Abstract
Pancreatic cancer is a leading cause of cancer-related death in the western world, and in most patients, current chemotherapies have negligible survival benefit. Evaluation of targeted therapies, however, is a relatively recent development. Paradoxically, mutations in KRAS, and in genes involved in one if its major effector pathways, the PI3K/Akt pathway, are often found simultaneously in human tumors. Accounting for this, we have recently found that activated PI3K/Akt signaling results in a weak senescence that actually impairs the stronger Ras-induced senescence. We showed that loss of Pten and thus activation of PI3K/Akt/mTOR signaling leads to acceleration of PDAC progression in mouse. Similarly, in humans, activation of PI3K/Akt/mTOR signaling correlated with poor patient survival. Importantly, these patients represent a discrete subpopulation of this disease in which PI3K/Akt/mTOR inhibitors might be effective. Reactivating senescence has recently emerged as a realistic outcome of cancer therapy. Clearly, promising treatments may work only in certain tumor subsets, or only as part of combinatorial approaches. Thus, careful consideration should be taken before selecting preclinical models and patient populations in which to test new agents.
Key words: pancreatic cancer, Ras, PI3K, Pten, Akt, mTOR, senescence
Pancreatic cancer is one of the leading causes of cancer-related death in the western word. Every year over 200,000 cases are diagnosed worldwide and, in about 90% of cases, the tumor is too advanced for resection at the time of diagnosis.1 Even in patients with surgically resectable tumors, the probability of recurrence is high.1 Tumors are also extremely resistant to chemotherapy and despite significant advances in the treatment of other tumors, current therapies have a negligible survival benefit in this cancer type. The prognosis of those diagnosed with the disease is very poor and the 5-y survival rate is less than 5%.2 Consequently, the development of more effective strategies to combat pancreatic cancer is of paramount importance.
Pancreatic ductal adenocarcinoma (PDAC) arises from pre-malignant precursor lesions, collectively known as pancreatic intra-epithelial neoplasms (PanINs), that are grouped into three stages based on increasing levels of architectural and nuclear atypia.3 Progression through these stages to the resultant invasive adenocarcinoma is accompanied by the accumulation of a number of mutations.3 Activating mutations of KRAS are found in over 90% of invasive PDAC, and are thought to be the driver mutations,4 while inactivation of a number of tumor suppressor genes including CDKN2A, TP53, SMAD4 and BRCA2, occurs and increases in progressively higher PanIN stages.3 Many more pathways are deregulated by changes at expression level, albeit less frequently. For example, one pathway that is frequently misregulated in pancreatic cancer and is gaining interest as a potential target for therapy is the Pten/PI3K/Akt/mTOR pathway.
In the last decade there has been a considerable expansion in the use of genetically modified mouse models for the study of pancreatic cancer.5 These models have become more sophisticated in their design, allowing the introduction of mutations in a conditional and targeted way so that animals develop spontaneous pancreatic cancer that more closely reflects both the histological and genetic changes that occur in humans. Hingorani and colleagues have developed a genetically engineered mouse model in which expression of activated KrasG12D at the endogenous allele is targeted to the mouse pancreas via the Pdx1 gene promoter, using Cre-Lox technology.6 The mice develop preinvasive PanIN lesions that fail to progress quickly unless combined with other genetic lesions such as mutant p53R172H, in which case lesions progress rapidly to invasive and metastatic pancreatic cancer.7
Cellular senescence is an irreversible proliferation arrest activated by various cellular and molecular stresses, including activated oncogenes, such as mutated Ras.8 Considering that KRAS mutations occur in many different tumor types, it seems paradoxical that activating KRAS mutations induce a senescence program in normal human and mouse cells in culture.9 However oncogene-induced senescence (OIS) in vivo is now believed to represent a major barrier against tumorigenesis. In particular, recent studies have now shown that induction of senescence can restrain progression of premalignant lesions in vivo.10–13 We recently reported that although KrasG12D is one of the major oncogenic drivers of PDAC, the PanIN lesions that develop in the Pdx1-Cre, LSL-KrasG12D mouse model exhibit many features of senescence.14 Consistent with observations in vitro, inactivation of tumor suppressor genes, for example, Ink4a allowed rapid progression of these premalignant lesions to PDAC.14 This progression was consistent with failed senescence of premalignant lesions, suggesting that long-term growth arrest or senescence occurs in KrasG12D-induced PanINs and that this must be overcome in order for gross tumor development to occur. In human PanINs too, features of senescence have been observed, including senescence-associated β-galactosidase, low proliferative index and telomere shortening.12,15
These observations have enabled us recently to address another paradox in tumor biology. The Pten/PI3K/Akt/mTOR pathway is activated downstream of Ras signaling and likely represents a major mediator of Ras-driven oncogenesis. It is somewhat surprising then, that mutations in KRAS and in genes involved in Pten/PI3K/Akt/mTOR signaling are often found together in the same human tumor. For example, mutations in KRAS and in genes encoding molecules involved in PI3K signaling occur simultaneously in endometrial cancer, thyroid cancer and acute lymphoblastic leukemia.16 In human colon cancer, 25% of tumors have been shown to carry mutations both in KRAS and in multiple PI3K-associated genes.17 In human pancreatic cancer, in which the ‘driver’ mutation is almost universally KRAS,4 deregulation of the PI3K pathway is found in the majority of tumors. For example, loss of function of the tumor suppressor PTEN, due to mutation, deletion or epigenetic silencing has been observed in about 60% of human PDAC.18 Activation or overexpression, of AKT1 itself has been reported in 20–70% of human pancreatic tumors,19,20 while AKT2 is amplified or overexpressed in 10–20% of human PDACs.18,21 In the mouse, inactivation of Pten in the pancreas induces ductal metaplasia,22 and recent work has shown that overexpression of constitutively active Akt in pancreatic progenitor cells induces proliferation and expansion of the ductal epithelium and expression of progenitor cell markers resulting in the development of pre-neoplastic lesions and late onset malignant transformation.23
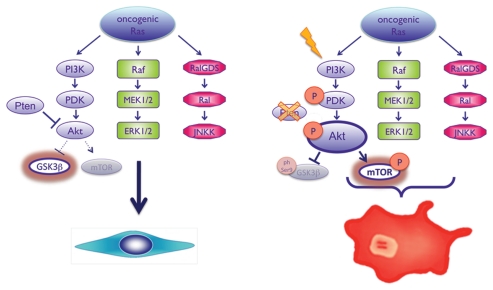
We have now found that activated PI3K signaling induces a ‘weak’ senescence, compared with the robust senescence induced by activated Ras. Intriguingly, simultaneous activation of Ras and PI3K/Akt dampens Ras-induced senescence. Thus, not all oncogenes are equally capable of inducing senescence, and in the case of PI3K/Akt and Ras, the weaker inducer of senescence is dominant over the stronger24 (Fig. 1). We hypothesized that in tumors, the advantage of mutations coexisting in both KRAS and in PI3K-associated genes could be the suppression of Ras-induced senescence. Indeed when mice harboring a conditional knockout allele of Pten were crossed with Pdx1-Cre KrasG12D mice we found that, consistent with our in vitro observations, activation of PI3K/Akt signaling bypassed the Ras-induced senescence normally observed in PanINs in this model.24 Crucially, loss or deficiency of Pten (and consequent activation of Akt) led to rapid acceleration of PDAC progression.24 Importantly, when human PDAC samples were analyzed, approximately 20% of tumors exhibited activation of PI3K/Akt/mTOR signaling, and this correlated significantly with a poorer prognosis.24
Figure 1.
Activated Ras signaling induces a robust senescence phenotype in vitro and in vivo. Hyper-activation of PI3K signaling induces a weak senescence when compared with Ras, but simultaneous activation of Ras and PI3K/Akt actually dampens Ras-induced senescence, allowing proliferation and tumor formation in vivo.
These findings suggest that it may be possible for human PDACs to be divided into subsets based on the pathways misregulated, and that treatment should be selected accordingly. For example mTOR inhibitors may prove effective in tumors harboring mutations in both PI3K pathway members and in KRAS. IGF-1 inhibitors could also inhibit tumor growth and spread in these cases, since IGF-1 enhances pancreatic cancer cell proliferation and invasiveness through stimulation of the PI3K/Akt pathway.25 Conversely, tumors harboring mutations in other tumor suppressor genes, with ‘normal’ PI3K signaling may not respond to these targeted therapies.
The existence of a tumor suppressing senescent brake also provides an intriguing new avenue to explore with regard to cancer therapy. While induction of apoptosis in tumor cells has long been seen as an attractive option for cancer treatment, reactivation of senescence has only recently been proposed. Senescence is now being increasingly recognized as a possible outcome of both drug and radiation therapy. Recent findings suggest that reactivation of senescence too could be a realistic option in the treatment of cancer. Indeed reactivation of p53 in vivo has been shown to elicit senescence, in addition to apoptosis.26
Pandolfi and coworkers have suggested that blocking prostate cancer progression using pro-senescence therapy may be possible. They found that oncogenic signaling through the PI3K/Akt pathway, triggered by loss of Pten, resulted in a form of p53-dependent senescence in vivo that they termed Pten-loss-induced cellular senescence (PICS). Pten loss in a mouse model of prostate cancer resulted in the occurrence of senescent lesions, with tumors only arising with long latency. When senescence was blocked by inactivation of p53 however, Pten loss was able to rapidly drive tumorigenesis in this model, highlighting the crucial role played by ‘driver’ mutations.11 In the case of human prostate cancer, where most tumors have lost only one allele of PTEN, the authors postulate that inhibition of the second copy could trigger senescence.27 Indeed, Pten inhibition triggered senescence and inhibited tumorigenesis in a xenograft model of human prostate cancer.
In the last few years, animal models have been extremely valuable in furthering our understanding of novel putative cancer treatments. Additional work in these models is still required to fully assess the utility of pro-senescence therapies in cancer, and also the relationship between cancer genotype and therapeutic response; some promising targeted therapies might work well in a subset of tumors harboring particular mutations, but not in others. Tailored mouse models of pancreatic cancer should be used to analyze subsets of tumors that have particular dependency on specific signaling pathways, and to determine if inhibition of these pathways can improve survival in these groups. Careful selection of the appropriate in vivo models in which to test new drugs, and importantly, combinatorial chemotherapeutic approaches, is vital. These approaches could lead to new personalized therapies for patients with pancreatic cancer.
Abbreviations
- PI3K
phosphatidylinositol-3-kinase
- mTOR
mammalian target of rapamycin
- PDAC
pancreatic ductal adenocarcinoma
- PanIN
pancreatic intraepithelial neoplasm
- OIS
oncogene induced senescence
- IGF1
insulin-like growth factor 1
- PICS
Pten-loss-induced cellular senescence
Extra View to: Kennedy AL, Morton JP, Manoharan I, Nelson DM, Jamieson NB, Pawlikowski JS, et al. Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Mol Cell. 2011;42:36–49. doi: 10.1016/j.molcel.2011.02.020.
References
- 1.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/S1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 4.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 5.Ding Y, Cravero JD, Adrian K, Grippo P. Modeling pancreatic cancer in vivo: from xenograft and carcinogen-induced systems to genetically engineered mice. Pancreas. 2010;39:283–292. doi: 10.1097/MPA.0b013e3181c15619. [DOI] [PubMed] [Google Scholar]
- 6.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/S1535-6108(03)00309-X. [DOI] [PubMed] [Google Scholar]
- 7.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Adams PD. Healing and hurting: molecular mechanisms, functions and pathologies of cellular senescence. Mol Cell. 2009;36:2–14. doi: 10.1016/j.molcel.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/S0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 10.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 13.Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 14.Morton JP, Timpson P, Karim SA, Ridgway RA, Athineos D, Doyle B, et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci USA. 2010;107:246–251. doi: 10.1073/pnas.0908428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Heek NT, Meeker AK, Kern SE, Yeo CJ, Lillemoe KD, Cameron JL, et al. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol. 2002;161:1541–1547. doi: 10.1016/S0002-9440(10)64432-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeang CH, McCormick F, Levine A. Combinatorial patterns of somatic gene mutations in cancer. FASEB J. 2008;22:2605–2622. doi: 10.1096/fj.08-108985. [DOI] [PubMed] [Google Scholar]
- 17.Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L, et al. Colorectal cancer: mutations in a signalling pathway. Nature. 2005;436:792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- 18.Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NFkappaB and c-Myc in pancreatic cancer cells. Oncogene. 2004;23:8571–8580. doi: 10.1038/sj.onc.1207902. [DOI] [PubMed] [Google Scholar]
- 19.Semba S, Moriya T, Kimura W, Yamakawa M. Phosphorylated Akt/PKB controls cell growth and apoptosis in intraductal papillary-mucinous tumor and invasive ductal adenocarcinoma of the pancreas. Pancreas. 2003;26:250–257. doi: 10.1097/00006676-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Michl P, Downward J. Mechanisms of disease: PI3K/AKT signaling in gastrointestinal cancers. Z Gastroenterol. 2005;43:1133–1139. doi: 10.1055/s-2005-858638. [DOI] [PubMed] [Google Scholar]
- 21.Ruggeri BA, Huang L, Wood M, Cheng JQ, Testa JR. Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Mol Carcinog. 1998;21:81–86. doi: 10.1002/(SICI)1098-2744(199802)21:2<81::AID-MC1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 22.Stanger BZ, Stiles B, Lauwers GY, Bardeesy N, Mendoza M, Wang Y, et al. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005;8:185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Elghazi L, Weiss AJ, Barker DJ, Callaghan J, Staloch L, Sandgren EP, et al. Regulation of pancreas plasticity and malignant transformation by Akt signaling. Gastroenterology. 2009;136:1091–1103. doi: 10.1053/j.gastro.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy AL, Morton JP, Manoharan I, Nelson DM, Jamieson NB, Pawlikowski JS, et al. Activation of the PIK3CA/AKT Pathway Suppresses Senescence Induced by an Activated RAS Oncogene to Promote Tumorigenesis. Mol Cell. 2011;42:36–49. doi: 10.1016/j.molcel.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A, Takahashi H, et al. IGF-1 mediates PTEN suppression and enhances cell invasion and proliferation via activation of the IGF-1/PI3K/Akt signaling pathway in pancreatic cancer cells. J Surg Res. 2010;160:90–101. doi: 10.1016/j.jss.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Sharpless NE, DePinho RA. Cancer biology: gone but not forgotten. Nature. 2007;445:606–607. doi: 10.1038/nature05567. [DOI] [PubMed] [Google Scholar]
- 27.Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC, et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest. 2010;120:681–693. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]