Abstract
Mice showing mosaic expression of an appropriate marker gene that is activated during development provide simple tools for investigating cell lineages. We used the mosaic β-galactosidase staining patterns in adrenal cortices of 21OH/LacZ transgenic mice to study both organogenesis and maintenance of the adult tissue. Randomly orientated mosaic patterns present in embryonic day 14.5 (E14.5) adrenals changed progressively during the perinatal period from discrete spots, via patches and radial arrays, to radial stripes, which first emerged between postnatal days 0 and 7 (P0 and P7). The mosaic radial stripe pattern was fully established by P21 and remained unchanged throughout the adult period (8–52 weeks). The mouse adrenal gland grew continuously between E14.5 and P21, including the period during which stripes emerge. Ki67-positive, proliferative cells in the adrenal cortex were mainly localized to the outer cell layers between E18.5 and P3. By P10, cell proliferation had increased, and the proliferative region had expanded but was still mainly confined to the outer cortex. Correlation of changes in mosaic patterns in 21OH/LacZ adrenal cortices with the locations of adrenocortical cell proliferation suggest that the radial stripes arise by edge-biased growth during the perinatal period, even if they are maintained by stem cells in adults. The stability of the adult stripe pattern suggests that stem cell function is unchanged between 8 and 52 weeks.
Key words: adrenal cortex, development, organogenesis, edge-biased growth, mosaic, lineage, 21OH/LacZ transgene
Introduction
The mammalian endocrine adrenal gland is composed of two different tissues with distinct developmental origins: the mesoderm-derived outer steroidogenic cortex and the neuroectoderm-derived chromaffin cells of the inner medulla, which produce catecholamines and neuropeptides. The adult adrenal cortex is surrounded by a mesenchymal capsule and subdivided into three principal concentric biochemically and morphologically distinct zones of steroid-synthesizing cells. The outer zona glomerulosa (ZG) synthesizes the mineralocorticoid aldosterone; the middle zona fasciculata (ZF) produces the glucocorticoid cortisol (corticosterone in mice and rats), and the inner zona reticularis (ZR) bordering the medulla makes C19 steroids, the so-called adrenal androgens in humans and some primates, though not in rats and mice.
In common with other species, the three principal adult mouse adrenocortical zones each display a characteristic morphological cellular arrangement, although zonal boundaries can sometimes be indistinct, and their thickness, particularly of the ZR, varies considerably between different mouse strains.1–3 The small basophilic cells of the ZG have little cytoplasm and are arranged in globular arched-like structures. Eosinophilic cells of the ZF contain abundant cytoplasmic lipid droplets, the substrate for steroid hormone synthesis, and are arranged in columns. Cells of the ZR retain some lipid but display a characteristically compacted morphology. Early studies identified a morphologically distinct zona intermedia (ZI) in the rat adrenal cortex, located at the junction between the ZG and ZF.4,5 Subsequently, this zone was shown to lack expression of the terminal steroidogenic enzymes 11β-hydroxylase (Cyp11b1) and aldosterone synthase (Cyp11b2) and has therefore been termed the undifferentiated zone (ZU).5–7 In mouse, however, the presence of a ZU has yet to be reliably demonstrated.
Development of the murine adrenal gland has been well-described in references 3 and 8–15 and progresses through the following stages: (1) formation of an adrenogenital primordium from a group of condensing coelomic epithelial cells expressing the key steroidogenic transcription factor SF-1 (mouse embryonic days 8.5–9.5; E8.5–E9.5); (2) dorso-medial separation of distinct adrenal and gonadal primordia (mouse E10.5–E11.5); (3) commencement of fetal adrenal growth and expression of SF-1-dependent adrenal-specific steroidogenic enzymes, e.g., steroid 21-hydroxylase, Cyp21a1 (mouse E11.5–E12.5); (4) migration of neural crest cells into the developing adrenal gland to form the medulla (mouse E12.5–E13.0); (5) adrenal capsule formation from mesenchymal cells (mouse E13.5–E16.5); (6) expansion of the fetal cortex from inner fetal cortical progenitors and initiation of the definitive (adult) cortex from peripherally located fetal cortical progenitor cells, which give rise to a distinct adult lineage from around E14.5; (7) resolution of the three definitive adrenocortical zones (late gestation from around E16.5 onwards in mice); (8) formation of the relatively short-lived X zone from the fetal zone adjacent to the medulla (from mouse postnatal day 10; P10); (9) degeneration of the X zone (at puberty in male mice, during the first pregnancy in females and later in virgin females); (10) normal physiological function of the adrenal cortex from early adulthood at around P40–50.
Though currently less well-defined for the mouse, the adult rat adrenal cortex is maintained by a balance of cell proliferation in the outer cortex, centripetal displacement of cells16,17 and cell death in the ZR close to the medulla boundary.10,18,19 Nevertheless, it is still not clear whether (1) the adult adrenocortical zones are maintained by independent proliferation of cells within a zone, (2) the ZG and ZF/ZR are maintained separately by stem cells located at the border between the ZG and ZF (ZI/ZU in the rat), or (3) the entire cortex is maintained by centripetal migration of cells from a peripheral stem cell population located either within the capsule, or the subcapsular region of the ZG.20,21
A recent study has suggested a variant of the third possibility involving two populations of embryonic adrenocortical progenitors, both of which are capable of generating all the steroidogenic cell types in the adrenal cortex. This new model suggests that the primary progenitor cells are relatively undifferentiated steroidogenic cells located in the outer cortex, expressing Shh. These primary progenitors induce non-steroidogenic cells in the adjacent capsule and mesenchyme that express Gli1 (which encodes a transcription factor whose activity is regulated by Shh) to become secondary progenitors.22 Lineage analysis with tamoxifen-inducible reporters showed that labeling of Shh-expressing cells in the outer cortex at E14.5 gave rise to postnatal clones of labeled cells extending from the periphery almost to the medulla by 28 d after reporter induction. Crucially, however, while cell labeling was initiated at E14.5, shortly after the adult adrenocortical lineage becomes established,15 this is likely to be well before adult adrenocortical stem cells are activated. Thus, it is possible that clonal lineages, spanning most of the cortex, include cells produced both before and after stem cells are activated. Therefore, it will be important to determine whether the radial pattern of clonal lineages is established before or after centripetal cell migration and adult tissue maintenance begins.
Chimeras and mosaics provide a simple means of marking two genetically distinct populations of cells early in development and, so, offer the opportunity to investigate cell lineages both during organogenesis and in the adult. Experiments with chimeric rats have shown that, although the discrete adult adrenocortical zones are arranged concentrically around the central medulla, radial lineages cross all three zones and appear as stripes of labeled cells.23,24 Similar radial stripes have also been seen in mouse chimeras and X-inactivation mosaics25,26 as welll as in 21OH/LacZ transgenic mice.25 In the 21OH/LacZ transgenic mouse, the proximal 6.4 kb mouse steroid 21OH A gene promoter directs mosaic expression of a LacZ reporter in steroidogenic cells to give a radial stripe pattern of β-galactosidase (β-gal) staining spanning all three zones of the adrenal cortex.27 Mosaic transgene expression in these mice probably involves a stochastic “position effect” mechanism, such that the transgene is activated during development in only a proportion of the cells expressing the endogenous 21OH promoter.28 Thus, descendents of transgene-expressing (β-gal-positive) and transgene-silent (β-gal-negative) cells form clonal cell lineages, which can be distinguished by β-gal reporter staining (blue or unstained, respectively). The qualitative and quantitative similarity between patterns in adult adrenals between mouse chimeras, X-inactivation mosaics and 21OH/LacZ mosaics25 suggests that 21OH/LacZ mosaicism is also lineage-based and can be used to study steroidogenic cell lineage relationships in the mouse adrenal cortex, with the advantage that only steroidogenic cells will be labeled by the LacZ reporter.25
Previous work on adrenal organogenesis with 21OH/LacZ transgenic mice showed that the β-gal-stained cells in adrenal cortices were arranged as randomly orientated mosaic patterns at fetal stages (E15.5–16.5) rather than radial stripes, as seen in adults,27 implying that a patch-to-stripe transition occurs at some time during development. The lineage analysis experiments described above in reference 22, showed that cells in the outer cortex labeled at E14.5 can produce radial lineages spanning the cortex. However, it is not known whether radial adrenocortical cell lineages are established during organogenesis (as described for the retinal pigment epithelium in mosaics and chimeras29–32), or if radial stripes only arise as a consequence of stem cell activation (as described for the corneal epithelium33). It is important to distinguish between these possibilities for the adrenal cortex, because it has implications for the different hypothetical mechanisms proposed for organogenesis and maintenance of the three biochemically distinct adrenocortical zones. The aims of this study were, therefore, to identify when and how radial adrenocortical clonal lineages are established. We also sought to determine whether radial stripe patterns, once established, are maintained in the adult adrenal cortex, or if age-related changes occur that might suggest a decline in stem cell function similar to that reported for the corneal epithelium.34
Results
Changes in mosaic β-gal staining patterns during 21OH/LacZ adrenal gland development.
Typical examples of mosaic β-gal staining patterns in developing (E14.5 to P21) and adult 21OH/LacZ adrenal glands are shown in Figure 1. The “classical” adult pattern of radial β-gal stripes was fully established by P21 but was preceded by a succession of mosaic staining pattern combinations, covering the transition from embryonic patches to adult stripes. These staining patterns were classified according to shape, symmetry and orientation: (1) single spot, (2) non-radial patch, (3) radial array of spots, (4) radially elongated patch and (5) radial stripe, as described in the Materials and Methods.
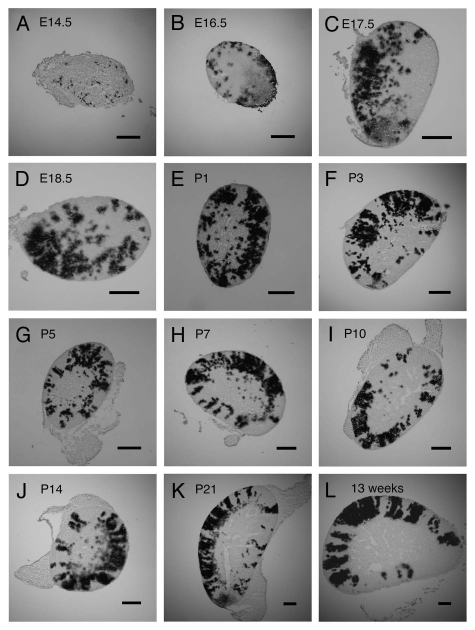
Figure 1.
Mosaic β-gal staining patterns in 21OH/LacZ transgenic fetal and postnatal adrenal glands from E14.5 to P21 and adult. Typical mosaic β-gal staining patterns seen in longitudinal mid-sections of 21OH/LacZ adrenal glands at (A) E14.5 (not sexed), (B) E16.5 (not sexed), (C) E17.5 female, (D) E18.5 female, (E) P1 female, (F) P3 female, (G) P5 female, (H) P7 female, (I) P10 female, (J) P14 female (K) P21 female, (L) adult female (13 weeks). Adrenal glands were frozen-sectioned and stained for β-gal activity as described in Materials and Methods. Males and females were analyzed separately from E17.5 and showed similar patterns of β-gal staining at each stage studied, so only results for females are illustrated. Dark areas in the photographs were stained blue (β-gal-positive). Scale bars = 200 µm.
At E14.5, mosaic β-gal staining patterns appeared primarily as randomly orientated single spots (Fig. 1A). This changed first to clusters of spots and non-radial patches, with the addition of radial arrays of spots and radially elongated patches at later fetal stages leading up to birth (E16.5–E18.5; Fig. 1B–D). Radially elongated patches became progressively more apparent during the first few days after birth and predominated by P5, while radial stripes (defined as uninterrupted radially orientated elongated areas of staining crossing much of the cortex) were first observed on postnatal day 1 (Fig. 1E–G). At P7 and P10, radially elongated patches and stripes were predominant (Fig. 1H and I), while stripes spanning the entire cortex were first observed at P7 (Fig. 1H). From P14 the β-gal staining pattern consisted primarily of radial stripes (Fig. 1J) and, by P21, appeared identical to the adult mosaic stripe pattern, with mostly complete radial stripes spanning the cortex (Fig. 1K and L).
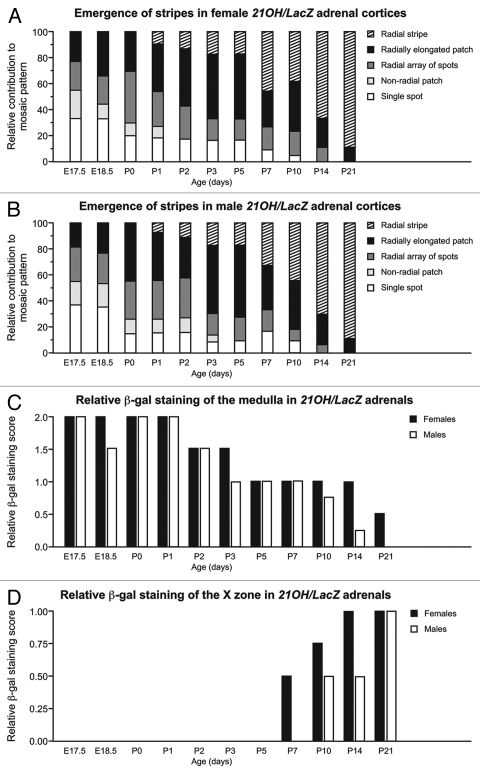
Figure 2A and B show semi-quantitative estimates of the relative contributions of each of the five types of individual staining patterns to the overall mosaic patterns from E17.5 to P21, analyzed separately for males and females (see Materials and Methods). Comparison of adrenocortical β-gal staining patterns revealed that radial stripes emerged during the first postnatal week in both males and females. The proportion of stripes (vs. other staining patterns) increased similarly over the next 14 d for both sexes until P21, when the pattern was indistinguishable from the adult radial striped pattern (Fig. 2A and B).
Figure 2.
Semi-quantitative analysis of changes in the mosaic β-gal staining patterns in 21OH/LacZ transgenic adrenal glands from E17.5 to P21. (A and B) changes in the contributions of different types of β-gal-positive staining to the overall mosaic pattern of the adrenal cortex with age in (A) female and (B) male 21OH/LacZ transgenic mice. (Bar charts A and B are shaded according to type of staining; see text for details). (C) Change in β-gal staining patterns in the adrenal medulla. (D) Change in β-gal staining patterns in the X zone. 21OH/LacZ adrenocortical β-gal staining was classified separately for left and right adrenals as described in Materials and Methods. The average scores for left and right adrenals are shown.
Two additional areas of β-gal staining were noted in 21OH/LacZ adrenal glands (Fig. 2C and D). First, significant numbers of β-gal-stained cells were seen in the central region of developing glands in the region where the medulla forms from E14.5 to P1 (Figs. 1A–E and 2C), but then declined gradually from P2 (Fig. 2C). Initially, the rate of decline was similar in adrenals of both sexes but then became more rapid in males from around P10, such that β-gal-stained cells were essentially absent from the medulla region of male adrenal glands by P21 (Fig. 2C). In contrast, β-gal-stained cells were still abundant in the female adrenal medulla at P14 (Fig. 1J), only starting to decline in number around P21 (Fig. 2C).
Second, a region of diffuse β-gal staining was first observed in postnatal 21OH/LacZ adrenals at the boundary of the cortex and medulla in females at P7 (Fig. 1H) and in males at P10 in the region where the morphological X zone develops at around P14. This staining became more clearly delineated in both sexes at P14 and P21, though more prominent in female adrenals (Fig. 2D) and coincided with the now visibly developing morphological X zone (Fig. 1J and K).
Qualitative analysis of radial stripes in adult 21OH/LacZ transgenic adrenals.
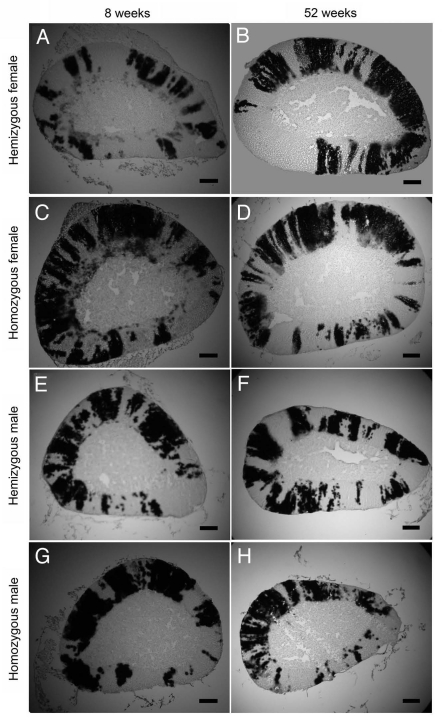
Adrenal sections from male and female 21OH/LacZ mice aged 8, 13, 26, 39 and 52 weeks, displayed clear radial stripes with well-defined boundaries spanning the adrenal cortex without any obvious differences in stripe patterns among ages. For brevity, only β-gal staining patterns at 8 and 52 weeks are shown in Figure 3. The only qualitative age-related difference noted among adrenals was the disappearance of pale β-gal staining seen in the X zone of female adrenals at 8 weeks (Fig. 3A and C), by 52 weeks of age (Fig. 3B and D), consistent with the short-lived nature of the X zone.14
Figure 3.
Representative images of β-gal-positive and β-gal-negative stripes in adult 21OH/LacZ transgenic adrenal cortex sections. (A and B) hemizygous female, (C and D) homozygous female, (E and F) hemizygous male and (G and H) homozygous male 21OH/LacZ transgenic mice at 8 (A, C, E and G) and 52 (B, D, F and H) weeks of age. Adrenal glands were frozen-sectioned and stained for β-gal. Dark stripes in the photographs were stained blue (β-gal-positive). Scale bar, 0.2 mm.
Some hypotheses of adrenocortical maintenance have proposed that different zones are maintained independently (see Introduction), which could result in discontinuous stripes. To investigate this possibility, stripes were examined in 6–10 serial sections taken from the middle of each of 200 adult adrenal glands. In only three cases did this reveal discontinuities that could not be explained by the plane of section being at an angle to the stripe (data not shown). Each of these cases displayed a broad area at the outer edge of the cortex with several layers of β-gal-negative cells without obvious zonal morphology, underneath which the rest of the inner cortex comprised the usual mixture of β-gal-positive and β-gal-negative radial stripes. The reverse pattern of an outer layer of β-gal-positive cells overlying an inner β-gal-negative cortex was never observed. This suggests that these rare discontinuous stripes might be attributed to a structural anomaly, such as a thickening of the capsule around an arteriole, or an area of undifferentiated neoplastic growth, which occurs only very rarely in mouse adrenals on the (C57BL/6 x CBA/Ca) F1 genetic background. Thus, in adults, the radial stripes in tissue sections represent predominantly three-dimensional, radial columns of cells spanning the entire adrenal cortex.
Quantitative analysis of radial stripes in adult 21OH/LacZ transgenic adrenals.
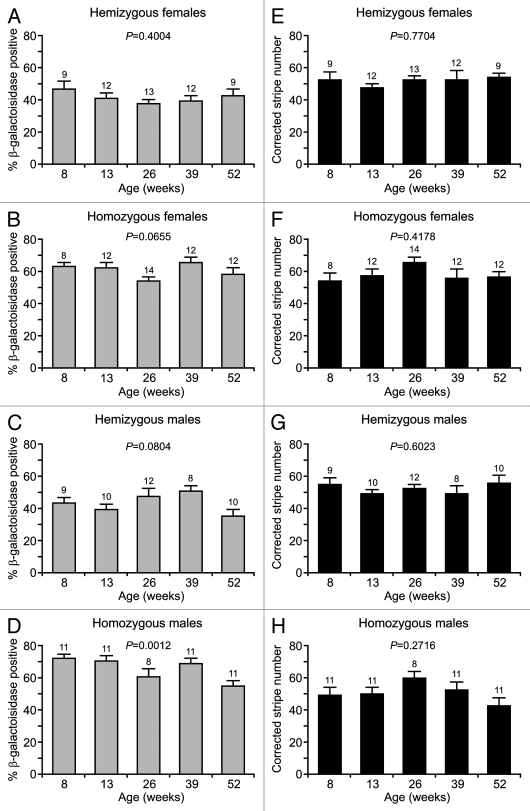
The percentage of β-gal-positive cells in the adrenal cortex was similar in males and females (Fig. 4A–D), although homozygotes had higher percentages of β-gal-positive cells compared with hemizygotes, suggesting that expression of at least one of the two transgenic alleles in homozygotes occurred more frequently than expression of the single allele in hemizygotes.
Figure 4.
Percentages of β-gal-positive cells and corrected stripe numbers in 21OH/LacZ adrenal cortices. (A–D) The mean percentage of β-gal-positive cells (±SEM) is shown at 8, 13, 26, 39 and 52 weeks for (A) hemizygous females, (B) homozygous females, (C) hemizygous males, (D) homozygous males. Variation among ages was only significant for homozygous males (p = 0.0012; ANOVA). Scheffe's post hoc tests showed significant differences in percentage of β-gal-negative cells between 8 and 52 (p = 0.0124), 13 and 52 (p = 0.0228) and 39 and 52 weeks (p = 0.0382), but other comparisons were not significant. (E–H) The mean corrected stripe number (±SEM), calculated as described in the Materials and Methods, is shown at 8, 13, 26, 39 and 52 weeks for (E) hemizygous females, (F) homozygous females, (G) hemizygous males, (H) homozygous males. No significant variation in mean corrected stripe number occurred with age for any of the four groups. The p-values shown in figure are all for ANOVA tests. Sample numbers are shown above each bar.
One-way ANOVA showed no significant differences in the percentage of β-gal-positive adrenocortical cells at different ages for hemizygous females, hemizygous males or homozygous females (Fig. 4A–C). Although there were differences among homozygous males (Fig. 4D), post-hoc tests indicated that this was attributable solely to a significantly lower percentage of β-gal-positive cells at 52 weeks. The absence of an overall trend across the groups toward continuous reduction in transgene expression with age implies that 21OH/LacZ transgene expression is not being progressively inactivated and is maintained in adults over the period analyzed.
As outlined in the Materials and Methods, the corrected stripe number in adrenocortical mid-sections is likely to be proportional to the number of active stem cell clones, so this analysis was used to compare putative stem cell function in the different groups. Comparison of the mean corrected adrenocortical stripe number in the four groups of 21OH/LacZ mice at five different ages (Fig. 4E–H) showed that the corrected stripe number was generally similar (close to 50 per section) for both homozygous and hemizygous 21OH/LacZ genotypes in both males and females at all ages.
The mean corrected stripe number per section was significantly higher in homozygous females vs. homozygous males at 52 weeks (p = 0.013) and in homozygous females vs. hemizygous females at 13 and 26 weeks (p = 0.031 and p = 0.022, respectively). This trend for homozygous females to have more corrected stripes is not adequately explained by increased adrenal size, because adrenals from homozygous females were not significantly heavier than adrenals from hemizygous females (p = 0.534 by two-way ANOVA). Although female adrenals were heavier than male adrenals at each age, for both homozygotes (p < 0.0001) and hemizygotes (p < 0.0001), hemizygous males and females had similar mean corrected stripe numbers per section. Any differences in corrected stripe numbers associated with sex and genotype were small, being significant at only a few ages and lacking a trend, suggesting that they are unlikely to be biologically significant. Critically, the absence of any age-related reduction in the corrected stripe number suggests that mouse adrenocortical stem cell function does not decline over the 8–52 weeks age range analyzed.
Adrenal growth during perinatal stripe formation.
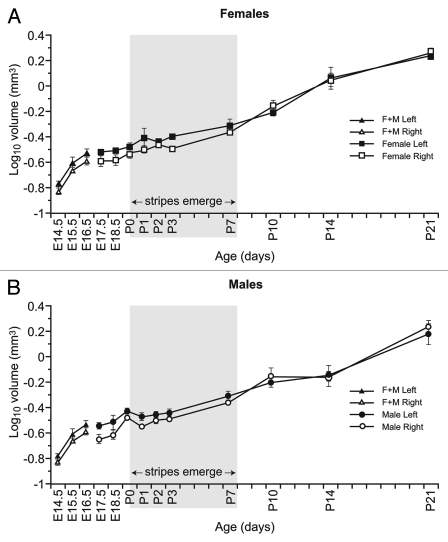
Adrenal size in both males and females increased rapidly between P14.5 and P16.5 and also after P3, with an apparently slower phase of net growth between E16.5 and P3 (Fig. 5A and B). Additional statistical modeling showed that neither a cubic nor a quadratic model provided a significantly better fit than the linear model of growth using log10-transformed data. The lack of evidence for a plateau in the growth curve between E16.5 and P3 or a steady increase in the slope (of log10-transformed adrenal size data) suggests that the adrenal gland continued to grow at all stages examined, crucially including stages P0–P7 when mosaic stripe patterns first emerge. The general trend was for adrenals to be slightly larger in females than males and, in both cases, for the left adrenal to be larger than right, but these differences were not consistently statistically significant for both length and width at every stage examined.
Figure 5.
Growth of 21OH/LacZ adrenal from E14.5 to P21. Semi-log plots of changes in estimated adrenal volume (estimated from separate measurements of adrenal length and width as described in the Materials and Methods) with age for (A) females and (B) males. 3–4 mice of each sex were analyzed from E17.5 to P21, but E14.5–E16.5 fetuses were not sexed and are included in both (A and B). The shaded area (P0–P7) is to highlight size changes during the period when the stripes emerge (Fig. 2). F, female; M, male.
Cell proliferation in the adrenal cortex during the period when stripes emerge perinatal stripe formation.
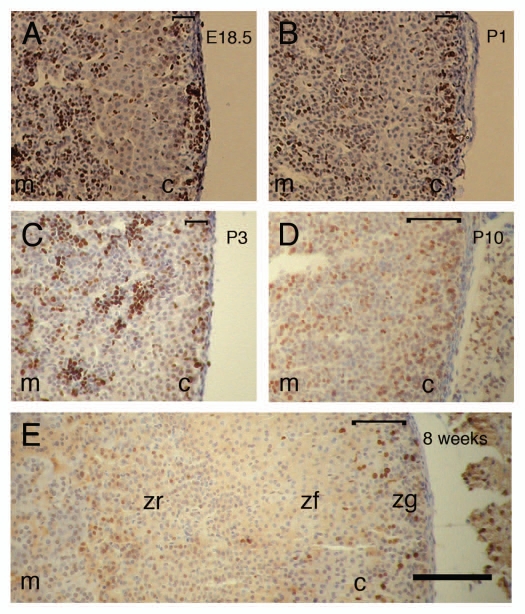
Histological staining of (C57BL/6 x CBA/Ca)F1 adrenal sections with hematoxylin and eosin (H&E) at E18.5 identified 1–3 layers of basophilic ZG cells, distinguished from cells of the ZF by their small size and sparse cytoplasm, which at P1 and P3 showed the beginnings of the classical ZG arch-like cellular organization, confirming appropriate adrenocortical development at these stages (data not shown). Ki67-immunopositive (proliferating) cells were distributed similarly in both the cortex and medulla of males and females at all ages examined; the results for females are illustrated in Figure 6. Most of the proliferating cells in the cortex were located in the outer three subcapsular cell layers (all within the developing ZG) at E18.5, P1 and P3 (Fig. 6A–C and Table 1).
Figure 6.
Locations of proliferating cells in female adrenal glands at different stages of development. Immunohistochemical staining for Ki67 nuclear antigen (brown nuclear staining) at (A) E18.5, (B) P1, (C) P3, (D) P10 and (E) 8 weeks. Scale bar = 100 µm (A–E are at the same magnification). Staining in the adrenal cortex is primarily in the outer cortex at all stages but more tightly subcapsular at E18.5-P3, while staining in the medulla at E18.5, P1 and P3 is somewhat patchy, as described in the text. Results for Ki67 immunohistochemistry were similar for males and females at each stage studied, so only results for females are illustrated. c, cortex; m, medulla; zf, zona fasciculata; zg, zona glomerulosa; zr, zona reticularis. The square bracket at the top right of each photograph shows the extent of the major Ki67-positive region in the outer cortex at each stage. Brown nuclear staining appears dark in greyscale prints of the figure.
Table 1.
Distribution of Ki67-positive cells during adrenal gland development
| Age | Main location of Ki67-positive cells in cortex | Frequency of Ki67-positive cells (subjective score) | ||
| Outer cortex | Inner cortex | Medulla | ||
| E18.5 | 1–3 subcapsular cell layers* | +++ | + | +++ |
| P1 | 2–3 subcapsular cell layers (ZG) | +++ | + | +++ |
| P3 | 2–3 subcapsular cell layers (ZG) | +++ | + | +++ |
| P10 | ZG + outer half of ZF | ++++ | + | +++ |
| Adult (8 weeks) | ZG + outer third of ZF | ++ | + | ++ |
The subcapsular region is the most peripheral part of the outer cortex (three cell layers closest to capsule).
By P10 all three definitive cortical zones and the boundary between the cortex and medulla could be distinguished clearly by H&E staining (data not shown). The arch-like structure of the ZG, the columnar morphology of the ZF and the compacted morphology of the ZR were fully formed and appeared morphologically similar to the adult. At P10, the number of Ki67-positive cells in the adrenal cortex was increased, and their distribution had expanded to include the outer half of the ZF (Fig. 6D and Table 1). In the adult adrenal cortex, Ki67-positive cell numbers were decreased, and positive cells were scattered throughout the outer cortex (ZG and outer ZF; Fig. 6E). Although at all stages of development examined, the majority of Ki67-positive cells were located in the outer cortex, a small number of Ki67-positive cells were always present in the inner cortex. From E18.5 to P10, the medulla also displayed many Ki67-positive cells, indicative of rapid cell division but fewer positive cells in the adult, suggesting that the rate of cell division had slowed (Fig. 6 and Table 1).
Discussion
In the present study, we have combined analysis of changes in the mosaic patterns of 21OH/LacZ adrenocortical β-gal reporter expression with alterations in the distribution of proliferating cells observed during adrenal gland growth during a period (E18.5-P10) not previously reported in mouse. This showed that radial striped patterns arise initially during the perinatal period by edge-biased growth (where most proliferation is at the edge of the tissue). By P10, the proliferative region in the outer adrenal cortex has broadened and is similar to that found in the adult. The radial striped patterns remain stable in the adult adrenal cortex to at least to one year of age. Our results provide a link between previous studies of mouse adrenocortical organogenesis and adult adrenocortical maintenance.
Growth and cell proliferation in the developing adrenal cortex.
The period (P0–P21) when patches are replaced by continuous stripes in 21OH/LacZ mosaics coincides with extensive remodelling of the developing adrenal gland (the cortex and medulla separate; adrenocortical zonation occurs, and the X zone appears). By comparing cell proliferation patterns with the morphology of the developing adrenal gland, our results showed that at perinatal stages, cell proliferation was largely confined to the outer three subcapsular cell layers of the cortex, in the region of the developing ZG, implying that cell lineages expand predominantly at the growing edge of the cortex with little cell mixing.31 By P10, the proliferative region had expanded to include the entire ZG and the outer part of the ZF, and this pattern was maintained in the adult. Adrenocortical cell proliferation patterns determined by BrdU labeling and PCNA immunohistochemistry have been reported previously for various embryonic and postnatal stages in rat10 and mouse35 but not at mouse perinatal stages. These latter authors described a change from a diffuse pattern of proliferation at E13.5 and E15.5 throughout the developing mouse adrenal gland to a more restricted subcapsular proliferative region at E17.5, which supports the idea of a switch from interstitial growth to edge-biased growth by the time 21OH/LacZ stripes emerge. Our results extend these observations by showing that proliferation driving edge-biased growth remains focused in the subcapsular region perinatally at E18.5, P1 and P3. However, this has changed by P10 and in the adult, where the main proliferative region is no longer immediately below the capsule but in a broader region encompassing both the outer ZF and ZG.
β-gal staining during development of the adrenal medulla, fetal adrenal cortex (X zone lineage) and definitive adult cortex.
As expected for a transgene driven by the 21OH gene promoter, which is active in both adult and fetal steroidogenic adrenocortical cells,8 the capsule showed little if any β-gal staining (Fig. 1I and J). The few cases where staining apparently extended to section edges can probably be explained by incomplete encapsulation at the earlier fetal stages examined or damage during tissue dissection or sectioning.
Changes in the relative proportion of β-gal-stained cells during embryonic growth (E14.5-P0) most likely result from the changing proportion of cortex vs. medulla at different stages rather than changes in transgene expression per se. For example, the low proportion of β-gal-stained cells at E14.5 probably reflects the low proportion of differentiated steroidogenic cells interspersed with undifferentiated and/or medullary cells at this stage. The presence of β-gal-stained cells in the center of mouse adrenal glands at E14.5 to E18.5 is consistent with the observed intermingling of cortical steroidogenic and medullary chromaffin cell precursors in the embryonic rat and mouse adrenal glands before zonation occurs.10,15 This intermingling is gradually resolved after birth.
Our experiments were designed to determine when and how radial striped patterns first emerge during development of 21OH/LacZ mosaic adrenal cortices but may also be informative about development of the adrenal X zone. It has recently become clear from lineage studies, using a fetal adrenal enhancer to direct LacZ reporter transgene expression specifically to the fetal adrenal cortex, that the X zone is derived from cells that comprise most of the adrenal cortex at fetal stages.14 Lineage tracing has also shown that the adult adrenal cortex is derived directly from fetal adrenocortical cells, forming a distinct adult lineage from around E14.5.15 The diffuse pale blue β-gal staining, observed in postnatal 21OH/LacZ adrenals at the cortical-medullary boundary in females at P7 and in males at P10 may mark the condensing X zone before it can be identified morphologically. This is consistent with the idea that the involuting fetal adrenocortical zone, which also expresses 21-hydroxylase,8 forms the postnatal X zone.14 The greater degree of staining observed in female adrenals is consistent with reports of more prominent female X zone development.12,36
Emergence of radial stripes in the developing 21OH/LacZ adrenal cortex.
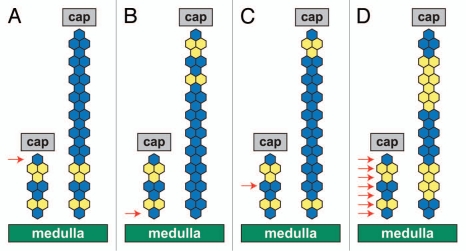
Analysis of the changes in the mosaic pattern helps distinguish between various hypothetical possibilities of adrenocortical growth illustrated in Figure 7 and identifies when and how adrenocortical stripes emerge in mosaic 21OH/LacZ adrenal cortices. The emergence of stripes cannot be explained by stochastic increases or decreases in the proportion of cells expressing the 21OH/LacZ transgene (as this would produce patches rather than stripes) or the hypothetical proliferation pattern illustrated in Figure 7D. The restriction of cell proliferation to a few outer subcapsular cell layers of the adrenal cortex over the perinatal period strongly supports the hypothesis that the emergence of mosaic stripes from P1 in mosaic 21OH/LacZ adrenal cortices is driven by edge-biased growth (illustrated in Fig. 7A), so excluding the possibilities shown in Figure 7B and C. This edge-biased growth is presumed to cause expansion of the definitive adult adrenocortical lineage, which surrounds the fetal adrenocortical/X zone lineage.
Figure 7.
Hypothetical patterns of adrenocortical growth in the developing 21OH/LacZ adrenal cortex. Each diagram (A–D) represents an alternative pattern of blue (β-gal-positive) and yellow (β-gal-negative) cells that would be produced by cell proliferation localized to specific regions in the developing 21OH/LacZ adrenal cortex. In each diagram, the group of hexagons on the left represents a column of cells spanning the adrenal cortex (from the medulla boundary at the bottom to the capsule at the top) shortly before birth. The arrows indicate the location of proliferating cell(s) in each example, and the taller groups of hexagons on the right represent the enlarged column of cells at a later stage, following adrenocortical growth. In (A–C), proliferation is restricted to a specific region. (A) Only cells in the outer row proliferate. In the example, the outer blue cell proliferates to produce a blue stripe, leaving the original mosaic region in the inner cortex. This is equivalent to strict edge growth, but a similar pattern would be expected with edge-biased growth if almost all cell divisions were at the edge. (B) Only cells adjacent to the medulla proliferate, and so an inner blue cell produces a blue stripe, which leaves the original mosaic region confined to the outer cortex (top). (C) proliferation is restricted to a more central region. Proliferating blue cells produce a blue stripe with mosaic regions in both the inner and outer cortex. (D) Proliferation occurs throughout the adrenal cortex, so no long continuous stripe is produced. cap, capsule. In greyscale prints of the figure, blue hexagons appear dark and yellow hexagons appear light.
Edge-biased growth at the periphery of the cortex predicts that the original randomly orientated mosaic pattern would persist in the inner cortex (Fig. 7A) but not the center or periphery of the cortex (Fig. 7B and C). Although there is evidence for the persistence of randomly orientated mosaic patterns in the X zone in 8-week-old females (discussed below), the β-gal staining patterns (Fig. 1) did not provide sufficient resolution at earlier stages to test this prediction adequately. This may be partly because the pattern was confused by the continued intermingling of medulla cells, which do not express the transgenic β-gal reporter, with β-gal-positive and-negative cortical lineages until 2–3 weeks after birth (Fig. 2C). However, the evidence for edge-biased growth discussed above is sufficient to exclude the models shown in Figure 7B–D. We, therefore, conclude that radial stripes in the mosaic 21OH/LacZ transgenic mouse adrenal cortex are produced by edge-biased growth during development (Fig. 7A).
Final transition to full radial stripes in the 21OH/LacZ adrenal cortex.
In adults, radial stripes span the whole adrenal cortex, from the capsule to the medulla, so it is necessary to explain how developing stripes extend into older cortical regions displaying randomly orientated mosaic patterns and eventually replace them. This could occur if the stripes were composed entirely of newer tissue, while older non-proliferative cells within the randomly orientated mosaic tissue died. Stripes formed by edge-biased growth (Fig. 7A) would extend toward the medulla if apoptosis occurred near the boundary with the medulla, as is the case in the P10 and adult rat adrenal cortex.10,37 Apoptosis would gradually eliminate residual randomly orientated mosaic tissue by progressively eroding it from the inner part of the cortex.
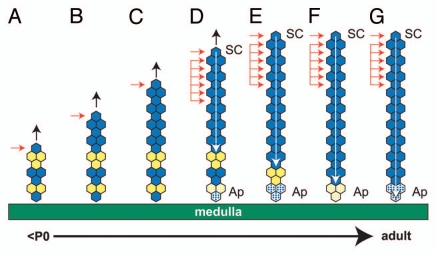
In addition, much of the randomly orientated mosaic tissue adjacent to the medulla is likely to have originated from expansion of the fetal adrenocortical lineage, so would be sequestered to the condensing X zone and eliminated later. If stripe formation begins in the fetal adrenal gland by edge-biased proliferation of a peripherally located adult adrenocortical lineage, stripes may span most or all of the adult adrenal cortex from the outset. In some cases, the staining pattern in the X zone of 8-week females resembles randomly orientated patches (e.g., top left in Fig. 3C), as predicted if the X zone was formed from the original fetal adrenocortical lineage (Figs. 7A and 8) and not displaced by stripes extending centripetally. However, some stripes do appear to extend across both the adult adrenal cortex and X zone (e.g., top right in Fig. 3A).
Figure 8.
Hypothetical formation of a blue (β-gal-positive) stripe spanning the whole adult adrenal cortex by a combination of edge-biased growth and centripetal cell displacement. Diagram showing initial edge-biased growth (A–C) followed by transition (D) to stem cell maintenance (E–G) in the adrenal cortex. (A–C) show the production of a blue (β-gal-positive) stripe in the outer adrenal cortex, driven by the onset of edge-biased growth (as in Fig. 7A), leaving a mosaic region in the inner cortex (perhaps in the X zone). The horizontal arrows indicate the proliferating cell(s), and the vertical black arrows indicate increased radial growth. (E–G) show extension of the blue stripe toward the medulla by production of new cells in the outer cortex, balanced by loss of cells in the inner cortex following the onset of tissue maintenance. Activated stem cells in the outer cortex (single horizontal arrow) self-renew, produce more differentiated daughter cells (probably equivalent to transient amplifying cells), which move centripetally, divide in the outer third of the cortex and displace existing cells toward the medulla, where they finally die by apoptosis (indicated by stippled shading of the bottom three cells). The grouped horizontal arrows represent the region of the cortex where cells proliferate (see text). The white arrow shows the direction of cell displacement and points to the same cell as it is displaced centripetally until it undergoes apoptosis. This process erodes the original mosaic pattern remaining in the inner cortex and replaces it with a continuous blue stripe, which spans the whole cortex. (D) shows a hypothetical transition stage between adrenal growth and maintenance, where the adrenal cortex is still growing, but stem cells have become activated in the outer cortex. some daughter cells also divide in the outer half of the cortex (so broadening the proliferative zone to include the ZG and the outer ZF), and cell death begins in the inner cortex, adjacent to the medulla. Ap, apoptosis (cell death); SC, stem cell. In greyscale prints of the figure, blue hexagons appear dark, and yellow hexagons appear light.
There is evidence that somatic stem cells later become activated in the outer cortex20,21 and produce proliferative cells (equivalent to transient amplifying cells), which move centripetally to maintain the adult adrenal cortex.16 Thus, a stem cell specified within a pre-existing β-gal-positive stripe will maintain the stripe by producing a clone of β-gal-positive cells that extends centripetally, eventually reaching the medulla by displacing the original β-gal-positive cells in the stripe. Figure 8 illustrates both elimination of residual randomly orientated mosaic tissue at the medulla boundary by apoptosis and centripetal movement by displacement of cells derived from stem cells in the outer cortex. This is consistent with a recent lineage analysis study demonstrating centripetal extension of clones derived from progenitor cells located in the outer cortex and/or capsule.22 In this experiment, cells labeled at E14.5 produced clusters of labeled cells near the periphery of the mouse adrenal cortex after 5 d (∼P0) and radial stripes of labeled cells, extending from the periphery almost to the medulla by 28 d (∼P23). This implies that radial extension of clones is not evident by P0, but by P23, the radial clones span almost the whole cortex. This is in good agreement with our observation that in 21OH/LacZ mosaics, stripes emerge from P0, and the adult pattern of complete radial stripes is formed by P21. As the clones were labeled at E14.5, it is likely that their initial expansion was by edge-biased growth before stem cells were activated.
Maintenance of radial stripes in the adult 21OH/LacZ transgenic adrenal cortex.
No significant qualitative differences in adult adrenocortical stripe patterns were identified between 8 and 52 weeks, suggesting that stem cells continue to maintain the adrenal cortex to at least one year of age and are not replaced by other modes of tissue maintenance. Quantitative analysis showed that corrected stripe numbers did not differ significantly with age for any of the four groups examined, predicting that adrenocortical stem cell function does not decline with age, as has been suggested by a similar analysis of the corneal epithelium of X-inactivation mosaics over the same age range.34 It remains possible that alterations in stem cell function may occur either before or after the 8–52 week age range examined in this study. It is also possible that the higher rate of cell turnover in the corneal epithelium depletes the stem cell pool earlier than in the adrenal cortex, or that the adrenal cortex has greater reserves of stem cells.
Different models of adrenocortical maintenance by stem cells.
Our evidence for adrenocortical growth by edge-biased growth is relevant to the issue of whether the three principal zones of the adult adrenal cortex are maintained by one or more stem cell populations (see Introduction). The radial stripes spanning all three cortical zones in chimeric or mosaic adrenals represent clonal lineages, which implies that adjacent regions of the ZG, ZF and ZR have a common origin.23,24,27 This common lineage relationship has been used to support the concept of centripetal migration of adrenocortical cells from a peripherally located stem cell population,20,21 and recent lineage analysis experiments are also most simply interpreted to suggest that the radial labeled clones represent cells derived from stem cells located in the periphery of the adrenal cortex.22 However, our results, implying that the adrenal grows by edge-biased growth, leave open the possibility that different adult adrenocortical zones could be maintained independently without disrupting the full span of the stripes. If radial stripes are established in the developing adrenal cortex before somatic stem cells are activated, it is possible that two or even three stem cell populations are specified along the radial axis as the adrenal cortex grows by edge-biased growth. Thus, different zones could have separate, radially aligned stem cell populations that are clonally related. If so, different zones would be maintained by different populations of stem cells, yet together, they would produce continuous radial stripes that span the cortex. This question could be resolved by repeating the previous Cre-loxP transgenic lineage analysis experiment22 but inducing labeled clones with tamoxifen in the adult rather than at E14.5.
Conclusions
Elegant transgenic lineage analyses of mouse adrenocortical organogenesis and maintenance have shown (1) that distinct groups of fetal adrenocortical cells with a common origin form the X zone and definitive adult adrenal cortex14,15 and (2) that progenitor cells labeled at E14.5 can produce clones of cells that span the cortex and produce all the steroidogenic cell types of the adrenal cortex.22 Our present results bridge these two observations and show that perinatal adrenal cortical growth occurs by edge-biased growth. This presumably represents the expansion of the population of cells in the fetal adrenal, forming a distinct adult lineage from around E14.515 that develops into the adult adrenal cortex (rather than the X zone). This period of edge-biased growth coincides with the first signs of radial stripes in mosaic 21OH/LacZ, implying that the initial adrenocortical stripes are formed by edge-biased growth and so resembles stripe formation during development of the retinal pigment epithelium in mosaics and chimeras.29–32 However, once established the adult stripes are likely to be maintained by stem cells in the outer cortex.20–22 Radial stripe patterns were unchanged with age in adults up to 52 weeks, predicting that mouse adrenocortical stem cell function remains stable during this period, in contrast to results for the mouse corneal epithelium.34
Materials and Methods
Mice.
Hemizygous 21OH/LacZ transgenic mice were produced by crossing homozygotes from line 791127 maintained on a mixed (C57BL/6 x CBA/Ca) background to non-transgenic, wild-type (C57BL/6 x CBA/Ca)F1 hybrid mice. Wild-type mice for histology and Ki67 immunohistochemistry were produced from crosses between C57BL/6 females and CBA/Ca males. Fetal age was timed from the day of the vaginal plug, which was taken to be embryonic day (E) 0.5. The day of birth was taken as postnatal day (P) 0. Mice were bred and maintained in the Medical Faculty Animal Area, University of Edinburgh and the Biological Research Facility at Little France Phase 1 (BRF-LF1), University of Edinburgh. Animal work was performed in accordance with institutional guidelines and UK Home Office regulations.
Adrenal size.
Adrenal glands were dissected, trimmed of fat and placed on a moist paper towel for measuring. The lengths and widths of adrenal gland were measured with a Wild M5A dissecting microscope fitted with a calibrated eyepiece graticule. Adrenal glands of different species vary in shape, and the mouse adrenal is approximately ellipsoid. Adrenal volume was estimated from the equation: depth) by assuming that adrenal glands are ellipsoid in shape and that the depth is equal to the measured width. While this is an over-simplification, it proved adequate for investigating whether growth continued throughout the experimental period.
Histology.
Hematoxylin and eosin (H&E) staining was routinely used for histological examination of the adrenal cortex. Adrenal glands were dissected and immediately fixed for 2 h (up to P10) or 4 h (adult adrenals) in 4% (w/v) paraformaldehyde (PFA) in PBS at 4°C and embedded in paraffin wax by standard procedures. Longitudinal 7 µm tissue sections were cut and mounted on microscope slides, dewaxed in xylene (2 × 10 min), washed in 100% (v/v) ethanol (2 × 5 min) and rehydrated through a graded ethanol series to water. Sections were then stained with Gill's hematoxylin (Vector, H3401) and eosin Y (Surgipath) by standard procedures, dehydrated through a graded ethanol series, cleared in xylene and mounted under coverslips with DPX mounting medium.
Ki67 immunohistochemistry.
Immunohistochemistry for Ki67 nuclear antigen detects cells that are in late G1, S, G2 and M but not G0 phases of the cell cycle.38 Adrenal glands were dissected, fixed and sectioned as described above but mounted on Polysine slides (VWR laboratory supplies), then sections were dewaxed and washed in ethanol. Endogenous peroxidase activity was blocked by immersing sections in 3% (w/v) hydrogen peroxide in methanol for 30 min in the dark at room temperature followed by rehydration in 70% (v/v) aqueous ethanol (2 × 5 min) and washing in phosphate buffered saline (PBS; 2 × 5 min). To unmask antigenic sites, sections were microwaved at full power (800 Watts) for 4 × 5 min in 0.01 M sodium citrate buffer, pH 6.0,39 and then cooled and washed in PBS (5 min). Sections were treated with blocking serum [20% (v/v) normal rabbit serum in 1x Tris-buffered saline, pH 7.6 (TBS)] for 30 min then with primary anti Ki67 antibody (Novocastra Lab Ltd.; NCL-Ki67-MM1; 5 µl/ml in blocking serum) for 2 h, rinsed then washed in TBS (2 × 5 min). Biotinylated secondary antibody (rabbit antimouse IgG biotin, Dako E0354; 5 µl/ml in blocking serum) was applied for 30 min and sections washed in TBS (2 × 5 min), treated with avidin-biotin-horseradish peroxidase solution (ABComplex/HRP kit; Dako K0355) for 30 min at room temperature and washed in TBS (2 × 5 min). Sections were finally incubated for 3 min with 3,3′-diaminobenzidine solution (DAB isopac, Sigma D9015), comprising 25 µl DAB (2 mg/ml) + 5.9 mls 0.2 M TBS + 1 µl 30% (w/v) H2O2, and rinsed in water to stop the reaction, which produces a stable, dark brown insoluble precipitate at sites of antibody localization. After lightly counterstaining with hematoxylin (Vector, H3401), slides were rinsed, dehydrated through graded ethanols, cleared in xylene and mounted under coverslips with DPX mounting medium.
X-gal staining of 21OH/LacZ transgenic adrenals.
Adrenal glands were dissected from 21OH/LacZ mice, measured as described previously and orientated in OCT-embedding medium (Tissue-Tek) in plastic molds without fixation, frozen on dry ice and either cryosectioned immediately or stored at −70°C. Longitudinal 10 µm frozen tissue sections were cut with a cryostat (Leica CM 1900), transferred to Polysine slides (VWR Laboratory Supplies) and then either stored in a frost-free freezer at −20°C or immediately fixed and stained for β-gal activity.40 After staining, slides were washed in wash buffer (2 × 5 min), counterstained in neutral red by standard procedures and mounted under coverslips with Histoclear mounting medium. Sections were examined with a Zeiss Axioplan 2 compound microscope, and images were captured with a Nikon Coolpix 995 digital camera.
Semi-quantitative analysis of β-gal staining patterns during adrenal cortex organogenesis.
Routinely, for each age point (E14.5-P21) and sex, five serial longitudinal sections cut from the middle region of a minimum of three left and three right adrenal glands were stained, photographed and analyzed. However, fetuses were not sexed at E14.5–E16.5. Areas of β-gal-positive staining in 21OH/LacZ adrenal cortices were classified according to their shape, symmetry and orientation: (1) A “single spot” was defined as a small, discrete area of staining representing either one or more cells (diffusion of β-gal reaction product during the staining and fixation processes makes it difficult to distinguish between these alternatives). (2) A “non-radial patch” was defined as an area of staining larger than a single cell that was not elongated along the radial axis of the adrenal cortex (i.e., it is either symmetrical or elongated in a non-radial direction). (3) A “radial array of spots” was defined as a grouping of discrete spots elongated along the radial axis of the adrenal cortex. (4) A “radially elongated patch” was defined as an area of staining larger than a single cell that was elongated along the radial axis of the adrenal cortex. (5) A “radial stripe” was defined as an uninterrupted radially orientated elongated area of staining crossing much of the cortex. The frequencies of each type of staining from E17.5 to P21 were initially scored separately for left and right adrenals of each sex according to a subjective five-point scale (with values of 0–4) and then averaged for left and right adrenals. The relative contribution of each of the five classes of staining to the overall mosaic pattern at each stage was estimated by expressing the scores as a percentage of all the scores for the same age and sex. For example, the average score for single spots in left and right P0 female adrenals was 1.0, and the total of the mean scores for all five classes was 5.0, so the relative contribution of single spots was scored as 20.0% (1.0 × 100/5.0).
The degree of β-gal staining in the X zone was scored as negative (0), weak (0.5) or positive (1), and in the medulla it was scored as negative (0), weak (0.5) or positive (with values of 1–4). Mean scores were calculated for left and right adrenals for each age and sex.
Measurement of radial stripe widths in adult 21OH/LacZ transgenic adrenal cortices.
The three-dimensional arrangements of radial columns of β-gal-positive and β-gal-negative cells in the intact adult tissue are represented as a two-dimensional pattern of radial stripes in longitudinal, adrenocortical mid-sections. As most radial stripes extend continuously across the full width of the adrenal cortex, the two-dimensional radial stripe pattern in sections can be analyzed as a one-dimensional pattern of stripe widths by measuring around the adrenocortical circumference. To allow for variation in stripe width at different depths, the stripe width is expressed as a proportion of the circumference.
Calibrated digital images of β-gal-stained adrenal sections were analyzed using UTHSCSA Image Tool software for Microsoft Windows.41 To measure the widths of the radial stripes, a circumferential line was drawn within the zona fasciculata parallel to the capsular-cortical boundary at a depth equivalent to 33% of the distance from the capsule to the inner margin of cortex (excluding any X zone). Starting at one radial boundary between a blue-stained, β-gal-positive stripe and an unstained, β-gal-negative stripe, the width of each stripe was measured following the line in a clockwise direction around the cortex back to the start position. This allowed a list of all the β-gal-positive and β-gal-negative stripes and their widths to be assembled for each adrenal cortex analyzed, from which the total measured circumference, the proportion of β-gal-positive cells and the “corrected stripe number” (Section 2.7) were calculated.33,34,42 Any diffuse pale blue staining flanking β-gal-positive adrenocortical stripes was included with the unstained β-gal-negative tissue, because this was considered to be a technical artifact resulting either from smearing of β-gal-positive cells during sectioning or diffusion of reaction product from neighboring β-gal-positive regions during staining.
Calculation of adult corrected stripe numbers for comparison of stem cell function.
It is not feasible to estimate the actual number of active stem cells from either two-dimensional or three-dimensional mosaic patterns, but they can provide information about relative stem cell function, which allows different groups to be compared. In practice, stripe widths measured in two-dimensional, mid-sections can be used for these comparisons after correction for differences in proportions of β-gal-positive cells, as described below.
There is a finite probability in mosaic tissues that two independent clonal cell lineages of the same phenotype may be positioned next to each other. Therefore, a single visible, β-gal-positive radial stripe in sections of 21OH/LacZ adrenal cortices may consist of several adjacent β-gal-positive radial coherent clones, and the probability of this “clone aggregation” effect increases with the proportion of β-gal-positive cells in the tissue. This can be allowed for by calculating a “corrected stripe number” which is the total number of stripes after correcting for the effect of the proportions of β-gal-positive and β-gal-negative cells on the mean number of clones per stripe.33,34,42 Crucially, therefore, unlike the observed stripe number, the corrected stripe number is independent of the relative proportions of β-gal-positive and β gal-negative cells in mosaic tissues. Furthermore, the corrected stripe number is likely to be proportional, even if not equal, to the total number of radial coherent clones (β-gal-positive plus β-gal-negative clones) located around the adrenocortical circumference in the histological section analyzed. In turn, the number of radial coherent clones within the cortex should be equal to the number of active coherent clones of stem cells in that section.33,34,42 Therefore, although the corrected stripe number does not provide a direct estimate of numbers of active stem cells, it is likely to be proportional to the number of active coherent clones of stem cells and, because it is unaffected by the proportion of β-gal-positive cells in the tissue, it can be used to make quantitative comparisons of stem cell function between different groups of mosaic adrenal glands.
The measurements of individual stripe widths for each adrenal section allowed calculation of both the proportion of β-gal-positive cells (p) around the measured circumference and the mean β-gal-positive stripe width. The observed mean β-gal-positive stripe width was then corrected for the probability that stripes would contain multiple adjacent β-gal-positive clones by dividing the mean stripe width by the correction factor 1/(1 - p) (where p is the proportion of β-gal-positive cells around the circumference) to give the “corrected mean stripe width.” This correction factor predicts the mean number of β-gal-positive clones per stripe width, and the assumptions inherent in the use of this correction are discussed in references 33 and 34. In practice, for a series of radial stripes that form a complete circle, the corrected mean stripe width will be the same for both β-gal-positive and β-gal-negative stripes. Therefore, the reciprocal of the corrected mean stripe width, expressed as the proportion of the circumference of the measured line, is the total “corrected stripe number” for all the β-gal-positive plus β-gal-negative stripes forming the circle. The corrected stripe numbers were then used to compare stem cell function between 21OH/LacZ adrenal cortices at different ages.
Statistical analysis.
Adrenal growth curves were analyzed using SPSS software (SPSS Inc.) as described in the text. Other statistical tests were performed using Microsoft Excel or StatView (SAS Institute Inc.) software.
Acknowledgments
We thank Dr. Robert Elton (Rob Elton Consulting) for help in analyzing the growth curves and Maureen Ross, Denis Doogan and staff at BRR, University of Edinburgh for expert animal husbandry and specialized technical services. We are grateful to Ronnie Grant and Ted Pinner for assistance with the illustrations, Drs. Chris Kenyon and Linda Mullins for helpful comments on the manuscript and to the late Keith Parker for helpful discussions on the work. This work was supported in part by a Tenovus Scotland project grant award E04/6 to J.D.W. and S.D.M. S.P.C. is grateful for an award from the University of Edinburgh Common Bursaries Fund, and J.J.M. was the recipient of a Wellcome Trust Principal Research Fellowship, 053646/Z/98/C.
Abbreviations
- ANOVA
analysis of variance
- E
embryonic day
- H&E
haematoxylin and eosin
- P
postnatal day
- PBS
phosphate buffered saline
- PFA
paraformaldehyde
- TBS
tris-buffered saline
- ZF
zona fasciculata
- ZG
zona glomerulosa
- ZI
zona intermedia
- ZR
zona reticularis
- ZU
undifferentiated zone
- β-gal
β-galactosidase
Disclosure of Potential Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Tanaka S, Matsuzawa A. Comparison of adrenocortical zonation in C57BL/6J and DDD mice. Exp Anim. 1995;44:285–291. doi: 10.1538/expanim.44.285. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka S, Nishimura M, Kitoh J, Matsuzawa A. Strain difference of the adrenal cortex between A/J and SM/J mice, progenitors of SMXA recombinant inbred group. Exp Anim. 1995;44:127–130. doi: 10.1538/expanim.44.127. [DOI] [PubMed] [Google Scholar]
- 3.Nyska A, Maronpot RR. Adrenal gland. In: Maronpot RR, Boorman GA, Gaul BW, editors. Pathology of the Mouse Reference and Atlas. Vienna, IL: Cache River Press; 1999. pp. 509–536. [Google Scholar]
- 4.Cater DB, Lever JD. The zona intermedia of the adrenal cortex—a correlation of possible functional significance with development, morphology and histochemistry. J Anat. 1954;88:437–454. [PMC free article] [PubMed] [Google Scholar]
- 5.Mitani F, Suzuki H, Hata J, Ogishima T, Shimada H, Ishimura Y. A novel cell layer without corticosteroid-synthesizing enzymes in rat adrenal cortex: histochemical detection and possible physiological role. Endocrinology. 1994;135:431–438. doi: 10.1210/en.135.1.431. [DOI] [PubMed] [Google Scholar]
- 6.Mukai T, Kusaka M, Kawabe K, Goto K, Nawata H, Fujieda K, et al. Sexually dimorphic expression of Dax-1 in the adrenal cortex. Genes Cells. 2002;7:717–729. doi: 10.1046/j.1365-2443.2002.00556.x. [DOI] [PubMed] [Google Scholar]
- 7.Mitani F, Mukai K, Miyamoto H, Suematsu M, Ishimura Y. The undifferentiated cell zone is a stem cell zone in adult rat adrenal cortex. Biochim Biophys Acta. 2003;1619:317–324; PMID:12573491; DOI:10.1016/S0304-4165(02)00490-7. doi: 10.1016/s0304-4165(02)00490-7. [DOI] [PubMed] [Google Scholar]
- 8.Raschellà G, Smets G, Claeys A, Verdood P, Romeo A, Hooghepeters EL. Transcriptional pattern of 21-hydroxylase gene (P-450c21) during embryonic development, before and after birth in mice as determined by in situ hybridization. J Histochem Cytochem. 1989;37:751–756. doi: 10.1177/37.5.2784812. [DOI] [PubMed] [Google Scholar]
- 9.Wotus C, Levay-Young BK, Rogers LM, Gomez-Sanchez CE, Engeland WC. Development of adrenal zonation in fetal rats defined by expression of aldosterone synthase and 11beta-hydroxylase. Endocrinology. 1998;139:4397–4403. doi: 10.1210/en.139.10.4397. [DOI] [PubMed] [Google Scholar]
- 10.Mitani F, Mukai K, Miyamoto H, Suematsu M, Ishimura Y. Development of functional zonation in the rat adrenal cortex. Endocrinology. 1999;140:3342–3353. doi: 10.1210/en.140.7.3342. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto M, Takemori H. Differentiation and zonation of the adrenal cortex. Curr Opin Endocrinol Diabetes. 2000;7:122–127. [Google Scholar]
- 12.Keegan CE, Hammer GD. Recent insights into organogenesis of the adrenal cortex. Trends Endocrinol Metab. 2002;13:200–208. doi: 10.1016/S1043-2760(02)00602-1. [DOI] [PubMed] [Google Scholar]
- 13.Bland ML, Desclozeaux M, Ingraham HA. Tissue growth and remodeling of the embryonic and adult adrenal gland. Ann NY Acad Sci. 2003;995:59–72. doi: 10.1111/j.1749-6632.2003.tb03210.x. [DOI] [PubMed] [Google Scholar]
- 14.Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol. 2006;26:4111–4121. doi: 10.1128/MCB.00222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zubair M, Parker KL, Morohashi K. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol. 2008;28:7030–7040. doi: 10.1128/MCB.00900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zajicek G, Ariel I, Arber N. The streaming adrenal cortex: direct evidence of centripetal migration of adrenocytes by estimation of cell turnover rate. J Endocrinol. 1986;111:477–482. doi: 10.1677/joe.0.1110477. [DOI] [PubMed] [Google Scholar]
- 17.Kataoka Y, Ikehara Y, Hattori T. Cell proliferation and renewal of mouse adrenal cortex. J Anat. 1996;188:375–381. [PMC free article] [PubMed] [Google Scholar]
- 18.Carsia RV, Macdonald GJ, Gibney JA, Tilly KI, Tilly JL. Apoptotic cell death in the rat adrenal gland: an in vivo and in vitro investigation. Cell Tissue Res. 1996;283:247–254. doi: 10.1007/s004410050535. [DOI] [PubMed] [Google Scholar]
- 19.Breidert M, Bottner A, Moller S, Herberg L, Bornstein S. Apoptosis in the adrenal gland of non-obese diabetic (NOD) mice. Exp Clin Endocrinol Diabetes. 1998;106:478–483. doi: 10.1055/s-0029-1212020. [DOI] [PubMed] [Google Scholar]
- 20.Kim AC, Hammer GD. Adrenocortical cells with stem/progenitor cell properties: Recent advances. Mol Cell Endocrinol. 2007;265-6:10–16. doi: 10.1016/j.mce.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim AC, Barlaskar FM, Heaton JH, Else T, Kelly VR, Krill KT, et al. In search of adrenocortical stem and progenitor cells. Endocr Rev. 2009;30:241–263. doi: 10.1210/er.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci USA. 2009;106:21185–21190. doi: 10.1073/pnas.0909471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iannaccone PM. The study of mammalian organogenesis by mosaic pattern analysis. Cell Differ. 1987;21:79–91. doi: 10.1016/0045-6039(87)90415-5. [DOI] [PubMed] [Google Scholar]
- 24.Iannaccone PM, Weinberg WC. The histogenesis of the rat adrenal cortex—a study based on histologic analysis of mosaic pattern in chimeras. J Exp Zool. 1987;243:217–223. doi: 10.1002/jez.1402430207. [DOI] [PubMed] [Google Scholar]
- 25.Morley SD, Chang SP, Tan SS, West JD. Validity of the 21-OH/LacZ transgenic mouse as a model for studying adrenocortical cell lineage. Endocr Res. 2004;30:513–519. doi: 10.1081/ERC-200043607. [DOI] [PubMed] [Google Scholar]
- 26.West JD. Genetic studies with mouse chimaeras. In: Reeve ECR, editor. Encyclopedia of Genetics. London and Chicago: Fitzroy Dearborn; 2001. pp. 293–302. [Google Scholar]
- 27.Morley SD, Viard I, Chung BC, Ikeda Y, Parker KL, Mullins JJ. Variegated expression of a mouse steroid 21-hydroxylase/β-galactosidase transgene suggests centripetal migration of adrenocortical cells. Mol Endocrinol. 1996;10:585–598. doi: 10.1210/me.10.5.585. [DOI] [PubMed] [Google Scholar]
- 28.Dobie K, Mehtali M, McClenaghan M, Lathe R. Variegated gene expression in mice. Trends Genet. 1997;13:127–130. doi: 10.1016/S0168-9525(97)01097-4. [DOI] [PubMed] [Google Scholar]
- 29.Bodenstein L, Sidman RL. Cell patterning in vertebrate development: Models and model systems. Curr Top Dev Biol. 1987;21:1–29. doi: 10.1016/S0070-2153(08)60131-3. [DOI] [PubMed] [Google Scholar]
- 30.Bodenstein L, Sidman RL. Growth and development of the mouse retinal pigment epithelium. II. Cell patterning in experimental chimeras and mosaics. Dev Biol. 1987;121:205–219. doi: 10.1016/0012-1606(87)90153-9. [DOI] [PubMed] [Google Scholar]
- 31.Bodenstein L. A dynamic simulation model of tissue growth and cell patterning. Cell Differ. 1986;19:19–33. doi: 10.1016/0045-6039(86)90022-9. [DOI] [PubMed] [Google Scholar]
- 32.Bodenstein L, Sidman RL. Growth and development of the mouse retinal pigment epithelium. I. Cell and tissue morphometrics and topography of mitotic activity. Dev Biol. 1987;121:192–204. doi: 10.1016/0012-1606(87)90152-7. [DOI] [PubMed] [Google Scholar]
- 33.Collinson JM, Morris L, Reid AI, Ramaesh T, Keighren MA, Flockhart JH, et al. Clonal analysis of patterns of growth, stem cell activity and cell movement during the development and maintenance of the murine corneal epithelium. Dev Dyn. 2002;224:432–440. doi: 10.1002/dvdy.10124. [DOI] [PubMed] [Google Scholar]
- 34.Mort RL, Ramaesh T, Kleinjan DA, Morley SD, West JD. Mosaic analysis of stem cell function and wound healing in the mouse corneal epithelium. BMC Dev Biol. 2009;9:4. doi: 10.1186/1471-213X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulte DM, Shapiro I, Reincke M, Beuschlein F. Expression and spatio-temporal distribution of differentiation and proliferation markers during mouse adrenal development. Gene Expr Patterns. 2007;7:72–81. doi: 10.1016/j.modgep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Howard-Miller E. A transitory zone in the adrenal cortex which shows age and sex relationships. Am J Anat. 2005;40:251–293. doi: 10.1002/aja.1000400204. [DOI] [Google Scholar]
- 37.Wyllie AH, Kerr JF, Currie AR. Cell death in the normal neonatal rat adrenal cortex. J Pathol. 1973;111:255–261. doi: 10.1002/path.1711110406. [DOI] [PubMed] [Google Scholar]
- 38.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 39.Norton AJ, Jordan S, Yeomans P. Brief, high-temperature heat denaturation (pressure cooking)—a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol. 1994;173:371–379. doi: 10.1002/path.1711730413. [DOI] [PubMed] [Google Scholar]
- 40.McGreggor G, Nolan G, Fiering S, Roederer M, Herzenberg L. Use of E. coli LacZ (β-galactosidase) as a reporter gene. In: Murray E, Walker J, editors. Methods in Molecular Biology. Gene Expression In Vivo. Vol. 7. Clifton, NJ: Humana Press Inc.; 1991. pp. 2–19. [Google Scholar]
- 41.Wilcox C, Dove S, McDavid W, Greer D. UTHSCSA ImageTool Version 3.0. Available from: http://ddsdx.uthscsa.edu/dig/itdesc.html.
- 42.Collinson JM, Chanas SA, Hill RE, West JD. Corneal development, limbal stem cell function and corneal epithelial cell migration in the Pax6+/ mouse. Invest Ophthalmol Vis Sci. 2004;45:1101–1108. doi: 10.1167/iovs.03-1118. [DOI] [PubMed] [Google Scholar]