Abstract
GTPases are central hubs for directing cytoskeletal reorganization and cell migration. The DOCK family enforces positive regulation of certain GTPases, leading to their activation in discrete areas of the cell. ELMO, a well-known DOCK180 binding partner, has been cast with the role of potentiating DOCK180-mediated Rac activation. Exactly how ELMO contributes to Rac signaling is only beginning to be understood. Here, we discuss our most recent research investigating ELMO regulation of the DOCK180/Rac pathway. Interestingly, we found that ELMO is autoinhibited via intramolecular contacts at basal levels and we explore the novel domains that we identified at the heart of the auto-regulatory switch. We propose that the closed ELMO molecule masks protein-protein interfaces or domains with novel uncharacterized function; cell stimulation and GTPase binding to ELMO is proposed to activate (open) the protein and/or target the ELMO/DOCK180 complex to the cell membrane. In this manner, promiscuous signaling/activity downstream of ELMO/DOCK180 can be controlled for both spatially and temporally. Additionally, we report new data highlighting that DOCK proteins can form heterodimers, and we discuss possible mechanisms that could be implicated in controlling the ELMO activation state.
Key words: DOCK180, DOCK5, ELMO, autoinhibition, RBD, cell migration, cytoskeletal reorganization, GEF
Introduction
Dynamic remodeling of the actin cytoskeleton is indispensable for several biological tasks including cell polarity and directional migration, formation of a phagosome during bacterial/apoptotic cell engulfment and in myoblast:myoblast membrane fusion throughout the development of muscle fibers.1,2 Rho GTPases emerge as key components that filter signals from cell surface receptors and transduce the information to key regulators of the actin cytoskeleton. Among the Rho superfamily, Rac proteins, which are well recognized for their roles in shaping membrane ruffles and lamellipodia, are found at the crossroad of several pathways leading to actin cytoskeleton reorganization.
Like most small G proteins, Rac members cycle between GDP- and GTP-loaded states: only in their active GTP-loaded state are they in a conformation permissive for coupling to specific effectors, and, therefore, capable of initiating downstream signaling cascades. Two large classes of regulatory proteins take control of the activation state of Rho GTPases. First, the GTPase activating proteins (GAPs) bind to GTP-loaded Rho proteins to enhance their GTPase activity and thus favoring the inactive Rho-GDP state. Second, and conversely, Rho guanine exchange factors (GEFs), support nucleotide dissociation and as such promote reloading of the GTPases with GTP. The 84 GEFs for Rho GTPases can be subdivided into Dbl and DOCK families.3–6 The DOCK proteins consists of 11 atypical GEFs that are potent and highly specific activators of Rac or Cdc42.1,3 Recent studies in mice have uncovered important in vivo roles for DOCK180, DOCK5 and DOCK2 in myoblast fusion, cardiovascular development, osteoclast-mediated bone resorption and immune homeostasis.7–11 Recent human data has also closely linked deficiencies in DOCK8 to hyper IgE syndromes that can be corrected by transplantation of healthy hematopoietic stem cells in afflicted patients.12,13 Similarly, studies in cancer cells have highlighted that DOCK3, via its Rac GEF activity, promotes a mesenchymal type of movement14 while DOCK10, via its Cdc42 GEF activity, supports amoeboid migration.15 Clearly, these GEFs appear to play important biological functions and future studies will be important to fully understand the role of these GTPases regulators in development and diseases.
ELMO Proteins in the DOCK180/Rac Pathway
Initially, Ced-12 (worm ortholog of ELMO) was identified in a C. elegans screen aiming to uncover genes controlling engulfment of both necrotic and apoptotic cells.16 Later, Ced-12 was cloned and shown to participate in the same genetic cascade as Ced-2, Ced-5 and Ced-10 in mediating phagocytosis of apoptotic bodies and gonadal distal tip cell migration.17 The Ced-12 protein is evolutionarily conserved and is represented by 3 mammalian members: Engulfment and Motility (ELMO)-1, ELMO2 and ELMO3. Studies in mammalian cells suggest that DOCK180 and ELMO interact biochemically and cooperate to activate Rac for optimal phagocytosis of apoptotic cells and cell migration.18 ELMO proteins are characterized by a C-terminal atypical PH domain and proline-rich motifs that are implicated in binding to DOCK proteins (see below). At the N-terminus, ELMO proteins had, until recently (see below), no recognizable domains, but this portion of the protein was reported to bind activated RhoG, ERM proteins and BAI1.19–21 Located in the central region of ELMO proteins is a highly evolutionarily conserved “ELMO” domain (which we renamed ELM domain to avoid confusion with the protein nomenclature) of unknown function.
ELMO and DOCK180: Coming Together for Rac GTP-Loading?
Early studies in cell lines suggested that DOCK180 might not be a functional GEF by itself; instead, only when co-expressed with ELMO was robust Rac GEF activity observed.22–24 Mechanistically, the PH domain was proposed as a key structural feature in ELMO that does not bind to DOCK180 but instead cooperates in trans with the DOCK180 DHR-2 GEF domain by facilitating DOCK180:Rac interactions, and as such, promotes Rac GEF activity. One study also suggested that overexpression of ELMO1 in a plasmacytoma cell line enhanced Rac GTP-loading supporting the idea that ELMO can unmask Rac GEF activity.25 In marked contrast, several other studies demonstrated that expression of DOCK180 by itself, or its purified DHR-2 GEF domain, was sufficient for mediating GDP/GTP exchange on Rac.4,26,27 Likewise, other DOCK family members do not appear to require ELMO binding for their activity.4,5,28,29 Several reasons can account for these discrepancies. For one, coexpression of ELMO with DOCK180 greatly facilitates the accumulation of the DOCK180 protein in cells23 by preventing its degradation by the ubiquitin-proteasome system.30 This can explain in part why higher Rac activity can be observed when both ELMO and DOCK180 are coexpressed.23 Another justification could be that ELMO facilitates the co-localization of DOCK180 and Rac at the membrane and therefore indirectly endorses Rac GEF activity of this complex. The N-termini of ELMO proteins were indeed reported to harbor such membrane-targeting activity.18
The contradicting evidences in the literature have made it difficult to reconcile whether DOCK180 activity as a Rac GEF is ELMO-dependent (bipartite GEF model) or if ELMO plays additional vital roles during Rac signaling events downstream of the DOCK180/ELMO complex. To address this issue, we recently characterized in detail the mechanism of interaction between DOCK180 and ELMO1 and developed novel mutants of these proteins that fail to interact biochemically.31 Our mapping studies underlined a stretch of hydrophobic amino acids located on an atypical α-helical N-terminal extension of the ELMO1 PH domain that specifically interact with high affinity with hydrophobic residues positioned in a predicted α-helical region of DOCK180 flanking the SH3 domain. This domain in ELMO that binds DOCK180 directly was also reported to be active in promoting Rac activity by acting in trans to DOCK180.24 A second interaction interface was found to involve the SH3 of DOCK180 and the extreme C-terminal proline-rich motifs in ELMO. With the design of these novel mutants of ELMO incapable of binding DOCK180, we re-visited the role of ELMO in Rac GTP-loading. Our data distinctly suggest that ELMO is not required for Rac activation. Nevertheless, we found that the formation of an ELMO/DOCK180 physical complex is vital for Rac-induced cell polarization and migration. How then is ELMO factoring into DOCK180-mediated Rac signaling?
New Modular Domains in ELMO: Defining A Novel Autoregulatory Switch
In our pursuit to answer the question on how ELMO operates in Rac signaling, we took a reductionist approach and aimed to identify which fragments of ELMO are necessary to synergize with DOCK180 during Rac-induced cell polarization. Having established the C-terminal portion of ELMO1, encompassing amino acids 532–727, as the DOCK180-binding region, we and others found that this shorter fragment of ELMO is incapable of cooperating with DOCK180 in cell polarization (data not shown and reviewed in ref. 18), even when total Rac activation levels are comparable in all conditions. Since a panel of ELMO mutants lacking portions of the N-terminus also failed to promote cell polarization when co-expressed with DOCK180 (data not shown), we came to the conclusion that the whole N-terminus may contain the “activity” important for efficient Rac signaling downstream of DOCK/ELMO. We next explored in detail if any protein domains may be present in the ELMO N-terminus. As extensively described in our original report,32 we used multiple bioinformatics tools that analyze for structural features in ELMO proteins and this work disclosed three novel evolutionarily conserved domains in the ELMO superfamily.
Our first instructive observation from these in silico studies was the exposing of five predicted Armadillo Repeats (ARMs, approximately between residues 80–303 of ELMO1) that share homology to ARMs found in Dia-related formins (DRFs). Defined as central actin nucleators, the activity of DRFs is tightly regulated by a well-designed autoinhibition switch.33 Critical for this repression are the ARMs of DRFs, known as the Diaphanous Inhibitory Domain (DID), which directly interacts with a short C-terminal Diaphanous Auto-regulatory Domain (DAD) to maintain formins inactive. Our studies also led us to uncover a short DAD-like protein sequence in the ELMO family. The presence of domains sharing striking similarity to the auto-regulatory domains of Dia proteins led us to test the hypothesis that ELMO proteins may likewise be regulated via intramolecular contacts. Indeed, our experimental evidence supports a model whereby the N-terminal ARMs, renamed ELMO Inhibitory Domain (EID), can directly and specifically interact with the C-terminal region described above termed the ELMO Autoregulatory Domain (EAD). Furthermore, via the development of a mono-molecular biosensor, we found that disrupting the EID/EAD interaction in the context of full length ELMO resulted in important conformational changes in the protein.32 Together, these data led us to propose that ELMO proteins may exist in a “closed” conformation at basal state.
An additional defining structural feature of a subset of Dia-family proteins is the presence of a GTPase-binding activity flanking the DID domain on its N-terminal side.33 These domains can differ in terms of structure and effector binding in formins. In comparison to Dia1 binding to RhoA/C, FHOD1 has only been shown to bind with high affinity to Rac.34,35 These domains come in two flavors in DRFs, namely a GTPase-Binding Domain (GBD) for Dia and a Ras-Binding Domain (RBD) in FHOD family members. In these DRFs, these modules can collaborate during the autoinhibition switch of the formin's actin nucleation activity by specifically releasing this catalytic function in a space and timedependent manner. Release of the formin DID/DAD connection is proposed to come in the form of GTPase-binding to the formin's GTPase-binding region, although some recent data highlight that such interactions may only lead to partial release of autoinhibition (i.e., RhoA-binding to Dia1),36,37 or not at all (i.e., Rac1-GTP engaging to FHOD1).38
In the case of ELMO proteins, Katoh and colleagues had previously identified a RhoG-binding activity in a region flanking the DID domain,19 therefore suggesting that ELMO activation state may be regulated by engaging small GTPase(s) much like in formins. We found that the RhoG-binding site in ELMO structurally resembles the RBD of FHOD1 but not the GBD of Dia1. The RBD of ELMO also shows similarity to the RBDs present in c-Raf and PI3-Kinase. Endorsing the evolutionarily conserved nature of the RBD in ELMO proteins, we found that this RBD superfold is also present in its orthologs in Drosophila and C. elegans.39 In summary, ELMO proteins are equipped with RBD, EID and EAD domains that may confer them auto-regulatory properties.
Functionally, we demonstrated that disrupting the activity of either the EID or EAD in ELMO enhances Rac-dependent signaling.32 Interestingly, we also observed that abolishing the GTPase-binding activity of the ELMO RBD prevents membrane recruitment and Rac signaling. These data suggest that the ELMO RBD may play a function in both relieving autoinhibition and localization of the ELMO/DOCK complex. Such a scenario is reminiscent of Rac engaging to the RBD of FHOD1 to localize and activate the formin.38 One important future avenue of investigation will be to generate in vivo models where this regulatory switch is disturbed in order to fully appreciate the importance of the tight control it may provide to Rac signaling.
How is ELMO Activated in Cells?
While we demonstrated that “opening” of ELMO is integral for cell elongation and migration, and that RhoG binds the RBD and localizes ELMO to the membrane, it remains to be determined what signals lead to the unhinging of the closed ELMO conformation. At this time, the biological meaning of the RhoG/ELMO interaction is restricted to phagocytosis.40 Interestingly, RhoG is not required for DOCK180/Rac-mediated cell migration.41 Therefore, it is quite possible that a repertoire of unidentified GTPases is waiting to be unmasked as novel ELMO RBD partners. Multiple effector binding is a feature of many GBDs. As yet, no signature has been revealed that will allow us to identify which GTPase(s) will bind a given GBD. Therefore, a screen of all known Ras family GTPases against the ELMO RBD may uncover vital partners involved in opening the intramolecular ELMO complex. Currently, we are examining the entire Ras superfamily of GTPases against the ELMO1 RBD and ELMO1 full-length proteins via yeast two-hybrid experiments to identify potential candidates. Studies with isolated RBD domains can produce misleading data since additional domains in the effector protein are sometimes required for RBD engagement via the GTPase. This point is exemplified by the RBD of Tiam1 which additionally requires the presence of the DH-PH module to efficiently bind Rap.42 In this way, other domains of ELMO may be required by binding partners in order to engage the RBD.
For FHOD1, RBD engagement does not appear to directly promote the release of the DID/DAD interaction and additional experimental evidence suggests that this association does not result in the exposure of its actin nucleation catalytic activity.38,43 Instead, the major role of the RBD in FHOD1 appears to position this formin at the membrane where it will be fully activated by an unknown mechanism. This is also partly true for the formin Dia, where only a partial release of autoinhibition is seen with GTPase binding. It is suggested that additional factors are required to fully activate the formin. Therefore, it is possible that in ELMO, the RBD serves only to localize the protein to discrete areas of the cell and binding partners in the vicinity of the EID or EAD serve to maximally release the autoinihibited molecule. An example of this form of regulation is portrayed by the formin DAAM1 which binds Dvl at its C-terminus.44 One hypothesis would be that ELMO and DOCK180 exist as individual proteins that enter in a physical complex upon cell stimulation. However, we have found that endogenous ELMO and DOCK180 are always in a complex either in non-stimulated, integrin-stimulated or EGF-treated cells (Fig. 1 and data not shown). Therefore, we suggest that, endogenously in these complexes, ELMO bound to DOCK180 is awaiting a specific signal for autoinhibition relief. As such, it is possible that yet unidentified binders of the C-terminal region of ELMO will serve as a release mechanism. Moreover, it would be interesting to test already identified partners whose binding sites overlap with the ELMO RBD and EID regions (i.e.: IpgB1, BAI1 and ERM proteins21,45,46) for promotion of conformational changes in ELMO that induce or contribute toward ELMO autoinhibition relief. Finally, ELMO has been shown to be phosphorylated downstream of HCK signaling.47 It would be interesting to see whether ELMO phosphorylation could also lead to autoinhibition relief.43 The identification of novel ELMO partners, as well as, investigation into its post-translational modification(s) will increase our understanding of ELMO regulation.
Figure 1.

Constitutive interaction between DOCK180 and ELMO. Endogenous DOCK180 was immunoprecipitated using a DOCK180-specific antibody in suspension and fibronectin (FN)-stimulated serum-starved CHO LR73 cells at various timepoints and bound-endogenous ELMO was detected using an ELMO-specific antibody. p130Cas was immunoprecipitated as a control for adhesion-dependent stimulation and phosphorylated p130Cas was visualized with a phospho-specific antibody (pY).
One possibility, yet unexplored, is that the RBD of ELMO is acting as a sensor for selected GTPase(s) activation. In this model, the RBD would be poorly accessible, or exist in a low affinity state for binding its preferred GTPases, such as RhoG, when ELMO is in a closed conformation. When the concentration of active GTPases would reach a threshold level, they could then engage the RBD and fully expose a high affinity module that can then tightly anchor the ELMO/DOCK180 GEF complex at the membrane and trigger maximal Rac signaling. This type of regulation would also prevent promiscuous activation of the ELMO/DOCK180 by GTPases that are not themselves being actively activated by their own GEF or signaling event. It will be important in the future to measure the binding affinity of the RBD in both closed and open conformations of ELMO to test this hypothesis.
Open vs. Closed: What is ELMO Hiding?
The human network consists of six ELMO members divided into two groups based on primary sequence similarity and biological function(s). The one common feature between members is the ELM domain. While a subset of these proteins display DOCK180-binding and a role in Rac signaling pathways (ELMO1–3), the other half are suggested to display GAP activity toward Arf GTPases, specifically Arl2 (ELMOD1–3). Although not clearly demonstrated, this latter family's Arf GAP activity is likely to involve the ELM domain.48 This domain in the ELMO1–3 members shows no activity on Arf GTPases and has yet to demonstrate any GAP activity at all; thus far, not many GTPases have been tested against potential ELMO GAP activity.
Intriguingly, while mammalian, Drosophila and C. elegans ELMO orthologs have always been correlated with promoting migratory events, in Dictyostelium discoideum, the ELMO-like protein ElmoA is associated with a negative regulatory role during phagocytosis and cell migration.49 In terms of phylogeny, ElmoA lacks a PH domain and is thus closer to the ELMOD orthologs than to mammalian, fly and worm ELMOs.50 The reason for this deviant physiological output may reside in the fact that ElmoA could contain a yet undiscovered GAP activity that shuts off important GTPase(s) that stimulate cell polarization. One attractive model is that the autoinhibitory regulation of ELMO may be a means to mask regions host to untapped GAP activity (i.e., ELM domain). It is also possible that these masked areas have a completely novel role, such as, binding of Rac effectors or other proteins important for cytoskeletal transformation. Probing for specific ELM domain binding partners would answer some of the questions we have on the functions of ELMO.
We are particularly interested in the ELM region of ELMO because its primary sequence is highly conserved from worms to humans, and points to evolutionarily conserved functions. The fact that its binding surface is very likely masked at dormant states by autoregulation makes it even more intriguing. Clearly, the next big step will be to investigate the ELM domain with closer scrutiny; we postulate that discovering the function(s) of the ELMO ELM domain will be essential for understanding how the ELMO/DOCK180 complex regulates directed cell motility.
Potentiating the ELMO/DOCK Signaling Cascade
Research into DOCK proteins has brought forth the idea that oligomerization of these GEFs can lead to potentiation of GTPase activation. DOCK9 was the first of these to report dimerization in both endogenous and exogenous proteins.51 The dimerization is documented to occur through the DHR-2 domain in each molecule. The DHR-2 is the integral GEF activity domain, and Meller and colleagues determined that each molecule in the DOCK9 dimer is able to bind and GTP-load a separate Cdc42 GTPase.51 Most recently, the crystallization of the DOCK9 DHR-2 domain unveiled that homodimerization sites are distinct from Cdc42-binding sites leaving the GEF openly accessible to activate the GTPase.6 DOCK180 also demonstrates dimerization in cellulo.51 However, it has yet to be determined whether, as in DOCK9, the DHR-2 is responsible for dimer formation and if there is any functional consequences of dimerization.
Intriguingly, our work revealed that DOCK5 co-immunoprecipitates robustly with DOCK180 in cells, and is thus the first documented case of heterodimerization of DOCK proteins (Fig. 2). Moreover, via mass spectrometry of DOCK180-specific immunoprecipitates, we identified DOCK5 as a binding partner for DOCK180, making heterodimerization between DOCK GEFs highly probable at the endogenous level. This interaction needs to be investigated further to de-mystify its functional relevance. One possibility is that DOCK180/DOCK180, DOCK180/DOCK5 and DOCK5/DOCK5 complexes display different levels of GEF activity. Nonetheless, the fact that these DOCK families GEFs are able to oligomerize supports a model where DOCK proteins can “aggregate” in distinct areas of the cell and form multiprotein complexes with ELMO (Fig. 3). This accumulation of DOCK/ELMO complexes can possibly lead to a potentiation of localized GTPase activation and cytoskeletal restructuring. Interestingly, bringing together these heterodimeric DOCK GEFs would also increase the local concentration of ELMO proteins; one can imagine that they could start to interact in trans and regulate themselves their open/closed conformation (Fig. 3).
Figure 2.
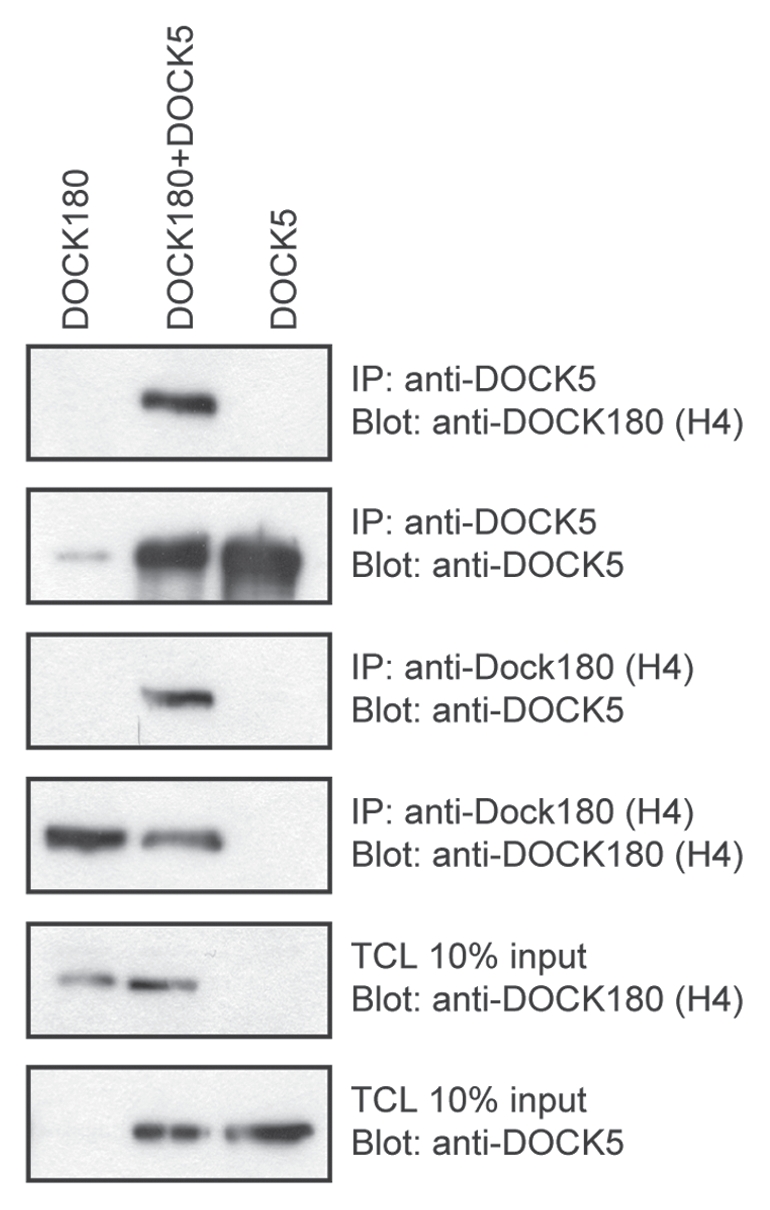
Heterodimerization of DOCK180 and DOCK5 GEFs. DOCK180 and DOCK5 were immunoprecipitated using a DOCK180-specific and DOCK5-specific antibody in HEK 293T cells lysates. Co-precipitated DOCK partners were detected using the indicated antibodies.
Figure 3.
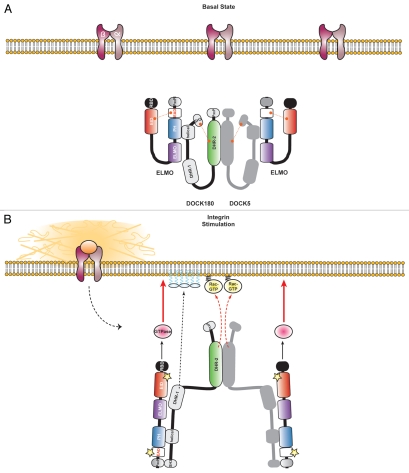
Proposed model for regulation of the ELMO/DOCK180 complex. (A) ELMO and DOCK are autoinhibited at basal levels. ELMO forms a constitutive complex with DOCK180 through its primary interface between the ELMO PH/DOCK180 helical regions. Additionally, at basal levels, ELMO is autoinhibited due to intramolecular EID/EAD interactions. Also proposed here is that, in unstimulated conditions, DOCK180 GEF activity is hindered through a DHR-2/SH3 interaction as described by Ravichandran and colleagues. Another level of complexity is added to the DOCK/ELMO complex by the possibility that DOCK proteins can oligomerize. This may permit for the “aggregation” of ELMO proteins and potentiation of signaling downstream of the DOCK180/ELMO complex. (B) Activating the ELMO/DOCK180 complex. Relief of ELMO autoinhibition occurs through cell stimulation and ELMO RBD engagement (via a GTPase or other novel binding partner). We propose that cell stimulation will lead to ELMO conformational changes that now facilitate DOCK180 SH3/ELMO PxxP interactions: this new interaction would compete with the SH3/DHR-2 interaction and facilitate the Rac GEF activity in DOCK proteins. The ELMO/DOCK180 complex is then anchored to the membrane for localized Rac activation (where DOCK180 DHR1-mediated membrane anchorage via phosphoinositides is vital). It is also possible that cell stimulation leads to ELMO binding of yet unknown partners that bind the EID or EAD and relieve autoinhibition (yellow stars). Thus, masked regions of ELMO, the ELM domain, become exposed. A few probable roles of the ELM include: (i) binding of Rac effectors or (ii) GAP activity on yet unidentified GTPases that control cell migration and/or directionality.
Conclusions and Perspectives
Study of the DOCK180/ELMO interaction has revealed an intricate relationship between the two proteins. It appears that tight regulation of the activity of the ELMO1/DOCK180 complex by masking protein-protein interaction sites or other functional domains is required to prevent promiscuous Rac signaling.
An interesting proposition involves the steric-inhibition model for DOCK180. This model suggests that a DOCK180 SH3/DHR-2 interaction blocks its Rac GEF activity at basal levels, and cell stimulation and ELMO binding to DOCK180 relieves this autoinhibition.52 We have shown here that ELMO and DOCK proteins are found to be in a stable complex and it is thus unlikely that the formation of new associations between the proteins would regulate their activity. Since the SH3 domain of DOCK180 additionally interacts with the proline-rich motifs of ELMO proteins, this interaction would be in direct competition with the steric inhibition created by DOCK180 SH3/DHR-2 domains. In our model, we suggest that in unstimulated conditions, ELMO and DOCK180 are already in a complex through its primary interaction interface, ELMO PH/DOCK180 80–153 (helical region) (Fig. 3). Additionally, DOCK proteins are able to oligomerize and accumulate ELMO and other binding partners in its vicinity. A stimulus will then lead to ELMO opening: this could potentially expose the proline-rich region of ELMO and allow it to bind the SH3 domain of DOCK180 resulting in concomitant relief of autoinhibition in the ELMO scaffold and the DOCK180 GEF (Fig. 3).
Acknowledgements
We thank Mélanie Laurin for critical reading of the manusctipt. We apologize to colleagues whose work we could not cite due to space constraints. J.F.C. is a recipient of a Junior Investigator award from the Fonds en Recherches et Santé du Québec (FRSQ). This work was funded by grants from the Canadian Institute of Health Research (CIHR) and Canadian Cancer Society to J.F.C.
Extra View to: Patel M, Margaron Y, Fradet N, Yang Q, Wilkes B, Bouvier M, et al. An evolutionarily conserved autoinhibitory molecular switch in ELMO proteins regulates Rac signaling. Curr Biol. 2010;20:2021–2027. doi: 10.1016/j.cub.2010.10.028.
References
- 1.Côté JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 2007;17:383–393. doi: 10.1016/j.tcb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell. 2009;17:310–322. doi: 10.1016/j.devcel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 4.Côté JF, Vuori K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J Cell Sci. 2002;115:4901–4913. doi: 10.1242/jcs.00219. [DOI] [PubMed] [Google Scholar]
- 5.Meller N, Irani-Tehrani M, Kiosses WB, Del Pozo MA, Schwartz MA. Zizimin1, a novel Cdc42 activator, reveals a new GEF domain for Rho proteins. Nat Cell Biol. 2002;4:639–647. doi: 10.1038/ncb835. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Zhang Z, Roe SM, Marshall CJ, Barford D. Activation of Rho GTPases by DOCK exchange factors is mediated by a nucleotide sensor. Science. 2009;325:1398–1402. doi: 10.1126/science.1174468. [DOI] [PubMed] [Google Scholar]
- 7.Laurin M, Fradet N, Blangy A, Hall A, Vuori K, Cote JF. The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Proc Natl Acad Sci USA. 2008;105:15446–15451. doi: 10.1073/pnas.0805546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanematsu F, Hirashima M, Laurin M, Takii R, Nishikimi A, Kitajima K, et al. DOCK180 is a Rac activator that regulates cardiovascular development by acting downstream of CXCR4. Circ Res. 2010;107:1102–1105. doi: 10.1161/CIRCRESAHA.110.223388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukui Y, Hashimoto O, Sanui T, Oono T, Koga H, Abe M, et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412:826–831. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 10.Kunisaki Y, Nishikimi A, Tanaka Y, Takii R, Noda M, Inayoshi A, et al. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J Cell Biol. 2006;174:647–652. doi: 10.1083/jcb.200602142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vives V, Laurin M, Cres G, Larrousse P, Morichaud Z, Noel D, et al. The Rac1 exchange factor Dock5 is essential for bone resorption by osteoclasts. J Bone Miner Res. 2011;26:1099–1110. doi: 10.1002/jbmr.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046–2055. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barlogis V, Galambrun C, Chambost H, Lamoureux-Toth S, Petit P, Stephan JL, et al. Successful allogeneic hematopoietic stem cell transplantation for DOCK8 deficiency. J Allergy Clin Immunol. 2011;128:420–422.e2. doi: 10.1016/j.jaci.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 15.Gadea G, Sanz-Moreno V, Self A, Godi A, Marshall CJ. DOCK10-mediated Cdc42 activation is necessary for amoeboid invasion of melanoma cells. Curr Biol. 2008;18:1456–1465. doi: 10.1016/j.cub.2008.08.053. [DOI] [PubMed] [Google Scholar]
- 16.Chung S, Gumienny TL, Hengartner MO, Driscoll M. A common set of engulfment genes mediates removal of both apoptotic and necrotic cell corpses in C. elegans. Nat Cell Biol. 2000;2:931–937. doi: 10.1038/35046585. [DOI] [PubMed] [Google Scholar]
- 17.Wu YC, Tsai MC, Cheng LC, Chou CJ, Weng NY. C. elegans CED-12 acts in the conserved crkII/DOCK180/Rac pathway to control cell migration and cell corpse engulfment. Dev Cell. 2001;1:491–502. doi: 10.1016/S1534-5807(01)00056-9. [DOI] [PubMed] [Google Scholar]
- 18.Grimsley CM, Kinchen JM, Tosello-Trampont AC, Brugnera E, Haney LB, Lu M, et al. Dock180 and ELMO1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J Biol Chem. 2004;279:6087–6097. doi: 10.1074/jbc.M307087200. [DOI] [PubMed] [Google Scholar]
- 19.Katoh H, Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424:461–464. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
- 20.Elliott MR, Zheng S, Park D, Woodson RI, Reardon MA, Juncadella IJ, et al. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature. 2010;467:333–337. doi: 10.1038/nature09356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimsley CM, Lu M, Haney LB, Kinchen JM, Ravichandran KS. Characterization of a novel interaction between ELMO1 and ERM proteins. J Biol Chem. 2006;281:5928–5937. doi: 10.1074/jbc.M510647200. [DOI] [PubMed] [Google Scholar]
- 22.Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/S0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 23.Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol. 2002;4:574–582. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- 24.Lu M, Kinchen JM, Rossman KL, Grimsley C, deBakker C, Brugnera E, et al. PH domain of ELMO functions in trans to regulate Rac activation via Dock180. Nat Struct Mol Biol. 2004;11:756–762. doi: 10.1038/nsmb800. [DOI] [PubMed] [Google Scholar]
- 25.Gotoh K, Tanaka Y, Nishikimi A, Inayoshi A, Enjoji M, Takayanagi R, et al. Differential requirement for DOCK2 in migration of plasmacytoid dendritic cells versus myeloid dendritic cells. Blood. 2008;111:2973–2976. doi: 10.1182/blood-2007-09-112169. [DOI] [PubMed] [Google Scholar]
- 26.Côté JF, Vuori K. In vitro guanine nucleotide exchange activity of DHR-2/DOCKER/CZH2 domains. Methods Enzymol. 2006;406:41–57. doi: 10.1016/S0076-6879(06)06004-6. [DOI] [PubMed] [Google Scholar]
- 27.Kiyokawa E, Hashimoto Y, Kobayashi S, Sugimura H, Kurata T, Matsuda M. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998;12:3331–3336. doi: 10.1101/gad.12.21.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watabe-Uchida M, John KA, Janas JA, Newey SE, Van Aelst L. The Rac activator DOCK7 regulates neuronal polarity through local phosphorylation of stathmin/Op18. Neuron. 2006;51:727–739. doi: 10.1016/j.neuron.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Namekata K, Enokido Y, Iwasawa K, Kimura H. MOCA induces membrane spreading by activating Rac1. J Biol Chem. 2004;279:14331–14337. doi: 10.1074/jbc.M311275200. [DOI] [PubMed] [Google Scholar]
- 30.Makino Y, Tsuda M, Ichihara S, Watanabe T, Sakai M, Sawa H, et al. Elmo1 inhibits ubiquitylation of Dock180. J Cell Sci. 2006;119:923–932. doi: 10.1242/jcs.02797. [DOI] [PubMed] [Google Scholar]
- 31.Komander D, Patel M, Laurin M, Fradet N, Pelletier A, Barford D, et al. An alpha-helical extension of the ELMO1 pleckstrin homology domain mediates direct interaction to DOCK180 and is critical in Rac signaling. Mol Biol Cell. 2008;19:4837–4851. doi: 10.1091/mbc.E08-04-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel M, Margaron Y, Fradet N, Yang Q, Wilkes B, Bouvier M, et al. An evolutionarily conserved autoinhibitory molecular switch in ELMO proteins regulates Rac signaling. Curr Biol. 2010;20:2021–2027. doi: 10.1016/j.cub.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schonichen A, Geyer M. Fifteen formins for an actin filament: a molecular view on the regulation of human formins. Biochim Biophys Acta. 2010;1803:152–163. doi: 10.1016/j.bbamcr.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Gasteier JE, Madrid R, Krautkramer E, Schroder S, Muranyi W, Benichou S, et al. Activation of the Rac-binding partner FHOD1 induces actin stress fibers via a ROCK-dependent mechanism. J Biol Chem. 2003;278:38902–38912. doi: 10.1074/jbc.M306229200. [DOI] [PubMed] [Google Scholar]
- 35.Westendorf JJ. The formin/diaphanous-related protein, FHOS, interacts with Rac1 and activates transcription from the serum response element. J Biol Chem. 2001;276:46453–46459. doi: 10.1074/jbc.M105162200. [DOI] [PubMed] [Google Scholar]
- 36.Lammers M, Rose R, Scrima A, Wittinghofer A. The regulation of mDia1 by autoinhibition and its release by Rho*GTP. EMBO J. 2005;24:4176–4187. doi: 10.1038/sj.emboj.7600879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose R, Weyand M, Lammers M, Ishizaki T, Ahmadian MR, Wittinghofer A. Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature. 2005;435:513–518. doi: 10.1038/nature03604. [DOI] [PubMed] [Google Scholar]
- 38.Schulte A, Stolp B, Schonichen A, Pylypenko O, Rak A, Fackler OT, et al. The human formin FHOD1 contains a bipartite structure of FH3 and GTPase-binding domains required for activation. Structure. 2008;16:1313–1323. doi: 10.1016/j.str.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Patel M, Margaron Y, Fradet N, Yang Q, Wilkes B, Bouvier M, et al. An evolutionarily conserved auto-inhibitory molecular switch in ELMO proteins regulates Rac signaling. Curr Biol. 2010;20:2021–2027. doi: 10.1016/j.cub.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.deBakker CD, Haney LB, Kinchen JM, Grimsley C, Lu M, Klingele D. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr Biol. 2004;14:2208–2216. doi: 10.1016/j.cub.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 41.Meller J, Vidali L, Schwartz MA. Endogenous RhoG is dispensable for integrin-mediated cell spreading but contributes to Rac-independent migration. J Cell Sci. 2008;121:1981–1989. doi: 10.1242/jcs.025130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167:111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeya R, Taniguchi K, Narumiya S, Sumimoto H. The mammalian formin FHOD1 is activated through phosphorylation by ROCK and mediates thrombin-induced stress fibre formation in endothelial cells. EMBO J. 2008;27:618–628. doi: 10.1038/emboj.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W, Sato A, Khadka D, Bharti R, Diaz H, Runnels LW, et al. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci USA. 2008;105:210–215. doi: 10.1073/pnas.0707277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Handa Y, Suzuki M, Ohya K, Iwai H, Ishijima N, Koleske AJ, et al. Shigella IpgB1 promotes bacterial entry through the ELMO-Dock180 machinery. Nat Cell Biol. 2007;9:121–128. doi: 10.1038/ncb1526. [DOI] [PubMed] [Google Scholar]
- 46.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 47.Yokoyama N, deBakker CD, Zappacosta F, Huddleston MJ, Annan RS, Ravichandran KS, et al. Identification of tyrosine residues on ELMO1 that are phosphorylated by the Src-family kinase Hck. Biochemistry. 2005;44:8841–8849. doi: 10.1021/bi0500832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowzard JB, Cheng D, Peng J, Kahn RA. ELMOD2 is an Arl2 GTPase-activating protein that also acts on Arfs. J Biol Chem. 2007;282:17568–17580. doi: 10.1074/jbc.M701347200. [DOI] [PubMed] [Google Scholar]
- 49.Isik N, Brzostowski JA, Jin T. An Elmo-like protein associated with myosin II restricts spurious F-actin events to coordinate phagocytosis and chemotaxis. Dev Cell. 2008;15:590–602. doi: 10.1016/j.devcel.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Brzostowski JA, Fey P, Yan J, Isik N, Jin T. The Elmo family forms an ancient group of actin-regulating proteins. Commun Integr Biol. 2009;2:337–340. doi: 10.4161/cib.2.4.8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meller N, Irani-Tehrani M, Ratnikov BI, Paschal BM, Schwartz MA. The novel Cdc42 guanine nucleotide exchange factor, zizimin1, dimerizes via the Cdc42-binding CZH2 domain. J Biol Chem. 2004;279:37470–37476. doi: 10.1074/jbc.M404535200. [DOI] [PubMed] [Google Scholar]
- 52.Lu M, Kinchen JM, Rossman KL, Grimsley C, Hall M, Sondek J, et al. A Steric-inhibition model for regulation of nucleotide exchange via the Dock180 family of GEFs. Curr Biol. 2005;15:371–377. doi: 10.1016/j.cub.2005.01.050. [DOI] [PubMed] [Google Scholar]