmacological space. Nat Biotech. 2006;24(7):805–815. doi: 10.1038/nbt1228. [DOI] [PubMed] [Google Scholar]
- 18.Wenlock MC, Austin RP, Barton P, Davis AM, Leeson PD. A comparison of physiochemical property profiles of development and marketed oral drugs. J Med Chem. 2003;46:1250–1256. doi: 10.1021/jm021053p. [DOI] [PubMed] [Google Scholar]
- 19.Johnson TW, Dress KR, Edwards M. Using the golden triangle to optimize clearance and oral absorption. Bioorg Med Chem Lett. 2009;19:5560–5564. doi: 10.1016/j.bmcl.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 20.Ritchie TJ, Macdonald SJF. The impact of aromatic ring count on compound developability—are too many aromatic rings a liability in drug design? Drug Disc Today. 2008;14(21/22):1011–1020. doi: 10.1016/j.drudis.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Peters J, Schnider P, Mattei P, Kansy M. Pharmacology promiscuity: dependence on compound properties and target specificity in a set of recent roche compounds. Chem Med Chem. 2009;4:680–686. doi: 10.1002/cmdc.200800411. [DOI] [PubMed] [Google Scholar]
- 22.Hughes JD, Blagg J, Price DA, Bailey S, DeCrescenzo GA, Devraj RV, Ellsworth E, Fobian YM, Gibbs ME, Gilles RW, Greene N, Huang E, Krieger-Burke T, Joesel J, Wager T, Whiteley L, Zhang Y. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg & Med Chem Lett. 2008;18:4872–4875. doi: 10.1016/j.bmcl.2008.07.071. [DOI] [PubMed] [Google Scholar]
- 23.Wager TT, Chandrasekaran RY, Hou X, Troutman MD, Verhoest PR, Villalobos A, Will Y. Defining desirable central nervous system drug space through the alignment of molecular properties, in vitro ADME, and safety attributes. ACS Chem Neurosci. 2010;1:420–434. doi: 10.1021/cn100007x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wager TT, Hou X, Verhoest PR, Villalobos A. Moving beyond Rules: the development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. ACS Chem Neurosci. 2010;1:435–449. doi: 10.1021/cn100008c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abad-Zapatero C, Metz JT. Ligand efficiency indices as guideposts for drug discovery. Drug Disc Today. 2005;10(7):464–469. doi: 10.1016/S1359-6446(05)03386-6. [DOI] [PubMed] [Google Scholar]
- 26.Jarvis MF. Contributions of P2X3 homomeric and heteromeric channels to acute and chronic pain. Expert Opin Ther Targets. 2003;7:513–522. doi: 10.1517/14728222.7.4.513. [DOI] [PubMed] [Google Scholar]
- 27.Inoue K, Tsuda M, Tozaki-Saitoh H. Modification of neuropathic pain sensation through microglial ATP receptor. Purinergic Sig. 2007;3:311–316. doi: 10.1007/s11302-007-9071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martucci C, Trovato AE, Costa B, Borsani E, Franchi S, Magnaghi V, Panerai AE, Rodella LF, Valsecchi AE, Sacerdote P, Colleoni M. The purinergic antagonist PPADS reduces pain related behaviors and interleukin-1beta, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain. 2008;137:81–95. doi: 10.1016/j.pain.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Lewis CJ, Surprenant A, Evans RJ. 2′,3′-O-(2,4,6-trinitrophenyl)ATP- a nanomolar affinity antagonist at rat mesenteric artery P2X receptor ion channels. Br J Pharmacol. 1998;124:1463–1466. doi: 10.1038/sj.bjp.0702001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 31.Donnelly-Roberts D, McGaraughty S, Shieh C-C, Honore P, Jarvis MF. Painful purinergic receptors. J Pharm Exp Ther. 2008;324(2):409–415. doi: 10.1124/jpet.106.105890. [DOI] [PubMed] [Google Scholar]
- 32.Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci USA. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGaraughty S, Wismer CT, Zhu CZ, Mikusa J, Honore P, Chu KL, Lee CH, Faltynek CR, Jarvis MF. Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol. 2003;140:1381–1388. doi: 10.1038/sj.bjp.0705574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakagawa T, Wakamatsu K, Zhang N, Maeda S, Minami M, Satoh M, Kaneko S. Intrathecal administration of ATP produces long-lasting allodynia in rats: differential mechanisms in the phase of the induction and maintenance. Neuroscience. 2007;147:445–455. doi: 10.1016/j.neuroscience.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 35.Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford APDW. Pharmacology of P2X channels. Eur J Physiol. 2006;452:513–537. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 36.Carter DS, Alam M, Cai H, Dillon MP, Ford APDW, Gever JR, Jahangir A, Lin C, Moore AG, Wagner PJ, Zhai Y. Identification and SAR of novel diaminopyrimidines. Part 1: the discovery of RO-4, a dual P2X3/P2X2/3 antagonist for the treatment of pain. Bioorg Med Chem Lett. 2009;19:1628–1631. doi: 10.1016/j.bmcl.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Burgey CS (2011) Discovery of P2X3 receptor antagonists for the treatment of chronic pain. In: 241st ACS national meeting & exposition, Anaheim, CA, 27–31 March. MEDI-15
- 38.Cantin L, Bayrakdarian M, Buon C, Hu Y, Kennedy V, Laplante M, Leung C, Luo X, Popovic N, Rene O, Santhakumar V, Butterworth J, Godbout C, Gosselin M, Grazzini E, Labrecque J, Pare M, Projean D, Yu XH, Tomaszewski MJ (2010) Pyrrolopyrimidine-based P2X3 antagonists for the treatment of pain. In: 240th ACS national meeting, Boston, MA, 2–26 August. MEDI-474
- 39.Gever JR, Soto R, Henningsen RA, Martin RS, Hackos DH, Panicker S, Rubas W, Oglesby IB, Dillon MP, Mila ME, Burnstock G, Ford APDW. AF-353 a novel potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br J Pharmacol. 2010;160:1387–1398. doi: 10.1111/j.1476-5381.2010.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raouf R, Chabot-Doré AJ, Ase AR, Blais D, Séguéla P. Differential regulation of microgial P2X4 and P2X7 ATP receptors following LPS-induced activation. Neuropharmacol. 2007;53:496–504. doi: 10.1016/j.neuropharm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther. 2006;109:210–226. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009;29:998–1005. doi: 10.1523/JNEUROSCI.4595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuda M, Toyomitsu E, Komatsu T, Masuda T, Kunifusa E, Nasu-Tada K, Koizumi S, Yamamoto K, Ando J, Inoue K. Fibronectin/integrin system is involved in P2X(4) receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia. 2008;56:579–585. doi: 10.1002/glia.20641. [DOI] [PubMed] [Google Scholar]
- 44.Tsuda M, Tozaki-Saitoh H, Masuda T, Toyomitsu E, Tezuka T, Yamamoto T, Inoue K. Lyn tyrosine kinase is required for P2X4 receptor upregulation and neuropathic pain after peripheral nerve activation. Glia. 2008;56:50–58. doi: 10.1002/glia.20591. [DOI] [PubMed] [Google Scholar]
- 45.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-Regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 47.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, Koninck P, Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 48.Trang T, Beggs S, Wan X, Salter MW. P2X4 receptor mediated synthesis and release of brain derived neurotrophic factor in microglia is dependent on calcium and p38-MAPK. J Neurosci. 2009;29:3518–3528. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernier LP, Ase AR, Chevallier S, Blais D, Zhao Q, Boué-Grabot E, Logothetis D, Séguéla P. Phosphoinositides regulate P2X4 ATP-gated channels through direct interactions. J Neurosci. 2008;28:12938–12945. doi: 10.1523/JNEUROSCI.3038-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jarvis MF, Khakh BS. ATP-gated P2X cation-channels. Neuropharmacol. 2009;56:208–215. doi: 10.1016/j.neuropharm.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 51.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 52.Muller CE. Emerging structures and ligands for P2x3 AND P2X4 receptors—towards novel treatments of neuropathic pain. Purinergic Signalling. 2010;6:145–148. doi: 10.1007/s11302-010-9182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrari D, Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, DiVirgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 54.MacKenzie A, Wilson HL, Kiss-Tosh E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity. 2001;8:825–835. doi: 10.1016/S1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 55.Perregaux DG, Gabel CA. Interleukin-1β maturation and release in response to ATP and nigericin. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 56.DiVirgilio F. Purinergic signalling between axons and microglia. In: Chadwick DJ, Goode J, editors. Purinergic signalling in neuron-glia interactions. No. 276. Chichester: Wiley; 2006. pp. 253–262. [Google Scholar]
- 57.Pelegrin P, Surprenant A. The P2X7 receptor-pannexin connection to dye uptake and IL-1beta release. Purinergic Sig. 2009;5:129–137. doi: 10.1007/s11302-009-9141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 59.Chessell IP, Hatcher J, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CBA, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 60.Wolf G, Yirmiya R, Goshen I, Iverfeldt K, Holmlund L, Takeda K, Shavit Y. Impairment of interleukin-1 (IL-1) signaling reduces basal pain sensitivity in mice: genetic, pharmacological and developmental aspects. Pain. 2004;104:471–480. doi: 10.1016/S0304-3959(03)00067-8. [DOI] [PubMed] [Google Scholar]
- 61.Honore P, Wade CL, Zhong C, Harris RR, Wu C, Ghayur T, Iwakura Y, Decker MW, Faltynek C, Sullivan J, Jarvis MF. Interleukin-1ab gene-deficient mice show reduced nociceptive sensitivity in models of inflammatory and neuropathic pain but not post-operative pain. Behav Br Res. 2006;167:355–364. doi: 10.1016/j.bbr.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 62.Guile SD, Alcaraz L, Birkinshaw TN, Bowers KC, Ebden MR, Furber M, Stocks MJ. Antagonists of the P2X7 receptor. From lead identification to drug development. J Med Chem. 2009;52:3123–3141. doi: 10.1021/jm801528x. [DOI] [PubMed] [Google Scholar]
- 63.Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007;151:571–579. doi: 10.1038/sj.bjp.0707265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donnelly-Roberts D, Namovic MT, Han P, Jarvis MF. Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br J Pharmacol. 2009;157:1203–1214. doi: 10.1111/j.1476-5381.2009.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beigi RD, Kertesy SB, Aquilina G, Dubyak GR. Oxidized ATP (oATP) attenuates proinflammatory signaling via P2 receptor independent mechanisms. Br J Pharmacol. 2003;140:507–519. doi: 10.1038/sj.bjp.0705470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jo S, Bean BP. Inhibition of neuronal voltage-gated sodium channels by brilliant blue g. Mol Pharmacol. 2011;80(2):247–257. doi: 10.1124/mol.110.070276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, Harris R, Medrano AP, Carroll W, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-740003 (N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl) acetamide, a novel and selective P2X7 receptor antagonist dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- 68.Nelson DW, Gregg RJ, Kort ME, Perez-Medrano A, Voight EA, Wang Y, Namovic MT, Grayson G, Donnelly-Roberts DL, Niforatos W, Honore P, Jarvis MF, Faltynek CR, Carroll WA. Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem. 2006;49:3659–3666. doi: 10.1021/jm051202e. [DOI] [PubMed] [Google Scholar]
- 69.McGaraughty S, Chu KL, Namovic MT, Donnelly-Roberts DL, Harris RR, Zhang X-F, Shieh C-C, Wismer CT, Zhu CZ, Gauvin DM, Fabiyi AC, Honore P, Nelson DW, Gregg RJ, Carroll WA, Faltynek CR, Jarvis MF. P2X7-related modulation of pathological nociception in rats. Neuroscience. 2007;146:1817–1828. doi: 10.1016/j.neuroscience.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 70.Broom DC, Matson DJ, Bradshaw E, Buck ME, Meade R, Coombs S, Matchett M, Ford KK, Yu W, Yuan J, Sun SH, Ochoa R, Krause JE, Wustrow DJ, Cortright DN. Characterization of N-(adamantan-1-ylmethyl)-5-((3R-amino-pyrrolidin-1-yl)methyl)-2-chloro-benzamide, a P2X7 antagonist in animal models of pain and inflammation. J Pharm Exper Ther. 2008;327(3):620–633. doi: 10.1124/jpet.108.141853. [DOI] [PubMed] [Google Scholar]
- 71.Furber M, (2008) P2X7 antagonists in a rheumatoid arthritis pain model. In: ACS national meeting, Philadelphia, PA. MEDI 237
- 72.Honore P, Donnelly-Roberts D, Namovic M, Zhong C, Wade C, Chandran P, Zhu C, Carroll W, Perez-Medrano A, Iwakura Y, Jarvis MF. The anti-hyperalgesic effects of a selective P2X7 receptor antagonist are lost in IL-1ab knockout mice. Behav Br Res. 2009;204:77–81. doi: 10.1016/j.bbr.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 73.Hansen RR, Nielsen CK, Nasser A, Thomsen SIM, Eghorn LF, Pham Y, Schulenburg C, Syberg S, Ding M, Stojilkovic SS, Jorgensen NR, Heegaard AM. P2X7 receptor deficient mice are susceptible to bone cancer pain. Pain. 2011;152:1766–1776. doi: 10.1016/j.pain.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pajouhesh H, Lenz GR. Medicinal chemical properties of successful central nervous system drugs. NeuroRx. 2005;2:541–553. doi: 10.1602/neurorx.2.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Friden M, Winiwarter S, Jerndal G, Bengtsson O, Wan H, Bredberg U, Hammarlund-Udenaes M, Antonsson M. Structure-brain exposure relationships in rat and human using a novel data set of unbound drug concentrations in brain interstitial and cerebrospinal fluids. J Med Chem. 2009;52:6233–6243. doi: 10.1021/jm901036q. [DOI] [PubMed] [Google Scholar]
- 76.Hitchcock SA. Blood-brain barrier permeability considerations for CNS-targeted compound library design. Curr Op Chem Biol. 2008;12:318–323. doi: 10.1016/j.cbpa.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 77.Blakemore DC, Bryans JS, Carnell P, Chessum NEA, Field MJ, Kinsella N, Kinsora JK, Osborne SA, Williams SC. Synthesis and in vivo evaluation of 3-substituted gababutins. Bioorg Med Chem Lett. 2010;20(1):362–365. doi: 10.1016/j.bmcl.2009.10.089. [DOI] [PubMed] [Google Scholar]
- 78.Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DF, Yu SS, Anderson GD, Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC. Synthesis and biological evaluation of the 1,5,-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4(5-(4-Methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzemesulfonamide (SC-58635, Celecoxib) J Med Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 79.Riendeau D, Percival MD, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, Evans J, Falgueyret JP, Ford-Hutchinson AW, Gordon R, Greig G, Gresser M, Guay J, Kargman S, Léger S, Mancini JA, O’Neill G, Ouellet M, Rodger IW, Thérien M, Wang Z, Webb JK, Wong E, Chan CC, et al. Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor. Br J Pharmacol. 1997;121(1):105–117. doi: 10.1038/sj.bjp.0701076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei Q, Zhou DH, Shen QX, Chen J, Chen LW, Wang TL, Pei G, Chi ZQ. Human mu-opioid receptor overexpressed in Sf9 insect cells functionally coupled to endogenous Gi/o proteins. Cell Res. 2000;10(2):93–102. doi: 10.1038/sj.cr.7290039. [DOI] [PubMed] [Google Scholar]
- 81.Cashman JR, Ghirmai S. Inhibition of serotonin and norepinephrin reuptake and inhibition of phosphodiesterase by multi-target inhibitors as potential agents for depression. Bioorg Med Chem. 2009;17:6890–6897. doi: 10.1016/j.bmc.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 82.Jarvis MF, Khakh BS. ATP-gated P2X cation channels. Neuropharmacology. 2009;56:208–215. doi: 10.1016/j.neuropharm.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 83.Jacobson KA. P2X and P2Y receptors. Tocris Biosci Sci Rev Ser. 2010;1:15. [Google Scholar]
- 84.Gunosewoyo H, Kassiou M. P2X purinergic receptor ligands: recently patented compounds. Exp Op Ther Pat. 2010;20(5):625–646. doi: 10.1517/13543771003702424. [DOI] [PubMed] [Google Scholar]
- 85.Jahangir A, Alam M, Carter DS, Dillon MP, Bois DJ, Ford APDW, Gever JR, Lin C, Wagner PJ, Zhai Y, Zira J. Identification and SAR of novel diaminopyrimidines. Part 2: the discovery of RO-51, a potent and selective, dual P2X3/P2X2/3 antagonist for the treatment of pain. Bioorg Med Chem Lett. 2009;19:1632–1635. doi: 10.1016/j.bmcl.2009.01.097. [DOI] [PubMed] [Google Scholar]
- 86.Brotherton-Pleiss CE, Dillon MP, Ford APDW, Gever JR, Carter DS, Gleason SK, Lin CJ, Moore AG, Thompson AW, Villa M, Zhai Y. Discovery and optimization of RO-85, a novel drug-like, potent, and selective P2X3 receptor anatagonist. Bioorg Med Chem Lett. 2010;20:1031–1036. doi: 10.1016/j.bmcl.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 87.Prous (2009) 659156. Available at http://.integrity.thomson-pharma.com. Drug Data Report 31(6):538
- 88.Prous (2010) 669469, 690966, 703690. Available at http://integrity.thomson-pharma.com. Drug Data Report 32(3):241
- 89.Shieh C-C, Jarvis MF, Lee C-H, Perner RJ. P2X receptor ligands and pain. Exp Op Ther Pat. 2006;16(8):1113–1127. doi: 10.1517/13543776.16.8.1113. [DOI] [PubMed] [Google Scholar]
- 90.Jiang L-H, Mackenzie AB, North RA, Surprenant A. Brilliant Blue G selectively blocks ATP-gated rat P2X7 receptors. Mol Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- 91.Romagnoli R, Baraldi PG, Carrion MD, Cara CL, Preti D, Cruz-Lopez O, Tabrizi MA, Moorman AR, Gessi S, Fogli E, Sacchetto V, Borea PA. From tyrosine to glycine: synthesis and biological activity of potent antagonists of the purinergic P2X7 receptor. J Med Chem. 2007;50(15):3706–3715. doi: 10.1021/jm070443e. [DOI] [PubMed] [Google Scholar]
- 92.Romagnoli R, Baraldi PG, Cruz-Lopez O, Lopez-Cara C, Preti D, Borea PA, Gessi S. The P2X7 receptor as a therapeutic target. Exp Op Ther Targ. 2008;12(5):647–661. doi: 10.1517/14728222.12.5.647. [DOI] [PubMed] [Google Scholar]
- 93.Prous (2010) entry 669469. Available at http://integrity.thompsonpharma.com. Exp Pharmacol Rep
- 94.Chen X, Pierce B, Naing W, Grapperhaus ML, Phillion DP. Discovery of 2-chloro-N-((4,4-difluoro-1-hydroxycyclohexyl)methyl)-5-(5-fluoropyrimidin-2-yl)benzamide as a potent and CNS penetrable P2X7 receptor antagonist. Bioorg Med Chem Lett. 2010;20(10):3107–3111. doi: 10.1016/j.bmcl.2010.03.094. [DOI] [PubMed] [Google Scholar]
- 95.Prous (2010) entry 703690. Available at http://integrity.thompsonpharma.com. Exp Pharmacol Rep