Abstract
Pharmacological manipulation of P2X and P2Y receptors has been critical to the elucidation of the biological roles of these receptors within a multitude of physiological and pathological processes. Initial purinergic signalling research made use of compounds based on pyridoxal phosphate, suramin and nucleotide analogues; recently developed compounds are often derivatives of these early tools. Tocris Bioscience first entered the field of purinergic signalling reagents with the commercial release of the pyridoxal phosphate derivative, iso-PPADS. During the past two decades, Tocris has assembled a collection of over 50 compounds for P2 receptor modulation, including research tools commercialised from both academic and industrial laboratories. Recently, a number of P2X subtype-selective compounds have been generated by pharmaceutical company medicinal chemistry programmes, supplementing our range of P2Y-selective compounds. Here, we detail the current, commercially available agonists and antagonists of P2X1,2/3,3,4,7 and P2Y1,6,11,12 receptors; considered together, they form the foundations of a comprehensive P2 receptor pharmacological ‘toolkit’.
Keywords: P2X, P2Y receptors, Chemical inhibitors, Antagonists, Agonists, Allosteric modulators, Tocris Bioscience
Introduction
Over the last 30 years, the P2 receptors for extracellular nucleotides have been described at the molecular level [1]. Seven fast acting P2X ion channels (P2X1–7) and eight G-protein coupled P2Y receptors (P2Y1,2,4,6,11,12,13 and P2Y14) have been cloned and characterised in terms of their endogenous nucleotide and nucleoside preference [2, 3]. P2 receptor signalling participates in the immediate modulation of cellular processes such as neurotransmission, metabolism, adhesion, migration and activation and affects longer term responses including proliferation, differentiation and apoptosis. Some of the P2 receptors have also been implicated in pathologies such as atherosclerosis, inflammatory conditions and neuropathic pain.
A key part in understanding the signalling mediated by these receptors has come through the use of pharmacological tools. From the prototypic P2 receptor antagonists (e.g.pyridoxalphosphate-6-azophenyl-2′,5′-disulphonic acid (PPADS), KN-62) and agonists (e.g. 2′(3′)-O-(4-benzoylbenzoyl) adenosine 5′-triphosphate (BzATP)) to more recent compounds from pharmaceutical companies and other drug discovery programmes, chemical tools are widely employed to differentiate the various roles of these receptors in tissue distribution and function [4–6]. In this article, we take a brief historical and largely chemical view of the development of the modern purinergic researcher’s pharmacological tool collection. The main purpose of this article is to highlight the origin and commercial development of some of the most relevant pharmacological research compounds for elucidating P2 receptor signalling that have been made available by Tocris Bioscience.
First compound(s)
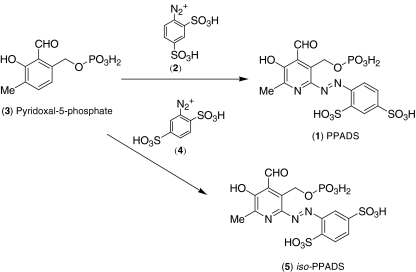
The field of purinergic signalling first came to the attention of Tocris in 1992, when Professor Richard Cookson, the founder of Cookson Chemicals, wrote to Günther Lambrecht, at that time a researcher of purinergic signalling at the University of Frankfurt, about an interesting new research compound named PPADS (1) that had recently appeared in the European Journal of Pharmacology [7]. Cookson had established Cookson Chemicals a few years earlier upon his retirement from the chemistry department at the University of Southampton, and while its main business was the custom synthesis of otherwise unobtainable chemicals, Cookson realised that there might be ‘off-the-shelf’ demand from biological scientists for research chemicals that appeared in scientific literature. Lambrecht agreed that PPADS was a particularly effective molecule, and they made plans to commercialise it. A method for the compound synthesis was sent to Cookson Chemicals (Fig. 1). The diazonium salt of aniline 2,4-disulphonic acid (2) was reacted with a suspension of the sodium salt of pyridoxal-5-phosphate (3), and the product precipitated out by pouring the mixture into acetone. This synthesis, while straightforward, was a little cumbersome, with the final product obtained as a tetrasodium salt with approximately 20% water present. Further purification of this compound was impractical and analysis of the final product proved to be rather difficult, so Cookson sent the PPADS to Germany for direct comparison with the original compound. It transpired that Cookson’s PPADs had to all intents and purposes the same biological activity as the original, and therefore, the compound began to be sold as a novel chemical tool, providing a brisk trade.
Fig. 1.
Reaction scheme for the synthesis of PPADS and iso-PPADS
Approximately 1 year later, when PPADS was ready to be resynthesised, Cookson Chemicals discovered that instead of using aniline 2,4-disulphonic acid, they had used aniline 2,5-disulphonic acid (4) and had in fact produced an isomeric compound with a different structure (5). Consequently, Cookson wrote to all the customers that had purchased PPADS, notifying them of the error with sincere apologies. Surprisingly, these customers responded with requests for Cookson to supply more of the 2,5 isomer, which actually exhibited an activity that was comparable to or even superior to the original 2,4. Consequently, Cookson Chemicals renamed the 2,5 isomer iso-PPADS, and this compound has continued in its use as a research tool to this day and is generally more potent than PPADS at P2X receptors [8].
Development of a P2 receptor compound range
Shortly after this inauspicious start, in 1994, Cookson Chemicals and Tocris Neuramin merged into one company, Tocris Cookson. This allied Cookson’s strong chemistry skills and fledgling catalogue range with Tocris’ wide collection of glutamate receptor compounds, many of which originated directly from the laboratory of Jeff Watkins at Bristol University and were unavailable elsewhere, thus in high demand [9]. Even at this time, the idea of making and selling compounds reported in scientific literature was considered rather unusual, with academics usually relying on drug companies or each other for their supplies. At the newly created Tocris, sales of glutamate compounds were very healthy; however, the company recognised the customers’ need for research tools in many other areas of preclinical research. The field of Purinergics was certainly seen as a potential growth area, not least because by then PPADS had recovered from its shaky start to become a widely used tool as one of the first agents able to discriminate between P2X and P2Y receptors.
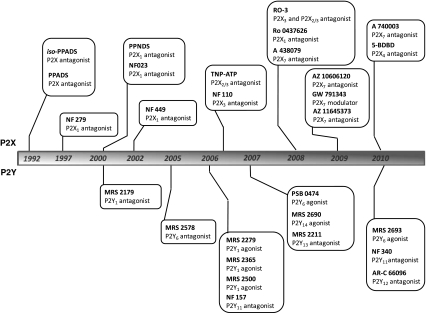
Tocris has continued to seek out new, useful and, where possible, selective compounds for the field of purinergics. Over the years, we have collected many standard tools such as α,β-MeATP, BzATP and 2-methylthioADP, which are widely regarded and commonly used in this research area [10]. After the initial introduction of PPADS and iso-PPADS, between 1997 and 2007, Tocris began to introduce novel compounds that are generally derived from pyridoxal phosphate, suramin (6), and endogenous nucleotides (such as adenosine 5′-triphosphate (ATP) (7) and uridine diphosphate (UDP) (8)). The approximate timeline during which Tocris introduced a wide variety of purinergic compounds is illustrated in Fig. 2. These compounds were generally unavailable elsewhere at the time of introduction, except from the originating laboratory.
Fig. 2.
Timeline of P2 receptor compounds released by Tocris Bioscience
Four distinct categories of tool compounds
The Tocris catalogue pages for the P2 receptors show a diverse collection of pharmacological agents that are currently widely employed in purinergic signalling research [11, 12]. Broadly speaking, there are four distinct categories of molecules within this P2 receptor ‘toolkit’: compounds based on (1) pyridoxalphosphate, (2) naphthalene sulphonic acid and other dyes, (3) endogenous ligands (adenosine diphosphate (ADP), ATP and uridine 5′-triphosphate (UTP)) and, finally the most structurally diverse category, (4) compounds that arose from medicinal chemistry programmes. The following discussion highlights some of the key compounds in each of the categories.
Pyridoxal phosphate derivatives
Purinergic researchers began to synthesise novel compounds using PPADS as a reference drug, with the intention of discovering one which was more potent and selective between the different P2 isotypes. After PPADS and iso-PPADS, one of the first purinergic compounds added to the Tocris range was the P2X1 antagonist PPNDS (9), a naphthylazo-6-nitro-4′,8′-disulphonate derivative (Fig. 3). Unlike PPADS, PPNDS was selective for P2X over P2Y and was seven times more potent at P2X1 [13]. A 2-chloro-5-nitrophenylazo derivative of iso-PPADS initially described by Kim et al. [14] named MRS 2211 (from the Molecular Recognition Section at the NIH) (10), was later introduced in 2007. This compound is a competitive antagonist which is >20-fold selective for P2Y13 over P2Y1 and P2Y12. MRS 2211 was originally found to inhibit ADP-induced IP3 production 45 times more potently than PPADS in human 1321N1 astrocytoma cells and is widely used as a P2Y13 antagonist in a variety of models [14].
Suramin and other organic dye inspired compounds
Fig. 3.
PPADS and derivatives
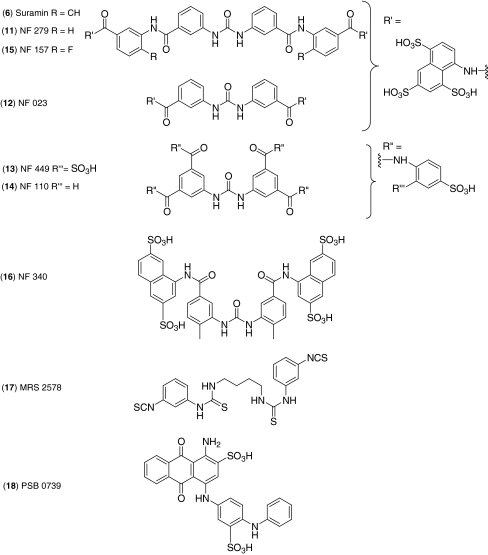
Originally developed by Bayer in 1916, and despite its venerable age and lack of selectivity, suramin (6, Fig. 4) continues to be in-demand as a P2 antagonist. This polysulphonated naphthylurea has been used as a useful ‘chemical lead’ for developing P2X subtype-selective compounds [15]. NF 279 (11) was one of the first selective P2X1 antagonists to be discussed in the literature and was first commercialised under an agreement with Professors Lambrecht and Nickel and made widely available to the research community by Tocris in 1999.
Fig. 4.
Suramin derivatives and novel P2Y receptor antagonists
NF 279 exhibited significantly different antagonistic properties to its parent compound, suramin, with the greatest selectivity between P2X and P2Y receptors seen at that time [16]. NF 279 was also unique for its fast binding (unlike PPADS) and inability to be degraded by ectonucleotidases (unlike TNP-ATP), making it particularly useful for functional experiments within native tissues that contained these enzymes [17]. Shortly after, in June 2000, another competitive P2X antagonist with two arylsulphonic acid residues, NF 023 (12) was introduced that was subtype-selective for P2X1 with an IC50 value of 0.21 μM compared to 28.9, >50 and >100 μM for P2X3, P2X2 and P2X4, respectively [18]. This was followed in May 2002 by a more potent compound, NF 449 (13), the most widely used P2X1 antagonist that Tocris offers [19, 20]. The tetravalent compound, NF 449 was described in 2001 as the most potent, selective P2X1 antagonist available, with an IC50 value of 0.28 nM for the rat P2X1 receptor. In comparison to suramin and other divalent analogues, NF 449 is more than 3,000-fold more potent than its parent, and 800- and 60-fold more potent than NF 023 and NF 279, respectively.
Research into sulphonic acid suramin derivatives as selective P2 antagonists continued at the Universities of Bonn and further commercial agreements led to new additions to the Tocris range in 2006. The first compound was a high-affinity P2X3 antagonist, NF 110 (14), an antitumour compound with no discernible activity at P2Y receptors (IC50 > 10 μM). NF 110 was unique amongst all other suramin derivatives in its selectivity profile with maximal selectivity at rat P2X2/3 and P2X3 over P2X1 [21]. The elucidation of this suramin analogue with different binding specificities highlighted the importance of the precise structural position of sulphonic acid groups within the molecule having a direct effect on its P2 receptor binding properties. The second compound was a potent, selective P2Y11 antagonist NF 157 (15) [22]. With the exception of suramin itself, NF 157 was the only known P2Y11 antagonist at that time. The compound exhibited nanomolar potency and up to 650-fold selectivity over P2Y1 and P2Y2 and more than 67-fold selectivity over P2X2,3,4 and P2X7. The disadvantages of NF 157 are its equipotency at P2Y11 and P2X1 receptors and the fact that it is a large molecule with six sulphonic acid groups. Recently, Tocris was granted permission to make available the sulphonic acid derivative, NF 340 (16), an additional P2Y11 antagonist which is a smaller molecule with no significant activity at P2X receptors and is 4-fold more potent than NF 157 at P2Y11 [23].
Structurally novel P2 receptor chemical compounds include the P2Y6 antagonist, MRS 2578 (17) and the P2Y12 antagonist, PSB 0739 (18). MRS 2578 is a symmetrical di-isothiocyanate with a distinctly un-purine, suramin or pyridoxal like structure [24]. Despite lacking these antecedents, this compound is an important purinergic research tool as one of the first selective P2Y6 antagonists; inhibiting the response of UDP at the rat P2Y6 receptor with no activity at P2Y1,2,4 and P2Y11. PSB 0739, for which Tocris was recently granted a licence by the University of Bonn, is based on the anthraquinone dye Reactive Blue 2 [25]. This compound should prove to be a very useful tool, since unlike clopidogrel, it does not require metabolism by cytochrome P450 to release the active compound. PSB 0739 represents an important step forward for pharmacological tools since it is currently one of the most potent (Ki = 25 nM), metabolically stable and selective competitive antagonists available for study of the P2Y12 receptor [26].
ADP, ATP and UDP mimetics
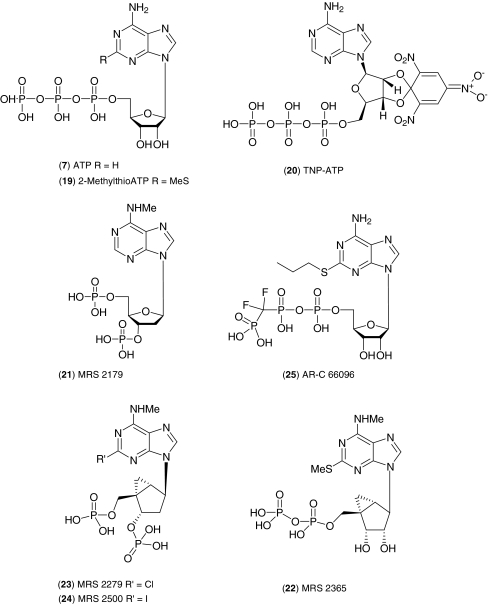
The widely used stable ATP analogue, 2-methylthioATP (19, Fig. 5) was added by Tocris in 1997 [27]. 2-MethylthioATP is a non-selective, potent agonist at P2Y receptors and several P2X receptors, particularly P2X1 and P2X3. The trinitrophenyl-substituted analogue, 2′,3′-O-(2,4,6-trinitrophenyl)adenosine-5′-triphosphate (TNP-ATP) (20), a selective P2X2/3 antagonist was introduced in 2006 [28]. The compound has an IC50 value of 3–6 nM for rat and human P2X2/3 receptors and is 1,000-fold selective over P2X2, P2X4 and P2X7. TNP-ATP is particularly useful in vitro as it provides a direct demonstration of P2X receptor heterogeneity in sensory neurons; however, the compound may be susceptible to enzymatic breakdown in multicellular preparations [29].
Fig. 5.
ATP nucleotide derivatives
P2Y1 antagonist MRS 2179 (21), first appeared in the literature in 1998 [30], and after 2 years (spent firstly obtaining a licence from the NIH and secondly synthesising the molecule), Tocris were able to launch it in October 2000. MRS 2179 was one of the first compounds to have a high affinity and selectivity for P2Y1, with no significant activity at other P2Y receptor subtypes, and it has established itself as a widely used probe. As a result of another licensing agreement, Tocris were able to introduce the three closely structurally related and synthetically extremely challenging P2Y1-targeted compounds, namely MRS 2365 (22), MRS 2279 (23) and MRS 2500 (24) [31–33]. These compounds originate from the NIH Molecular Recognition Section headed by Ken Jacobson, and include the ‘methanocarba’ modification, a ring-constrained combination of cyclopropane and cyclopentane rings which enables retention of high affinity for P2Y1 that is lost in other ATP analogues [31]. MRS 2365 is an agonist with an EC50 of 0.4 nM for the human P2Y1 receptor whilst MRS 2279 and MRS 2500 are P2Y1 antagonists. MRS 2500 has greater potency for P2Y1 than MRS 2279 and remains one of the most potent, selective antagonists for P2Y1 reported [33].
The ATP analogue, AR-C 66096 (25), which was originally developed in the early nineties, is a selective and reversible antagonist targeting the P2Y12 receptors with no activity at other P2Y or P2X receptors [34]. This compound is commonly used as a potent antagonist of ADP-induced platelet aggregation and has aided the characterization of P2Y12 function in platelets. Tocris has released AR-C 66096 under a licensing agreement with AstraZeneca.
Several P2Y receptors are also activated by uracil nucleotides and, in particular, UDP (Fig. 6). In 2007, Tocris introduced the UDP-based selective P2Y6 agonist PSB 0474 (26) [35]. The elucidation of a 5-iodo-UDP, MRS 2693 (27), provided a potent and selective agonist at the human P2Y6 receptor, demonstrating an EC50 value of 15 nM in vitro and no activity at other P2Y subtypes [36]. This compound contains a large phenylacyl substituent which enhances its selectivity for P2Y6 over P2Y2 and P2Y4 which may also be activated by UDP derivatives. In 2008, MRS 2690 (28) was added to the Tocris range. This P2Y14 receptor agonist has a potency 7-fold higher than UDP-glucose for P2Y14 [37]
Drug discovery programme compounds
Fig. 6.
UDP nucleotide derivatives
Clinically relevant drugs and pharmacological tool compounds can have a different set of chemical criteria to make them useful [38, 39]. For example, successful drugs may require a degree of polypharmacology to have a therapeutic effect, whilst chemical probes require a degree of selectivity for meaningful interpretation of the experimental data (e.g. suramin versus the NF compounds mentioned previously) [40]. However, medicinal chemistry programmes do provide many of the compounds for research from both high-throughput campaigns and by optimising and then abandoning chemical series. Of course, patents eventually expire and some older drugs find new life as research tools. By closely monitoring pharmaceutical company patent activity and fostering suitable licensing arrangements, Tocris has been able to commercialise a number of the best tool compounds as a result of medicinal chemistry programmes whilst respecting intellectual property (Fig. 7).
Fig. 7.
Novel compounds for P2X receptors from drug discovery campaigns
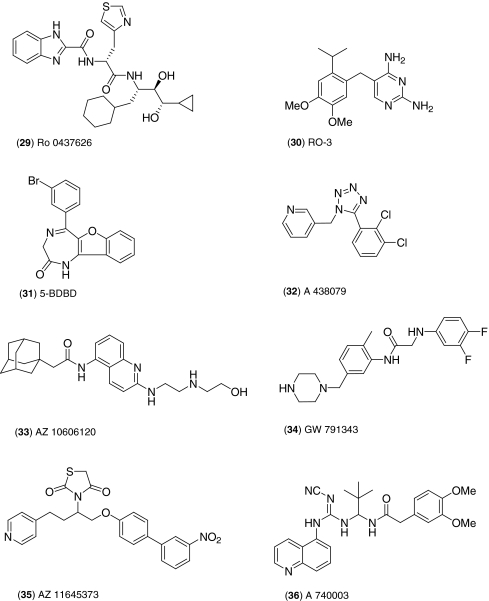
As described in 1 to 3, P2Y receptors and P2X1 are fairly well covered for selective tool compounds generated by academic labs working on series inspired the three classes of compound described. Until recently, some P2X receptors lacked selective reagents. Two of the first drug discovery compounds commercialised as research tools for the P2X receptors came from the Roche Neuroscience and Medicinal Chemistry group at Palo Alto and were released by Tocris in late 2008. Ro 0437626 (29), a benzimidazole-2-carboxamide derivative, has been shown to be selective for the P2X1 receptors (demonstrates >30-fold selectivity over the P2X2, P2X3 and P2X2/3 receptors). This compound represented the first drug-like structure for a P2X1 antagonist and showed improved pharmacokinetic properties over the more potent compounds, PPNDS and NF 449 [41]. The second Roche compound to be introduced into the Tocris range during the same year was RO-3 (30), which is an early example of the diaminopyrimidine series that Roche have currently licensed to Afferent Pharmaceuticals for the development of treatments for neuropathic pain. RO-3 selectively targets the P2X2 and P2X2/3 receptors (pIC50 = 7.0 and 5.9, respectively), and although it has moderate potency, the compound is both brain penetrant and orally available [42]. The introduction of a diazepinone derivative, 5-BDBD (31), which was originally described only in the patent literature, provided the first P2X4 antagonist. This compound has been shown to inhibit P2X4-mediated currents in Chinese hamster ovary cells with an IC50 value of 0.5 μM [42].
Until recently, the only commercially available P2X7 antagonists were the non-selective sulphonic acid derivatives, KN-62 [43] or Evans Blue dye [44, 45]. In the last decade, much interest has been focussed upon the P2X7 receptor because of its potential as a novel target for chronic inflammation, neurodegeneration and neuropathic pain [46, 47]. As a result, several large pharmaceutical companies instigated high-throughput screening campaigns in an attempt to discover new P2X ligands [48]. The first selective and P2X7 antagonist to be made as a commercially available research tool by Tocris in 2008 was the Abbott laboratories tetrazole-based compound, A 438079 (32) [49]. This competitive antagonist displays no activity at other P2 receptors (IC50 > 10 μM), and with sub-micromolar potency at the human and rat P2X7 receptors, it has proved to be a useful tool for the in vitro study of the P2X7 receptor, particularly in models of neuropathic and inflammatory pain. Other compounds in this tetrazolyl series may yet prove to have greater utility in animal models [50].
Allosteric modulators of P2X7 were first reported in the patent literature in the year 2000, although the first published characterisation was by researchers at GlaxoSmithKline (GSK) in 2008 [51, 52]. The AstraZeneca adamantyl quinolinyl derivative, AZ 10606120 (33), is a potent non-competitive antagonist and negative allosteric modulator at both the human and rat P2X7 receptors in functional studies. The compound binds at a site distinct from the ATP binding site of P2X7 [51, 52]. Michel et al. [53] also characterised the compounds, GW 791343 (34) and AZ 11645373 (35). Like AZ 10606120, GW 791343 is a non-competitive antagonist and negative allosteric modulator at the human P2X7 receptor; however, this compound also acts as a positive allosteric modulator at the rat P2X7 receptor [52]. Again, the compound shows limited interaction with the ATP binding site of P2X7 receptors; however, it may potentially reduce ATP binding ability. These compounds were released by Tocris in 2009 under long standing licence agreements with GSK and AstraZeneca. A third compound for P2X7, AZ 11645373 was also introduced at the same time. AZ 11645373 is a selective antagonist at the human P2X7 receptor with no activity at the rat receptor or any other P2X subtype (KB = 5 nM at human P2X7 and >10,000 nM at rat P2X7) [53].
Perhaps, the most potent, competitive P2X7 antagonist tool compound released by Tocris in recent years is a cyanoguanidine derivative from Abbott, A 740003 (36) [54]. This compound inhibited both rat and human P2X7 receptors (IC50 values are 18 and 40 nM, respectively) and showed a high degree of selectivity over other P2 receptors and ion channels in the CEREP selectivity assay panel. This compound demonstrated increased oral bioavailability over A 438079, and demonstrated in vivo efficacy and antinociceptive effects in models of neuropathic pain [54–56]. A 740093 provides a good example of the commercialisation of the medicinal chemistry programme outputs to generate potent and specific tool compounds.
Tocris website: linking tool compounds to pharmacological targets
Early versions of the Tocris catalogue classified many of the compounds described above simply as ‘Purinergics’. The Tocris website has recently evolved to take into account the greater molecular clarity around the classification of receptors such that today, a search for P2Y or P2X (or the gene names P2RX, P2RY) will generate a listing of all the available compounds that act on these receptors. Receptors and ion channels are two such categories, separated on the basis of guidelines established by the International Union of Pharmacology and the British Journal of Pharmacology. Within this system of categorisation, P2X and P2Y channels fall under their respective target type (ligand-gated ion channels and 7-TM receptors). If compounds have other known activities, a link is provided to the related target pages. In this way, it is a simple matter to identify selective chemical tools.
In addition to pharmacological target, compounds on the Tocris website (www.tocris.com) are also categorised by cellular process (e.g. apoptosis) or by the broader research area. Hence, P2X receptors are also contained within ‘Pain and Inflammation’ research due to their involvement in nociception and pain models, whilst the P2Y receptors are listed within ‘Cardiovascular Disease’ research, due to their involvement in platelet aggregation. Cross referencing of research areas and targets in such a way enables a number of different approaches to be used to locate a compound with the desired activity. Gene data for these targets are provided on the website wherever possible, along with related literature which provides context and perspective across various different research areas [57].
Final thoughts
It is obvious from a comparison of the timeline shown in Fig. 2 with the literature citations for many of the compounds in the Tocris catalogue that new product introductions are constrained to compounds which can be commercialised whilst respecting intellectual property. By demonstrating our conscientious approach in disseminating materials otherwise unobtainable to researchers, and the subsequent growth in areas that may result (that otherwise might not), we have found the pharmaceutical companies and other holders of intellectual property around research tools to be increasingly willing to licence these important reagents to us. The main purpose of this article is to highlight the most useful of the commercially available compounds that researchers require as a P2 receptor signalling pharmacological toolkit. In addition, we hope to illustrate Tocris’ long tradition of working alongside scientists—bringing new discoveries out of the lab and into the commercial arena. Over the past two decades, Tocris has strived to provide up-to-date, novel purinergic research tools and continues to be a key player in the introduction of useful compounds, in the knowledge that availability of new tools creates new demand by stimulating new research.
Glossary
- 5-BDBD
5-(3-Bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one
- α,β-MeATP
α,β-Methyleneadenosine 5′-triphosphate
- A 438079
3-[[5-(2,3-Dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine
- A 740003
N-[1-[[(Cyanoamino)(5-quinolinylamino)methylene]amino]-2,2-dimethylpropyl]-3,4-dimethoxybenzeneacetamide
- AR-C 66096
2-(Propylthio)adenosine-5′-O-(β,γ-difluoromethylene)triphosphate
- AZ 10606120
N-[2-[[2-[(2-Hydroxyethyl)amino]ethyl]amino]-5-quinolinyl]-2-tricyclo[3.3.1.13,7]dec-1-ylacetamide
- AZ 11645373
3-[1-[[(3′-Nitro[1,1′-biphenyl]-4-yl)oxy]methyl]-3-(4-pyridinyl)propyl]-2,4-thiazolidinedione
- BzATP
2′(3′)-O-(4-Benzoylbenzoyl)adenosine-5′-triphosphate
- GW 791343
2-[(3,4-Difluorophenyl)amino]-N-[2-methyl-5-(1-piperazinylmethyl)phenyl] acetamide
- iso-PPADS
Pyridoxalphosphate-6-azophenyl-2′,5′-disulphonic acid
- KN-62
4-[(2S)-2-[(5-isoquinolinylsulfonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl] phenyl isoquinolinesulphonic acid ester
- MRS 2179
2′-Deoxy-N6-methyladenosine 3′,5′-bisphosphate
- MRS 2211
2-[(2-Chloro-5-nitrophenyl)azo]-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-4-pyridinecarboxaldehyde
- MRS 2279
(1R,2S,4S,5S)-4-[2-Chloro-6-(methylamino)-9H-purin-9-yl]-2-(phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester
- MRS 2365
[[(1R,2R,3S,4R,5S)-4-[6-Amino-2-(methylthio)-9H-purin-9-yl]-2,3-dihydroxybicyclo[3.1.0]hex-1-yl]methyl] diphosphoric acid mono ester
- MRS 2500
(1R,2S,4S,5S)-4-[2-Iodo-6-(methylamino)-9H-purin-9-yl]-2-(phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester
- MRS 2578
N,N″-1,4-Butanediylbis[N′-(3-isothiocyanatophenyl)thiourea
- MRS 2690
Diphosphoric acid 1-α-d-glucopyranosyl ester 2-[(4′-methylthio)uridin-5″-yl] ester
- MRS 2693
5-Iodouridine-5′-O-diphosphate
- NF 023
8,8′-[Carbonylbis(imino-3,1-phenylenecarbonylimino)]bis-1,3,5-naphthalenetrisulphonic acid
- NF 110
4,4′,4″,4″′-[Carbonylbis[imino-5,1,3-benzenetriyl-bis(carbonylimino)]]tetrakisbenzenesulphonic acid
- NF 157
8,8′-[Carbonylbis[imino-3,1-phenylenecarbonylimino(4-fluoro-3,1-phenylene)carbonylimino]]bis-1,3,5-naphthalenetrisulphonic acid
- NF 279
8,8′-[Carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino)]bis-1,3,5-naphthalenetrisulphonic acid
- NF 340
4,4′-(Carbonylbis(imino-3,1-(4-methyl-phenylene)carbonylimino))bis(naphthalene-2,6-disulphonic acid)
- NF 449
4,4′,4″,4″′-[Carbonylbis(imino-5,1,3-benzenetriyl-bis(carbonylimino))]tetrakis-1,3-benzenedisulphonic acid
- PSB 0474
3-(2-Oxo-2-phenylethyl) uridine-5′-diphosphate
- PSB 0739
1-Amino-4-[4-phenylamino-3-sulfophenylamino]-9,10-dioxo-9,10-dihydroanthracene-2-sulphonic acid
- PPADS
Pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid
- PPNDS
Pyridoxal-5′-phosphate-6-(2′-naphthylazo-6′-nitro-4′,8′-disulphonate)
- Ro 0437626
N-[(1R)-2-[[(1S,2R,3S)-1-(Cyclohexylmethyl)-3-cyclopropyl-2,3-dihydroxypropyl]amino]-2-oxo-1-(4-thiazolylmethyl)ethyl]-1H-benzimidazole-2-carboxamide
- RO-3
5-[[4,5-Dimethoxy-2-(methylethyl)phenyl]methyl]-2,4-pyrimidinediamine
- Suramin
8,8′-[Carbonylbis[imino-3,1-phenylenecarbonylimino(4-methyl-3,1-phenylene)carbonylimino]]bis-1,3,5-naphthalenetrisulphonic acid
- TNP-ATP
2′,3′-O-(2,4,6-Trinitrophenyl)adenosine-5′-triphosphate
References
- 1.Burnstock G, Williams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Ther. 2000;295:862–869. [PubMed] [Google Scholar]
- 2.Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson KA, Jarvis MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson KA, Ivanov AA, Castro S, Harden TK, Ko H. Development of selective agonists and antagonists of P2Y receptors. Purinergic Signal. 2009;5:75–89. doi: 10.1007/s11302-008-9106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson KA, Boeynaems JM. P2Y nucleotide receptors; promise of therapeutic applications. Drug Discov Today. 2010;15:570–578. doi: 10.1016/j.drudis.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambrecht G, Friebe T, Grimm U, Windscheif U, Bungardt E, Hildebrandt C, Baumert HG, Spatz-Kumbel G, Mutschler E. PPADS, a novel functionally selective antagonist of P2 purinoceptor-mediated responses. Eur J Pharmacol. 1992;217:217–219. doi: 10.1016/0014-2999(92)90877-7. [DOI] [PubMed] [Google Scholar]
- 8.Kim YC, Camaioni E, Ziganshin AU, Ji XJ, King BF, Wildman SS, Rychkov A, Yoburn J, Kim H, Mohanram A, Harden TK, Boyer JL, Burnstock G, Jacobson KA. Synthesis and structure activity relationships of pyridoxal-6-arylazo-5’-phosphate and phosphonate derivatives as P2 receptor antagonists. Drug Dev Res. 1998;45:52–66. doi: 10.1002/(SICI)1098-2299(199810)45:2<52::AID-DDR2>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkins JC, Jane DE. The glutamate story. Br J Pharmacol. 2006;147(Suppl 1):S100–S108. doi: 10.1038/sj.bjp.0706444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziganshin AU, Ziganshina LE, Burnstock G. P2 receptors: theoretical background for the use in clinical practice. Bulletin of Exp Biol Med. 2002;134:313–317. doi: 10.1023/A:1021921824876. [DOI] [PubMed] [Google Scholar]
- 11.Tocris Bioscience webpage for purinergic (P2X) receptors (2011). http://www.tocris.com/pharmacologicalBrowser.php?ItemId=5081. Accessed 1 June 2011
- 12.Tocris Bioscience webpage for purinergic (P2Y) receptors (2011). http://www.tocris.com/pharmacologicalBrowser.php?ItemId=187653. Accessed 1 June 2011
- 13.Lambrecht G, Rettinger J, Bäumert HG, Czeche S, Damer S, Ganso M, Hildebrandt C, Niebel B, Spatz-Kümbel G, Schmalzing G, Mutschler E. The novel pyridoxal-5′-phosphate derivative PPNDS potently antagonizes activation of P2X1 receptors. Eur J Pharmacol. 2000;387:R19–R21. doi: 10.1016/S0014-2999(99)00834-1. [DOI] [PubMed] [Google Scholar]
- 14.Kim YC, Lee JS, Sak K, Mamedova L, Boeynaems JM, Jacobson KA. Synthesis of pyridoxal phosphate derivatives with antagonist activity at the P2Y13 receptor. Biochem Pharmacol. 2005;70:266–274. doi: 10.1016/j.bcp.2005.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bültmann R, Wittenburg H, Pause B, Kurz G, Nickel P, Starke K. P2-purinoceptor antagonists. III. Blockade of P2-purinoceptor subtypes and ecto-nucleotidases by compounds related to suramin. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:498–504. doi: 10.1007/BF00168442. [DOI] [PubMed] [Google Scholar]
- 16.Damer S, Niebel B, Czeche S, Nickel P, Ardanuy U, Schmalzing G, Rettinger J, Mutschler E, Lambrecht G. NF279: a novel potent and selective antagonist of P2X receptor-mediated responses. Eur J Pharmacol. 1998;350:R5–R6. doi: 10.1016/S0014-2999(98)00316-1. [DOI] [PubMed] [Google Scholar]
- 17.Rettinger J, Schmalzing G, Damer S, Muller G, Nickel P, Lambrecht G. The suramin analogue NF279 is a novel and potent antagonist selective for the P2X1 receptor. Neuropharmacology. 2000;39:2044–2053. doi: 10.1016/S0028-3908(00)00022-8. [DOI] [PubMed] [Google Scholar]
- 18.Rhee AM, Heijden MPA, Beukers MW, Ilzerman A, Soudijn W, Nickel P. Novel competitive antagonists for P2 purinoceptors. Eur J Pharmacol. 1994;268:1–7. doi: 10.1016/0922-4106(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 19.Kassack MU, Braun K, Ganso M, Nickel P, Boing B, Muller G, Lambrecht G. Structure-activity relationships of analogues of NF449 confirm NF449 as the most potent and selective known P2X1 receptor antagonist. Eur J Med Chem. 2004;39:345–357. doi: 10.1016/j.ejmech.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Rettinger J, Braun K, Hochmann H, Ullmann H, Nickel P, Schmalzing G, Lambrecht G. Profiling at recombinant homomeric and heteromeric rat P2X receptors identifies the suramin analogue NF449 as a highly potent P2X1 receptor antagonist. Neuropharmacology. 2005;48:461–468. doi: 10.1016/j.neuropharm.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Hausmann R, Rettinger J, Gerevich Z, Meis S, Kassack M, Illes P, Lambrecht G, Schmalzing G. The suramin analog 4,4′,4″,4″′-(carbonylbis(imino-5,1,3-benzenetriylbis(carbonylimino)))tetra-kis-benzenesulfonic acid (NF110) potently blocks P2X3 receptors: subtype selectivity is determined by location of sulfonic acid groups. Mol Pharmacol. 2006;69:2058–2067. doi: 10.1124/mol.106.022665. [DOI] [PubMed] [Google Scholar]
- 22.Ullmann H, Meis S, Hongwiset D, Marzian C, Wiese M, Nickel P, Communi D, Boeynaems JM, Wolf C, Hausman R, Schmalzing G, Kassack M. Synthesis and structure-activity relationships of suramin-derived P2Y11 receptor antagonists with nanomolar potency. J Med Chem. 2005;48:7040–7048. doi: 10.1021/jm050301p. [DOI] [PubMed] [Google Scholar]
- 23.Meis S, Hamacher A, Hongwiset D, Marzian C, Wiese M, Eckstein N, Royer HD, Communi D, Boeynaems JM, Hausmann R, Schmalzing R, Kassack MU. NF546 [4,4′-(carbonylbis(imino-3,1-phenylene-carbonylimino-3,1-(4-methyl-phenylene)-carbonylimino))-bis(1,3-xylene-α, α′-diphosphonic acid) tetrasodium salt] is a non-nucleotide P2Y11 agonist and stimulates release of interleukin-8 from human monocyte-derived dendritic cells. J Pharmacol Exp Ther. 2010;332:238–247. doi: 10.1124/jpet.109.157750. [DOI] [PubMed] [Google Scholar]
- 24.Mamedova LK, Joshi BV, Goa ZG, Kugelgen I, Jacobson KA. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol. 2004;67:1763–1770. doi: 10.1016/j.bcp.2004.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baqi Y, Atzler K, Kose M, Glanzel M, Müller CE. High-affinity, non-nucleotide-derived competitive antagonists of platelet P2Y12 receptors. J Med Chem. 2009;52:3784–3793. doi: 10.1021/jm9003297. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann K, Baqi Y, Morena MS, Glanzel M, Müller CE, Kugelgen I. Interaction of new, very potent non-nucleotide antagonists with Arg256 of the human platelet P2Y12 receptor. J Pharmacol Exp Ther. 2009;331:648–655. doi: 10.1124/jpet.109.156687. [DOI] [PubMed] [Google Scholar]
- 27.Burnstock G, Cusack NJ, Hills JM, MacKenzie I, Meghji P. Studies on the stereoselectivity of the P2-purinoceptor. Br J Pharmacol. 1983;79:907–913. doi: 10.1111/j.1476-5381.1983.tb10535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virginio C, Robertson G, Surprenant A, North RA. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3 and heteromeric P2X2/3 receptors. Mol Pharmacol. 1998;53:969–973. [PubMed] [Google Scholar]
- 29.Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Séguéla P, Voigt M, Humphrey PP. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- 30.Boyer JL, Mohanram A, Camaioni E, Jacobson KA, Harden TK. Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2’-deoxyadenosine 3’,5’-bisphosphate. Br J Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravi RG, Kim HS, Zimmermann H, Lee K, Boyer JL, Harden TK, Jacobson KA. Adenine nucleotide analogues locked in a northern methanocarba conformation: enhanced stability and potency as P2Y1 receptor agonists. J Med Chem. 2002;45:2090–2100. doi: 10.1021/jm010538v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nandanan E, Jang SY, Moro S, Kim HO, Siddiqui MA, Russ P, Marquez VE, Busson R, Herdewijn P, Harden TK, Boyer JL, Jacobson KA. Synthesis, biological activity, and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y1 receptor ligands. J Med Chem. 2000;43:829–842. doi: 10.1021/jm990249v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, Maddileti S, Marquez VE, Harden TK, Jacobson KA. 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a northern conformation: enhanced potency as P2Y1 receptor antagonists. J Med Chem. 2003;46:4974–4987. doi: 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphries RG, Tomlinson W, Ingall AH, Cage PA, Leff P. FPL 66096: a novel, highly potent and selective antagonist at human platelet P2T-purinoceptors. Br J Pharmacol. 1994;113:1057–1063. doi: 10.1111/j.1476-5381.1994.tb17100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Tayeb A, Qi A, Müller CE. Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4 and P2Y6 receptors. J Med Chem. 2006;49:7076–7087. doi: 10.1021/jm060848j. [DOI] [PubMed] [Google Scholar]
- 36.Besada P, Shin DH, Costanzi S, Ko H, Mathe C, Gagneron J, Gosselin G, Maddileti S, Harden TK, Jacobson KA. Structure-activity relationships of uridine 5′-diphosphate analogues at the human P2Y6 receptor. J Med Chem. 2006;49:5532–5543. doi: 10.1021/jm060485n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ko H, Fricks I, Ivanov AA, Harden TK, Jacobson KA. Structure-activity relationship of uridine 5′-diphosphoglucose analogues as agonists of the human P2Y14 receptor. J Med Chem. 2007;50:2030–2039. doi: 10.1021/jm061222w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frye SV. The art of the chemical probe. Nat Chem Biol. 2010;6:159–161. doi: 10.1038/nchembio.296. [DOI] [PubMed] [Google Scholar]
- 39.Workman P, Collins I. Probing the probes: fitness factors for small molecule tools. Chem Biol. 2010;17:561–577. doi: 10.1016/j.chembiol.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGeary RP, Bennett AJ, Tran QB, Cosgrove KL, Ross BP. Suramin: clinical uses and structure-activity relationships. Mini Rev Med Chem. 2008;8:1384–1394. doi: 10.2174/138955708786369573. [DOI] [PubMed] [Google Scholar]
- 41.Jaime-Figueroa S, Greenhouse R, Padilla F, Dillon MP, Gever JR, Ford APDW. Discovery and synthesis of a novel and selective drug-like P2X1 receptor. Bioorg Med Chem. 2005;15:3292–3295. doi: 10.1016/j.bmcl.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 42.Donnelly-Roberts D, McGaraughy S, Shieh CC, Honore P, Jarvis MF. Painful purinergic receptors. J Pharmacol Exp Ther. 2008;324:409–415. doi: 10.1124/jpet.106.105890. [DOI] [PubMed] [Google Scholar]
- 43.Tokumitsu H, Chijiwa T, Hagiwara M, Mizutani TM, Hidaka H. KN-62, 1-[N-O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1990;265:4315–4320. [PubMed] [Google Scholar]
- 44.Wittenburg H, Bultmann R, Pause B, Ganter C, Kurz G, Starke K. P2-purinoceptor antagonists: II blockade of P2-purinoceptor subtypes and ecto-nucleotidase by compounds related to Evans blue and trypan blue. Naunyn-Schmied Arch Pharmacol. 1996;354:491–497. doi: 10.1007/BF00168441. [DOI] [PubMed] [Google Scholar]
- 45.Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007;151:571–579. doi: 10.1038/sj.bjp.0707265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J. 2010;24:337–345. doi: 10.1096/fj.09-138883. [DOI] [PubMed] [Google Scholar]
- 48.Müller CE. Emerging structures and ligands for P2X3 and P2X4 receptors-towards novel treatments of neuropathic pain. Purinergic Signal. 2010;6:145–148. doi: 10.1007/s11302-010-9182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson DW, Gregg RJ, Kort ME, Perez-Medrano A, Voight EA, Wang Y, Grayson G, Namovic MT, Donelly-Roberts DL, Niforatos W, Honore P, Jarvis MF, Faltynek CR, Carroll WA. Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem. 2006;49:3659–3666. doi: 10.1021/jm051202e. [DOI] [PubMed] [Google Scholar]
- 50.Honore P, Donnelly-Roberts D, Namovic M, Zhong C, Wade C, Chandran P, Zhu C, Carroll W, Perez-Medrano A, Iwakura Y, Jarvis MF. The antihyperalgesic activity of a selective P2X7 receptor antagonist, A-839977, is lost in IL-1alphabeta knockout mice. Behav Brain Res. 2009;204:77–81. doi: 10.1016/j.bbr.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 51.Michel AD, Chambers LJ, Walter DS. Negative and positive allosteric modulators of the P2X7 receptor. Br J Pharmacol. 2008;153:737–750. doi: 10.1038/sj.bjp.0707625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michel AD, Clay WC, Ng SW, Roman S, Thompson K, Condreay JP, Hall M, Holbrook J, Livermore D, Senger S. Identification of regions of the P2X7 receptor that contribute to human and rat species differences in antagonist effects. Br J Pharmacol. 2008;155:738–751. doi: 10.1038/bjp.2008.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michel AD, Ng SW, Roman S, Clay WC, Dean DK, Walter DS. Mechanism of action of species-selective P2X7 receptor antagonists. Br J Pharmacol. 2009;156:1312–1325. doi: 10.1111/j.1476-5381.2009.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, Harris R, Medrano AP, Carroll W, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino)methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- 55.King BF. Novel P2X7 receptor antagonists ease the pain. Br J Pharmacol. 2007;151:565–567. doi: 10.1038/sj.bjp.0707266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donnelly-Roberts D, Namovic MT, Han P, Jarvis MF. Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br J Pharmacol. 2009;157:1203–1214. doi: 10.1111/j.1476-5381.2009.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobson (2010) P2X and P2Y receptors. http://www.tocris.com/pdfs/pdf_downloads/Purinergic_Receptors_Review.pdf. Accessed 1 June 2011