Abstract
Voltage-gated calcium channels are key mediators of depolarization induced calcium entry into electrically excitable cells. There is increasing evidence that voltage-gated calcium channels, like many other types of ionic channels, do not operate in isolation, but instead form complexes with signaling molecules, G protein coupled receptors, and other types of ion channels. Furthermore, there appears to be bidirectional signaling within these protein complexes, thus allowing not only for efficient translation of calcium signals into cellular responses, but also for tight control of calcium entry per se. In this review, we will focus predominantly on signaling complexes between G protein-coupled receptors and high voltage activated calcium channels, and on complexes of voltage-gated calcium channels and members of the potassium channel superfamily.
Key words: calcium channels, G proteins, potassium channels, calcium, GPCR
Introduction
Calcium ions serve as important mediators of cell signaling in both excitable and non-excitable tissues. Elevation in intracellular calcium levels triggers physiological responses that include cardiac muscle contraction, hormone secretion, neurotransmitter release, activation of calcium-dependent enzymes and calcium dependent gene transcription.1–5 In electrically excitable cells such as neurons and muscle cells, action potential evoked calcium entry is primarily due to the activation of both low and high voltage-gated calcium channels.6 Low voltage activated (LVA),7 and high voltage activated (HVA) calcium channels can be distinguished based on their threshold of activation, with LVA channels activating close to resting membrane potentials.8 In contrast, the family of HVA channels that includes subtypes such as L-, N-, P/Q- and R-types require larger membrane depolarizations in order to open.9 Individual calcium channel subtypes differ in their cellular and subcellular distributions, functional properties and pharmacological profiles.10–13 They also support distinct physiological functions. For example, T-type calcium channels are involved in regulating cellular excitability,14–16 N and P/Q-type channels mediate fast evoked neurotransmitter release,1 and L-type calcium channels mediate functions such as excitation contraction coupling in the heart and muscle, insulin secretion and calcium dependent gene transcription.17–21 Along these lines, different calcium channels isoforms are targeted by clinically active drugs such as analgesics, general anesthetics, antiepileptics and cardioactive drugs,12,15,22–24 and mutations in various calcium channel isoforms have been associated with conditions such as familial migraine, deafness, epilepsy, cardiac arrhythmias and ataxia (reviewed in refs. 25 and 26).
Common to all types of voltage-gated calcium channels is a pore forming Cavα1 subunit that contains four homologous membrane domains that are flanked by cytoplasmic N- and C-termini and connected by cytoplasmic linker regions.9 While T-type calcium channels are thought to contain only a Cavα1 subunit, members of the HVA calcium channel family also contain a cytoplasmic Cavβ subunit, as well as a larger extracellular membrane anchored Ca vα2δ subunit, and both of these subunits are represented by four different genes (reviewed in refs. 27 and 28). This coassembly with ancillary subunits is necessary in order to promote export of the channels from the endoplasmic reticulum,29 and also results in alteration of the functional properties of the channels.27,30 In addition, all of the known HVA channels co-assemble with calmodulin, a calcium binding protein that bestows calcium feedback regulation onto the channels in the form of calcium-dependent inactivation and facilitation.31–33 The incorporation of a calcium signaling protein into the channel complex is indicative of the notion that calcium channels do not operate in isolation, but tend to form larger signaling complexes that are not only designed to enhance coupling efficiency between calcium entry and downstream signaling events, but also provide for a means of regulating calcium channel activity per se.
There are numerous examples of cytoplasmic protein interactions with different types of voltage-gated calcium channels. For example, N and P/Q-type calcium channels tightly associate with proteins of the synaptic vesicle release machinery, such as syntaxin 1 and SNAP-25, thus effectively coupling calcium entry to fast vessel release, but also to confer feedback regulation onto channel activity.34–38 Small GTPases such as Rem, Gem and Rem2 associate with HVA channels to regulate channel surface expression (reviewed in ref. 39). A-Kinase anchoring proteins, protein kinase A, and phosphatase 2A associate with certain L-type channel isoforms to tightly control the regulation of channel activity by phosphorylation.40–43 However, it has only emerged recently that various types of calcium channels interact not only with cytoplasmic signaling molecules, but also with other membrane proteins such as receptors and ion channels to form physical signaling complexes that either allow for more efficient regulation of calcium channel activity or facilitate the specificity of calcium signaling.44 Here we will focus on two such examples: We will first discuss how protein interactions with G protein coupled receptors enhance calcium channel modulation and trafficking. We will then review how interactions between calcium channels and potassium channels bestow calcium regulation onto potassium channel function and thus modify neuronal output.
G Protein Coupled Receptor: Calcium ChannelComplexes
Most types of voltage-gated calcium channels are modulated following the activation of various types of G protein coupled receptors (GPCRs) (reviewed in refs. 45 and 46). Upon receptor activation, GDP bound constitutively to the Gα subunit is exchanged for GTP, resulting in a conformational change that either leads to a breakup of the Gαβγ trimer, or at the least in a conformational change within the trimer that results in the generation of two separate signaling entities—activated Gα and Gβγ dimers. Both Gα and Gβγ act on various effector molecules. For example, Gαs may stimulate the activity of adenylyl cyclase, leading to the production of cyclic AMP and the activation of protein kinase A.47 In the context of voltage-gated calcium channels, a classic example of such a soluble second messenger pathway is the regulation of L-type calcium channels by β-adrenergic receptors (βARs), which results in protein kinase A dependent phosphorylation of the channels in cardiac cells and a massive upregulation in channel activity that ultimately increases heart rate.48,49 While Gβγ subunits act to regulate various cell signaling pathways, they also can directly modulate certain types of ion channels via a membrane-delimited pathway. This was first described almost three decades ago for N-type calcium channels in sensory neurons.50 Activation of noradrenergic receptors was found to produce a rapid depression of N-type channel activity that was later found to be strongly voltage dependent, and to be mediated by direct binding of Gβγ to the N-type calcium channel α1 subunit.51–54 This binding interaction and the associated functional regulation appears to be destabilized by protein kinase C (PKC) dependent phosphorylation of the channel54–56 and there is evidence that PKC is in fact associated with the channel via an enigma homolog adaptor protein,57 suggesting the existence of a channel-kinase complex. Given that the Gβγ subunit is large and anchored to the inner leaflet of the plasma membrane and thus unlikely to diffuse quickly across large distances, one might expect that channels and receptors would have to be localized in close proximity to each other to account for the relatively rapid onset of G protein inhibition. This then raises the possibility that G protein coupled receptors and voltage-gated calcium channels may be located in close proximity either in the same lipid rafts, of perhaps even be physically associated.
L-Type Channels
The first description of a calcium channel-GPCR complex involved Cav1.2 L-type calcium channels and βARs. There are three known βAR subtypes expressed in the mammalian heart (β1AR, β2AR, β3AR),58 with the β1AR and β2AR subtypes able to activate PKA and stimulate calcium entry via L-type calcium channels into cardiac myocytes.59–61 It has been shown that β2ARs form macromolecular signaling complexes with Cav1.2 calcium channels.62 These complexes also contain other elements of the signaling machinery, such as G proteins and caveolin. The functional significance of these assemblies has not yet been demonstrated in cardiac myocytes, nor have the molecular determinants that underlie the interactions between L-type calcium channels and receptors been identified. The investigation into coupling between these receptors and the channels has been hampered by the fact that it has proven to be a challenge to reconstitute βAR signaling to L-type channels in heterologous systems—a problem that has only been solved recently.40 Nonetheless, the existence of receptor-Cav1.2 channel complexes suggests that they may help optimize the coupling between the receptor and the channels.
P/Q-Type Channels
Like N-type calcium channels, P/Q-type channels can be regulated by a membrane delimited Gβγ mediated pathway in response to activation of a number of different receptor subtypes. These channels are expressed at high levels in cerebellar Purkinje neurons where they are regulated by activation of metabotropic glutamate receptors.63–65 Coexpression of these receptors with Cav2.1 (P/Q-type) channels in heterologous expression systems was shown to reduce current amplitude in an agonist independent manner, suggesting a direct regulation of channel activity by the receptor itself.66 Furthermore, it was shown that receptors and channels colocalized to the same dendritic compartments in Purkinje neurons, and that they could be co-immunoprecipitated both from transfected cells and native tissue. This close spatial arrangement may serve to optimize receptor signaling to the P/Q-type channels and thus to fine tune synaptic transmission. Furthermore, the agonist independent modulation of the channel by these receptors could potentially provide a mechanism by which P/Q-type channel function may be regulated by changes in receptor density.66
N-Type Channels
The interaction between N-type channels and GPCRs has been extensively investigated. Like P/Q-type channels, N-type channels are heavily clustered at synaptic sites where they contribute to the release of neurotransmitters.67 Hence, any inhibition of N-type channels has the potential to inhibit neurotransmission. The significance of such regulation is underscored by the clinical use of morphine, a potent activator of µ-opioid receptors. Activation of these receptors results in the inhibition of N-type calcium channels at dorsal horn synapses, thus preventing the transmission of pain signals from primary afferent fibers.68
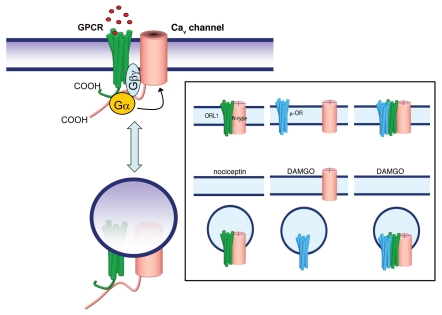
Numerous other types of GPCRs have been found to inhibit N-type channels via a Gβγ mediated, voltage dependent pathway, and in some cases a voltage-independent inhibition has been observed (but will not be considered further here).45 One of the hallmarks of the voltage-dependent modulation is that it can be reversed with strong membrane depolarizations or trains of action potentials.51,69 This then allows investigators to test for the tonic G protein modulation that may be experienced by the channel. Indeed, such tonic modulation was observed when the ORL1 receptor (a member of the extended opioid receptor family) was coexpressed with N-type channels in tsA-201 cells.70 With increasing receptor expression levels, the extent of tonic modulation became progressively augmented until it reached saturation. This effect could be attributed to the formation of a physical interaction between receptors and channels that allowed the channels to effectively sense Gβγ molecules that were available due to constitutive receptor activity (Fig. 1). This observation was not confined to expression systems as a similar effect occurred in acutely isolated dorsal root ganglion neurons. It is well established that many types of GPCRs undergo agonist induced internalization, and the ORL1 receptor is no exception.71,72 If the receptor is physically linked to a channel, then one might expect one of two scenarios. Either the channel prevents the internalization of the receptor, or the receptor and channel are co-internalized. Indeed, treatment of native or heterologously expressed channel/receptor complexes with the agonist nociceptin resulted in the internalization of the complexes into lysosomes, presumably for degradation73 (Fig. 1). At the same time, the coexpression of the N-type channel with ORL1 resulted in increased cell surface density of the channels. It is possible that the ORL1 receptor, when bound to the channel, occludes an ER retention motif, thus facilitating ER export and surface expression. This would fit with the observation that the ORL1 receptor binds to the channel's C-terminal region—a locus that has been implicated in the ER retention of the channel.74
Figure 1.
Physical interactions between certain types of GPCRS and voltage-gated calcium channels bring the receptors close to the channels, so that receptor/G protein activation results in effective coupling to the channels. In addition, N-type calcium channel interactions with either ORL1 receptors or dopamine receptors have been shown to support agonist dependent internalization of channel receptor complexes, and to facilitate insertion of complexes into the membrane after synthesis. Inset: ORL1 receptors mediate N-type channel internalization upon prolonged treatment with the agonist nociceptin. In contrast, the µ-opioid receptor agonist DAMGO results in internalization of these receptors, but not of the channels unless ORL1 receptors are also present (i.e., the ORL1 receptor acts as a bridge between the N-type channel and the µ-opioid receptor).
Unlike the ORL1 receptor, µ-opioid receptors do not appear to regulate N-type channel trafficking. Intriguingly, when coexpressed with the ORL1 receptor, the µ-opioid receptor subtype becomes capable of internalizing N-type channels. This is presumably due to the fact that opioid receptors can form heterodimers with ORL1, thus giving rise to a trimeric signaling complex with unique trafficking properties75 (Fig. 1, inset).
Two other types of GPCRs have since been shown to regulate N-type channel trafficking. Both D1 and D2 dopamine receptors interact with the N-type calcium channel α1 subunit, albeit at distinct sites.76,77 Nonetheless, in both cases, receptor coexpression increases the cell surface density of the channels, whereas receptor activation mediates N-type channel internalization. In the case of D1 receptors, it was shown that the receptor targets the channels to dendritic sites in prefrontal cortex neurons, thus effectively defining the subcellular localization of the channels.76 Hence, a GPCR is able to divert the N-type calcium channels from their normal presynaptic locus to mediate calcium signaling in dendrites.
At this point, it is not clear if other types of GPCRS are able to associate with N-type calcium channels to regulate their function and trafficking. However, efficient channel receptor coupling may necessitate a close spatial proximity between receptors and channels that may be best achieved through either direct physical interactions, or linkage via an intermediate protein.
In the above sections, we have discussed how the formation of calcium channel-GPCR complexes optimizes receptor-mediated control over calcium channel activity. Below, we will illustrate how the formation of protein complexes with potassium channels allows for better calcium channel mediated control of potassium channel function, and thus neuronal firing.
Calcium-Potassium Channel Complexes
Calcium channels have long been known to associate with calcium-dependent potassium (KCa) channels to control membrane excitability through activation of potassium efflux. KCa channels expressed in central neurons correspond either to big conductance (BK, mslo) or small conductance (SK1-3) channels.78,79 Past work has established the ubiquitous occurrence of calcium-KCa channel interactions across cells of the nervous system, but increasingly, it has become clear that coupling can be highly specific to particular channel isoforms in different cells. These interactions were typically first assessed at the level of a functional coupling between calcium channel subtypes and BK or SK potassium channels in relation to specific processes (i.e., spike discharge vs. subthreshold synaptic events) (reviewed in ref. 80). Although this interaction can reflect a close proximity between calcium and potassium channels, it may or may not reflect interactions at the molecular level within an ion channel complex. Recent work, however, has identified an association between different calcium and KCa channels that provides for highly localized and specific coupling between calcium influx and activation of a potassium channel within a protein complex. The first coupling between a calcium channel and a voltage-gated potassium channel was also reported in the form of a Cav3-Kv4 complex,81,82 significantly increasing the range of neuronal functions that will benefit from calcium-dependent regulation of potassium channel function.
Here we review examples of calcium-KCa channel coupling and the advantages conferred upon regulation of cell excitability in central neurons. We consider cases where potassium channel activation reflects a functional coupling as well as the existence of an actual ion channel complex between calcium and KCa channels, or to the Kv4/KChIP complex. For further information readers are referred to several excellent reviews on details of KCa and Kv4 channel activation.79,80,83–87
Control of Cell Excitability by Calcium-KCa Coupling
BK channels are activated through a complex and synergistic interplay between membrane voltage and the internal concentration of calcium.83 The channels are comprised of an extracellular N-terminus, seven transmembrane segments (S0–S6) and an internal C-terminus. Calcium sensitivity is mediated through a C-terminal “calcium bowl” and RCK1 domain, with response in the 1–10 µM range of internal calcium concentration.83,88 SKα subunits are encoded by four genes (SK1–4), with central neurons differentially expressing SK1–3 channels. SK channels have no intrinsic voltage-sensitivity and are thus only sensitive to changes in the concentration of internal calcium. Calcium dependence is achieved by interacting with calmodulin at a domain on the C-terminus, with the complex exhibiting greater sensitivity to internal calcium concentration than BK channels, with a half maximal activation at ∼0.3 µM calcium.80
The different means by which BK and SK channels achieve calcium-dependent activation with or without sensitivity to membrane voltage fluctuations enable these channels to influence widely different forms of neuronal activity. In general, BK channels are recognized for their role in repolarizing the fast falling phase of sodium spike discharge and a subsequent fast AHP of <10 msec.89–92 SK channels exhibit a time constant for activation of ∼5 msec but much slower deactivation that allows for the generation of a medium AHP (mAHP) that lasts in the order of a 100 msec.85,93,94 As such, SK-mediated AHPs can respond to longer time frames of cell activity than BK channels by sensing the levels of calcium accumulated during repetitive spike discharge. SK channels can thus alter firing rate gain89,94 or control the duration of calcium-dependent depolarizing or burst responses.95,96 The activation of BK or SK channels by spike discharge can further establish a baseline level of membrane excitability according to the rate of tonic background firing.97 Associating specific calcium channel subtypes with either BK or SK channels thus provides control over select aspects of neuronal activity.
The existence of toxins or drugs specific to different calcium channels and to KCa channels has helped to identify the source of calcium influx responsible for controlling KCa-mediated responses. The number of these studies conducted to date are too numerous to review here, but serve to indicate the existence of at least a functional coupling between different HVA or LVA calcium channels and BK or SK channels that are often specific to different cell types. Thus, N-type calcium channels were found to functionally couple to the activation of BK channels in CA1 pyramidal cells98 and SK channels in cells of deep cerebellar nuclei,97,99 thalamic reticular nucleus,95 and nucleus basalis.100 P-type calcium channels couple to the activation of BK channels101,102 or SK channels102 in cerebellar Purkinje cells. L-type calcium channels have been reported to activate BK channels in neocortical pyramidal cells103 and CA1 hippocampal pyramidal cells,90 as well as to SK channels in CA1 pyramidal cells.98 Finally, T-type calcium channels are at least functionally coupled to BK channel activation in medial vestibular neurons,104 and to activation of SK channel isoforms in dopaminergic neurons,105 thalamic reticular cells106 and nucleus basalis neurons.100
In some cases, calcium entry through more than one calcium channel isoform can activate the same KCa current in a given cell, as found for BK channel activation by both N- and L-type channels in neocortical pyramidal cells.103 Conversely, calcium entry through P-type channels can activate both BK and SK channels in Purkinje cells.102 These findings would suggest that a given cell can link different calcium channels to the same KCa channel subtype for selective activation, or that potassium channels are sensitive enough to respond to the domains of intracellular calcium increase produced by several different calcium channel subtypes. To resolve the issue of proximity of calcium and potassium channels, differences in the binding rate constants of EGTA or BAPTA can be used to distinguish between interactions that occur between channels positioned within a calcium microdomain (50–100 µm) or nanodomain (20–50 nm).88 This approach has shown that some cases of functional calcium-KCa channel coupling reflects a calcium sensing process at the micro-domain level, such as L-type calcium and SK channels in CA1 hippocampal cells98 or expressed Cav2.3 and BK channels.107 In several cells the Cav-KCa channel interaction proved to exhibit a differential block by BAPTA compared to EGTA, such as between BK channels and L-type,90,108 N-type, or P/Q-type98,102 calcium channels, suggesting the presence of a nanodomain interaction. These interactions are not obligatory however, in that both calcium and BK channels can be recorded in isolation in cells that also exhibit the Cav-BK complex.98 At this time no reports have surfaced on the potential for T-type calcium channels to form nanodomain interactions with either BK or SK channels.
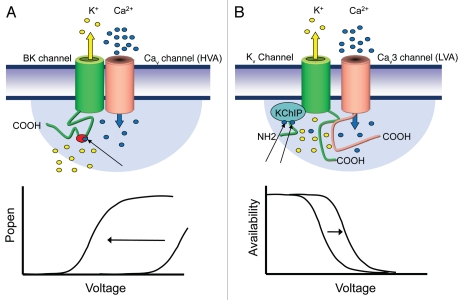
The mechanism by which a nanodomain interaction can take place between HVA calcium and BK channels was only recently resolved.107 In particular, all of Cav1.2 (L-type), Cav2.1 (P/Q-type) and Cav2.2 (N-type) (but not R-type) calcium channels could be coimmuoprecipitated with BK channel and β subunit proteins from rat brain or from lysates of expression systems. Expression of each of the HVA calcium channels with BK channels (and β-subunits) revealed that calcium-dependent activation of BK current was insensitive to internally perfused EGTA, but blocked by BAPTA, consistent with a nanodomain interaction. This interaction appeared to involve only the α-subunits of calcium and BK channels given the ability to coimmuoprecipitate these proteins in the absence of auxiliary subunits107 (Fig. 2A).
Figure 2.
(A) BK channels and certain types of HVA calcium channels physically interact to place the BK channels into a calcium nanodomain (see blue shaded area), allowing one or more calcium ions (black arrow) to effectively bind to a “calcium bowl” in the C-terminus of the BK channels (indicated in red), as well as possibly other sites in the channel.121 Hence, activation of calcium channels by membrane depolarization results in effective activation of adjacent BK channels, which then increase their open probability to mediate potassium efflux. The increase in calcium concentration mediated by voltage-gated channels in effect shifts the activation curve of the BK channels into a physiological voltage range (see bottom). (B) Kv4 channels and Cav3 T-type calcium channel form a macromolecular signaling complex that also includes the calcium binding protein KChIP. The channels interact via their C-termini, whereas KChIP binds to the N-terminus region of Kv4. Activation of T-type channels results in a calcium nanodomain that allows effective loading of KChIP/Kv4 complexes with up to two calcium ions (black arrows per KChIP). This then results in a depolarizing shift in the steady state inactivation curve of the Kv4 channel, thereby increasing the availability of the channel to open (bottom). Unlike in the case of BK channels that bind calcium directly, KChiPS are needed to confer calcium sensitivity onto the Kv4 channel.
The physiological relevance of an HVA-KCa interaction is several fold. By closely associating specific calcium channel subtypes to BK or SK channels, the activation of potassium current can be restricted to a given voltage response, or tailored to different activity patterns of a cell. Thus, spike frequency can be modulated according to the rate of spike repolarization and generation of an AHP, a process that would benefit from the nanodomain interaction between HVA calcium and BK channels. By comparison, microdomain interactions between T-type calcium and SK channels would be sufficient to control lower frequency oscillatory responses.95 The cell-specific interactions apparent between calcium and KCa channels also provide control over the voltage for potassium channel activation according to the associated calcium channel isoform. Thus, a comparison between the I-V plots for BK channels coexpressed with either L-type or P/Q-type calcium channels in Xenopus oocytes revealed distinctly different voltage ranges for potassium current activation, as conferred upon the potassium channel by the associated calcium channel.109 These distinctions were further shown to provide very different profiles of potassium current in response to spike-like voltage clamp commands of different half-widths. These differences would be expected to have significant influence on potassium current associated with spike discharge between different cells, and between soma and axon terminals, with consequent effects on transmitter release.
The current data thus reveal that interactions between calcium channels and either SK or BK channels can be highly specific to different cells, and tailored through the formation of protein complexes and differential calcium requirements to modify a wide range of neuronal outputs. While altered neuronal output will indirectly affect the activities of voltage-gated calcium channels (i.e., via changes in membrane potential), it remains to be determined if the physical association with these potassium channel family members may directly alter calcium channel function.
Cav-Kv Interactions
Most recently, the range of possible interactions between calcium and potassium channels was greatly extended by reports of a new interaction between LVA Cav3 T-type calcium channels and Kv4 A-type potassium channels.81,82 This interaction is unique in representing the first identification of an association between a calcium channel and a purely voltage-gated potassium channel. T-type calcium and A-type potassium currents share the properties of fast activation and inactivation, near complete inactivation at rest, and recovery from inactivation during membrane hyperpolarizations.87,110 This extensive overlap in the voltage-dependent and kinetic properties of Cav3 calcium and Kv4 potassium channels thus appears ideally suited to associating these channel types as part of a single complex. Periods of inhibition in cells expressing these channels will then increase the availability of both A-type and T-type currents, allowing for their transient activation upon return to resting potential. The consequence of this combination of inward and outward current activation was illustrated in cerebellar stellate cells, where an A-type mediated increase in first spike latency (FSL) was transformed to a decrease in FSL over the slightly shifted voltage range for recovery of T-type channels from inactivation.111 Further investigation of this interaction revealed that Cav3 and Kv4.2 channels coimmunoprecipitate from brain lysate, with pull-down experiments indicating an association at the molecular level between Kv4.2 channels and Cav3 C-termini81,82 (Fig. 2B).
The existence of this association was instrumental in resolving a long-standing issue of the potential function for a previously defined association between Kv4 channels and K+ Channel Interacting Proteins (KChIPs). First discovered in yeast-two hybrid screens searching for Kv4 associated proteins, KChIPs bind Kv4 channels at two sites: a hydrophobic N-terminal segment and at the T1 assembly domain loop.112 By way of binding, KChIPs dramatically change the voltage-dependent and kinetic properties of Kv4 channels. Specifically, KChIP binding promotes the trafficking of Kv4 channels to the plasma membrane, hastens the rate of IA recovery from inactivation, slows the rate of IA inactivation, and causes shifts in the voltage-dependence of activation and inactivation.87 Importantly, KChIPs are calcium binding proteins that belong to the neuronal calcium sensor (NCS) superfamily.113 Similar to other NCS family members, KChIPs contain four EF-hand binding motifs, two of which (EF-3 and EF-4) bind calcium.112 Initial mutagenesis studies revealed that KChIP1 molecules that were incapable of binding calcium could still associate with Kv4 channels but lost the ability to modulate Kv4 properties,114 suggesting a role for calcium in Kv4 function. Indeed, a study investigating the effects of intracellular calcium on Kv4.3-KChIP2d (a minimal KChIP isoform which only contains one EF-hand) demonstrated that chelation of intracellular calcium abolished the established KChIP effects on gating.115 Conversely, recent structural studies of the Kv4.3-KChIP1 complex have revealed that some KChIP1 EF-hand mutants lose the ability to bind Kv4.2 channels, highlighting the important role calcium binding plays in structural maintenance of KChIP proteins.112
While isolated studies had shown that neuronal IA can be modulated by fluctuations in intracellular calcium,115,116 identifying a calcium source capable of regulating Kv4 properties had remained elusive. However, it was recently determined that Cav3-mediated calcium influx in cerebellar stellate cells serves to modulate the voltage-dependence of Kv4 inactivation (Vh) at the nanodomain level81 (Fig. 2B). Heterologous expression studies further showed that KChIP3 (but not KChIP1, 2 or 4) was the calcium sensor involved in the modulation of Kv4 Vh.81 Similarly, internal perfusion of specific antibodies directed at KChIP proteins in stellate cells revealed that KChIP3 was the critical link between Cav3-mediated calcium influx and the calcium-dependent modulation of Kv4 function.81 Moreover, without the actions of the Cav3-Kv4 complex, the voltage range for Kv4 channel inactivation would be shifted far enough leftward as to essentially fall outside of the physiological range of usual membrane potential fluctuations. The Cav3-Kv4 interaction thus appears to be instrumental to the contribution of A-type Kv4 potassium channels to neuronal activity. Although the number of physiological processes that could be affected by the Cav3-Kv4 complex remain to be fully explored, the current data set reveal that the Cav3-Kv4 complex is key in controlling stellate cell excitability by modifying the gain of firing frequency. Given the prominent expression of these channel types117–120 and KChIP3 proteins,119 it is predicted that calcium-dependent modulation of Kv4 availability and thereby neuronal excitability will be a widespread phenomenon throughout central and peripheral neurons. As in the case of calcium activated potassium channels, it remains to be seen if KChIPs or Kv4 channels can directly alter calcium channel function/membrane expression levels.
Concluding Remarks
Here, we have highlighted two general examples of how cell signaling can be optimized through the formation of specific signaling complexes between membrane proteins. While receptor heterodimerization has been a well-established phenomenon, the emergence of singling complexes between receptors and ion channels, or between two different types of ion channels is a more recent concept. The notion that channels and receptors may exist as macromolecular signaling complexes makes intuitive sense, as this not only allows maximizing the efficiency of coupling and crosstalk between the individual players, but may also allow for unique signaling properties that are specific to a particular subcellular locus. Although the examples of physically signaling complexes involving different types of channels and receptors are still relatively limited, it is likely that more such examples will emerge in the near future.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) (Ray W. Turner and Gerald W. Zamponi) and studentship support (Dustin Anderson) through Alberta Innovates—Health Solutions (AIHS), T. Chen Fong award, Achievers in Medical Sciences award, and an I. Walton Killam Scholarship. Gerald W. Zamponi is an AIHS Scientist and Canada Research Chair and Ray W. Turner is an AIHS Scientist.
References
- 1.Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 2.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 3.Cribbs L. T-type calcium channel expression and function in the diseased heart. Channels (Austin) 2010;4:447–452. doi: 10.4161/chan.4.6.12870. [DOI] [PubMed] [Google Scholar]
- 4.Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kuhbandner S, et al. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem. 2000;275:39193–39199. doi: 10.1074/jbc.M006467200. [DOI] [PubMed] [Google Scholar]
- 5.Comunanza V, Marcantoni A, Vandael DH, Mahapatra S, Gavello D, Carabelli V, et al. CaV1.3 as pacemaker channels in adrenal chromaffin cells: specific role on exo- and endocytosis? Channels (Austin) 2010;4:440–446. doi: 10.4161/chan.4.6.12866. [DOI] [PubMed] [Google Scholar]
- 6.Bean BP. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- 7.Pongs O, Leicher T, Berger M, Roeper J, Bahring R, Wray D, et al. Functional and molecular aspects of voltage-gated K+ channel beta subunits. Ann NY Acad Sci. 1999;868:344–355. doi: 10.1111/j.1749-6632.1999.tb11296.x. [DOI] [PubMed] [Google Scholar]
- 8.Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 9.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 10.McKay BE, McRory JE, Molineux ML, Hamid J, Snutch TP, Zamponi GW, et al. Cav3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons. Eur J Neurosci. 2006;24:2581–2594. doi: 10.1111/j.1460-9568.2006.05136.x. [DOI] [PubMed] [Google Scholar]
- 11.Westenbroek RE, Hell JW, Warner C, Dubel SJ, Snutch TP, Catterall WA. Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron. 1992;9:1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- 12.Belardetti F, Zamponi GW. Linking calcium-channel isoforms to potential therapies. Curr Opin Investig Drugs. 2008;9:707–715. [PubMed] [Google Scholar]
- 13.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 14.Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, et al. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca2+ channels. Neuron. 2001;31:35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 15.Khosravani H, Zamponi GW. Voltage-gated calcium channels and idiopathic generalized epilepsies. Physiol Rev. 2006;86:941–966. doi: 10.1152/physrev.00002.2006. [DOI] [PubMed] [Google Scholar]
- 16.Cain SM, Snutch TP. Contributions of T-type calcium channel isoforms to neuronal firing. Channels (Austin) 2010;4:475–482. doi: 10.4161/chan.4.6.14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, et al. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 18.Drews G, Krippeit-Drews P, Dufer M. Electrophysiology of islet cells. Adv Exp Med Biol. 2010;654:115–163. doi: 10.1007/978-90-481-3271-3_7. [DOI] [PubMed] [Google Scholar]
- 19.Fossat P, Dobremez E, Bouali-Benazzouz R, Favereaux A, Bertrand SS, Kilk K, et al. Knockdown of L calcium channel subtypes: differential effects in neuropathic pain. J Neurosci. 2010;30:1073–1085. doi: 10.1523/JNEUROSCI.3145-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanabe T, Mikami A, Numa S, Beam KG. Cardiac-type excitation-contraction coupling in dysgenic skeletal muscle injected with cardiac dihydropyridine receptor cDNA. Nature. 1990;344:451–453. doi: 10.1038/344451a0. [DOI] [PubMed] [Google Scholar]
- 21.Koschak A. Impact of gating modulation in Cav1.3 L-type calcium channels. Channels (Austin) 2010;4:523–525. doi: 10.4161/chan.4.6.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J, Luo ZD. Calcium channel functions in pain processing. Channels (Austin) 2010;4:510–517. doi: 10.4161/chan.4.6.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orestes P, Todorovic SM. Are neuronal voltage-gated calcium channels valid cellular targets for general anesthetics? Channels (Austin) 2010;4:518–522. doi: 10.4161/chan.4.6.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamponi GW, Lewis RJ, Todorovic SM, Arneric SP, Snutch TP. Role of voltage-gated calcium channels in ascending pain pathways. Brain Res Rev. 2009;60:84–89. doi: 10.1016/j.brainresrev.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietrobon D. Cav2.1 channelopathies. Pflugers Arch. 2010;460:375–393. doi: 10.1007/s00424-010-0802-8. [DOI] [PubMed] [Google Scholar]
- 26.Striessnig J, Bolz HJ, Koschak A. Channelopathies in Cav1.1, Cav1.3 and Cav1.4 voltage-gated L-type Ca2+ channels. Pflugers Arch. 2010;460:361–374. doi: 10.1007/s00424-010-0800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buraei Z, Yang J. The ss subunit of voltage-gated Ca2+ channels. Physiol Rev. 2010;90:1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 29.Jarvis SE, Zamponi GW. Trafficking and regulation of neuronal voltage-gated calcium channels. Curr Opin Cell Biol. 2007;19:474–482. doi: 10.1016/j.ceb.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Yasuda T, Chen L, Barr W, McRory JE, Lewis RJ, Adams DJ, et al. Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur J Neurosci. 2004;20:1–13. doi: 10.1111/j.1460-9568.2004.03434.x. [DOI] [PubMed] [Google Scholar]
- 31.Minor DL, Jr, Findeisen F. Progress in the structural understanding of voltage-gated calcium channel (Cav) function and modulation. Channels (Austin) 2010;4:459–474. doi: 10.4161/chan.4.6.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tadross MR, Dick IE, Yue DT. Mechanism of local and global Ca2+ sensing by calmodulin in complex with a Ca2+ channel. Cell. 2008;133:1228–1240. doi: 10.1016/j.cell.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Yang PS, Yang W, Yue DT. Enzyme-inhibitor-like tuning of Ca2+ channel connectivity with calmodulin. Nature. 2010;463:968–972. doi: 10.1038/nature08766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheng ZH, Rettig J, Takahashi M, Catterall WA. Identification of a syntaxin-binding site on N-type calcium channels. Neuron. 1994;13:1303–1313. doi: 10.1016/0896-6273(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 35.Sheng ZH, Yokoyama CT, Catterall WA. Interaction of the synprint site of N-type Ca2+ channels with the C2B domain of synaptotagmin I. Proc Natl Acad Sci USA. 1997;94:5405–5410. doi: 10.1073/pnas.94.10.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bezprozvanny I, Scheller RH, Tsien RW. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature. 1995;378:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- 37.Jarvis SE, Barr W, Feng ZP, Hamid J, Zamponi GW. Molecular determinants of syntaxin 1 modulation of N-type calcium channels. J Biol Chem. 2002;277:44399–44407. doi: 10.1074/jbc.M206902200. [DOI] [PubMed] [Google Scholar]
- 38.Jarvis SE, Zamponi GW. Interactions between presynaptic Ca2+ channels, cytoplasmic messengers and proteins of the synaptic vesicle release complex. Trends Pharmacol Sci. 2001;22:519–525. doi: 10.1016/s0165-6147(00)01800-9. [DOI] [PubMed] [Google Scholar]
- 39.Flynn R, Zamponi GW. Regulation of calcium channels by RGK proteins. Channels (Austin) 2010;4:434–439. doi: 10.4161/chan.4.6.12865. [DOI] [PubMed] [Google Scholar]
- 40.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal. 2010;3:70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall DD, Feekes JA, Arachchige Don AS, Shi M, Hamid J, Chen L, et al. Binding of protein phosphatase 2A to the L-type calcium channel Cav1.2 next to Ser1928, its main PKA site, is critical for Ser1928 dephosphorylation. Biochemistry. 2006;45:3448–3459. doi: 10.1021/bi051593z. [DOI] [PubMed] [Google Scholar]
- 43.Xu H, Ginsburg KS, Hall DD, Zimmermann M, Stein IS, Zhang M, et al. Targeting of protein phosphatases PP2A and PP2B to the C-terminus of the L-type calcium channel Cav1.2. Biochemistry. 2010;49:10298–10307. doi: 10.1021/bi101018c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altier C, Zamponi GW. Signaling complexes of voltagegated calcium channels and G protein-coupled receptors. J Recept Signal Transduct Res. 2008;28:71–81. doi: 10.1080/10799890801941947. [DOI] [PubMed] [Google Scholar]
- 45.Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev. 2006;58:837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- 46.Currie KP. G protein modulation of Cav2 voltage-gated calcium channels. Channels (Austin) 2010;4:497–509. doi: 10.4161/chan.4.6.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilman AG. G proteins and dual control of adenylate cyclase. Cell. 1984;36:577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- 48.Reuter H, Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977;264:49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown HF, DiFrancesco D, Noble SJ. How does adrenaline accelerate the heart? Nature. 1979;280:235–236. doi: 10.1038/280235a0. [DOI] [PubMed] [Google Scholar]
- 50.Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 52.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 53.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 54.Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G proteins and protein kinase C mediated by the calcium channel alpha1 subunit. Nature. 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- 55.Hamid J, Nelson D, Spaetgens R, Dubel SJ, Snutch TP, Zamponi GW. Identification of an integration center for cross-talk between protein kinase C and G protein modulation of N-type calcium channels. J Biol Chem. 1999;274:6195–6202. doi: 10.1074/jbc.274.10.6195. [DOI] [PubMed] [Google Scholar]
- 56.Cooper CB, Arnot MI, Feng ZP, Jarvis SE, Hamid J, Zamponi GW. Cross-talk between G-protein and protein kinase C modulation of N-type calcium channels is dependent on the G-protein beta subunit isoform. J Biol Chem. 2000;275:40777–40781. doi: 10.1074/jbc.C000673200. [DOI] [PubMed] [Google Scholar]
- 57.Maeno-Hikichi Y, Chang S, Matsumura K, Lai M, Lin H, Nakagawa N, et al. A PKC epsilon-ENH-channel complex specifically modulates N-type Ca2+ channels. Nat Neurosci. 2003;6:468–475. doi: 10.1038/nn1041. [DOI] [PubMed] [Google Scholar]
- 58.Devic E, Xiang Y, Gould D, Kobilka B. Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol Pharmacol. 2001;60:577–583. [PubMed] [Google Scholar]
- 59.Skeberdis VA, Jurevicius J, Fischmeister R. Beta-2 adrenergic activation of L-type Ca++ current in cardiac myocytes. J Pharmacol Exp Ther. 1997;283:452–461. [PubMed] [Google Scholar]
- 60.Zhang ZS, Cheng HJ, Ukai T, Tachibana H, Cheng CP. Enhanced cardiac L-type calcium current response to beta2-adrenergic stimulation in heart failure. J Pharmacol Exp Ther. 2001;298:188–196. [PubMed] [Google Scholar]
- 61.Chen-Izu Y, Xiao RP, Izu LT, Cheng H, Kuschel M, Spurgeon H, et al. G(i)-dependent localization of beta(2)-adrenergic receptor signaling to L-type Ca2+ channels. Biophys J. 2000;79:2547–2556. doi: 10.1016/S0006-3495(00)76495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, et al. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- 63.Hillman D, Chen S, Aung TT, Cherksey B, Sugimori M, Llinas RR. Localization of P-type calcium channels in the central nervous system. Proc Natl Acad Sci USA. 1991;88:7076–7080. doi: 10.1073/pnas.88.16.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991;349:760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- 65.Wang SS, Denk W, Hausser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- 66.Kitano J, Nishida M, Itsukaichi Y, Minami I, Ogawa M, Hirano T, et al. Direct interaction and functional coupling between metabotropic glutamate receptor subtype 1 and voltage-sensitive Cav2.1 Ca2+ channel. J Biol Chem. 2003;278:25101–25108. doi: 10.1074/jbc.M303266200. [DOI] [PubMed] [Google Scholar]
- 67.Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons and nerve terminals. J Neurosci. 1998;18:6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei ZY, Karim F, Roerig SC. Spinal morphine/clonidine antinociceptive synergism: involvement of G proteins and N-type voltage-dependent calcium channels. J Pharmacol Exp Ther. 1996;278:1392–1407. [PubMed] [Google Scholar]
- 69.Brody DL, Patil PG, Mulle JG, Snutch TP, Yue DT. Bursts of action potential waveforms relieve G-protein inhibition of recombinant P/Q-type Ca2+ channels in HEK 293 cells. J Physiol. 1997;499:637–644. doi: 10.1113/jphysiol.1997.sp021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beedle AM, McRory JE, Poirot O, Doering CJ, Altier C, Barrere C, et al. Agonist-independent modulation of N-type calcium channels by ORL1 receptors. Nat Neurosci. 2004;7:118–125. doi: 10.1038/nn1180. [DOI] [PubMed] [Google Scholar]
- 71.Spampinato S, Di Toro R, Alessandri M, Murari G. Agonist-induced internalization and desensitization of the human nociceptin receptor expressed in CHO cells. Cell Mol Life Sci. 2002;59:2172–2183. doi: 10.1007/s000180200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corbani M, Gonindard C, Meunier JC. Ligand-regulated internalization of the opioid receptor-like 1: a confocal study. Endocrinology. 2004;145:2876–2885. doi: 10.1210/en.2004-0062. [DOI] [PubMed] [Google Scholar]
- 73.Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, et al. ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci. 2006;9:31–40. doi: 10.1038/nn1605. [DOI] [PubMed] [Google Scholar]
- 74.Altier C, Garcia-Caballero A, Simms B, You H, Chen L, Walcher J, et al. The Cavbeta subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nat Neurosci. 2011;14:173–180. doi: 10.1038/nn.2712. [DOI] [PubMed] [Google Scholar]
- 75.Evans RM, You H, Hameed S, Altier C, Mezghrani A, Bourinet E, et al. Heterodimerization of ORL1 and opioid receptors and its consequences for N-type calcium channel regulation. J Biol Chem. 2010;285:1032–1040. doi: 10.1074/jbc.M109.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kisilevsky AE, Mulligan SJ, Altier C, Iftinca MC, Varela D, Tai C, et al. D1 receptors physically interact with N-type calcium channels to regulate channel distribution and dendritic calcium entry. Neuron. 2008;58:557–570. doi: 10.1016/j.neuron.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Kisilevsky AE, Zamponi GW. D2 dopamine receptors interact directly with N-type calcium channels and regulate channel surface expression levels. Channels (Austin) 2008;2:269–277. doi: 10.4161/chan.2.4.6402. [DOI] [PubMed] [Google Scholar]
- 78.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 2005;57:463–472. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 79.Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, et al. Molecular diversity of K+ channels. Ann NY Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 80.Berkefeld H, Fakler B, Schulte U. Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev. 2010;90:1437–1459. doi: 10.1152/physrev.00049.2009. [DOI] [PubMed] [Google Scholar]
- 81.Anderson D, Mehaffey WH, Iftinca M, Rehak R, Engbers JD, Hameed S, et al. Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat Neurosci. 2010;13:333–337. doi: 10.1038/nn.2493. [DOI] [PubMed] [Google Scholar]
- 82.Anderson D, Rehak R, Hameed S, Mehaffey WH, Zamponi GW, Turner RW. Regulation of the Kv4.2 complex by Cav3.1 calcium channels. Channels (Austin) 2010;4 doi: 10.4161/chan.4.3.11955. [DOI] [PubMed] [Google Scholar]
- 83.Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci. 2009;66:852–875. doi: 10.1007/s00018-008-8609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pedarzani P, Stocker M. Molecular and cellular basis of small—and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol Life Sci. 2008;65:3196–3217. doi: 10.1007/s00018-008-8216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vogalis F, Storm JF, Lancaster B. SK channels and the varieties of slow after-hyperpolarizations in neurons. Eur J Neurosci. 2003;18:3155–3166. doi: 10.1111/j.1460-9568.2003.03040.x. [DOI] [PubMed] [Google Scholar]
- 86.Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- 87.Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004;27:343–369. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 88.Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron. 2008;59:873–881. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 89.Viana F, Bayliss DA, Berger AJ. Multiple potassium conductances and their role in action potential repolarization and repetitive firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol. 1993;69:2150–2163. doi: 10.1152/jn.1993.69.6.2150. [DOI] [PubMed] [Google Scholar]
- 90.Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- 92.Golding NL, Jung HY, Mickus T, Spruston N. Dendritic calcium spike initiation and repolarization are controlled by distinct potassium channel subtypes in CA1 pyramidal neurons. J Neurosci. 1999;19:8789–8798. doi: 10.1523/JNEUROSCI.19-20-08789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stocker M, Hirzel K, D'Hoedt D, Pedarzani P. Matching molecules to function: neuronal Ca2+-activated K+ channels and afterhyperpolarizations. Toxicon. 2004;43:933–949. doi: 10.1016/j.toxicon.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 94.Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci USA. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cueni L, Canepari M, Lujan R, Emmenegger Y, Watanabe M, Bond CT, et al. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nat Neurosci. 2008;11:683–692. doi: 10.1038/nn.2124. [DOI] [PubMed] [Google Scholar]
- 96.Swensen AM, Bean BP. Ionic mechanisms of burst firing in dissociated Purkinje neurons. J Neurosci. 2003;23:9650–9663. doi: 10.1523/JNEUROSCI.23-29-09650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alvina K, Khodakhah K. Selective regulation of spontaneous activity of neurons of the deep cerebellar nuclei by N-type calcium channels in juvenile rats. J Physiol. 2008;586:2523–2538. doi: 10.1113/jphysiol.2007.148197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- 99.Tadayonnejad R, Anderson D, Molineux ML, Mehaffey WH, Jayasuriya K, Turner RW. Rebound discharge in deep cerebellar nuclear neurons in vitro. Cerebellum. 2010;9:352–374. doi: 10.1007/s12311-010-0168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Williams S, Serafin M, Muhlethaler M, Bernheim L. Distinct contributions of high- and low-voltage-activated calcium currents to afterhyperpolarizations in cholinergic nucleus basalis neurons of the guinea pig. J Neurosci. 1997;17:7307–7315. doi: 10.1523/JNEUROSCI.17-19-07307.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci. 2004;24:8818–8822. doi: 10.1523/JNEUROSCI.2915-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J Physiol. 2003;548:53–69. doi: 10.1113/jphysiol.2002.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun X, Gu XQ, Haddad GG. Calcium influx via L- and N-type calcium channels activates a transient large-conductance Ca2+-activated K+ current in mouse neocortical pyramidal neurons. J Neurosci. 2003;23:3639–3648. doi: 10.1523/JNEUROSCI.23-09-03639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smith MR, Nelson AB, Du Lac S. Regulation of firing response gain by calcium-dependent mechanisms in vestibular nucleus neurons. J Neurophysiol. 2002;87:2031–2042. doi: 10.1152/jn.00821.2001. [DOI] [PubMed] [Google Scholar]
- 105.Wolfart J, Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci. 2002;22:3404–3413. doi: 10.1523/JNEUROSCI.22-09-03404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cueni L, Canepari M, Adelman JP, Luthi A. Ca2+ signaling by T-type Ca2+ channels in neurons. Pflugers Arch. 2009;457:1161–1172. doi: 10.1007/s00424-008-0582-6. [DOI] [PubMed] [Google Scholar]
- 107.Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, et al. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- 108.Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berkefeld H, Fakler B. Repolarizing responses of BKCa-Ca v complexes are distinctly shaped by their Cav subunits. J Neurosci. 2008;28:8238–8245. doi: 10.1523/JNEUROSCI.2274-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- 111.Molineux ML, Fernandez FR, Mehaffey WH, Turner RW. A-type and T-type currents interact to produce a novel spike latency-voltage relationship in cerebellar stellate cells. J Neurosci. 2005;25:10863–10873. doi: 10.1523/JNEUROSCI.3436-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pioletti M, Findeisen F, Hura GL, Minor DL., Jr Three-dimensional structure of the KChIP1-Kv4.3 T1 complex reveals a cross-shaped octamer. Nat Struct Mol Biol. 2006;13:987–995. doi: 10.1038/nsmb1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Burgoyne RD. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci. 2007;8:182–193. doi: 10.1038/nrn2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 115.Patel SP, Campbell DL, Strauss HC. Elucidating KChIP effects on Kv4.3 inactivation and recovery kinetics with a minimal KChIP2 isoform. J Physiol. 2002;545:5–11. doi: 10.1113/jphysiol.2002.031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang X, Bao J, Zeng XM, Liu Z, Mei YA. Elevation of intracellular Ca2+ modulates A-currents in rat cerebellar granule neurons. J Neurosci Res. 2005;81:530–540. doi: 10.1002/jnr.20576. [DOI] [PubMed] [Google Scholar]
- 117.Kollo M, Holderith NB, Nusser Z. Novel subcellular distribution pattern of A-type K+ channels on neuronal surface. J Neurosci. 2006;26:2684–2691. doi: 10.1523/JNEUROSCI.5257-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- 119.Rhodes KJ, Carroll KI, Sung MA, Doliveira LC, Monaghan MM, Burke SL, et al. KChIPs and Kv4 alpha subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci. 2004;24:7903–7915. doi: 10.1523/JNEUROSCI.0776-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Piskorowski R, Aldrich RW. Calcium activation of BKCa potassium channels lacking the calcium bowl and RCK domains. Nature. 2002;420:499–502. doi: 10.1038/nature01199. [DOI] [PubMed] [Google Scholar]