Abstract
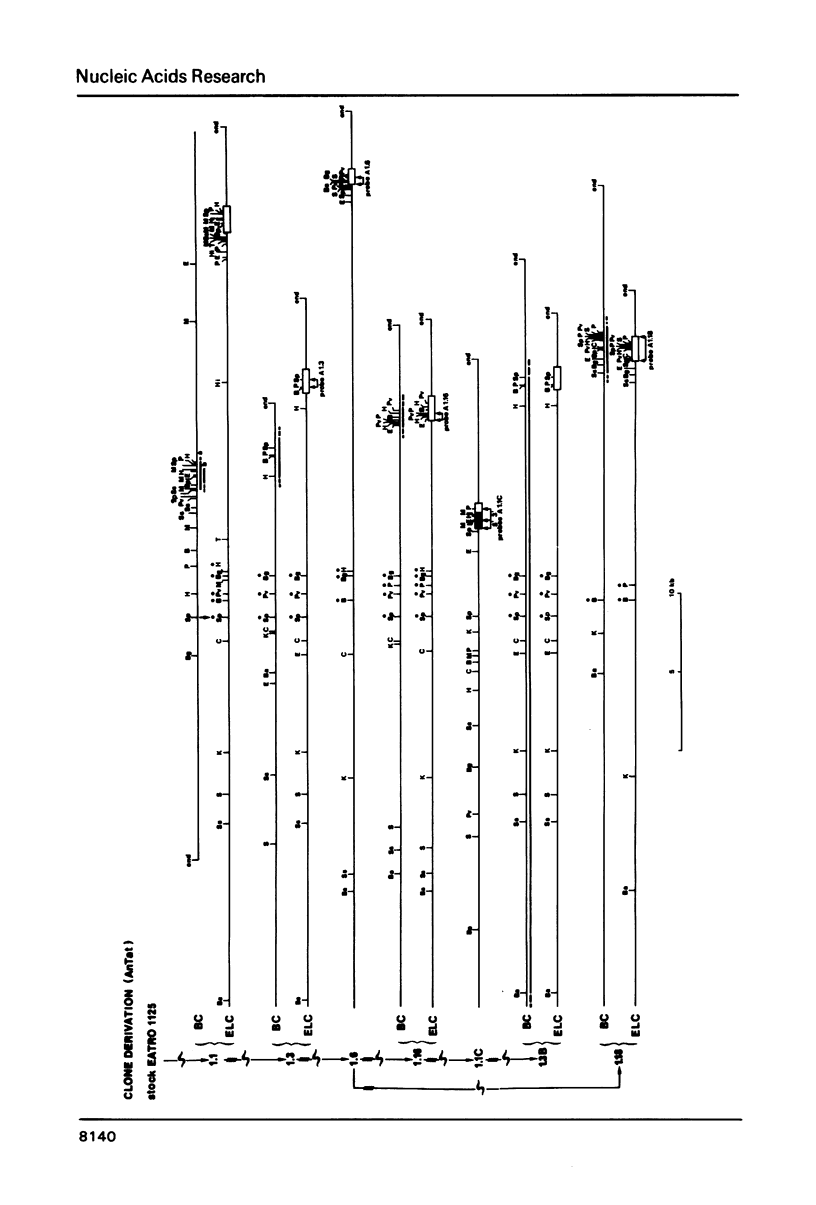
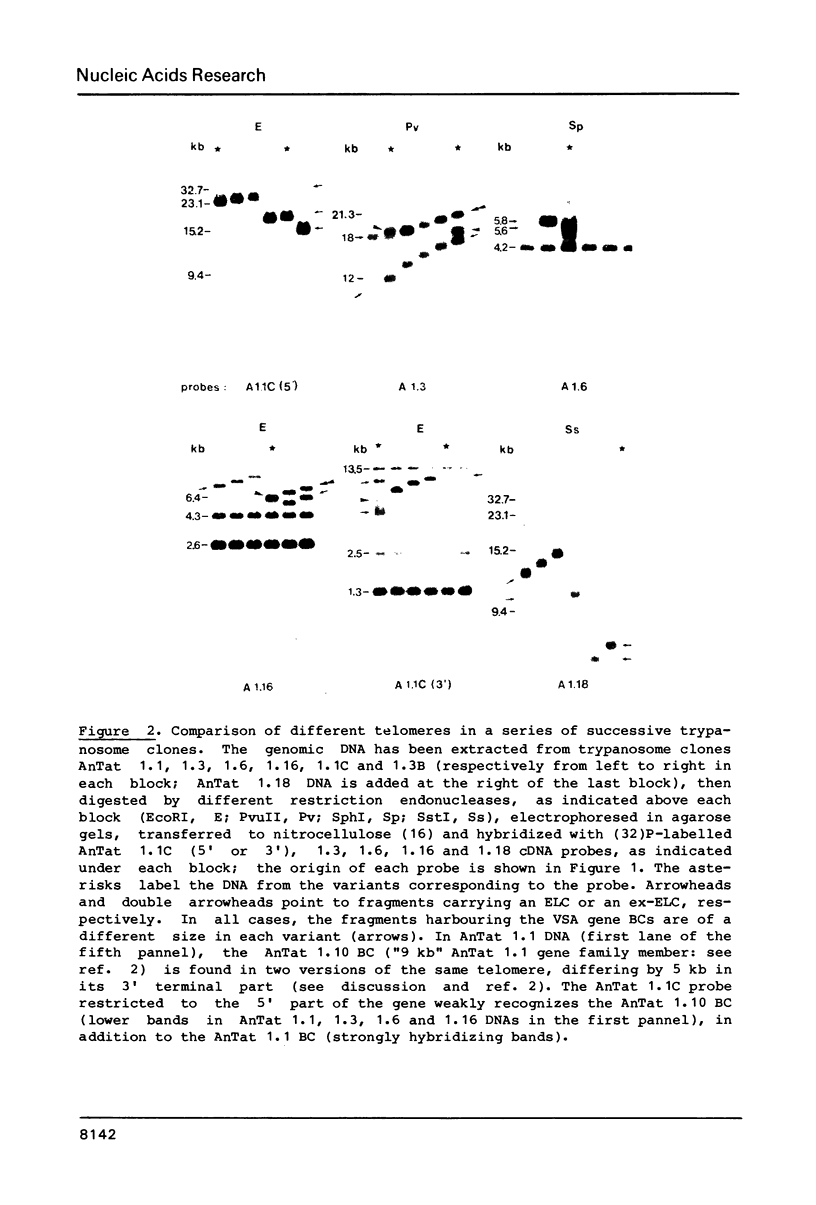
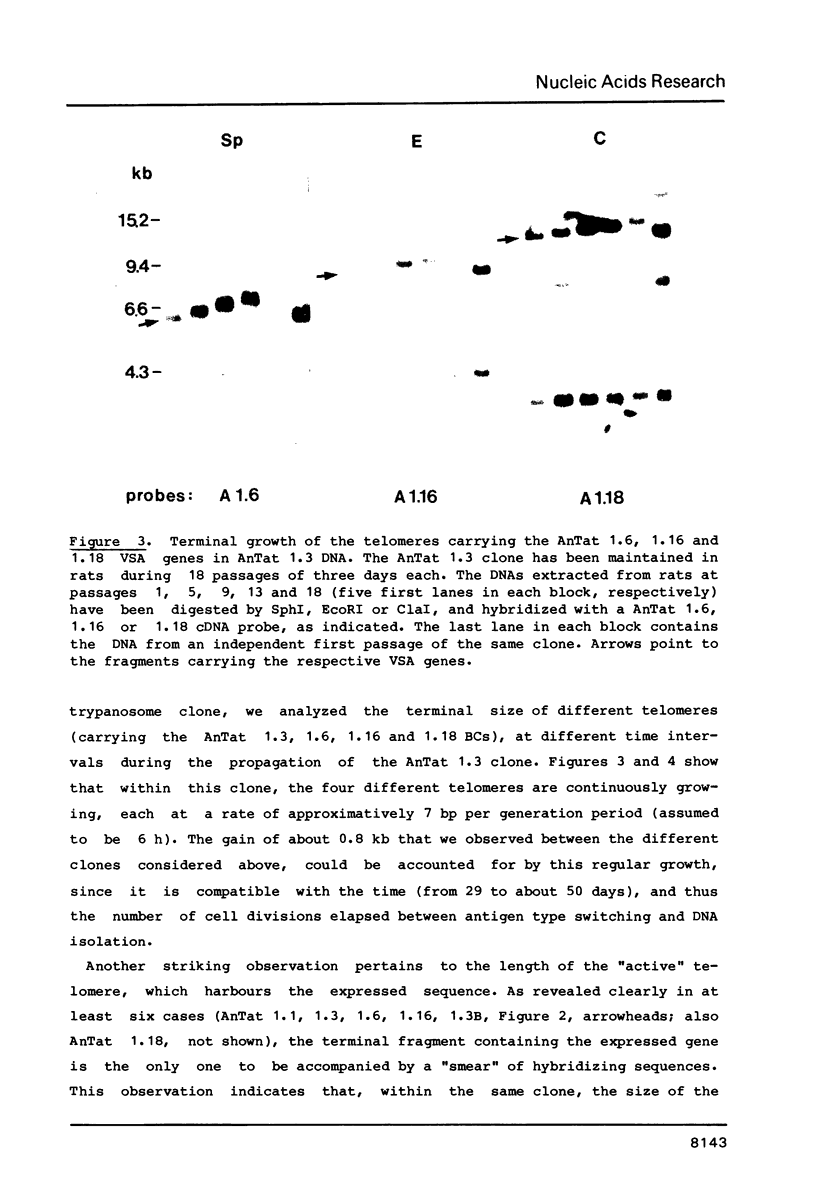
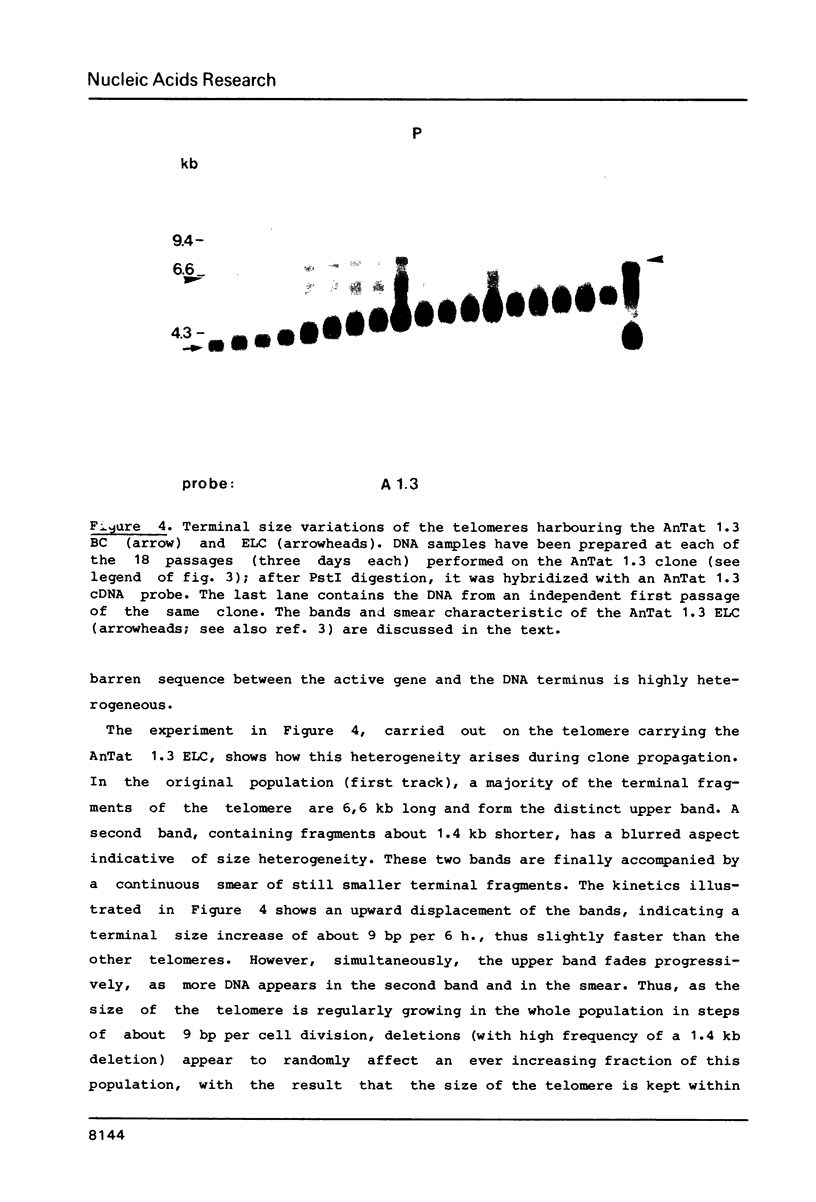
We have studied the genes coding for the variant-specific surface antigen (VSA) in a series of seven trypanosome clones derived from AnTat 1.1: 1.1 leads to 1.3 leads to 1.6 leads to 1.16 leads to 1.1C leads to 1.3B leads to 1.18 These genes are all telomeric (1-5), and their surrounding, although sometimes similar, differs in each case. The length between these antigen genes and the corresponding DNA end appears to increase at each antigenic switch, with however occasional sharp size reductions, often linked to the involvement of the telomere in gene expression. This increase is due to a constant "growth" of the telomeres, at a rate of about 28 bp per day in at least four cases and probably linked to chromosome duplication. The telomere harbouring the transcribed VSA gene is growing slightly faster (about 36 bp per day), and it is the only one whose size reduction is progressive, leading to a terminal length heterogeneity within a clone. As a result, the active VSA gene is found in a population of telomeres which, as the trypanosomes divide, becomes increasingly heterogeneous, with however a preferred discrete size class about 1.4 kb smaller. The fact that the "active" telomere is the only one in a chromatin conformation highly sensitive to DNAaseI (1-4, 6), suggests that chromatin structure influences the rate and extent of both size increase and shortening of telomeres.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bateman A. J. Letter: Simplification of palindromic telomere theory. Nature. 1975 Jan 31;253(5490):379–380. doi: 10.1038/253379a0. [DOI] [PubMed] [Google Scholar]
- Bernards A., Michels P. A., Lincke C. R., Borst P. Growth of chromosome ends in multiplying trypanosomes. Nature. 1983 Jun 16;303(5918):592–597. doi: 10.1038/303592a0. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H., Gall J. G. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978 Mar 25;120(1):33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- Borst P., Cross G. A. Molecular basis for trypanosome antigenic variation. Cell. 1982 Jun;29(2):291–303. doi: 10.1016/0092-8674(82)90146-5. [DOI] [PubMed] [Google Scholar]
- Capbern A., Giroud C., Baltz T., Mattern P. Trypanosoma equiperdum: etude des variations antigéniques au cours de la trypanosomose experimentale du lapin. Exp Parasitol. 1977 Jun;42(1):6–13. doi: 10.1016/0014-4894(77)90055-8. [DOI] [PubMed] [Google Scholar]
- De Lange T., Borst P. Genomic environment of the expression-linked extra copies of genes for surface antigens of Trypanosoma brucei resembles the end of a chromosome. Nature. 1982 Sep 30;299(5882):451–453. doi: 10.1038/299451a0. [DOI] [PubMed] [Google Scholar]
- King B. O., Yao M. C. Tandemly repeated hexanucleotide at Tetrahymena rDNA free end is generated from a single copy during development. Cell. 1982 Nov;31(1):177–182. doi: 10.1016/0092-8674(82)90417-2. [DOI] [PubMed] [Google Scholar]
- Klar A. J., Strathern J. N., Hicks J. B. A position-effect control for gene transposition: state of expression of yeast mating-type genes affects their ability to switch. Cell. 1981 Aug;25(2):517–524. doi: 10.1016/0092-8674(81)90070-2. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Swanton M. T., Donini P., Prescott D. M. All gene-sized DNA molecules in four species of hypotrichs have the same terminal sequence and an unusual 3' terminus. Proc Natl Acad Sci U S A. 1981 May;78(5):3015–3019. doi: 10.1073/pnas.78.5.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent M., Pays E., Magnus E., Van Meirvenne N., Matthyssens G., Williams R. O., Steinert M. DNA rearrangements linked to expression of a predominant surface antigen gene of trypanosomes. Nature. 1983 Mar 17;302(5905):263–266. doi: 10.1038/302263a0. [DOI] [PubMed] [Google Scholar]
- Michiels F., Matthyssens G., Kronenberger P., Pays E., Dero B., Van Assel S., Darville M., Carvador A., Steinert M., Hamers R. Gene activation and re-expression of a Trypanosoma brucei variant surface glycoprotein. EMBO J. 1983;2(7):1185–1192. doi: 10.1002/j.1460-2075.1983.tb01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y., Shiota S., Nakai S., Nishida Y., Okubo S. Inverted terminal repeat sequence in the macronuclear DNA of Stylonychia pustulata. Gene. 1980 Sep;10(4):301–306. doi: 10.1016/0378-1119(80)90150-x. [DOI] [PubMed] [Google Scholar]
- Pays E., Lheureux M., Steinert M. The expression-linked copy of a surface antigen gene in Trypanosoma is probably the one transcribed. Nature. 1981 Jul 16;292(5820):265–267. doi: 10.1038/292265a0. [DOI] [PubMed] [Google Scholar]
- Pays E., Van Assel S., Laurent M., Darville M., Vervoort T., Van Meirvenne N., Steinert M. Gene conversion as a mechanism for antigenic variation in trypanosomes. Cell. 1983 Sep;34(2):371–381. doi: 10.1016/0092-8674(83)90371-9. [DOI] [PubMed] [Google Scholar]
- Pays E., Van Assel S., Laurent M., Dero B., Michiels F., Kronenberger P., Matthyssens G., Van Meirvenne N., Le Ray D., Steinert M. At least two transposed sequences are associated in the expression site of a surface antigen gene in different trypanosome clones. Cell. 1983 Sep;34(2):359–369. doi: 10.1016/0092-8674(83)90370-7. [DOI] [PubMed] [Google Scholar]
- Pays E., Van Meirvenne N., Le Ray D., Steinert M. Gene duplication and transposition linked to antigenic variation in Trypanosoma brucei. Proc Natl Acad Sci U S A. 1981 May;78(5):2673–2677. doi: 10.1073/pnas.78.5.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Van Meirvenne N., Janssens P. G., Magnus E. Antigenic variation in syringe passaged populations of Trypanosoma (Trypanozoon) brucei. 1. Rationalization of the experimental approach. Ann Soc Belg Med Trop. 1975;55(1):1–23. [PubMed] [Google Scholar]
- Williams R. O., Young J. R., Majiwa P. A. Genomic environment of T. brucei VSG genes: presence of a minichromosome. Nature. 1982 Sep 30;299(5882):417–421. doi: 10.1038/299417a0. [DOI] [PubMed] [Google Scholar]