Abstract
BRAF and RAS are often mutated in cutaneous melanoma and both mutations stimulate the MAPK pathway. However the biological consequences of BRAF and NRAS mutations are different because when RAS is mutated in melanoma, cells use CRAF rather than BRAF to activate MEK/ERK. The mechanism of this BRAF to CRAF isoform switching in response to oncogenic RAS has recently been described. Activation of the MAPK pathway, which results from a mutation of NRAS, induces phosphorylation of BRAF on serine 151 by ERK which prevents its binding to NRAS. To circumvent this negative feedback inhibition of BRAF, melanoma cells containing a mutation of RAS use CRAF to activate MEK/ERK. However, because the cAMP pathway in melanocytes constitutively inhibits CRAF, RAF isoform switching in melanoma is accompanied by an inhibition of the cAMP pathway. This inhibition is due to an increase in phosphodiesterase activity, which degrades cAMP thereby preventing inhibition of CRAF by PKA. These data highlight the importance of CRAF downstream of oncogenic Ras in tumor development.
Key words: RAS, BRAF, CRAF, cAMP, melanoma, PDE, ERK, therapy
Melanocytes are pigment-producing cells localized in the basal layer of the epidermis. Their proliferation, differentiation and migration are regulated by several signaling pathways, simultaneously activated by growth factors and hormones released into the local skin micro-environment. Two major signaling pathways that are activated simultaneously in melanocytes are the cyclic AMP (cAMP) pathway and the MAPK (mitogen activated protein kinase) pathway and interactions between these pathways are essential for regulating melanocyte fate.
cAMP is a second messenger produced, in melanocytes, by the binding of melanocytic hormones such as α-MSH (α-melanocyte stimulating hormone) to the melanocortin receptor type 1 (MC1R). MC1R is a seven-transmembrane domain receptor coupled to hetero-trimeric G proteins. Activation by its ligands induces an increase in cAMP content in melanocytes, which activates the protein kinase A (PKA), which in turn phosphorylates and activates the transcription factor CREB. CREB stimulates the transcription of microphthalmia (MITF), a transcription factor that plays a key role in the differentiation of melanocytes through induction of the transcription of many genes associated with melanin synthesis or melanosome function (TYR, TYRP1, DCT, RAB27A and GPR143).1,2 The cAMP pathway is regulated in space and time by phosphodiesterases (PDE), enzymes that degrade cAMP. Among the 11 different families of PDE, 8 are capable of hydrolyzing cAMP. Each family comprises several genes that, due to alternative splicing, generate over 30 different isoforms. Several kinases phosphorylate PDE enzymes to regulate their activity, allowing the PDEs to play a central role in the interaction between the cAMP pathway and other intracellular signaling pathways.3,4
In physiological conditions, the MAPK pathway is activated by growth factors binding to their surface receptor tyrosine kinase (RTK) and the transmission of signals through the small GTPase RAS. In its active form, bound to GTP, RAS proteins activate a number of effectors and in particular, the serine/threonine kinases of the RAF family. There are three RAF isoforms: ARAF, BRAF and CRAF (also known as RAF1), which activate MAP kinase kinases (MEK), which in turn activate the MAP kinases (ERK). ERK has many substrates, which are mainly involved in regulating the proliferation of melanocytes.5,6 While the MAPK pathway is activated, in melanocytes, by growth factors such as SCF (Stem Cell Factor), EGF (Epidermal Growth Factor), FGF (Fibroblast Growth Factor) or HGF (Hepatocyte Growth Factor), this pathway is constitutively activated in melanoma due to the presence of activating mutations of BRAF or NRAS. BRAF and NRAS oncogenes are mutated in respectively 50% and 20% of cutaneous melanoma. These mutations are mutually exclusive because their oncogenic activity is, in both cases, linked to stimulation of the MAPK pathway.7,8
Because both cAMP and MAP kinase pathways are activated simultaneously in melanocytes under physiological conditions, these cells provide an excellent model for studying the interaction between both pathways.9 Constitutive activation of the cAMP pathway in melanocytes leads to phosphorylation and inactivation of CRAF by PKA, which is essential to suppress the oncogenic potential of CRAF in these cells.10 Therefore activation of RTKs by growth factors stimulates the MAP kinase pathway through BRAF in melanocytes. The importance of BRAF in melanocytic cells is emphasized by the fact that 50% of melanoma contains an oncogenic mutation of this kinase, whereas the other RAF family kinases are never mutated in melanoma.11,12 Although these data suggest that CRAF does not play a major role in melanoma, recent results have highlighted the importance of CRAF in the activation of the MAPK pathway in melanoma, under three conditions.
Melanoma Containing a Low Activity BRAF Mutant
Although most studies on the role of BRAF in melanoma have focused on the BRAF V600E mutation, several other mutations in the BRAF gene have been identified.13 Many of these mutants show a lower BRAF kinase activity than that of the V600E BRAF mutant. Although, these mutants were classified as having low activity, when expressed in COS-1 cells these low activity mutants are able of activating the MAPK pathway by directly binding to and activating CRAF.14 Interestingly, targeting CRAF in melanoma cells expressing a low activity BRAF mutant induces their death by apoptosis suggesting that CRAF is a potential therapeutic target in this group of melanoma.15
Mechanism of Resistance to BRAF Inhibitors
With the prevalence of the BRAF V600E mutation in several tumors including melanoma, specific inhibitors of the mutant protein V600E BRAF have recently been developed. Inhibitors of BRAF such as PLX4032 (Roche/Plexxikon) and GSK2118436 (GlaxoSmithKline) have had impressive results on metastatic melanoma carrying the V600E BRAF mutation.16,17 However, these exciting clinical results were weakened by the fact that resistance appeared quickly and therefore therapeutic responses were only transient. Several groups have identified numerous mechanisms of resistance to BRAF inhibitors through in vitro experiments. Interestedly, this resistance is never associated with additional mutations in BRAF but are linked to alterations of either other oncogenes (NRAS, PDGFR, IGF-1R…) within the MAPK pathway or proteins involved in other signaling pathways (PTEN).18–21 Interestingly, a proportion of this resistance to BRAF inhibitors involves activation of the CRAF kinase, which is relatively resistant to these inhibitors. Therefore, targeting CRAF and BRAF together in melanoma could delay the emergence of resistance to BRAF inhibitors.
Melanoma Containing a Mutation of RAS
Although BRAF activates the MAPK pathway in melanocytes, in melanoma harboring a mutation of RAS there is a switch of RAF isoform usage and thus CRAF activates the MAPK pathway.10 The inability of BRAF to activate the MAPK pathway in RAS mutated melanoma is due to a permanent negative feedback regulation preventing its association with RAS. BRAF is phosphorylated on S151 near its RAS Binding Domain inhibiting the RAS/BRAF complex interaction.22 Ritt and colleagues have shown that BRAF is a substrate of activated ERK which phosphorylates it on four sites: S151, T401, S750 and T753. Whereas S151 phosphorylation promotes the dissociation of the RAS/BRAF complex, T401, S750 and T753 phosphorylation control CRAF/BRAF hetero-dimerization.23,24 The negative feedback regulation of BRAF by ERK described by Ritt et al. prevents BRAF over-activation after mitogen activation. Phosphorylated BRAF is recycled to a signaling competent state by the combined action of PP2A and Pin-1 prolyl-isomerase.24,25 However, in melanoma bearing an oncogenic RAS, BRAF phosphorylation on S151 is very stable and persists for several hours after inhibition of ERK, suggesting that ERK phosphorylated BRAF is not recycled. This persistence of phosphorylated BRAF seems specific of melanoma cells as, in COS cells, co-expression of oncogenic NRAS with BRAF induces dephosphorylation of BRAF on S151, allowing it to bind NRAS. Remarkably, CRAF, which binds RAS and activates the MAPK pathway in melanoma cells, seems insensitive to feedback inhibition.22 It seems that PP2A or Pin1 target BRAF and CRAF differently in melanoma. To overcome this inhibition of BRAF by ERK, melanoma cells have developed two strategies: (i) approximately 50% of melanoma cells have acquired an activating mutation in the BRAF gene (usually V600E), which makes the BRAF kinase activity independent of RAS. (ii) In melanoma bearing a mutation in RAS (approximately 20% of melanoma), the cells switch RAF isoform usage, using CRAF to activate the MAPK pathway. In consequence, inhibition of CRAF in melanocytes greatly reduces the ability of oncogenic RAS to transform them.22 However, to use CRAF to activate the MAPK pathway, these melanoma cells need to inactivate the cAMP pathway, which, in melanocytes, constitutively inhibits CRAF. In melanocytes transformed by oncogenic RAS and in melanoma cell lines mutated on RAS, melanocytic hormones such as α-MSH can no longer activate the cAMP pathway.10 However, this inhibition can be overcome when PDE activity is inhibited and in particular the activity of enzymes of the PDE4 family.22 Inhibiting the expression or activity of PDE4 enzymes greatly reduces the ability of oncogenic RAS to transform melanocytes highlighting the physiological importance of PDE4 in melanocyte transformation.
An important consequence of these results is that inhibition of the cAMP pathway is necessary for proliferation of melanoma cells mutated on RAS. Indeed, reactivation of the cAMP pathway using rolipram (a PDE4 inhibitor) in combination with a low dose of forskolin (an activator of adenylyl cyclase) induced a marked decrease in the proliferation of melanoma cell lines but not of melanocytes, associated with induction of apoptosis.22 These data suggest that enzymes of the PDE4 family are interesting new therapeutic targets in melanoma mutated on RAS. This hypothesis is interesting because this group of melanoma is resistant to BRAF inhibitors currently in clinical development.26–29 In addition these melanoma are relatively resistant to MEK inhibitors.30 However, there is currently much interest in the development of selective PDE4 inhibitors for the treatment of asthma, lung inflammation, rheumatoid arthritis and brain tumors.31,32 These inhibitors could be used to increase intracellular cAMP in melanoma and hence inhibit proliferation of melanoma cells mutated on RAS and, more generally, of all melanoma cells using CRAF to activate the MAPK pathway.
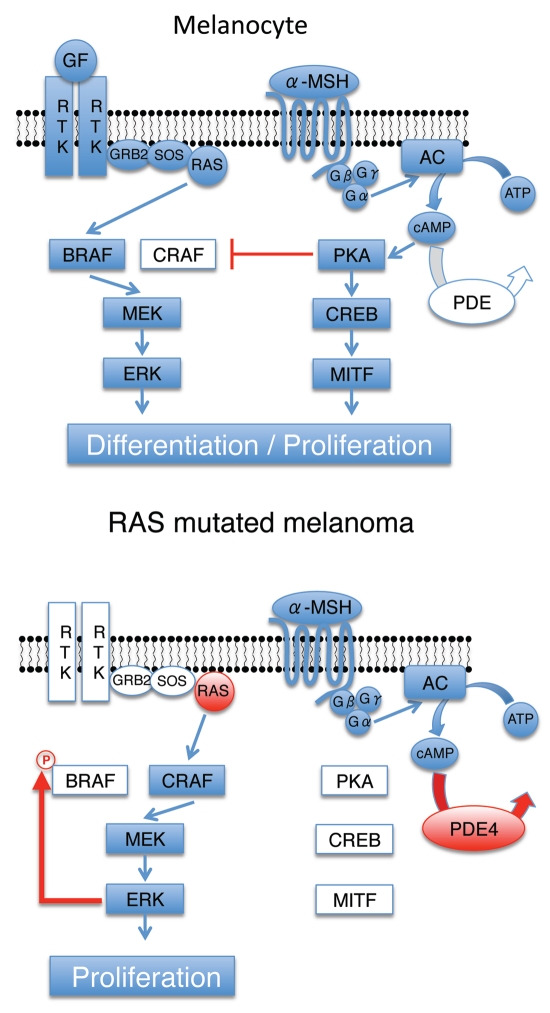
Until recently, the role of the individual RAF kinases in cancer cells bearing oncogenic RAS had not been fully explored. Two recent papers agree with our data in highlighting the importance of CRAF downstream of oncogenic RAS in tumor development. Using Cre-recombinase/loxP technology, two groups were able to selectively delete BRAF or CRAF in the lungs of mice expressing oncogenic KRAS.33,34 Both papers showed that deletion of BRAF did not affect tumor formation, whereas CRAF deletion significantly caused a reduction in tumor development.33,34 Therefore in RAS mutated melanoma and lung cancers, BRAF is unable to compensate for CRAF in relaying signals from oncogenic RAS to MEK. Altogether these data provide evidence that CRAF is a therapeutic target in oncogenic RAS driven cancer, supporting the need for the development of CRAF selective drugs as therapeutic agents (Fig. 1).
Figure 1.
Change in RAF isoform usage in response to a RAS mutation. In melanocytes, activation of cAMP not only stimulates melanogenesis but also inhibits the kinase CRAF. Therefore, BRAF activates the MAPK pathway downstream of RTKs in these cells. In melanomas containing a mutation of RAS, BRAF kinase is phosphorylated by ERK inhibiting its interaction with RAS. Hence, it is CRAF that activates the MAPK pathway downstream of RAS. Overexpression of PDE4 degrades cAMP preventing inhibition of CRAF by PKA. α-MSH: melanocyte stimulating hormone; MC1R: melanocortin-1 receptor, AC: adenylyl cyclase, PDE: phosphodiesterase, PKA: protein kinase A, CREB: cAMP responsive element binding protein, MITF: microphtalmia associated transcription factor; GF: growth factor; RTK: receptor tyrosine kinase.
Acknowledgements
This work was funded by the French Institut National de la Santé et de la Recherche Médicale, Université Paris Diderot, Sorbonne Paris Cité, Société Française de Dermatologie, Ligue Contre le Cancer (Comité du Val de Marne) and the French Institut National du Cancer (INCa 2007-1-PL7).
Extra View to: Marquette A, André J, Bagot M, Bensussan A, Dumaz N. ERK and PDE4 cooperate to induce RAF isoform switching in melanoma. Nat Struct Mol Biol. 2011;18:584–591. doi: 10.1038/nsmb.2022.
References
- 1.Lomas J, Martin-Duque P, Pons M, Quintanilla M. The genetics of malignant melanoma. Front Biosci. 2008;13:5071–5093. doi: 10.2741/3065. [DOI] [PubMed] [Google Scholar]
- 2.Cheli Y, Ohanna M, Ballotti R, Bertolotto C. Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment Cell Melanoma Res. 2009;23:27–40. doi: 10.1111/j.1755-148X.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- 3.Baillie GS, Scott JD, Houslay MD. Compartmentalisation of phosphodiesterases and protein kinase A: opposites attract. FEBS Lett. 2005;579:3264–3270. doi: 10.1016/j.febslet.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 4.Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Maurer G, Tarkowski B, Baccarini M. Raf kinases in cancer-roles and therapeutic opportunities. Oncogene. 2011;30:3477–3488. doi: 10.1038/onc.2011.160. [DOI] [PubMed] [Google Scholar]
- 6.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 7.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 8.Platz A, Egyhazi S, Ringborg U, Hansson J. Human cutaneous melanoma; a review of NRAS and BRAF mutation frequencies in relation to histogenetic subclass and body site. Mol Oncol. 2008;1:395–405. doi: 10.1016/j.molonc.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumaz N, Marais R. Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the anniversary prize of the Gesellschaft fur Biochemie und Molekularbiologie Lecture delivered on 5 July 2003 at the Special FEBS Meeting in Brussels. FEBS J. 2005;272:3491–3504. doi: 10.1111/j.1742-4658.2005.04763.x. [DOI] [PubMed] [Google Scholar]
- 10.Dumaz N, Hayward R, Martin J, Ogilvie L, Hedley D, Curtin JA, et al. In Melanoma, RAS Mutations Are Accompanied by Switching Signaling from BRAF to CRAF and Disrupted Cyclic AMP Signaling. Cancer Res. 2006;66:9483–9491. doi: 10.1158/0008-5472.CAN-05-4227. [DOI] [PubMed] [Google Scholar]
- 11.Emuss V, Garnett M, Mason C, Marais R. Mutations of C-RAF are rare in human cancer because C-RAF has a low basal kinase activity compared with B-RAF. Cancer Res. 2005;65:9719–9726. doi: 10.1158/0008-5472.CAN-05-1683. [DOI] [PubMed] [Google Scholar]
- 12.Marquette A, Bagot M, Bensussan A, Dumaz N. Recent discoveries in the genetics of melanoma and their therapeutic implications. Arch Immunol Ther Exp (Warsz) 2007;55:363–372. doi: 10.1007/s00005-007-0043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garnett MJ, Marais R. Guilty as charged; B-RAF is a human oncogene. Cancer Cell. 2004;6:313–319. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Garnett MJ, Rana S, Paterson H, Barford D, Marais R. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol Cell. 2005;20:963–969. doi: 10.1016/j.molcel.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Smalley KS, Xiao M, Villanueva J, Nguyen TK, Flaherty KT, Letrero R, et al. CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations. Oncogene. 2009;28:85–94. doi: 10.1038/onc.2008.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vultur A, Villanueva J, Herlyn M. Targeting BRAF in advanced melanoma: a first step toward manageable disease. Clin Cancer Res. 2011;17:1658–1663. doi: 10.1158/1078-0432.CCR-10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang CC, Lai F, Thorne RF, Yang F, Liu H, Hersey P, et al. MEK-independent survival of B-RAFV600Emelanoma cells selected for resistance to apoptosis induced by the RAF inhibitor PLX4720. Clin Cancer Res. 2011;17:721–730. doi: 10.1158/1078-0432.CCR-10-2225. [DOI] [PubMed] [Google Scholar]
- 19.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paraiso KHT, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, et al. PTEN Loss Confers BRAF Inhibitor Resistance to Melanoma Cells through the Suppression of BIM Expression. Cancer Res. 2011;71:2750–2760. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 2011;29:3085–3096. doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marquette A, Andre J, Bagot M, Bensussan A, Dumaz N. ERK and PDE4 cooperate to induce RAF isoform switching in melanoma. Nat Struct Mol Biol. 2011;18:584–591. doi: 10.1038/nsmb.2022. [DOI] [PubMed] [Google Scholar]
- 23.Brummer T, Naegele H, Reth M, Misawa Y. Identification of novel ERK-mediated feedback phosphorylation sites at the C-terminus of B-Raf. Oncogene. 2003;22:8823–8834. doi: 10.1038/sj.onc.1207185. [DOI] [PubMed] [Google Scholar]
- 24.Ritt DA, Monson DM, Specht SI, Morrison DK. Impact of Feedback Phosphorylation and Raf Heterodimerization on Normal and Mutant B-Raf Signaling. Mol Cell Biol. 2010;30:806–819. doi: 10.1128/MCB.00569-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dougherty MK, Muller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD, et al. Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell. 2005;17:215–224. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 26.Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, et al. PLX4032, a selective BRAFV600Ekinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAFWT melanoma cells. Pigment Cell Melanoma Res. 2010;23:190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 28.Heidorn SJ, Milagre M, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-Dead BRAF and Oncogenic RAS Cooperate to Drive Tumor Progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Byrne PM, Gauvreau G. Phosphodiesterase-4 inhibition in COPD. Lancet. 2009;374:665–667. doi: 10.1016/S0140-6736(09)61538-5. [DOI] [PubMed] [Google Scholar]
- 32.Sengupta R, Sun T, Warrington NM, Rubin JB. Treating brain tumors with PDE4 inhibitors. Trends Pharmacol Sci. 2011;32:337–344. doi: 10.1016/j.tips.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blasco RB, Francoz S, Santamaria D, Canamero M, Dubus P, Charron J, et al. c-Raf, but not B-Raf, is essential for development of K-Ras oncogene-driven non-small cell lung carcinoma. Cancer Cell. 2011;19:652–663. doi: 10.1016/j.ccr.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karreth FA, Frese KK, DeNicola GM, Baccarini M, Tuveson DA. C-Raf Is Required for the Initiation of Lung Cancer by K-RasG12D. Cancer Discovery. 2011;1:128–133. doi: 10.1158/2159-8290.CD-10-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]