Abstract
Introduction:
Smoking tobacco during pregnancy results in exposure to the fetal neuroteratogen nicotine. The current study evaluated if the offspring of smokers show abnormalities in maternal ratings of executive function, prevalence of Attention Deficit Hyperactivity Disorder (ADHD), and academic performance. A secondary objective was to determine the utility of online data collection.
Methods:
Mothers (N = 357) completed the parent form of the Behavioral Rating Inventory of Executive Function (BRIEF) and provided information about smoking during pregnancy.
Results:
The internal consistency of the BRIEF when administered electronically was quite satisfactory (Cronbach’s α = .98). As anticipated, ADHD was more frequently diagnosed in the offspring of women that smoked at least 10 cigarettes/day (odds ratio [OR] = 2.64, 95% CI = 1.22–5.71). Higher (i.e., more problematic) ratings relative to unexposed children (p < .01) were only identified on the total BRIEF score, the Metacognition Index, and on the Initiate, Plan/Organize, and Monitor scales among children exposed to ≥10 cigarettes/day. Nicotine-exposed children were also more likely to perform less well than their classmates in math (OR = 2.78, 95% CI = 1.59–4.87) and reading (OR = 2.00, 95% CI = 1.10–3.63), and these academic effects were independent of maternal education levels.
Conclusions:
This report provides preliminary evidence that the BRIEF has adequate psychometric properties when administered electronically and that mothers who smoke have offspring with lower executive function proficiency. These findings contribute to a larger literature that indicates that smoking during pregnancy results in adverse reproductive outcomes and, possibly, subtle but enduring deficits in prefrontal function.
Introduction
The adverse consequences of cigarette smoking on reproductive outcomes and on the neonate are well established. Nicotine exposure causes dose-dependent increases in the risk of stillbirth (Strandberg-Larsen, Tinggaard, Nybo Andersen, Olsen, & Grønbaek, 2008, Wisborg, Kesmodel, Henriksen, Olsen, & Secher, 2001), low birth weight/decreased head circumference (Jaakkola, Jaakkola, & Zahlsen, 2001; Roza et al., 2007; Winzer-Serhan, 2008), and sudden infant death syndrome (Alm et al., 1998; Wisborg, Kesmodel, Henriksen, Olsen, & Secher, 2000). In contrast, relatively less is definitively known about the long-term sequelae of in utero nicotine on neurobehavioral function (Batty, Der, & Deary, 2006; Knopik, 2009; Linnet et al., 2003; Shea & Steiner, 2008). Many, but not all, case–control and cross-sectional studies have found that the rates of Attention Deficit Hyperactivity Disorder (ADHD) are elevated (odds ratio [OR] = 2 to 4) among the offspring of smokers, even after accounting for group differences in maternal education and socioeconomic status (Langley, Rice, van den Bree, & Thapar, 2005; Linnet et al., 2003; Schmitz et al., 2006). Hyperkinetic disorder, the International Classification of Diseases equivalent of ADHD, frequency was similarly increased in children with a history of prenatal nicotine (Obel et al., in press). However, other investigations using novel methodologies have challenged the perspective that early developmental nicotine causes ADHD symptomology (Thapar et al., 2009), deficits in intelligence (Gilman, Gardener, & Buka, 2008), or academic difficulties (Lambe, Hultman, Torrång, Maccabe, & Cnattingius, 2006).
Executive functions are responsible for guiding, directing, and managing cognitive and emotional behaviors, especially when solving novel problems, and are mediated by a network of forebrain structures including the prefrontal cortex (Gioia, Isquith, Guy, & Kenworthy, 2000). The behavioral endpoints that have been reported to be sensitive to in utero smoking include auditory sustained attention (Kristjansson, Fried, & Watkinson, 1989) and perseverative errors on the Wisconsin Card Sorting Test (Cornelius, Ryan, Day, Goldschmidt, & Willford, 2001). Although both attentional vigilance and the ability to override a previously learned rule are elements of executive function, some studies indicate that executive function is not a simple unitary process (Huijbregts, Warren, de Sonneville, & Swaab-Barneveld, 2008, Miyake et al., 2000). Furthermore, there may be distinct developmental trends for different executive function component processes and their integration (Pennequin, Sorel, & Fontaine, 2010; Piper, Li, Eowiz, Kobel, Benice, Chu, et al., 2011). Therefore, a benefit of the Behavioral Rating Inventory of Executive Function (BRIEF) is that this instrument can assess various nonoverlapping aspects of executive functioning (e.g., inhibition, self-monitoring, working memory, and emotional control) in a variety of ecologically valid contexts (home, school, play). Although this measure has been used previously with women that used illicit drugs during pregnancy (Piper, Acevedo, Kolchugina, Butler, Corbett, Honeycutt,et al., 2011), to our knowledge, no prior investigations have evaluated the offspring of tobacco users.
Online survey administration is becoming an increasingly common methodology in the substance abuse field with studies of adult alcohol (Collins, Logan, & Neighbors, 2010; Kypri, Paschall, Langley, Baxter, & Bourdeau, 2010), cannabis (Mullens, Young, Dunne, & Norton, 2010), methamphetamine (Hirshfield, Remien, Humberstone, Walavalkar, & Chiasson, 2004; Hirshfield, Remien, Walavalkar, & Chiasson, 2004), ecstasy (Gamma, Jerome, Liechti, & Sumnall, 2005; Rodgers et al., 2006), prescription stimulant (McCabe & Teter, 2007), and nicotine (Heffernan, Ling, Parrott, Buchanan, Scholey, & Rodgers, 2005) users. To our knowledge, no online investigations have been conducted in the neurotoxicology and teratology field. This is unfortunate for two reasons. First and foremost, with the appropriate safeguards and confidentiality protections, sensitive/illegal behaviors may be more readily disclosed in electronic surveys, which minimize the risk of interviewer judgments (Hirshfield, Remien, Humberstone, et al., 2004; Hirshfield, Remien, Walavalkar, et al., 2004). Second, the individual items on computerized questionnaires can be tailored automatically to each respondent based on prior responses. This could involve the administration of additional questions about the timing and extent of drug use only if the respondent reported lifetime use. Additionally, automated data collection and processing is faster and more efficient than more traditional (i.e., paper and pencil or computer-aided interviews) methods and is acceptable to various populations (Gamma, Jerome, Liechti, & Sumnall, 2005; Heffernan et al., 2005; Shakeshaft, Bowman, & Sanson-Fisher, 1998). The present online study examines maternally rated executive function using the BRIEF in children exposed to nicotine during pregnancy. As abnormalities in executive function may contribute to deficits in school performance as well as a diagnosis of ADHD, these endpoints were also evaluated. Since this is the first Internet-based study to use the BRIEF, some psychometric properties were also determined.
Methods
Participants
Mothers (N = 357) of children aged 5–18 years were recruited for a child behavior investigation. Flyers were posted on community boards throughout Doernbecher Children’s Hospital, Oregon Health and Science University (OHSU), the Portland metro area, western Oregon, and western Washington (e.g., grocery stores, libraries, coffee shops). Links to the study were also displayed on the community and volunteer sections of Craigslist (craigslist.org) as well as on message boards for parents (e.g., iVillage.com). The majority of respondents were from the northwest (68.3% from Oregon, Washington, or Idaho) with other participants mostly from adjacent states of California and Montana. This anonymous online survey was administered through Research Electronic Data Capture (REDCap), version 1.3.9, a web-based application for building and managing online databases with maximal security for sensitive information (Harris, Taylor, Thielke, Payne, Gonzalez, & Conde, et al., 2009). The Institutional Review Board at OHSU approved all procedures.
Measures
After providing an online consent to participate in this study, the parents began the survey, which typically took about 20 min to complete. The items on the first half were organized from less to more personal and included questions about maternal and child demographics (e.g., age, sex, ethnicity), academic performance (e.g., “Please rate your child’s performance in math with relation to their scores on the state’s standardized test.” with options of below, at, or above grade level), and child/maternal neurological or psychiatric conditions (e.g., Has your child been diagnosed with any of the following? with options of ADHD, fetal alcohol syndrome [FAS], and brain trauma). Items on maternal drug use (nicotine, alcohol, marijuana, cocaine, and the opiates) were organized into two periods: during pregnancy and specifically during the third trimester. If the respondent answered in the affirmative then additional item(s) about the extent of use (e.g., how many cigarettes did the biological mother smoke each day?) were displayed.
The BRIEF accounted for the remaining 86 items. The BRIEF is a widely employed parental rating instrument for the clinical evaluation of children aged 5–18 years with inherited and acquired neurobehavioral conditions focusing on the child’s everyday activities at home and at school. Each behavior is rated as never, sometimes, or often a problem (1–3 points, respectively) in the last six months. The eight BRIEF scales form two measures of executive functioning (Metacognition and Behavioral Regulation), and these are summed to form an overall measure of executive functioning (the Global Executive Composite or GEC). The Metacognition Index comprises the following five scales: (a) Initiate, the capacity to act independently to produce ideas, responses, or problem-solving strategies; (b) Working Memory, the ability to hold information to complete a task; (c) Plan/Organize, the capability to anticipate future events, form goals, and construct the appropriate steps to complete an objective; (d) Organization of Materials, the degree of orderliness of work and play spaces; and (e) Monitor, self-monitoring habits scales. The Behavioral Regulation Index consists of three scales: (a) Inhibit, the ability to regulate one’s behavior at the appropriate time and not act on impulse; (b) Shift, the ability to switch attention and change focus; and (c) Emotional Control, the capacity to regulate emotional responses. Standardized T50 scores were determined from raw scores based on age/sex norms with higher scores indicating greater severity. The inconsistency scale is obtained by calculating the difference between 10 pairs of items (range = 0–20) with a score ≥9 interpreted as inconsistent. For example, a response of often (3 points) to “Has explosive, angry outbursts” and never (1 point) to “Has outbursts for little reason” would contribute two points to the inconsistency score.
The paper and pencil BRIEF has excellent internal consistency (Cronbach’s α = .97) and good test–retest reliability over two weeks (r = .86 for the GEC, .88 for Metacognition Index, and .84 for the Behavioral Regulation Index) in a normative sample. Importantly, Gioia et al. noted that the education level of the rater showed small, but significant, negative correlations with some BRIEF scales, that is, lower education was associated with more problematic ratings which accounted for as much as 5% of the variance. Good convergent and divergent validity with other standard parent report measures are described elsewhere (Gioia, Isquith, Guy, & Kenworthy, 2000).
Statistical Methods
Statistical analyses were completed with the Statistical Package for the Social Sciences, version 16.0, with data expressed as mean (±SEM) and p < .05 considered statistically significant. Exclusion criteria were child age (<5 or >18), incomplete/unfinished questionnaires (N = 138), responses from any other source besides the biological mother (father, grandparent, adoptive/foster parent, N = 80), or children with brain trauma (N = 3; Sesma, Slomine, Ding, & McCarthy, 2008) or FAS (N = 7; Chasnoff, Wells, Telford, Schmidt, & Messer, 2010). Categorical-level analyses were completed with the χ2, or, if the N/cell was < 5, likelihood ratios. As no online research with the BRIEF has been completed previously, three quality checks were conducted. First, the internal consistency (Cronbach, 1951) of the instrument was determined and compared with prior data from paper administration (Gioia et al., 2000). Second, the percentage of responses that met the inconsistent criteria was examined. Third, as the BRIEF is one of many instruments used in the diagnosis of ADHD (Gioia et al., 2000; McCandless & O’Laughlin, 2007), a comparison of unexposed ADHD+ versus ADHD− was completed to evaluate test validity. A BRIEF T50 ≥ 65, 1.5 SDs above the mean, is interpreted as a clinically significant (Gioia et al., 2000). The OR of clinically significant problems with the 95% CI was also determined for nicotine, education, and income (all coded dichotomously: exposed relative to unexposed, less than or equal to high school relative to above, <$10K/year during pregnancy relative to above). Analysis of covariance was also completed with covariates selected empirically based on variables in Table 1 that statistically (p < .05) differentiated women that did (NIC+) and did not (NIC−) use nicotine during pregnancy. Additional analyses were also conducted with the NIC+ divided into a low (1–9 cigarettes/day) and high (10+ cigarettes/day) groups. This categorization has been used previously by others (Huijbregts, Warren, Sonneville, & Swaab-Barneveld, 2008; Olds, Henderson, & Tatelbaum, 1994; Sexton, Fox, & Hebel, 1990; Thapar et al., 2009).
Table 1.
Characteristics of Women and Their Children by Nicotine Use During Pregnancy (low is 1–9 cigarettes/day and high is 10+ cigarettes/day)
| Nicotine |
Dose |
|||
| − (N = 272) | + (N = 85) | Low (N = 52) | High (N = 33) | |
| Maternal | ||||
| Age when child born (SEM) | 26.3 (0.4) | 23.9 (0.7)b | 24.1 (1.0)a | 23.8 (1.1)a |
| Ethnicity (% non-White) | 12.5 | 7.1 | 9.6 | 3.1 |
| Current income (<25K, %) | 22.6 | 42.7c | 38.8 | 48.5 |
| Education (≤ high school, %) | 23.6 | 41.7b | 39.2a | 45.5a |
| Northwest resident (%) | 69.9 | 75.9 | 75.5 | 76.7 |
| ADHD (%) | 9.1 | 7.1 | 7.7 | 6.1 |
| Pregnancy | ||||
| Income (% < 10K) | 10.7 | 40.7c | 35.4c | 48.5c |
| Prenatal vitamins (%) | 78.2 | 69.4 | 73.1 | 63.6 |
| Number of cigarettes/day (SEM) | NA | 9.4 (0.8) | 5.3 (0.6) | 15.7 (0.9) |
| Alcohol (%) | 16.8 | 22.4 | 21.2 | 24.2 |
| Marijuana (%) | 7.6 | 23.5c | 21.2c | 27.3c |
| Cocaine (%) | 0.4 | 5.9b | 3.8a | 9.1b |
| Methamphetamine (%) | 1.1 | 14.1c | 11.5c | 18.2c |
| Third trimester | ||||
| Nicotine (%) | 0.0 | 83.5c | 78.8c | 90.9c |
| Alcohol (%) | 9.6 | 10.6 | 9.6 | 12.1 |
| Marijuana (%) | 3.3 | 16.5c | 17.3 | 15.2 |
| Cocaine (%) | 0.0 | 2.4a | 1.9 | 3.0 |
| Methamphetamine (%) | 0.0 | 7.1c | 5.8c | 9.1c |
| Child | ||||
| Age (SEM) | 10.3 (0.2) | 11.0 (0.4) | 10.4 (0.5) | 12.0 (0.8) |
| Sex (% female) | 48.7 | 58.8 | 53.8 | 66.7 |
| Ethnicity (% non-White) | ||||
| Premature | 13.3 | 22.6a | 21.6 | 24.2 |
| ADHD (%) | 17.8 | 29.4a | 25.0 | 36.4a |
| Math (% below) | 17.3 | 36.7c | 28.6 | 50.0c |
| Reading (% below) | 15.9 | 27.5a | 28.0a | 26.7 |
Note. ADHD = Attention Deficit Hyperactivity Disorder; NA = not applicable.
ap < .05, bp < .005, or cp < .0005 versus nicotine−.
Results
Cronbach’s α was .98 for GEC, .97 for the Metacognition Index, and .96 for the Behavioral Regulation Index (corresponding values for the paper and pencil version were .97, .96, and .94, see Supplementary Figure 1 for further details). Furthermore, only one respondent met the criteria for inconsistent responding (0.3%). As the Metacognition Index has been shown previously to differentiate ADHD+ and ADHD− children (McCandless & O’Laughlin, 2007), this pattern was evaluated and verified with online BRIEF administration, ADHD− = 54.8 ± 0.8, ADHD+ = 69.5 ± 1.6, t(271) = 7.91, p < .0005. Overall, these three internal checks for the reliability and validity of online BRIEF administration all indicated very satisfactory psychometric properties.
Demographics, substance use patterns, and academic performance are depicted in Table 1. Women that did (NIC+) and did not (NIC−) use cigarettes during pregnancy did not differ in ethnicity, frequency of ADHD, or alcohol use. However, NIC+ women were younger and had lower income (both during pregnancy and currently), less education, were more likely to also use marijuana, cocaine, and methamphetamine during pregnancy and specifically in the third trimester. The NIC− and NIC+ children did not differ based on age or sex, but NIC+ were more likely to be diagnosed with ADHD and to be behind their peers in math and reading.
Further analysis of the NIC+ group divided into low (1–9 cigarettes/day) versus high (10–30 cigarettes/day during pregnancy) revealed that maternal age and income were significantly different in the both the low and high groups relative to NIC−. Notably, these groups did not differ from each other. Relative to unexposed children, reading difficulties were present in the low-NIC group (OR = 2.05, 95% CI = 1.02–4.14), but math difficulties (OR = 4.80, 95% CI = 2.19–10.52) and ADHD (OR = 2.64, 95% CI = 1.22–5.71) showed significant elevations only among high NIC.
The GEC was significantly elevated by 0.5 of a SD in NIC+ (63.1± 1.5) relative to NIC− (57.8 ± 0.8) children, F(1,356) = 9.76, p < .005. This difference was retained with maternal education, age at pregnancy, pregnancy income, child ADHD diagnosis, maternal ADHD diagnosis, prenatal marijuana, cocaine, methamphetamine, or alcohol exposure, prematurity, or current income entered as covariates. Additional analyses noted some evidence for exposed boys exhibiting a more pronounced profile than girls. For example, the Behavioral Regulation Index was increased by eight points in males, NIC− = 57.9 ± 1.3, NIC+ = 65.9 ± 2.6, t(173) = 2.85, p ≤ .005, but only three points in females, NIC− = 55.7 ± 1.2, NIC+ = 58.6 ± 1.8, t(181) = 1.32, p = 0.19. In contrast, younger (<9.5) and older (>9.5) children were similarly affected (data not shown).
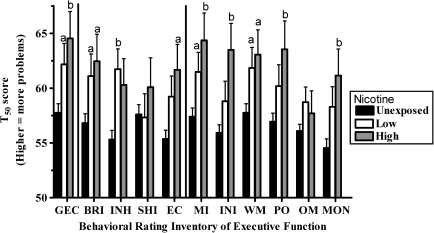
Figure 1 shows a comparison of each NIC group. Both the low (p < .05) and high (p < .01) exposed children differed from the unexposed on the GEC. Similarly, the low- and high-NIC groups were elevated on the Behavioral Regulation Index. The low-, but not high-, NIC children exhibited increases on the Inhibit scale with the reverse pattern noted for Emotional Control. The low (p < .05) and high (p < .01) groups had difficulties with Metacognition. There was partial evidence for dose-dependent exposure effect with significant (p < .01) increases for the Initiate, Plan/Organize, and Monitor scales for only the high-NIC children. On the other hand, there were no significant differences between low- and high-NIC groups.
Figure 1.
Standardized (T50) scores of maternal rating of executive function in children aged 5–18 years exposed to nicotine during pregnancy (low: 1–9 cigarettes/day and high: ≥10 cigarettes/day). GEC = Global Executive Composite, BRI = Behavioral Regulation Index, INH = Inhibit, SHI = Shift, MI = Metacognition Index, INI = Initiate, WM = Working Memory, PO = Plan/Organize, OM = Organization of materials, and MON = Monitor. ap < .05 or bp < .01 versus unexposed.
Table 2 shows the likelihood of having a clinically significant problem (T50 ≥ 65) on the BRIEF, having an ADHD diagnosis or being behind peers academically based on maternal education, income, or nicotine use during pregnancy. The prevalence of all these outcomes was uniformly increased in the offspring of women with no post-secondary education (note that ADHD did not fulfill the statistical cutoff with p = .053). Similarly, women with lower incomes during pregnancy generally showed a less generalized pattern of difficulties on these domains. Maternal nicotine use was associated with a more focused profile on the BRIEF including on the Inhibit, Emotional Control, Organization, and Monitor scales. Importantly, only math showed a nicotine effect, which was retained when the variance attributable to education as well as income was removed.
Table 2.
Odds Ratio (OR) and 95% CI for Clinically Significant Concerns on the Behavioral Rating Inventory of Executive Function (BRIEF), Diagnosis of Attention Deficit Hyperactivity Disorder (ADHD), and Performing Below Peers on Standardized Tests in Math and Reading by Maternal Education, Income, and Nicotine Use During Pregnancy
| Education |
Income |
Nicotine |
||||
| BRIEF | OR | CI | OR | CI | OR | CI |
| Global Executive Composite | 2.31b | 1.43–3.73 | 2.22a | 1.27–3.88 | 1.62 | 0.98–2.67 |
| Behavioral Rating Index | 2.87c | 1.76–4.66 | 1.74 | 0.99–3.07 | 1.50 | 0.90–2.50 |
| Inhibit | 2.65c | 1.62–4.34 | 1.37 | 0.76–2.47 | 1.97a | 1.18–3.29 |
| Shift | 1.90a | 1.17–3.10 | 1.14 | 0.64–2.04 | 1.01 | 0.60–1.71 |
| Emotional control | 2.80c | 1.70–4.61 | 2.23b | 1.25–3.97 | 1.81a | 1.07–3.05 |
| Metacognition Index | 2.44c | 1.50–3.95 | 1.80a | 1.03–3.16 | 1.45 | 0.88–2.41 |
| Initiate | 2.19b | 1.34–3.58 | 1.38 | 0.77–2.46 | 1.62 | 0.97–2.71 |
| Working memory | 2.05b | 1.27–3.30 | 1.28 | 0.73–2.26 | 1.55 | 0.94–2.56 |
| Plan/organize | 2.33b | 1.43–3.77 | 1.52 | 0.86–2.68 | 1.38 | 0.83–2.29 |
| Organization of materials | 1.70b | 1.02–2.81 | 1.05 | 0.57–1.95 | 1.72a | 1.02–2.90 |
| Monitor | 1.97a | 1.19–3.26 | 1.96a | 1.10–3.49 | 1.97a,d | 1.17–3.32 |
| ADHD | 1.71 | 0.99–2.95 | 1.64 | 0.87–3.09 | 1.92a | 1.10–3.36 |
| Math | 2.06a | 1.19–3.56 | 2.01a | 1.08–3.76 | 2.78c,e,f | 1.59–4.87 |
| Reading | 1.86a | 1.04–3.32 | 1.76 | 0.91–3.40 | 2.00a,d | 1.10–3.63 |
Note. ap < .05. bp < .005. cp < .0005 for logistic regression.
dp < .05 or ep < .005 for nicotine with education as a covariate.
fp < .005 for nicotine with income as a covariate.
Discussion
There are two key findings of this report. The first is that online survey administration verified and extended upon several findings that have been documented with other methodologies. Women that smoked during pregnancy were poorer, less educated, younger, and more likely to use other recreational drugs as well as give birth prematurely. All these demographic and perinatal findings are congruent with what has commonly been reported in earlier investigations of maternal smoking (Batty et al., 2006; Winzer-Serhan, 2008). Electronic BRIEF delivery resulted in equivalent internal consistency with the paper and pencil form (Gioia et al., 2000). Support for the validity of computerized administration of this executive function instrument was also identified with the anticipated (McCandless & O’Laughlin, 2007) elevation in the Behavioral Regulation Index among unexposed children with an ADHD diagnosis. Together, the methodological implications of these outcomes may be broadly relevant for others as we found that many mothers were quite willing to participate in online research. Instruments that have adequate psychometric properties when administered electronically could be employed for investigations focused on other drugs that are used less commonly than nicotine to examine the neurobehavioral profile of children exposed to nicotine cessation agents or to more efficiently recruit from rural populations. The web-based procedures could be incorporated in longitudinal investigations that include geographically mobile families or coupled with a medical record release forms to obtain more detailed perinatal information. Additional online studies with participants obtained from a national registry of research volunteers are also ongoing.
Second, nicotine-exposed children differ from their unexposed counterparts on several overlapping areas including psychiatric diagnoses, academic performance, and maternally rated executive function. Although our current understanding of the causes versus risk factors for ADHD is incomplete, identification of the extent that in utero nicotine is involved has been a highly active research area (Ball et al., 2010; Biederman, Monuteaux, Faraone, & Mick, 2009; Knopik, 2009; Kotimaa et al., 2003; Linnet et al., 2003; Milberger, Biederman, Faraone, Chen, & Jones, 1996; Obel et al., in press; Schmitz et al., 2006; Thapar et al., 2009).
Children whose mother smoked at least a half-pack per day were over twice as likely as unexposed children to have been diagnosed with ADHD. One confound of many of the prior investigations that have identified an increased frequency of ADHD among NIC-exposed children is that the rates of parental ADHD were also elevated (Biederman et al., 2009; Knopik, 2009; Milberger et al., 1996; Schmitz et al., 2006), which raises the possibility that a vulnerability to develop ADHD was inherited. Therefore, it is important to emphasize that the rates of maternal ADHD in this sample were equivalent in tobacco abstaining and tobacco using women. Another interesting finding from this investigation is that the offspring of women from lower educational backgrounds exhibited a nonsignificant tendency to more commonly be diagnosed with ADHD. If this finding is replicated in other community samples, future investigations should continue to pay close attention to this key potential confound (Langley et al., 2005). Although animal (Heath & Picciotto, 2009; Thomas, Garrison, Slawecki, Ehlers, & Riley, 2000) and, perhaps, human (Berlin et al., 2009) studies indicate that it is mechanistically plausible that early developmental smoking causes ADHD, as there was no nicotine-associated difference in ADHD frequency when adjusting for other variables, this would support the view that other socioeconomic and environmental factors have a larger contribution to the etiology of ADHD than does prenatal nicotine.
The current dataset documented that nicotine-exposed children were approximately two times as likely to be behind their peers on mathematics and reading, and these effects were not attributable to differences in maternal education or income, findings which are broadly concordant with Batstra, Hadders-Algra, and Neeleman (2003). Perhaps, the most definitive study to date to examine scholastic performance was conducted with a large (N = 50,000) sample of Swiss teenagers. Academic difficulties showed dose-dependent nicotine increases (OR = 2) within each of five maternal education levels. On the other hand, examination of siblings pairs where the mother smoked during one pregnancy but not the other also identified an elevation in school problems for both children which indicated that nonsmoking factors were responsible (Lambe et al., 2006). The same general pattern indicative of unmeasured genetic or environmental variables underlying intellectual performance deficits was subsequently replicated in an older all-male sample (Lundberg et al., 2010), indicating that the relationship between in utero nicotine exposure and school performance may be dependent on the sample characteristics.
There is currently no consensus whether prenatal nicotine causes or is only correlated with long-term reductions in academic success (Batstra et al., 2003; Knopik, 2009). Many cross-sectional and longitudinal investigations have identified significant decreases among the offspring of smokers on various measures of intelligence (Fried, Watkinson, & Gray, 2003; Gilman et al., 2008; Julvez et al., 2007; Mortensen, Michaelsen, Sanders, & Reinisch, 2004). A deficit in intellectual function on the Stanford–Binet was noted (Olds et al., 1994), and the pre-school–aged offspring of women that quit smoking during pregnancy, relative to those that persisted, had more difficulties on the verbal scale of the McCarthy assessment even after controlling for other prenatal and postnatal variables (Sexton et al., 1990). In contrast, nicotine-associated decrements in mathematics and reading on the Peabody Individual Achievement Test in children from the United States were nonsignificant when accounting for maternal education (Batty et al., 2006). Socioeconomic factors appear to be responsible for the nicotine group differences in reading but not mathematics or spelling in Dutch adolescents (Batstra et al., 2003).
Children with a history of in utero smoking exposure had more problems with maternally rated executive function. The BRIEF findings were quite robust with significant mean elevations in the NIC groups on the GEC, both indices, and six of the eight scales. Most notably, the difference between unexposed and nicotine-exposed children on the GEC were retained after removal of the variance attributable to several other potential confounds. The proportion of children scoring in the clinical range was also more frequent among NIC-exposed children on the Inhibit, Emotional Control, Organization, and Monitor scales. It should be reiterated that executive function is conceptualized by the BRIEF developers as a broad construct mediated by the frontal cortex with its associated cortical and subcortical connections that is responsible for intentional, goal-directed, problem-solving behaviors (Gioia et al., 2000). A fundamental strength of this instrument, unlike single laboratory executive function tests, is that this measure can be completed relatively quickly and can assess nonoverlapping aspects of executive functioning. Furthermore, although elevations in BRIEF ratings are well known among children with ADHD (Gioia et al., 2000; McCandless & O’Laughlin, 2007), abnormalities in executive function are certainly not unique to this condition and have also been identified in extremely low birth weight (Anderson & Doyle, 2004), FAS (Chasnoff et al., 2010), and children that experienced a traumatic brain injury (Sesma et al., 2008). While this is the first report to examine maternally assessed executive function in the offspring of smokers, there are prior studies with laboratory-based measures (Fried et al., 2003; Huizink & Mulder, 2006; Kristjansson et al., 1989), and the present finding of abnormalities in the offspring of smokers are generally concordant. Notably, the fact that no nicotine group differences on the clinically significant executive function measures (Table 2) survived after inclusion of prenatal income levels into the statistical models indicates that socioeconomic or other lifestyle factors are integral for the proportion of children meeting this criteria.
A potential limitation of this investigation is the reliance on retrospectively determined maternal drug use patterns. Although it is generally recognized that retrospective maternal drug use information is inferior to that which has been prospectively obtained (Huizink & Mulder, 2006), empirical examination of this issue with smokers does not lead to simplistic conclusions. The veracity of recall was repeatedly determined over a twenty-year period and found to be accurate for smoking (+ versus −) for the vast majority (94%) of women but correct classification of the number of packs smoked per day was lower (80%; Krall, Valadian, Dwyer, & Gardner, 1989). Furthermore, (Pickett, Kasza, Biesecker, Wright, & Wakschlag 2009) repeatedly evaluated urine cotinine during pregnancy and determined the correspondence with self-reported smoking measured during the second trimester as well as with smoking habits during pregnancy which were obtained over a decade later when the offspring were between the ages of 11 and 18. Among women whose urine tested positive for cotinine, the preponderance was classified correctly as smokers by both prospective (98.1%) and retrospective (95.6%) methods. Additionally, among women who prospectively denied smoking, approximately one quarter (22.7%) retrospectively reported nicotine use during pregnancy. (Pickett et al., 2009) concluded that retrospective measures may even be more informative than prospective ones for determining some smoking behaviors (e.g., packs per day during the first trimester). Additional prospective studies could incorporate a quantitative biomarker of smoking (Florescu et al., 2009) in conjunction with paternal, teacher, or self-ratings to further evaluate the generalizability, persistence, and potential strategies for remediation of the observed abnormalities in executive function and scholastic performance.
In conclusion, there is evidence possibly indicative of both a causal (Figure 1 and academic data of Table 1) and correlative (clinically significant problems in Table 2) relationship between in utero nicotine and subtle neurocognitive deficits. We suspect that future investigations will clarify that the type and strength of relationship depends not only on the domain measured but also upon on the extent and trimesters of maternal nicotine use, individual genetic differences (maternal and fetal), the ages participants are assessed, and the degree that confounds are present in different populations (Knopik, 2009). Ideally, an increased awareness of the reproductive and neurodevelopmental risks of nicotine will encourage more women to quit smoking prior to pregnancy.
Supplementary Material
Supplementary Figure 1 can be found online at http://www.ntr.oxfordjournals.org
Funding
This work was supported by the National Institute of Environmental Health Sciences (T32 ES007060-31A1), the Oregon Clinical Translational Research Institute (UL1 RR024140), the National Institute of Drug Abuse (L30 DA027582-01), the N.L. Tartar Trust to BJP, and the University of Puget Sound Richard Bangs Collier scholarship to SMC.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
Special thanks to Jacob Raber, Ph.D., as well as Reid Olsen, Julie Mitchell, M.S., and Ruth Rowland for their technical support; Jean O’Malley, M.P.H., for statistical advice; Joel T. Nigg, Ph.D., for reading an earlier version of this manuscript; and the mothers that took part in this study.
References
- Alm B, Milerad J, Wennergren G, Skjærven R, Øyen N, Norvenius G, et al. A case-control study of smoking and sudden infant death syndrome in the Scandinavian countries, 1992 to 1995. The Nordic epidemiological SIDS study. Archives of Disease in Childhood. 1998;78:329–334. doi: 10.1136/adc.78.4.329. doi:10.1136/adc.78.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PJ, Doyle LW. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics. 2004;114:50–57. doi: 10.1542/peds.114.1.50. doi:10.1542/peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- Ball SW, Gilman SE, Mick E, Fitzmaurice G, Ganz ML, Seidman LJ, et al. Revisiting the association between maternal smoking during pregnancy and ADHD. Journal of Psychiatric Research. 2010;44:1058–1062. doi: 10.1016/j.jpsychires.2010.03.009. doi:10.1016/j.jpsychires.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Batstra L, Hadders-Algra M, Neeleman J. Effect of antenatal exposure to maternal smoking on behavioural problems and academic achievement in childhood: Prospective evidence from a Dutch birth cohort. Early Human Development. 2003;75:21–33. doi: 10.1016/j.earlhumdev.2003.09.001. doi:10.1016/j.earlhumdev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Batty GD, Der G, Deary IJ. Effect of maternal smoking during pregnancy on offspring's cognitive ability: Empirical evidence for complete confounding in the US national longitudinal survey of youth. Pediatrics. 2006;118:943–950. doi: 10.1542/peds.2006-0168. doi:10.1542/peds.2006-0168. [DOI] [PubMed] [Google Scholar]
- Berlin I, Heilbronner C, Georgieu S, Meier C, Launay JM, Spreux-Varoquaux O. Reduced monoamine oxidase A activity in pregnant smokers and in their newborns. Biological Psychiatry. 2009;66:728–733. doi: 10.1016/j.biopsych.2009.05.029. doi:10.1016/j.biopsych.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Faraone SV, Mick E. Parsing the associations between prenatal exposure to nicotine and offspring psychopathology in a non-referred sample. Journal of Adolescent Health. 2009;45:142–148. doi: 10.1016/j.jadohealth.2008.12.003. doi:10.1016/j.jadohealth.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM, Telford E, Schmidt C, Messer G. Neurodevelopmental functioning in children with FAS, pFAS, and ARND. Journal of Developmental and Behavioral Pediatrics. 2010;31:199–201. doi: 10.1097/DBP.0b013e3181d5a4e2. doi:10.1097/DBP.0b013e3181d5a4e2. [DOI] [PubMed] [Google Scholar]
- Collins SE, Logan DE, Neighbors C. Which came first: The readiness or the change? Longitudinal relationships between readiness to change and drinking among college drinkers. Addiction. 2010;105:1899–1909. doi: 10.1111/j.1360-0443.2010.03064.x. doi:10.1111/j.1360-0443.2010.03064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Ryan CM, Day NL, Goldschmidt L, Willford JA. Prenatal tobacco effects on neuropsychological outcomes among preadolescents. Journal of Developmental and Behavioral Pediatrics. 2001;22:217–225. doi: 10.1097/00004703-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. doi:10.1007/BF02310555. [Google Scholar]
- Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: Focus on developmental toxicology. Therapeutic Drug Monitoring. 2009;31:14–30. doi: 10.1097/FTD.0b013e3181957a3b. doi:10.1097/FTD.0b013e3181957a3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicology and Teratology. 2003;25:427–436. doi: 10.1016/s0892-0362(03)00029-1. doi:10.1016/S0892-0362(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Gamma A, Jerome L, Liechti ME, Sumnall HR. Is ecstasy perceived to be safe? A critical survey. Drug & Alcohol Dependence. 2005;77:185–193. doi: 10.1016/j.drugalcdep.2004.08.014. doi:10.1016/j.drugalcdep.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Gardener H, Buka SL. Maternal smoking during pregnancy and children’s cognitive and physical development: A causal risk factor? American Journal of Epidemiology. 2008;168:522–531. doi: 10.1093/aje/kwn175. doi:10.1093/aje/kwn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavioral rating inventory of executive function: Professional manual. Lutz, FL: Psychological Assessment Resources; 2000. Retrieved from http://www4.parinc.com/Products/Product.aspx?ProductID=BRIEF. [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Information. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. doi:10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: Modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56:254–262. doi: 10.1016/j.neuropharm.2008.07.020. doi:10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan TM, Ling J, Parrott AC, Buchanan T, Scholey AB, Rodgers J. Self-rated everyday and prospective memory abilities of cigarette smokers and non-smokers: A web-based study. Drug & Alcohol Dependence. 2005;78:235–241. doi: 10.1016/j.drugalcdep.2004.11.008. doi:10.1016/j.drugalcdep.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Hirshfield S, Remien RH, Humberstone M, Walavalkar I, Chiasson MA. Substance use and high-risk sex among men who have sex with men: A national online study in the USA. AIDS Care. 2004;16:1036–1047. doi: 10.1080/09540120412331292525. doi:10.1080/09540120412331292525. [DOI] [PubMed] [Google Scholar]
- Hirshfield S, Remien RH, Walavalkar I, Chiasson MA. Crystal methamphetamine use predicts incident STD infection among men who have sex with men recruited online: A nested case-control study. Journal of Medical Internet Research. 2004;6:e41. doi: 10.2196/jmir.6.4.e41. doi:10.2196/jmir.6.4.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts SCJ, Warren AJ, de Sonneville LMJ, Swaab-Barneveld H. Hot and cool forms of inhibitory control and externalizing behavior in children of mothers who smoked during pregnancy: An exploratory study. Journal of Abnormal Child Psychology. 2008;36:323–333. doi: 10.1007/s10802-007-9180-x. doi:10.1007/s10802-007-9180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience & Biobehavioral Reviews. 2006;30:24–41. doi: 10.1016/j.neubiorev.2005.04.005. doi:10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Jaakkola JJ, Jaakkola N, Zahlsen K. Fetal growth and length of gestation in relation to prenatal exposure to environmental tobacco smoke assessed by hair nicotine concentration. Environmental Health Perspectives. 2001;109:557–561. doi: 10.1289/ehp.01109557. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1240335/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julvez J, Ribas-Fitó N, Torrent M, Forns M, Garcia-Esteban R, Sunyer J. Maternal smoking habits and cognitive development of children at age 4 years in a population-based birth cohort. International Journal of Epidemiology. 2007;36:825–832. doi: 10.1093/ije/dym107. doi:10.1093/ije/dym107. [DOI] [PubMed] [Google Scholar]
- Knopik VS. Maternal smoking during pregnancy and child outcomes: Real or spurious effect? Developmental Neuropsychology. 2009;34:1–36. doi: 10.1080/87565640802564366. doi:10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotimaa AJ, Moilanen I, Taanila A, Ebeling H, Smalley SL, McGough JJ, et al. Maternal smoking and hyperaticity in 8-year-old children. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:826–833. doi: 10.1097/01.CHI.0000046866.56865.A2. doi:10.1097/01.CHI.0000046866.56865.A2. [DOI] [PubMed] [Google Scholar]
- Krall EA, Valadian I, Dwyer JT, Gardner J. Accuracy of recalled smoking data. American Journal of Public Health. 1989;79:200–202. doi: 10.2105/ajph.79.2.200. Retrieved from http://ajph.aphapublications.org/cgi/content/abstract/79/2/200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson EA, Fried PA, Watkinson B. Maternal smoking during pregnancy affects children's vigilance performance. Drug & Alcohol Dependence. 1989;24:11–19. doi: 10.1016/0376-8716(89)90003-3. doi:10.1016/0376-8716(89)90003-3. [DOI] [PubMed] [Google Scholar]
- Kypri K, Paschall MJ, Langley JD, Baxter J, Bourdeau B. The role of drinking locations in university student drinking: Findings from a national web-based survey. Drug & Alcohol Dependence. 2010;111:38–43. doi: 10.1016/j.drugalcdep.2010.03.018. doi:10.1016/j.drugalcdep.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Lambe M, Hultman C, Torrång A, Maccabe J, Cnattingius S. Maternal smoking during pregnancy and school performance at age 15. Epidemiology. 2006;17:524–530. doi: 10.1097/01.ede.0000231561.49208.be. doi:10.1097/01.ede.0000231561.49208.be. [DOI] [PubMed] [Google Scholar]
- Langley K, Rice F, van den Bree MB, Thapar A. Maternal smoking during pregnancy as an environmental risk factor for attention deficit hyperactivity disorder behavior. A review. Minerva Pediatrics. 2005;57:359–371. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16402008. [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: Review of the current evidence. American Journal of Psychiatry. 2003;160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Lundberg F, Cnattinggius S, D’Onofrio B, Altman D, Lambe M, Hultman C, et al. Maternal smoking during pregnancy and intellectual performance in young Swedish male offspring. Peadiatric & Perinatal Epidemiology. 2010;24:79–87. doi: 10.1111/j.1365-3016.2009.01073.x. doi:10.1111/j.1365-3016.2009.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ. Drug use related problems among nonmedical users of prescription stimulants: A web-based survey of college students from a Midwestern university. Drug & Alcohol Dependence. 2007;91:69–76. doi: 10.1016/j.drugalcdep.2007.05.010. doi:10.1016/j.drugalcdep.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless S, O’Laughlin L. The clinical utility of the Behavior Rating Inventory of Executive Function (BRIEF) in the diagnosis of ADHD. Journal of Attention Disorders. 2007;10:381–389. doi: 10.1177/1087054706292115. doi:10.1177/1087054706292115. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? American Journal of Psychiatry. 1996;153:1138–1142. doi: 10.1176/ajp.153.9.1138. Retrieved from http://ajp.psychiatryonline.org/cgi/content/abstract/153/9/1138. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. doi:10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mortensen EL, Michaelsen KF, Sanders SA, Reinisch JM. A dose-response relationship between maternal smoking during late pregnancy and adult intelligence in male offspring. Paediatric Perinatalogy & Epidemiology. 2004;19:4–11. doi: 10.1111/j.1365-3016.2004.00622.x. doi:10.1111/j.1365-3016.2004.00622.x. [DOI] [PubMed] [Google Scholar]
- Mullens AB, Young RM, Dunne M, Norton G. The Cannabis Expectancy Questionnaire for men who have sex with men (CEQ-MSM): A measure of substance-related beliefs. Addictive Behaviors. 2010;3:616–619. doi: 10.1016/j.addbeh.2010.01.006. doi:10.1016/j.addbeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Obel C, Olsen J, Henriksen TB, Rodriguez A, Järvelin MR, Moilanen I, et al. Is maternal smoking during pregnancy a risk factor for hyperkinetic disorder?—Findings from a sibling design. International Journal of Epidemiology. 40:338–345. doi: 10.1093/ije/dyq185. in press. doi:10.1093/ije/dyq185. [DOI] [PubMed] [Google Scholar]
- Olds DL, Henderson CR, Tatelbaum R. Intellectual impairment in children of women who smoke cigarettes during pregnancy. Pediatrics. 1994;93:221–227. [PubMed] [Google Scholar]
- Pennequin V, Sorel O, Fontaine R. Motor planning between 4 and 7 years of age: Changes linked to executive functions. Brain & Cognition. 2010;74:107–111. doi: 10.1016/j.bandc.2010.07.003. doi:10.1016/j.bandc.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Kasza K, Biesecker G, Wright RJ, Wakschlag LS. Women who remember, women who do not: A methodological study of maternal recall of smoking during pregnancy. Nicotine & Tobacco Research. 2009;11:1166–1174. doi: 10.1093/ntr/ntp117. doi:10.1093/ntr/ntp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BJ, Acevedo SF, Kolchugina GK, Butler RW, Corbett SM, Honeycutt EB, et al. Abnormalities in parentally rated executive function in methamphetamine/polysubstance exposed children. Pharmacology, Biochemistry, and Behavior. 2011;98:432–439. doi: 10.1016/j.pbb.2011.02.013. doi:10.1016/j.pbb.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BJ, Li V, Eowiz MA, Kobel YV, Benice TS, Chu AM, et al. Executive function on the Psychology Experiment Building Language tests. Behavior Research Methods. 2011 doi: 10.3758/s13428-011-0096-6. doi:10.3758/s13428-011-0096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J, Buchanan T, Pearson C, Parrott AC, Ling J, Heffernan TM, et al. Differential experiences of the psychobiological sequelae of ecstasy use: Quantitative and qualitative data from an internet study. Journal of Psychopharmacology. 2006;20:437–446. doi: 10.1177/0269881105058777. doi:10.1177/0269881105058777. [DOI] [PubMed] [Google Scholar]
- Roza SJ, Verburg BO, Jaddoe VW, Hofman A, Mackenbach JP, Steegers EA, et al. Effects of maternal smoking in pregnancy on prenatal brain development. The Generation R Study. European Journal of Neuroscience. 2007;25:611–617. doi: 10.1111/j.1460-9568.2007.05393.x. doi:10.1111/j.1460-9568.2007.05393.x. [DOI] [PubMed] [Google Scholar]
- Schmitz M, Denardin D, Silva TL, Pianca T, Hutz MH, Faraone S, et al. Smoking during pregnancy and attention-deficit/hyperactivity disorder, predominately inattentive type: A case-control study. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:1338–1345. doi: 10.1097/S0890-8567(09)61916-X. doi:10.1097/S0890-8567(09)61916-X. [DOI] [PubMed] [Google Scholar]
- Sesma HW, Slomine BS, Ding R, McCarthy ML. Executive functioning in the first year after pediatric traumatic brain injury. Pediatrics. 2008;121:e1686–e1695. doi: 10.1542/peds.2007-2461. doi:10.1542/peds.2007-2461. [DOI] [PubMed] [Google Scholar]
- Sexton M, Fox NL, Hebel JR. Prenatal exposure to tobacco: II. Effects on cognitive functioning at age three. International Journal of Epidemiology. 1990;19:72–77. doi: 10.1093/ije/19.1.72. doi:10.1093/ije/19.1.72. [DOI] [PubMed] [Google Scholar]
- Shakeshaft AP, Bowman JA, Sanson-Fisher RW. Computers in community-based drug and alcohol clinical settings: Are they acceptable to respondents? Drug & Alcohol Dependence. 1998;50:177–180. doi: 10.1016/s0376-8716(98)00019-2. doi:10.1016/S0376-8716(98)00019-2. [DOI] [PubMed] [Google Scholar]
- Shea AK, Steiner M. Cigarette smoking during pregnancy. Nicotine & Tobacco Research. 2008;10:267–278. doi: 10.1080/14622200701825908. doi: 10.1080/14622200701825908. [DOI] [PubMed] [Google Scholar]
- Strandberg-Larsen K, Tinggaard M, Nybo Andersen AM, Olsen J, Grønbaek M. Use of nicotine replacement therapy during pregnancy and stillbirth: A cohort study. British Journal of Gynecology. 2008;115:1405–1410. doi: 10.1111/j.1471-0528.2008.01867.x. doi:10.1111/j.1471-0528.2008.01867.x. [DOI] [PubMed] [Google Scholar]
- Thapar A, Rice F, Hay D, Boivin J, Langley K, van den Bree M, et al. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: Evidence from a novel design. Biological Psychiatry. 2009;66:722–727. doi: 10.1016/j.biopsych.2009.05.032. doi:10.1016/j.biopsych.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Garrison ME, Slawecki CJ, Ehlers CL, Riley EP. Nicotine exposure during the neonatal brain growth spurt produces hyperactivity in preweanling rats. Neurotoxicology and Teratology. 2000;22:695–701. doi: 10.1016/s0892-0362(00)00096-9. doi:10.1016/S0892-0362(00)00096-9. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH. Long-term consequences of maternal smoking and developmental chronic nicotine exposure. Frontiers in Bioscience. 2008;13:636–649. doi: 10.2741/2708. doi:10.2741/2708. [DOI] [PubMed] [Google Scholar]
- Wisborg K, Kesmodel U, Henriksen TB, Olsen SF, Secher NJ. A prospective study of smoking during pregnancy and SIDS. Archives of Disease of Children. 2000;83:203–206. doi: 10.1136/adc.83.3.203. doi:10.1136/adc.83.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisborg K, Kesmodel U, Henriksen TB, Olsen SF, Secher NJ. Exposure to tobacco smoke in utero and the risk of stillbirth and death in the first year of life. American Journal of Epidemiology. 2001;154:322–327. doi: 10.1093/aje/154.4.322. doi:10.1093/aje/154.4.322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.