Abstract
Selective suppression of hyperactive sensory neurons is an attractive strategy for managing pathological pain. Blocking Na+ channels to eliminate action potentials and desensitizing transduction channels can both reduce sensory neuron excitability. The novel synthetic vanilloid ligand cap-ET preserves agonist activation of intracellular Ca2+ signals and large organic cation transport but loses effective electric current induction. Cap-ET can therefore be used to deliver the membrane impermeable Na+ channel blocker QX-314 to substantially inhibit voltage-activated Na+ currents. We explored, besides facilitating entry of organic cationic therapeutics, whether cap-ET can also produce receptor desensitization similar to the natural agonist capsaicin. Using the YO-PRO-1 based fluorescent dye uptake assay, we found that cap-ET effectively triggered Ca2+ dependent desensitization of TRPV1 when the receptor was pre-sensitized with the surrogate oxidative chemical phenylarsine oxide (PAO), suggesting an alternative use of permanently charged cationic capsaicinoids in differential neuronal silencing.
Key words: biased agonism, charged capsaicinoids, receptor desensitization, Ca2+, hyperalegesia, selective analgesia
Introduction
Drug-receptor activation typically elicits multiple downstream cellular events, while a partial agonist generally evokes reduced biological responses in all pathways. Partial agonists are useful for therapeutic inhibition without full ablation of receptor signaling. Biased agonism has become more recognized among new drugs. Biased agonists exhibit huge discrepancy in coupling efficiencies of distinct cellular pathways compared with full agonists. Even if they interact with the same ligand-binding domain, biased agonists can trigger such different conformational changes that only a subset of downstream signaling pathways are activated.1–4
Permanently charged cationic capsaicinoids are biased agonists in that they activate effective cellular Ca2+ signals and extracellular large cation transport, but poorly stimulate the electric currents mediated by TRPV1. We demonstrated that cap-ET, the most effective charged capsaicinoid tested so far, is potentially useful for preferential cytoplasmic delivery of the membrane impermeable Na+ channel blocker QX-314 into sensitized neurons to suppress their electric excitability.5 Another potential use of these cationic capsaicinoids is to desensitize TRPV1, thereby reducing the ability of this channel to transduce noxious signals.6–9
Results and Discussion
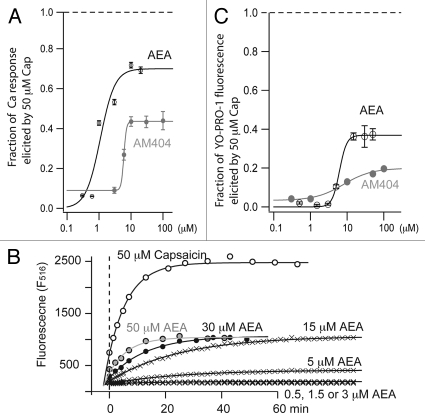
Capsaicin is well known to cause TRPV1 desensitization and suppress sensory neuron excitability in an extracellular-calcium dependent manner.10–13 We extended the fluorescent dye (YO-PRO-1) transport assay to evaluate whether charged capsaicinoids can be also useful for desensitization of TRPV1, and their efficacy in desensitizing the receptor compared to other TRPV1 partial agonists such as the endogenous lipid anandamide or the synthetic aminophenol AM404.14–16 In calcium imaging experiments, anandamide and AM404 are partial agonists displaying reduced efficacies compared to capsaicin or charged capsaicinoids (Fig. 1A). The reduction of agonist-induced YO-PRO-1 transport was also noted for anandamide and AM404 (Fig. 1B and C). Anandamide and AM404 are, therefore, low efficacy partial agonists for all three receptor functions downstream of ligand-induced TRPV1 pore opening.
Figure 1.
Anandamide (AEA) and AM404 are partial agonists of TRPV1 in Ca2+ imaging and YO-PRO-1 transport assays. (A) Ratiometric Fura-2 fluorescent signals normalized to maximal F340/F380 ratio elicited by 50 µM capsaicin. Each data point represents the mean value ± standard error from three independent wells. (B) Representative traces of anandamide-induced YO-PRO-1 cellular fluorescence at various agonist concentrations (n = 4 independent wells for each concentration). (C) The dose response curves of AEA and AM404 induced YO-PRO-1 entry, normalized to maximal fluorescence evoked by 50 µM capsaicin.
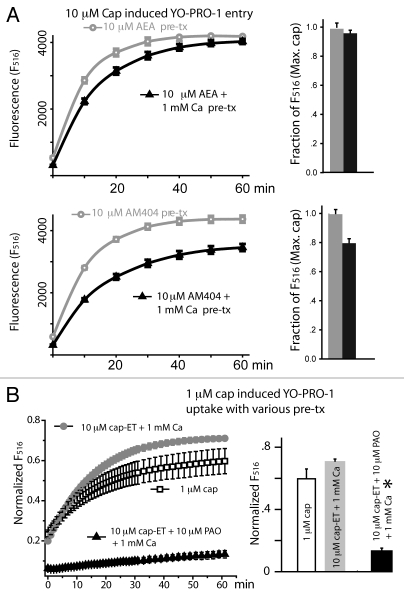
Given that the ligand induced channel desensitization requires Ca2+ entry to raise the intracellular Ca2+ level and stimulate the subsequent Ca2+ dependent pathways, we compared these partial agonists with capsaicin in inducing TRPV1 desensitization following prolonged agonist treatment. In the presence of 1 mM extracellular calcium, capsaicin (1 µM) pretreatment for 1 h led to pronounced inhibition of subsequent TRPV1-mediated YO-PRO-1 entry for all concentrations (1, 3 and 10 µM) of capsaicin tested (Fig. 2A). This inhibition is due to TRPV1 desensitization. Removal of Ca2+ alone for 1 h did not affect the ability of capsaicin to induce YO-PRO-1 entry; mean fluorescence values for cells incubated in a nominally Ca2+ free solution with or without 1 µM capsaicin were similar (2,390 ± 250 and 2,990 ± 220 respectively, n = 4 wells). Co-application of capsaicin with PAO reversed the inhibition introduced by capsaicin and calcium pretreatment (Fig. 2B), consistent with our earlier report that oxidative modification of TRPV1 channels is sufficient to override the calcium induced receptor desensitization initiated by agonist binding to TRPV1.17 However, after we incubated the cells in 10 µM anandamide or AM404 in the presence of 1 mM extracellular calcium, we observed only a small reduction of TRPV1-dependent YO-PRO-1 entry (Fig. 3A and p = 0.1 for AEA, 0.01 for AM404, unpaired t-test). This result indicates that anandamide and AM404, being low efficacy partial agonists, are also inefficient in their ability to induce TRPV1 desensitization. Given that 10 µM cap-ET could evoke a calcium response comparable to that induced by 10 µM AM404, we asked what would be the efficacy of cap-ET, a biased agonist with reduced potency, for induction of TRPV1 desensitization. TRPV1 cells were treated with 10 µM cap-ET with or without the sensitizing oxidative chemical PAO for 1 h and capsaicin-induced YO-PRO-1 entry was assessed. 10 µM cap-ET incubation for 1 h in a nominally Ca2+ free solution did not interfere with the subsequent capsaicin induced cytoplasmic YO-PRO-1 accumulation (normalized fluorescence value = 0.54 ± 0.01, n = 4 wells). Interestingly, without PAO pre-sensitization, incubation with cap-ET in the presence of extracellular calcium did not desensitize the subsequent TRPV1 activation either. In contrast, when the cells were sensitized by the oxidative chemical PAO, 10 µM cap-ET plus 1 mM calcium pretreatment for 1 h was sufficient to induce substantial receptor desensitization (Fig. 3B). PAO induced receptor sensitization was shown to enhance receptor activity in general. It was, nevertheless, noted that PAO sensitized capsaicin receptors still showed receptor desensitization, albeit at a much slower rate.17 These new results indicate that the new capsaicinoid cap-ET is capable of inducing TRPV1 desensitization, particularly under the condition when pro-inflammatory agents sensitize the receptor. Cap-ET like capsaicinoids thus can potentially reset the excitability of neurons bearing hyperactive TRPV1 by differentially desensitizing the receptors based on their initial activity level. The preferential desensitization of the hyperactive TRPV1 compared to the non-sensitized receptors by cap-ET suggests an additional use of cap-ET in clinical management of pain: a low dose of cap-ET may be given to patients and desensitize the TRPV1 receptor according to relative channel activity.
Figure 2.
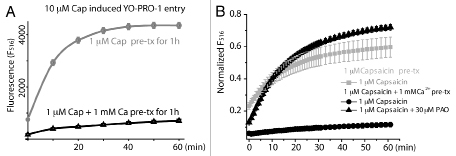
Desensitization of TRPV1 reduced the subsequent capsaicin-mediated YO-PRO-1 entry. (A) TRPV1 exposed to 1 µM capsaicin for 1 h became desensitized, provided that 1 mM extracellular Ca2+ was supplied during the pre-incubation phase (n = 4 wells for each treatment). (B) In a nominally Ca2+ free solution, addition of 10 µM PAO (filled triangles) reversed TRPV1 desensitization induced by one hour pretreatment with 1 µM capsaicin plus 1 mM extracellular Ca2+ (n = 4 for each treatment). 1 µM capsaicin was applied extracellularly in a nominally calcium free solution during the YO-PRO-1 entry phase for all three treatments (normalized to total cellular fluorescence after fixation).
Figure 3.
The biased agonist cap-ET under the PAO-sensitized condition effectively introduced Ca2+-dependent receptor desensitization. (A) AEA and AM404 induced less Ca2+-dependent desensitization of TRPV1 compared to capsaicin (n = 4 for each treatment). (B) Sensitization of TRPV1 by 10 µM PAO sufficiently increased the efficacy of 10 µM cap-ET for induction of Ca2+-dependent channel desensitization (*p < 5 × 10−4, unpaired t-test, n = 4 for each treatment).
Materials and Methods
Cell culture.
HEK (human embryonic kidney) 293 cells stably expressing rat TRPV1 were grown in DMEM with 10% newborn calf serum and antibiotics and cultured in a 5% CO2 aerated incubator at 37°C. Cells were trypsinized and plated on 96-well plates or matrix-coated coverslips for Ca2+ imaging and fluorescent plate-reader assays.
Ca2+ imaging.
HEK293 cells were loaded with 5 µM Fura-2 AM and 0.01% pluronic acid in Ca2+ imaging buffer for 3 h. The imaging solution contained 8.5 mM HEPES, 140 mM NaCl, 3.4 mM KCl, 1.7 mM MgCl2 and 1 mM CaCl2, pH to 7.4 with NaOH. 2x solution containing different concentrations of anandamide and AM404 was pipetted into individual wells for agonist delivery. The images were acquired every 5 seconds. The plots display mean ± SEM values of F340/F380 ratios (150 ms exposure time for each wavelength) from imaged fields. Each data point represented the mean of peak values from three independent wells.
YO-PRO-1 plate-reader assays.
Stably transfected HEK293 cells expressing TRPV1 receptors were seeded in a 96-well plate. When cells were confluent, they were assayed in the same solution used for Ca2+ imaging, except that extracellular Ca2+ was omitted. Various pharmacological treatments were given for 1 h as indicated in the text. Final YO-PRO-1 (Invitrogen) concentration was 5 µM for plate reading. Fluorescence intensity (485-nm excitation, 516-nm emission) was monitored by a Multi-detection Microplate Reader (BioTek). To normalize maximum TRPV1-mediated uptake for cell counts in each well, we fixed cells by adding 1% paraformaldehyde at the end of experiments. Total fluorescence from each well was then counted by adding 10% detergent (digitonin solution, MP biomedicals) with 5 µM YO-PRO-1. Traces were displayed as mean ± SEM fluorescence values or averaged normalized ratios to maximal fluorescence readings of individual wells from three or four independent wells.
References
- 1.Vaidehi N, Kenakin T. The role of conformational ensembles of seven transmembrane receptors in functional selectivity. Curr Opin Pharmacol. 2010;10:775–781. doi: 10.1016/j.coph.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Kenakin T. Functional selectivity through protean and biased agonism: Who steers the ship? Mol Pharmacol. 2007;72:1393–1401. doi: 10.1124/mol.107.040352. [DOI] [PubMed] [Google Scholar]
- 3.Kenakin T. G protein coupled receptors as allosteric proteins and the role of allosteric modulators. J Recept Signal Transduct Res. 2010;30:313–321. doi: 10.3109/10799893.2010.503964. [DOI] [PubMed] [Google Scholar]
- 4.Andresen BT. A pharmacological primer of biased agonism. Endocr Metab Immune Disord Drug Targets. 2011;11:92–98. doi: 10.2174/187153011795564179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Wang S, Chuang AY, Cohen BE, Chuang HH. Activity-dependent targeting of TRPV1 with a pore-permeating capsaicin analog. Proc Natl Acad Sci USA. 2011;108:8497–8502. doi: 10.1073/pnas.1018550108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toth A, Wang Y, Kedei N, Tran R, Pearce LV, Kang SU, et al. Different vanilloid agonists cause different patterns of calcium response in CHO cells heterologously expressing rat TRPV1. Life Sci. 2005;76:2921–2932. doi: 10.1016/j.lfs.2004.10.056. [DOI] [PubMed] [Google Scholar]
- 7.Walker JM, Krey JF, Chen JS, Vefring E, Jahnsen JA, Bradshaw H, Huang SM. Targeted lipidomics: Fatty acid amides and pain modulation. Prostaglandins Other Lipid Mediat. 2005;77:35–45. doi: 10.1016/j.prostaglandins.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 8.De Petrocellis L, Harrison S, Bisogno T, Tognetto M, Brandi I, Smith GD, et al. The vanilloid receptor (VR1)-mediated effects of anandamide are potently enhanced by the cAMP-dependent protein kinase. J Neurochem. 2001;77:1660–1663. doi: 10.1046/j.1471-4159.2001.00406.x. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Simon SA. The influence of removing extracellular Ca2+ in the desensitization responses to capsaicin, zingerone and olvanil in rat trigeminal ganglion neurons. Brain Res. 1998;809:246–252. doi: 10.1016/s0006-8993(98)00853-1. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Simon SA. Capsaicin-induced currents with distinct desensitization and Ca2+ dependence in rat trigeminal ganglion cells. J Neurophysiol. 1996;75:1503–1514. doi: 10.1152/jn.1996.75.4.1503. [DOI] [PubMed] [Google Scholar]
- 11.Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 13.Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- 14.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 15.Zygmunt PM, Chuang H, Movahed P, Julius D, Hogestatt ED. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol. 2000;396:39–42. doi: 10.1016/s0014-2999(00)00207-7. [DOI] [PubMed] [Google Scholar]
- 16.Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, et al. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuang HH, Lin S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc Natl Acad Sci USA. 2009;106:20097–20102. doi: 10.1073/pnas.0902675106. [DOI] [PMC free article] [PubMed] [Google Scholar]