Abstract
Signaling through the Rho family of small GTPases regulates a variety of cellular processes via changes in the actin cytoskeleton. Here we discuss recent findings that show the transcription factor p53 regulates the expression of several Rho pathway signaling molecules, and how mutation of p53 in cancer dramatically alters signaling output through this pathway.
Key words: Rho GTPases, RhoA, RhoB, RhoC, RhoE, LIMK, p53, miRNA, DNA damage, actin, cofilin, cytoskeleton
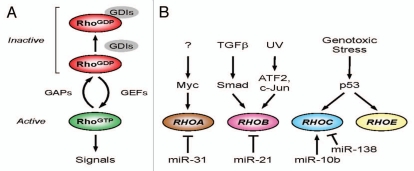
The Rho family of GTPases regulates a wide variety of cellular processes, including apoptosis, cell cycle progression and migration.1 Although this family of proteins is comprised of some 22 members, the best characterized are RhoA, Rac1 and Cdc42. Rho GTPases act as molecular ‘switches,’ cycling between an inactive GDP-bound form and an active GTP-bound form. Transition between these two states is regulated by different regulatory proteins: guanine nucleotide exchange factors (GEFs) stimulate the exchange of GDP for GTP and therefore increase Rho protein activity, whereas GTPase-activating proteins (GAPs) promote the hydrolysis of GTP leading to Rho inactivation (Fig. 1A). Rho GTPases are mainly located within the cytosol and can be post-translationally modified by prenylation of the C-terminus. This enables the Rho GTPase protein to associate with the plasma membrane and interact with its effector proteins. A group of inhibitory regulators called guanine dissociation inhibitors (GDIs) function by binding GDP-bound Rho proteins and sequestering them within the cytosol and away from the plasma membrane.
Figure 1.
Regulating Rho GTPase activity and expression. (A) Rho GTPases act as molecular switches to regulate downstream signal transduction pathways. Guanine nucleotide exchange factors (GEFs) promote GTP exchange, converting the GDP-bound form (RhoGDP) to the GTP-bound active form (RhoGTP). GTPase activating proteins (GAPs) promote GTP hydrolysis and return the GTPase to its inactive form. (B) Transcriptional regulation of Rho family GTPases. RHOA gene expression can be positively and negatively regulated by Myc and miR-31, respectively. TGFβ signaling via the Smad3,4 transcription factor complex leads to increased transcription of RHOB. RHOB mRNA expression is also upregulated by the ATF2 and c-Jun transcription factors in response to UV irradiation. Stabilization and activation of p53 by genotoxic stresses promotes the transcription of RhOC and RHOE. MicroRNAs (miRNAs) can also regulate Rho family gene expression by a variety of post-transcriptional mechanisms.
Given that Rho GTPases regulate such a wide range of cellular processes, it is not surprising that Rho protein activity is tightly regulated. However, Rho GTPase signaling may become de-regulated in cancer. Unlike the Ras GTPases, Rho GTPases are not mutated and constitutively activated in cancer, but both RhoA and RhoC expression are upregulated at the mRNA and protein levels in a number of human malignancies.2 The transcriptional mechanisms that control Rho GTPase gene expression under physiological and pathophysiological settings are only just beginning to be elucidated.
Transcriptional Regulation of Rho GTPase Expression
Cells respond to various cellular stresses by stabilizing and activating the transcription factor p53.3 In response, p53 acts as a sequence-specific transcription factor that binds to response elements (RE) within target genes.4 As such, p53 controls the expression of a plethora of genes involved in cell cycle arrest, metabolism, survival and apoptosis.
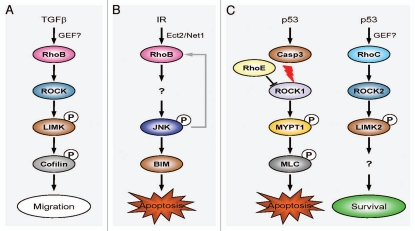
The first indication that p53 transcriptional activity could regulate the actin cytoskeleton was the identification of RhoE as a p53 target gene (Fig. 1B).5 RhoE (also known as Rnd3) belongs to the Rnd GTPase subfamily, which unlike other Rho proteins have very low intrinsic GTPase activity and are constitutively active. Ongusaha and co-workers showed that RhoE was induced in response to various DNA damaging agents resulting in the disassembly of actin stress fibers, which was dependent upon both RhoE and p53. Knockdown of RhoE not only prevented the disassembly of the actin cytoskeleton, but also promoted apoptosis. We previously showed that caspase-mediated cleavage and activation of ROCK1 occurs in the late stages of apoptosis, leading to cell contraction, membrane blebbing and nuclear disintegration.6,7 RhoE has been shown to bind the amino terminus of ROCK1 and inhibit its activity.8 RhoE induction in response to p53 activation was reported to promote cell survival by inhibiting ROCK1-dependent apoptosis (Fig. 2C). It should be noted, however, that inhibition of ROCK activity does not protect against apoptosis in all cell types,6 suggesting that additional factors (e.g., cell type, input from additional signaling pathways, etc.) likely contribute to the ultimate outcome.
Figure 2.
Context-dependent regulation of Rho GTPase signaling. (A) TGFβ-dependent regulation of RhoB activity. TGFβ upregulates RhoB expression by a MEK/ERK-dependent and Smad-dependent pathway. Once activated, RhoB signals through the ROCK-LIMK pathway to alter cell motility and migration. (B) RhoB-dependent cell death following DNA damage. Ionizing radiation (IR) triggers the DNA damage response to activate Ect2 and Net1. These GEFs promote the GTP exchange and activation of RhoB. Activated RhoB signals via the JNK pathway to induce the pro-apoptotic protein BIM and cell death. A positive feedback loop might allow JNK phosphorylation to further increase RHOB transcription and amplify RhoB-dependent cell death. (C) p53 regulates pro-survival functions of Rho GTPases. Caspase-3 is activated in response to genotoxic stress and cleaves and activates ROCK1. This kinase phosphorylates a number of substrates (including MYPT1 and MLC), which modify the actin cytoskeleton and ultimately generate contractile force within the cell. This increased contractility provides the force required for apoptotic membrane blebbing and disruption of the nucleus. RhoE can bind and inhibit ROCK1 activity, thereby promoting cell survival. A separate pro-survival pathway is triggered downstream of p53 by the upregulation and activation of RhoC and LIMK2. Signaling via an as yet unknown substrate of LIMK2 promotes cell survival.
Work from our laboratory also identified RhoC as a transcriptional target of p53 (Fig. 1B).9 Treatment of human tumor cells that express wild-type p53 with a variety of DNA-damaging agents increased the expression of RhoC mRNA, whereas RhoA mRNA levels were unaffected. Not only did genotoxic stress upregulate the expression of Rho protein but it also increased the levels of active GTP-bound Rho. Similar to Ongusaha and colleagues,5 we also observed elevated RhoE mRNA expression. However, we did not observe disassembly of the actin cytoskeleton following p53 activation, but instead found that actin stress fiber formation was increased.9 In addition, genotoxic stress failed to induce actin stress fibers in cells that express mutant p53, indicating that the cytoskeletal responses to genotoxic stress are predominantly due to activation of the p53 pathway. The discrepancy between the two studies is probably due to the increased expression and activation of RhoC signaling through ROCK2 to promote changes in the actin cytoskeleton generally being dominant over RhoE inhibition of ROCK1 (Fig. 2C; unpublished data).
Other transcription factors can also regulate Rho GTPase expression (Fig. 1B).10–12 Recently, Myc has been shown to cooperate with Skp2 to induce RhoA transcription by recruiting Miz1 and p300 to the RhoA promoter.10 Deficiency of the Myc-Skp2-Miz1-p300 complex not only resulted in impaired RhoA expression but also inhibited cell migration, invasion and breast cancer metastasis. RhoB, which shares 86% homology with RhoA, is readily inducible following exposure to growth factors, DNA damaging agents or cellular stress. Transforming growth factor-β (TGFβ) signaling through the TGFβ-1 receptor upregulates RhoB transcription via activation of cytoplasmic Smad3/4 and MEK/ERK pathways.11 Activation of the MEK/ERK pathway is required for Smad3 recruitment to a non-canonical binding site within the RhoB promoter. Interestingly, mutant p53 is also required to cooperate with the Smads to promote RhoB transcription. Activation of RhoB enables signaling through the ROCK-LIMK pathway to promote cell migration (Fig. 2A). RhoB expression is also upregulated following treatment with ultra violet (UV) light.12 However, in this case p38 MAPK regulates the recruitment of c-Jun and p300 to the RhoB promoter.
Small non-coding RNAs or microRNAs (miRNAs) can also regulate Rho GTPase gene expression (Fig. 1B) at the post-transcriptional level.13–16 Ma and colleagues reported that the Twist transcription factor induces miR-10b expression, which subsequently inhibits messenger RNA encoding homeobox D10 translation leading to increased RhoC gene expression.15 The microRNAs miR-31, miR-21 and miR-138 have been shown to suppress RhoA, RhoB and RhoC mRNAs, respectively.13,14,16 The microRNA miR-151 suppresses expression of RhoGDI A, resulting in increased basal activation of RhoA, Rac1 and Cdc42.17 Given the ability of p53 to regulate Rho expression, it will be interesting to determine whether microRNAs regulated at transcriptional and post-transcriptional levels by p53,18 comprise part of the mechanism that controls Rho GTPase expression and the expression of GAPs, GEFs and GDIs.
How is RhoC Activated in Response to Genotoxic Stress?
The GEF(s) responsible for activating RhoC following activation of the p53 pathway are currently unknown. Given that there are 85 GEFs in the human genome, an siRNA-based screening approach will be needed to functionally determine the identity of the GEF(s) that modulate RhoC-ROCK-LIMK2 signal transduction following genotoxic stress. Recent studies have highlighted the involvement of distinct nuclear-localized GEFs in the activation of other Rho proteins in response to genotoxic stress.19–21 Using an elegant assay to isolate activated GEFs, Scrougi and Burridge identified Ect2 and Net1 as the GEFs responsible for activating RhoB in response to DNA damage (Fig. 2B).21 Net1 has also been shown to promote RhoA activation in response to DNA damage caused by cytolethal distending toxin.19 Dubash and co-workers have shown using isolated nuclei that Net1 can activate a nuclear pool of RhoA.20 These findings suggest that signals from the cytoplasm are not required to activate both Net1 and RhoA in response to nuclear events.
How genotoxic stress activates nuclear GEFs still remains to be determined. GEF activity can be regulated by post-translational modifications. For instance, cytolethal distending toxin promotes Net1 activation by inducing the dephosphorylation of a critical inhibitory serine residue (S152).19 This dephosphorylation event could be due to the downregulation of a Net1-specific kinase or an increase in phosphatase activity. The Ataxia-telangiectasia mutated (ATM) kinase has been suggested to be a kinase that regulates GEF activity in response to DNA damage. It is also conceivable that microRNAs, possibly regulated by p53, may control the expression of GEFs or possibly the expression/function of GEF regulators. Further studies will be required to determine how GEF expression and activity are modulated in response to DNA damage.
p53-Dependent Activation of the Rho-ROCK-LIMK Pathway
Once activated, the Rho proteins (RhoA, RhoB and RhoC) recruit numerous effector proteins that ultimately cause re-organization of the actin cytoskeleton. Acting immediately downstream of the Rho proteins are the Rho-associated kinases ROCK1 and ROCK2, which phosphorylate and activate the LIM kinases (LIMK1 and LIMK2). Activated LIMK phosphorylates and inactivates the filamentous actin (F-actin) severing protein cofilin, thereby stabilizing actin filaments and promoting the formation of stress fibers. DNA damaging agents activate this pathway with increased levels of Rho-GTP, phosphorylated LIMK and phosphorylated cofilin all being apparent.9 By using selective inhibitors of Rho (Tat-C3) or ROCK kinases (Y-27632), we were able to show that DNA damaging agents activate Rho-ROCK signal transduction.
Other components of the Rho signaling pathway have also been shown to be regulated by wild-type p53.9,22,23 We and others have recently shown that LIMK2 is a p53 target gene.9,23 The human LIMK2 gene contains two distinct promoters that drive the expression of the transcript variants 2a and 2b, termed LIMK2a and LIMK2b, respectively. Alternative splicing within exon 16 of the LIMK2b transcript generates an additional variant, LIMK2v1. Interestingly, wild-type p53 activation upregulates LIMK2b and LIMK2v1 mRNA expression, whereas LIMK2a expression is repressed.9 This occurs because LIMK2a and LIMK2b contain independent p53 REs within their distinct first introns.9 The biological purpose of this differential expression of LIMK2 variants is currently not known. In unstressed cells, p53 levels are maintained at low levels by the ubiquitin ligase MDM2. Knockdown of p53 under these conditions has no effect on LIMK2 expression, suggesting that other transcription factors function to regulate basal levels of LIMK2 expression. As a result, p53 is able to regulate rearrangements of the actin cytoskeleton through a combination of increased Rho-pathway gene expression and Rho activation.
Modulation of Rho pathway signaling by p53 appears to be both cell and context dependent. In keratinocytes, Lefort and colleagues found that p53 can negatively regulate the expression of Rho and Cdc42 effectors ROCK1, ROCK2 and MRCKα.22 It does this via transcriptional regulation of Notch1, which negatively regulates ROCK and MRCKα gene expression.
Mutant p53 and Transcription
Mutations in the Tp53 gene occur in approximately 50% of human cancers, most of which are missense mutations that lead to single amino acid changes affecting residues within the DNA-binding domain of p53.3 As such, these mutations result in the production of full-length p53 protein (mutp53) that tends to accumulate in tumor cells at elevated levels. Although mutp53 loses wild-type functions, it acquires a number of gain-of-function (GOF) activities, including increased genomic instability, increased resistance to pro-apoptotic signals and increased cell migration and invasion.24
Recently, there has been a flurry of publications describing how p53 loss or mutation influences the invasive behavior of tumor cells.25 Gadea and coworkers reported that loss or mutation of p53 promotes a switch from a mesenchymal to an invasive, rounded (also termed amoeboid) cell morphology.26 Rounded invasive tumor cells possess elevated RhoA-ROCK signaling, with increased motility and invasion being dependent upon ROCK activity. Imaging of Rho activation in a live animal model of pancreatic cancer driven by mutant p53 using activation state-sensitive fluorescent probes revealed a discrete fraction of high RhoA activity at both the leading edge and rear of cells.27 The precise mechanisms that mutant p53 utilizes to hijack control of the Rho-ROCK pathway still remain to be worked out. Emerging evidence indicates that increased GEF expression and activity combine with reduced GAP activity to promote RhoA signaling.28,29 Other oncogenes are also likely to cooperate with mutp53. Indeed, activated Ras acts synergistically with mutp53 to stimulate RhoA activity and increase cell motility.29
There is accumulating evidence to suggest that mutant p53 can act as transcription factor and modulate gene promoter activity using its transactivation domain. Mutant p53 isoforms exert profound effects on gene expression, causing upregulation of pro-proliferative genes (such as Cyclin A, Cyclin B1 and cdk1), while repressing the transcription of pro-apoptotic (e.g., Caspase-3 and CD95/Fas/Apo1) and cell cycle arrest (p21) genes.24 These mutated p53 isoforms can also transcriptionally regulate the expression of components of the Rho signal transduction pathway. Mizurai and colleagues have demonstrated that mutant forms of p53 can transcriptionally upregulate the expression of GEF-H1, a GEF for RhoA.28 Moreover, we observed that although the LIMK2a p53RE was able to bind wild-type p53, its expression was repressed following p53 activation but increased by overexpression of a mutant form of p53 (273H).9 We found that wild-type p53 can regulate a pro-survival function that is dependent upon LIMK2b activity.9 It is tempting to speculate that the acquisition of mutant p53 would allow a cell to switch expression to the LIMK2a variant and thereby promote cell migration and invasion in response to RhoA activation.
Mutant p53 can also interact with the other p53 family members (p63 and p73) to alter their transcriptional activity and negatively regulate their function.25 The p63 and p73 isoforms that contain functional transactivation domains (TA isoforms) regulate gene expression patterns that overlap with wild-type p53. In addition, they can also regulate distinct activities that are separate from wild-type p53. TAp63 has recently been shown to suppress metastasis by direct transcriptional regulation of Dicer and miR-130b.30 It will be interesting to determine how mutp53 regulates microRNA function, and how this impinges on Rho signaling to regulate invasion and metastasis.
Summary
The additional layer of regulation of Rho GTPase activity through gene transcription has only recently been appreciated as a major contributory factor. These effects may be direct, or may involve post-transcriptional mechanisms such as microRNA modulation of Rho GTPases or regulatory proteins. Given that a number of proteins involved in Rho signal transduction have both cytoplasmic and nuclear distributions, an interesting area for future research will be determining how these proteins impact upon gene transcription, either directly or as part of feedback mechanisms.
Acknowledgments
This work was supported by Cancer Research UK.
References
- 1.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 2.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 3.Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 4.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 5.Ongusaha PP, Kim HG, Boswell SA, Ridley AJ, Der CJ, Dotto GP, et al. RhoE is a pro-survival p53 target gene that inhibits ROCK I-mediated apoptosis in response to genotoxic stress. Curr Biol. 2006;16:2466–2472. doi: 10.1016/j.cub.2006.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- 7.Croft DR, Coleman ML, Li S, Robertson D, Sullivan T, Stewart CL, et al. Actin-myosin-based contraction is responsible for apoptotic nuclear disintegration. J Cell Biol. 2005;168:245–255. doi: 10.1083/jcb.200409049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riento K, Guasch RM, Garg R, Jin B, Ridley AJ. RhoE binds to ROCK I and inhibits downstream signaling. Mol Cell Biol. 2003;23:4219–4229. doi: 10.1128/MCB.23.12.4219-4229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croft DR, Crighton D, Samuel MS, Lourenco FC, Munro J, Wood J, et al. p53-mediated transcriptional regulation and activation of the actin cytoskeleton regulatory RhoC to LIMK2 signaling pathway promotes cell survival. Cell Res. 2011;21:666–682. doi: 10.1038/cr.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan CH, Lee SW, Li CF, Wang J, Yang WL, Wu CY, et al. Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis. Nat Cell Biol. 2010;12:457–467. doi: 10.1038/ncb2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasilaki E, Papadimitriou E, Tajadura V, Ridley AJ, Stournaras C, Kardassis D. Transcriptional regulation of the small GTPase RhoB gene by TGF{beta}-induced signaling pathways. FASEB J. 2010;24:891–905. doi: 10.1096/fj.09-134742. [DOI] [PubMed] [Google Scholar]
- 12.Ahn J, Choi JH, Won M, Kang CM, Gyun MR, Park HM, et al. The activation of p38 MAPK primarily contributes to UV-induced RhoB expression by recruiting the c-Jun and p300 to the distal CCAAT box of the RhoB promoter. Biochem Biophys Res Commun. 2011;409:211–216. doi: 10.1016/j.bbrc.2011.04.121. [DOI] [PubMed] [Google Scholar]
- 13.Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szasz AM, Wang ZC, et al. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137:1032–1046. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Connolly EC, Van Doorslaer K, Rogler LE, Rogler CE. Overexpression of miR-21 promotes an in vitro metastatic phenotype by targeting the tumor suppressor RHOB. Mol Cancer Res. 2010;8:691–700. doi: 10.1158/1541-7786.MCR-09-0465. [DOI] [PubMed] [Google Scholar]
- 15.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 16.Jiang L, Liu X, Kolokythas A, Yu J, Wang A, Heidbreder CE, et al. Downregulation of the Rho GTPase signaling pathway is involved in the microRNA-138-mediated inhibition of cell migration and invasion in tongue squamous cell carcinoma. Int J Cancer. 2010;127:505–512. doi: 10.1002/ijc.25320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding J, Huang S, Wu S, Zhao Y, Liang L, Yan M, et al. Gain of miR-151 on chromosome 8q24.3 facilitates tumour cell migration and spreading through downregulating RhoGDIA. Nat Cell Biol. 2010;12:390–399. doi: 10.1038/ncb2039. [DOI] [PubMed] [Google Scholar]
- 18.Feng Z, Zhang C, Wu R, Hu W. Tumor suppressor p53 meets microRNAs. J Mol Cell Biol. 2011;3:44–50. doi: 10.1093/jmcb/mjq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerra L, Carr HS, Richter-Dahlfors A, Masucci MG, Thelestam M, Frost JA, et al. A bacterial cytotoxin identifies the RhoA exchange factor Net1 as a key effector in the response to DNA damage. PLoS One. 2008;3:2254. doi: 10.1371/journal.pone.0002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubash AD, Guilluy C, Srougi MC, Boulter E, Burridge K, Garcia-Mata R. The small GTPase RhoA localizes to the nucleus and is activated by Net1 and DNA damage signals. PLoS One. 2011;6:17380. doi: 10.1371/journal.pone.0017380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srougi MC, Burridge K. The nuclear guanine nucleotide exchange factors Ect2 and Net1 regulate RhoB-mediated cell death after DNA damage. PLoS One. 2011;6:17108. doi: 10.1371/journal.pone.0017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefort K, Mandinova A, Ostano P, Kolev V, Calpini V, Kolfschoten I, et al. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCK{alpha} kinases. Genes Dev. 2007;21:562–577. doi: 10.1101/gad.1484707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu FF, Lin TY, Chen JY, Shieh SY. p53-Mediated transactivation of LIMK2b links actin dynamics to cell cycle checkpoint control. Oncogene. 2010;29:2864–2876. doi: 10.1038/onc.2010.40. [DOI] [PubMed] [Google Scholar]
- 24.Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol. 2010;2:1107. doi: 10.1101/cshperspect.a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller PA, Vousden KH, Norman JC. p53 and its mutants in tumor cell migration and invasion. J Cell Biol. 2011;192:209–218. doi: 10.1083/jcb.201009059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadea G, de Toledo M, Anguille C, Roux P. Loss of p53 promotes RhoA-ROCK-dependent cell migration and invasion in 3D matrices. J Cell Biol. 2007;178:23–30. doi: 10.1083/jcb.200701120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timpson P, McGhee EJ, Morton JP, von Kriegsheim A, Schwarz JP, Karim SA, et al. Spatial regulation of RhoA activity during pancreatic cancer cell invasion driven by mutant p53. Cancer Res. 2011;71:747–757. doi: 10.1158/0008-5472.CAN-10-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizuarai S, Yamanaka K, Kotani H. Mutant p53 induces the GEF-H1 oncogene, a guanine nucleotide exchange factor-H1 for RhoA, resulting in accelerated cell proliferation in tumor cells. Cancer Res. 2006;66:6319–6326. doi: 10.1158/0008-5472.CAN-05-4629. [DOI] [PubMed] [Google Scholar]
- 29.Xia M, Land H. Tumor suppressor p53 restricts Ras stimulation of RhoA and cancer cell motility. Nat Struct Mol Biol. 2007;14:215–223. doi: 10.1038/nsmb1208. [DOI] [PubMed] [Google Scholar]
- 30.Su X, Chakravarti D, Cho MS, Liu L, Gi YJ, Lin YL, et al. TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature. 2010;467:986–990. doi: 10.1038/nature09459. [DOI] [PMC free article] [PubMed] [Google Scholar]