Abstract
Cognitive dysfunctions are common in major depressive disorder, but have been difficult to recapitulate in animal models. This study shows that Flinders sensitive line (FSL) rats, a genetic rat model of depression, display a pronounced impairment of emotional memory function in the passive avoidance (PA) task, accompanied by reduced transcription of Arc in prefrontal cortex and hippocampus. At the cellular level, FSL rats have selective reductions in levels of NMDA receptor subunits, serotonin 5-HT1A receptors and MEK activity. Treatment with chronic escitalopram, but not with an antidepressant regimen of nortriptyline, restored memory performance and increased Arc transcription in FSL rats. Multiple pharmacological manipulations demonstrated that procognitive effects could also be achieved by either disinhibition of 5-HT1AR/MEK/Arc or stimulation of 5-HT4R/MEK/Arc signaling cascades. Taken together, studies of FSL rats in the PA task revealed reversible deficits in emotional memory processing, providing a potential model with predictive and construct validity for assessments of procognitive actions of antidepressant drug therapies.
Keywords: antidepressant, BDNF, MAPK, passive avoidance, signal transduction, TrkB
Introduction
The clinical impact of emotional dysfunction on cognitive capacity is recognized but difficult to treat.1, 2, 3 Besides depressed mood, the DSM-IV criteria of major depressive disorder (MDD) involve cognitive aspects, that is, diminished ability to think and concentrate, or indecisiveness, with devastating effects on executive functions, short- and long-term learning and memory.2, 3 These cognitive impairments appear to involve alterations in the neuronal processing of emotional stimuli, that is, negative attentional bias including feelings of worthlessness or excessive guilt and recurrent thoughts of suicide.4 Multiple brain areas, involved in emotionality and cognition, that is, prefrontal cortex (PFC), hippocampus (HPC), parahippocampal region (PHR), amygdala and striatum, show altered functions in MDD.1, 2, 5 In particular, volume reduction and impaired functionality of hippocampus have been repeatedly reported in MDD.2, 6, 7
Studies in depressed patients have linked dysfunction of the central serotonergic system with abnormal cognitive processing of emotional stimuli, and indicated that an important component in antidepressant therapy is support of cognitive functions, which can be separated from elevation of mood.8 The clinical efficacy of selective serotonin reuptake inhibitors (SSRIs) in MDD is exerted by several of the 14 cloned 5-HT receptors, with 5-HT1AR being the most studied.9 Several studies have described alteration of 5-HT1AR in postmortem samples from patients with MDD.10, 11, 12, 13, 14, 15 Interestingly, 5-HT1AR antagonists facilitate learning and counteract memory impairments.9 In addition, 5-HT4R agonists may have antidepressant16 and procognitive effects in learning and memory tasks.17
The delayed onset of SSRIs and most other antidepressants implicate that therapeutic effects are mediated beyond elevations of monoamines, such as changes of receptor coupling to intracellular signaling cascades and cross talk with non-monoaminergic mechanisms. Several lines of evidence indicate that brain-derived neurotrophic factor (BDNF)-induced activation of TrkB mediate actions of antidepressants.18, 19, 20, 21 TrkB receptors, in turn, stimulate several intracellular cascades including the mitogen-activated protein kinase (MAPK), AKT and phospholipase C pathways.21, 22 In particular, the MAPK pathway is involved in behavioral effects of antidepressants and an important modulator of ionotropic receptor signaling, structural remodeling and consolidation of fear memories.23, 24, 25 Synaptic activation of MAPK induces, via CREB and Elk-1, expression of activity-related cytoskeletal-associated protein (Arc/Arg 3.1).23, 26, 27, 28 Arc expression is induced by long-term potentiation-inducing stimuli, requiring NMDAR,27, 29 involved in synaptic memory consolidation and enriched in hippocampal dendritic spines where its activation is required for acquisition of contextual and spatial learning.26, 30, 31 Arc expression is also activated by several other mechanism(s), including BDNF,28 protein kinase A,30 and pertussis-induced inhibition of Gi-mediated signaling.32 Accordingly, blockade of 5-HT1AR augments the effect of an acute dose of paroxetine on increasing transcription of Arc mRNA.33 Thus, Arc transcription may be a key nodal point in integration of monoaminergic antidepressant effects with glutamate-driven activity-dependent neuronal adaptations regulating gene transcription associated with information processing and associative learning.
The Flinders sensitive line (FSL) is a rat strain associated with distinct behavioral and neurochemical features of major depression, including face validity, that is, psychomotor retardation and increased rapid eye movement sleep. FSL rats display a genetic vulnerability to environmental stressors and have been successfully used for validation of antidepressant effects.34 Interestingly, promotion of hippocampal neuroplasticity, by means of intracerebroventricular injections of bone marrow mesenchymal stem cells, reverses the depressive-like phenotype of FSL rats.35 Long-term potentiation is also reduced in the cornu ammonis 1 (CA1) area of hippocampus, shown during in vivo recordings in anesthetized FSL rats36 paralleled by reduced NR1 subunits of the NMDAR in hippocampal synaptosomes.36 FSL rats also have reduced hippocampal volume and dendritic spines, which is reversed by antidepressants.37 These alterations may affect emotional memory processes that, indeed, are believed to depend on enduring remodeling of neuronal morphology and activity-dependent structural plasticity, particularly of glutamatergic dendritic spines, indicating possible cognitive impairments in FSL rats.
In the present study, we performed behavioral, pharmacological, biochemical and histological experiments to examine whether FSL rats exhibit impairments in emotional learning, which may respond differentially to various antidepressant therapies and/or selective manipulations of 5-HT neurotransmission.
Materials and methods
Animals and pharmacological treatments
Adult FSL and Flinders resistant line (FRL) rats were bred at the Department of Physiology and Pharmacology at Karolinska Institute, and were tested at 2–4 months of age. Rats were kept in standard cages (TypeIV Macrolon, Bayer Material Science, Leverkusen, Germany, 26 × 42 × 15 cm) with sawdust, at room temperature and relative humidity (45–55%) under a constant light/dark cycle (lights on at 0700 hours). Water and food pellets (LactaminR36, Stockholm, Sweden) were available ad libitum. Experimental procedures were approved by the local Animal Ethics Committee (Stockholms Norra Djurförsöksetiska Nämnd).
FRL and FSL rats were randomly assigned to groups given either escitalopram, nortriptyline or vehicle administered to standard rat pellets38, 39 (Supplementary Figure S4) (Lactamin AB). Escitalopram was given at 340 mg kg−1 pellets for 3 weeks, followed by 410 mg kg−1 pellets. Nortriptyline was administered at 330 mg kg−1 pellet.
NAD-299 ((R)-3-N,N-dicyclobutylamino-8-fluoro-3,4-dihydro-2H-1-benzopyran-5-carboxamide hydrogen (2R,3R)tartrate monohydrate; 1 mg kg−1 subcutaneous (s.c.); AstraZeneca R&D, Södertälje, Sweden) and RS67333 (1 mg kg−1 intraperitoneal (i.p.); Tocris, Bristol, UK) were administered 30 min before testing.40, 41 The (±)-8-OH-DPAT (8-hydroxy-2-(di-n-propylamino)tetralin; 0.3 mg kg−1 s.c.; Sigma, St Louis, MO, USA) and MK-801 ((+)-10,11-dihydro-5-methyl-5H-dibenzo[a,d]-cyclohepten-5,10-imine hydrogen maleate; 0.05 mg kg−1 s.c.; Sigma) were both administered 15 min before training.41 GR125487 (5-fluoro-2-methoxy-[1-[2-[(methylsulfonyl)amino]ethyl]-4-piperidinyl]-1H-indole-3-methylcarboxylate sulfamate; 10 mg kg−1 i.p.; Tocris) was given 60 min before training.17 Drugs were dissolved in 2 ml kg−1 of 0.9% NaCl. PD184161(Servier, Suresnes, France) was administered 60 min before testing at a dose of 30 mg kg−1 i.p. and dissolved in 1% Tween80 in 6 ml kg−1 of 0.9% NaCl.
Passive avoidance, forced-swim test, open field and elevated plus-maze
The step-through passive avoidance (PA) was performed as described earlier41 (see Supplementary Figure S1). During PA training, rats were placed in the bright compartment and allowed to explore for 120 s. Thereafter, the sliding door was opened and once the rat had entered the dark compartment, the sliding door was automatically closed and a weak electrical stimulus (0.4 mA, 2 s scrambled current) was delivered through the grid floor. After 24 h, the animal was again gently placed in the light compartment, and the latency to enter the dark compartment with all four paws was automatically measured (retention latency) with a cutoff time for testing after 9 min. See Supplementary information regarding the procedures for forced-swim test, open field and elevated plus-maze (Supplementary Figure S4).
Histological measurements of Arc and BDNF transcription and 5-HT receptors
For in situ hybridization experiments, rat brains were rapidly dissected after decapitation and immediately frozen at −80 °C. Fresh frozen (12 μm) coronal cryostat sections were prepared and hybridized with 35S-radiolabeled antisense riboprobes against Arc and BDNF according to a previous protocol.42 For receptor autoradiography procedure with [3H]8-OH-DPAT, [125I]cyanopindolol and [3H]GR113808, see Supplementary Figure S2. Densitometric measurements were obtained from autoradiograms using the NIH ImageJ 1.40 software (National Institute of Mental Health, Bethesda, MD, USA) after subtraction of non-specific binding.
Immunoprecipitation and immunoblotting of total protein, and their phosphorylation state
The levels of the studied proteins and their phosphorylation state were assessed by immunoblotting. To preserve protein phosphorylation, rats were sacrificed using focused microwave irradiation (Muromachi Kikai, Tokyo, Japan), with brain regions dissected and frozen at −80 °C. Alternatively, rats were decapitated, their head snap frozen, brain regions rapidly dissected out and processed to avoid dephosphorylation events. These procedures have been described earlier.42, 43 For detailed immunoprecipitation and immunoblotting protocols, see Supplementary Table S1.
Statistical analysis
The data were analyzed with one-way or two-way analysis of variances, with drug treatment, rat strain or brain region as factors in multiple comparisons. For each significant F-ratio, Newman–Keuls test was used for post hoc comparison of effects following one-way analysis of variance, whereas Bonferroni post hoc test was used after two-way analysis of variance. When only two groups were compared (FRL with FSL), a two-tailed unpaired Student's t-test was used. P<0.05 was considered statistically significant.
Results
Baseline performance in PA and Arc transcription in FSL and FRL rats
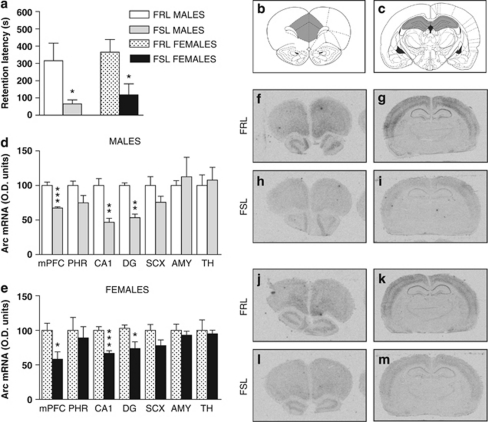
FSL rats of both sexes displayed reduced performance in the PA task compared with the control FRL rats (Figure 1a). To determine biochemical measures corresponding to the deficit in contextual memory, assessment of Arc mRNA levels was made in several brain regions. Arc mRNA expression was specifically reduced in medial PFC and in hippocampal CA1 and dentate gyrus subterritories, but not in parahippocampus, somatosensory cortex, amygdala or thalamus (Figures 1d–m).
Figure 1.
Male and female Flinders sensitive line (FSL) rats display emotional memory impairments and reduction of Arc mRNA expression specifically in brain regions implicated in cognitive processing. Passive avoidance (PA) emotional memory performance under baseline conditions in male (n=6–8 per group) and female (n=11 per group) Flinders resistant line (FRL) and FSL rats (a). Effects on step-through latency at the retention test performed 24 h after PA training. Atlas diagrams illustrating coronal sections of mPFC (b) and hippocampal subregions (c). Histograms of Arc mRNA in four to five male (d) and five female (e) FRL and FSL rats per group. Autoradiograms from in situ hybridization experiments against Arc mRNA in male FRL (f, g) and FSL (h, i) rats or female FRL (j, k) and FSL (l, m) rats. AMY, amygdaloid nuclei; CA1, cornu ammonis 1 of hippocampus; DG, dentate gyrus of hippocampus; mPFC, medial prefrontal cortex; PHR, parahippocampal region; SCX, primary sensory cortex; TH, thalamic nuclei. Data represent mean±s.e.m. *P<0.05; **P<0.01; and ***P<0.001 versus corresponding FRL control group.
Baseline regulation of glutamate and 5-HT receptors and signaling molecules
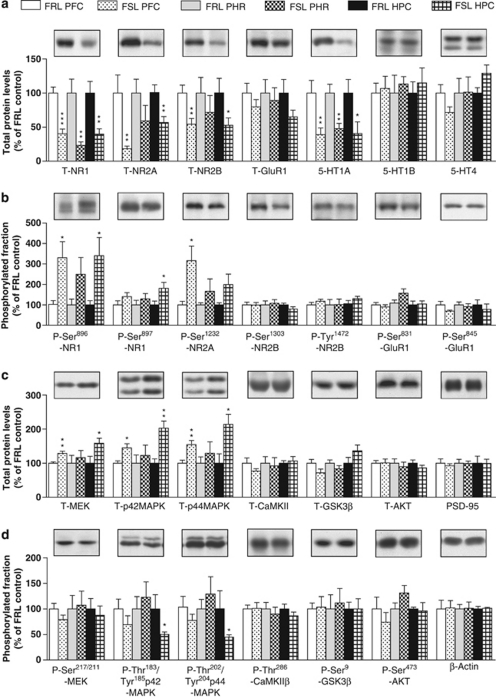
Arc mRNA expression is regulated by glutamate and serotonin neurotransmission, and, in accordance with reduced Arc mRNA, western blotting showed lower levels of NR1 subunits of the NMDAR complex in PFC, hippocampus and parahippocampus of FSL rats (Figure 2a). The compositions of the different NMDAR subunits determine gating and kinetic properties of the NMDAR, with important consequences for synaptic plasticity and memory.44, 45 The NR2B subunit is specifically involved in NMDAR interactions with the MAPK cascade.46 In FSL rats, NR2A and NR2B subunits were specifically lowered in PFC and hippocampus (Figure 2a). In none of the studied regions, GluR1 subunits of the AMPAR complex were different between FSL and FRL rats (Figure 2a). Serotonin 5-HT1AR, but not 5-HT1BR or 5-HT4R, were reduced in all studied regions in FSL rats (Figure 2a). Likewise, labeling of 5-HT1AR by [3H]8-OH-DPAT was reduced in hippocampal sections from male (Supplementary Figure S2A–C) and female (Supplementary Figure S2A, D and E) FSL rats, whereas there were no alterations in 5-HT1BR- or 5-HT4R-like binding using [125I]cyanopindolol and [3H]GR113808, respectively (Supplementary Figure S2F–O).
Figure 2.
Alterations of total proteins and phosphorylated forms of proteins in Flinders sensitive line (FSL) rats compared with Flinders resistant line (FRL) control rats. Top, illustration of labeling of bars. Histograms of quantification of total protein levels (a, c) and phosphorylated form of the protein normalized to the total level of the same protein (b, d) in prefrontal cortex (PFC), parahippocampal region (PHR) and hippocampal region (HPC). Representative western blots from hippocampal region are shown above each histogram. Values are mean±s.e.m. from four to eight animals per group. *P<0.05; **P<0.01; and ***P<0.001 versus corresponding FRL control group.
In contrast to the reduced levels of NMDAR subunits, their phosphorylation states appeared to be generally increased in FSL rats, possibly as a compensatory mechanism(s). Specifically, NR1 subunits showed significantly increased phosphorylation of Ser896, a PKC site, and Ser897, a protein kinase A site, in the hippocampus (Figure 2b). In prefrontal cortices, the phosphorylation at the NR1 subunit at site Ser896 was increased, and the NR2A subunit exhibited an increased phosphorylation at Ser1232, a cdk5 site (Figure 2b). The phosphorylation states of NR2B subunits at Ser1303, a CaMKII site, and Tyr1472, a SRC tyrosine kinase site, were not altered in FSL rats (Figure 2b). The phosphorylation levels of GluR1 subunits at Ser831, a PKC/CaMKII site, and Ser845, a protein kinase A site, were not altered (Figure 2b). The total levels of MEK, p42-MAPK and p44-MAPK were increased in PFC and hippocampus of FSL rats (Figure 2c). No changes in the phosphorylation of Ser217/221-MEK were found (Figure 2d). However, reduced phosphorylations at Thr183/Tyr185-p42 and Thr202/Tyr204-p44 in hippocampus were found, indicative of a reduced MEK activity in FSL rats (Figure 2d). In contrast, total levels or phosphorylation states of CaMKII, GSK-3β, AKT or in PSD-95, a postsynaptically enriched protein, were not altered (Figures 2c and d). These data suggest that MEK/MAPK signaling is particularly dysregulated in FSL rats and that decreased MEK activity, perhaps related to reduced NMDAR and 5-HT receptor-mediated transmission, parallels with memory impairments and reduced Arc mRNA expression in FSL rats.
Impairing effect on PA performance by NMDAR blockade in both FSL and FRL rats
NMDAR are critically implicated in the acquisition and consolidation of several types of memories including the PA task.9 In agreement with previous reports, treatment with an amnesic dose of MK-801, a non-competitive NMDAR antagonist, of FRL rats severely disrupted retention performance, mimicking the memory impairments seen in FSL rats (Supplementary Figure S3 and summary Supplementary Figure S7A–C).
Effects of escitalopram and nortriptyline on behavioral performance in PA, forced-swim test, open field, elevated plus-maze and transcription of Arc
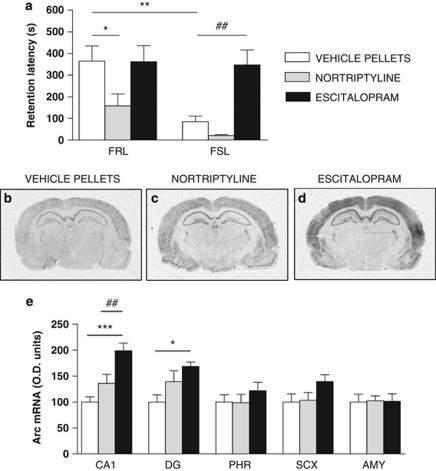
To test whether the impairment in memory performance in FSL rats could be reversed by antidepressants, FSL and FRL rats were treated chronically with vehicle, escitalopram or nortriptyline.38, 39 In FSL rats, escitalopram, but not nortriptyline, reversed the impairment of memory performance in the PA test (Figure 3a). In contrast, escitalopram was without effect on memory performance in FRL rats, comparable to lack of effects of paroxetine in Sprague–Dawley rats,47 whereas nortriptyline impaired performance (Figure 3a). Escitalopram, but not nortriptyline, specifically increased expression of Arc mRNA in the CA1 and dentate gyrus subregions of hippocampus of FSL rats (Figures 3b–e). Escitalopram reduced immobility in the forced-swim test in FSL rats.38 Similarly, nortriptyline decreased immobility of FSL rats in the forced-swim test (Supplementary Figure S4A) via mechanisms unrelated to alterations in locomotion (Supplementary Figure S4B and C). Notably, the differential effect of escitalopram and nortriptyline in the PA test could not be correlated to elevated plus-maze performance, as both agents induced anxiolytic-like effects in control FRL rats (Supplementary Figure S4D and E). FSL rats displayed a basal anxiolytic phenotype and nortriptyline was without effect, whereas escitalopram further reduced anxiety-like behavior (Supplementary Figure S4D and E).
Figure 3.
Differential effect of escitalopram and nortriptyline in restoration of emotional memory impairments and Arc transcription in Flinders sensitive line (FSL) rats. Effects on step-through latency at the retention test performed 24 h after passive avoidance training in male Flinders resistant line (FRL) and FSL rats (n=9–12 per group) (a). Autoradiograms from in situ hybridization experiments against Arc mRNA in rats receiving vehicle pellets (b), chronic nortriptyline (c) and chronic escitalopram (d). Histograms of quantification of the effects of antidepressants on Arc mRNA in six male FSL rats per group (e). AMY, amygdaloid nuclei; CA1, cornu ammonis 1 of hippocampus; DG, dentate gyrus of hippocampus; PHR, parahippocampal region; SCX, primary sensory cortex. Data represent mean±s.e.m. *P<0.05; **P<0.01; ***P<0.001 versus corresponding FRL control group; (a) ##P<0.01 FSL versus escitalopram; and (e) ##P<0.01 nortriptyline versus escitalopram.
Measurements of BDNF mRNA and protein, and phosphorylated TrkB under baseline conditions and following escitalopram and nortriptyline
Several antidepressant actions are mediated via BDNF and its predominant receptor TrkB, and BDNF signaling is reflected by the phosphorylation state of TrkB.18, 19, 20, 21, 22 BDNF signaling increases Arc mRNA and protein expression in neurons,28 and could potentially act upstream of Arc under baseline conditions and/or following escitalopram treatment in FSL rats. Immunoblotting revealed regional-specific differences of basal proBDNF, with reduced levels in the hippocampus of FSL rats when compared with FRL rats, but increased levels in the parahippocampal region and unaltered levels in the PFC (Supplementary Figure S5A). However, basal levels of mature BDNF, as well as total and phosphorylated TrkB, did not differ between the genotypes in the examined regions (Supplementary Figure S5A and B).
In response to both escitalopram and nortriptyline, FSL rats showed increased expression of BDNF mRNA in the dentate gyrus, but not in the CA1, of hippocampus (Supplementary Figure S5C–F). In comparison, no significant alterations of proBDNF or BDNF proteins were found in total hippocampal or parahippocampal homogenates from antidepressant-treated animals (Supplementary Figure S5G). Nortriptyline and escitalopram induced treatment-, region- and site-specific changes of TrkB phoshorylation, without affecting total TrkB. Specifically, both nortriptyline and escitalopram increased phosphorylation of TrkB at Tyr816, whereas nortriptyline, but not escitalopram, increased phoshorylation at Tyr705-TrkB, and none of these treatments regulated Tyr515-TrkB in hippocampus (Supplementary Figure S5H). No corresponding alterations were found in the parahippocampal region (Supplementary Figure S5H). It should be noted that the antisera toward phosphorylated TrkB can also detect phosphorylated TrkA, and both TrkB and TrkA are found in hippocampal extracts (Supplementary Figure S6A and B). In view of this, immunoprecipitations of TrkB were done from hippocampal brain tissue and, subsequently, analyzed for total TrkA and TrkB receptors as well as phosphorylated TrkB (Supplementary Figure S6C–E). No TrkA was found in TrkB immunoprecipitates (Supplementary Figure S6E). However, immunoreactivity of the antidepressant-responsive Tyr816-TrkB site was readily detected in TrkB immunoprecipitates (Supplementary Figure S6D). In response to treatment with escitalopram and nortriptyline, levels of Tyr816-TrkB were increased in hippocampal TrkB immunoprecipitates (Supplementary Figure S6D, F). Vice versa, in a confirmatory experiment, immunoprecipitation of Tyr816-TrkB resulted in higher levels of TrkB immunoreactivity in antidepressant-treated rats (Supplementary Figure S6C). Notably, no TrkA was found in Tyr816-TrkB immunoprecipitates (Supplementary Figure S6E), indicating that phosphorylation at this site in hippocampus occurs mainly at TrkB.
Effects of selective 5-HT1AR and 5-HT4R ligands, with and without a MEK inhibitor, on PA performance and transcription of Arc
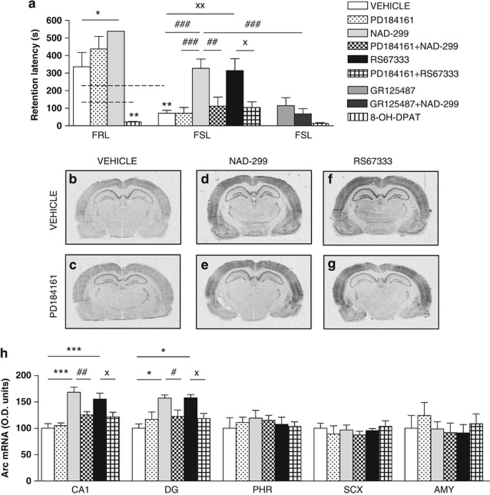
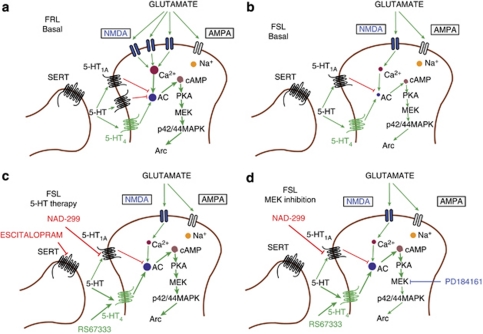
Based on the procognitive effects of escitalopram in the PA test in FSL rats and reduced 5-HT1AR levels, we studied 5-HT receptor subtypes in modulating memory performance in FSL rats. The 5-HT1AR agonist 8-OH-DPAT impaired performance in the PA test in FRL rats (Figure 4a). On the other hand, NAD-299, a 5-HT1AR antagonist, enhanced performance in the PA test in FRL rats and reversed the memory dysfunction in the FSL rats, reminiscent of the action of escitalopram (Figure 4a). Inhibition of presynaptic 5-HT1AR causes an increase in the firing rate of serotonin neurons, whereas inhibition of postsynaptic 5-HT1AR causes a decrease of inhibitory serotonin transmission in specific circuits.9 Next, we pretreated the rats with a 5-HT4R antagonist, known to mediate its actions at the postsynaptic site. GR125487 at a concentration where it had no intrinsic effect blocked the effect of NAD-299, indicating that NAD-299 exerts, at least, some of its effects via stimulation of 5-HT4R (Figure 4a). The 5-HT4R agonist, RS67333, also restored memory performance in the PA test (Figure 4a). Both 5-HT1AR9, 48 and 5-HT4R49, 50 have been shown to regulate the MEK/MAPK cascade, and as the biochemical data indicated that MEK/MAPK signaling is altered in FSL rats (Figures 2c and d), we investigated whether the MEK/MAPK signaling cascade is mediating procognitive actions of NAD-299 and/or RS67333. Rats were pretreated with a MEK inhibitor, PD184161,24 at a dose where it had no intrinsic effect, before treatment with either NAD-299 or RS67333. PD184161 completely blocked the enhancing effects of NAD-299 or RS67333 in the PA test (Figure 4a).
Figure 4.
Effects of 5-HT1AR or 5-HT4R agonists and antagonists, and involvement of MEK activity in restoration of emotional memory function and induction of Arc mRNA expression. Passive avoidance memory performance assessed by step-through latency at the retention test performed 24 h after training in male Flinders resistant line (FRL) and Flinders sensitive line (FSL) rats (n=7–18 per group) (a). The lack of error bar for FRL rats treated with NAD-299 ((R)-3-N,N-dicyclobutylamino-8-fluoro-3,4-dihydro-2H-1-benzopyran-5-carboxamide hydrogen (2R,3R)tartrate monohydrate) was because of that none of the rats returned to the conditioned compartment at testing. Autoradiograms from in situ hybridization experiments against Arc mRNA in rats receiving 5-HT ligands alone (upper row) or pretreatment with MEK inhibitor (lower row) or vehicle (b, c), 5-HT1AR antagonist (d, e) or 5-HT4R agonist (f, g). Primary mechanisms of action: PD184161—MEK inhibitor; NAD-299—5-HT1AR antagonist; RS67333—5-HT4R agonist; GR125487—5-HT4R antagonist; and 8-OH-DPAT (8-hydroxy-2-(di-n-propylamino)tetralin)—5-HT1AR agonist. Histograms of quantification of the effects on Arc mRNA transcription in six male FSL rats per group (h). AMY, amygdaloid nuclei; CA1, cornu ammonis 1 of hippocampus; DG, dentate gyrus of hippocampus; PHR, parahippocampal region; SCX, primary sensory cortex. Data represent mean±s.e.m. *P<0.05; **P<0.01; ***P<0.001 versus corresponding FRL control group; ##P<0.01; ###P<0.01 versus FSL NAD-299; and X P<0.05 versus FSL RS67333.
We also found that NAD-299 and/or RS67333 could mimic the region-specific stimulatory effects of escitalopram on Arc mRNA in the CA1 and dentate gyrus subregions of hippocampus (Figures 4d, f and h). Moreover, although PD184161 had no effects by itself on Arc transcription in FSL rats (Figures 4c and h), it significantly counteracted the actions of both NAD-299 and RS67333 on Arc mRNA (Figures 4e, g and h). It seems that MEK/MAPK signaling is a nodal point in mediating some behavioral and biochemical postsynaptic serotonergic effects that are critically involved in procognitive effects in the PA paradigm.
Discussion
A substantial proportion of depressed patients exhibits deficits in several memory tasks, including declarative verbal and recollection memory, executive functions and emotional word processing.2, 6, 8, 14, 21, 51, 52 Improvement of cognitive processing and emotional memory function in depression is believed to be important for the treatment outcome in MDD.2, 4 In the present study, we provide data indicating that the genetic FSL rat line of depression may be utilized as a model for studies of reversible impairments in emotional processing and memory, displaying predictive validity for assessment of drugs supporting cognitive performance.4, 53 The impairments of memory performance are in accordance with the findings of reduced Arc mRNA specifically in cortical and hippocampal subregions of FSL rats (Figures 1d–m), consistent with accumulating data indicating that Arc transcription is activated by neuronal stimuli particularly important for encoding of one-trial hippocampal-dependent learning and long-term neuroplasticity involved in consolidation of memories.26, 27, 31, 54
The marked alterations of glutamatergic receptors in the FSL rat line (Figure 2a, b) agree with a number of neuroimaging and postmortem histological studies showing glutamatergic abnormalities in MDD,55, 56 for example, magnetic resonance spectroscopy studies in patients with MDD demonstrated altered glutamate levels in several cortical subregions.57, 58, 59 Accordingly, NMDAR binding and subunits are decreased in cortical regions in depressed patients.60, 61, 62, 63, 64 Similarly, the core NR1 subunit of the NMDAR was markedly reduced in FSL rats in the PFC, PHR and HPC extracts, accompanied with reductions also of the NR2A and NR2B subunits in the PFC and hippocampus (Figure 2a). Selective reductions of several NMDAR subunits in corticolimbic brain regions in FSL rats further indicate that these alterations have severe consequences for synaptic plasticity and account for the impairments of long-term potentiation in FSL rats.36 Consistent with findings reported from MDD patients,55, 56 we observed only modest alterations of GluR1 levels. The phosphorylation state of NMDAR and AMPAR correlates with changes in synaptic strength.65 Despite the lower levels of NMDAR subunits found in FSL rats, phosphorylation levels were upregulated in several cases. Taken together, rather than subtype-specific alterations, regional-specific overall reductions of NMDAR partially compensated by hyperphosphorylation were found in FSL rats.
The specific reductions of 5-HT1AR-, but not 5-HT1BR- or 5-HT4R-like binding, using immunoblotting (Figure 2a) and autoradiography receptor binding in FSL rats (Supplementary Figure S2A–O) agree with a previous study66 The reduction of 5-HT1AR are consistent with several neuroimaging studies and postmortem data from patients with MDD.10, 11, 12, 13, 14 It has, however, been difficult to distinguish primary effects and confounders in view of variations of 5-HT1AR binding during onset and remission of depression, responses to antidepressants, differences in agonist versus antagonist labeling, with the mixed results most likely also reflecting the clinical heterogeneity of depressed patients, drug treatment and/or aging.12, 14, 15
After chronic antidepressant treatments, a discrepancy was found between escitalopram and nortriptyline on both behavioral outputs and on Arc mRNA expression (Figures 3a–e). The restoring effects on Arc transcription and emotional memory performance of FSL rats are consistent with clinical findings supporting a serotonergic component in recovery of cognitive aspects.4, 8, 67 Indeed, these results in FSL rats show predictive and face validity with data on clinical efficacy for escitalopram and nortriptyline, antidepressants that have been reported to be similar on three conventional depression-rating scales, but differentiate in a superior improvement of cognitive symptoms by escitalopram, whereas nortriptyline rather improved neurovegetative symptoms.68 Calculation of a proportional impairment ratio in humans were found to be three- to ninefold higher for tricyclic antidepressants, than those reported for escitalopram, on a range of different psychometric tests for cognitive symptoms. The varying drug-induced cognitive impairment caused by tricyclic agents is attributed to their anticholinergic and antihistaminergic properties, decreasing cognitive performance and arousal, respectively.67
Consistent with previous reports on antidepressant regimens, both escitalopram and nortriptyline increased BDNF mRNA in the dentate gyrus, but not CA1, of hippocampus (Supplementary Figure S5C–F).20, 21, 69 We could not detect any corresponding effects on protein levels of proBDNF and mature BDNF (Supplementary Figure S5G). As discussed previously (for reviews, see Martinowich et al.,20 Pittenger and Duman,21 and Castrén and Rantamäki69), a discrepancy between mRNA and protein expression of BDNF as found here may be due to several factors, including the omission of subregional analysis of hippocampal BDNF protein, retrograde/anterograde transport of BDNF protein transcribed in the dentate gyrus and the lack of distinct measures of intra- and extracellular BDNF protein.
BDNF-mediated signaling can be studied by measures of the phosphorylation state of the TrkB receptor. Binding of BDNF to the TrkB receptor induces its autophosphorylation at Tyr705-TrkB in the catalytic domain, which subsequently regulates the phosphorylation state at other tyrosine residues including Tyr515-TrkB, a Shc-binding site, and Tyr816-TrkB, a phospholipase Cγ1-binding site.69 The latter phosphorylation site of TrkB is also regulated via transactivation by G-protein-coupled receptors, independent of BDNF.70 In agreement with Rantamäki et al.18 and Saarelainen et al.19 we found that both a tricyclic antidepressant, nortriptyline, and a SSRI, escitalopram, increased phosphorylation at the phospholipase Cγ1-binding site Tyr816-TrkB, but not at the Shc-binding site Tyr515-TrkB in hippocampus (Supplementary Figure S5H). It is interesting that studies with phosphomutant TrkB mice have shown that the phospholipase Cγ1-binding site is more important for synaptic plasticity than the Shc-binding site,71 suggesting that the observed activation of Tyr816-TrkB by nortriptyline and escitalopram may be of functional significance in mediating long-term antidepressant actions. As the antisera toward phosphorylated TrkB can also detect phosphorylated TrkA and both these receptors are found in hippocampus (Supplementary Figure S6A and B), we performed additional experiments to confirm that antidepressants specifically increase phosphorylation at Tyr816 of TrkB. Indeed, in reciprocal immunoprecipitation experiments with antisera toward either TrkB or Tyr816-TrkB, both nortriptyline and escitalopram increased Tyr816 specifically at TrkB, without regulating TrkA (Supplementary Figure S6C–F). Using direct immunoblotting from snap frozen hippocampi, we also found that nortriptyline, but not escitalopram, increased Tyr705-TrkB at the studied time point, suggesting a more sustained and/or stronger activation of TrkB by nortriptyline (Supplementary Figure S5H). However, it should be noted that we sacrificed the rats during daytime when they consumed less drug-containing pellets, and previous work has demonstrated that the autophosphorylation at Tyr705-TrkB is transient and most pronounced within 1 h after an injection of a tricyclic or a SSRI antidepressant.18, 19
Taken together, the increased BDNF transcription and Tyr816-TrkB phosphorylation by both nortriptyline and escitalopram in FSL rats correlate well with the antidepressant and anxiolytic effects of these agents, but not with the differential effects on recovery of emotional memory in the PA paradigm. On the other hand, the discrepancy of nortriptyline and escitalopram on Arc transcription corresponds better with their effects on PA memory performance. Arc, rather than BDNF, transcription was therefore chosen as a biochemical correlate in the mechanistic studies on serotonin-mediated modulation of PA using selective ligands. However, it should be noted that a contribution of BDNF/TrkB signaling to the effects of escitalopram on Arc transcription and PA performance cannot be excluded.
Given the restoring effects of escitalopram on PA in FSL rats and the alterations of 5-HT1AR levels, we went on to investigate the effects of pharmacological modulation of specific 5-HT receptor subtypes. Stimulation of 5-HT1AR impairs learning and memory functions in a range of rodent cognitive tasks.9 This agrees with the pharmacological response to the 5-HT1AR agonist 8-OH-DPAT in FRL rats (Figure 4a and Supplementary Figure S7D), resembling the impaired memory performance of untreated FSL rats compared with control FRL rats (Figures 4a, 5a and b). The blunted response of FSL rats to 8-OH-DPAT is consistent with the findings of reduced postsynaptic 5-HT1AR density (Figure 2a). Conversely, the 5-HT1AR antagonist NAD-299 facilitates several types of learning in rodents, attributed to alleviation of 5-HT1AR-activated Gi/o-mediated signaling cascades in postsynaptic neurons (Figure 4a).9, 41 Thus, the effects of blockade of pre- and postsynaptic 5-HT1AR resembled the effects of increased 5-HT function by escitalopram (Figures 3a and 5c). Despite an important role of 5-HT1ARs in modulation of SSRI efficacy, chronic fluoxetine still has antidepressant-like effects in 5-HT1AR KO mice, emphasizing an involvement of other 5-HT receptor subtypes.72 Intriguingly, the 5-HT4R antagonist GR125487 blocked the facilitatory effects of NAD-299 on PA performance (Figure 4a), indicating that activation of postsynaptic 5-HT4Rs is necessary for the restoring effect of 5-HT1AR antagonism on PA performance in FSL rats. Likewise, direct agonist stimulation of 5-HT4R with RS67333 also restored PA performance (Figure 4a). Thus, these data indicate that not only does 5-HT1AR antagonism disinhibit postsynaptic neurons by alleviating a tonic inhibition mediated by 5-HT, but also enables a shift in endogenous 5-HT transmission toward activation of the 5-HT4R and downstream signaling cascades (Figure 5c). The effects of chronic SSRI administration on PA performance and Arc mRNA were thus replicated by either 5-HT1AR antagonism or 5-HT4R agonism (Figures 4a, d, f and h), further supporting that central 5-HT4R stimulation offers a potential target for improvement of emotional16 and cognitive processing.17
Figure 5.
Schematic representation of mechanisms involved in effects on emotional memory function and stimulation of Arc transcription. Basal level of transmission in control Flinders resistant line (FRL) rats (a). Baseline transmission in untreated Flinders sensitive line (FSL) rats (b). The 5-HT therapy restores emotional memory disturbances and increases Arc transcription (c). Pretreatment with a MEK inhibitor blocks the restoring effects of pharmacological 5-HT therapy on memory performance and expression of Arc mRNA (d). See text besides respective figures for further details.
Systemic pretreatment with the MEK inhibitor PD184161 fully blocked the ability of both NAD-299 and RS67333 to restore memory function (Figure 4a) and induce Arc mRNA expression (Figures 4e, g and h). These data implicate an involvement of the MAPK signaling pathway as a nodal point in restoration of emotional memory consolidation. There is support for Gi/o-protein- and Gs-protein-mediated receptor modulation of the MEK/MAPK cascade.9, 23, 48, 49, 50, 73 Local increase of cyclic adenosine monophosphate (cAMP) may trigger events that are involved in induction of short-term plasticity and early stages of memory formation, whereas activation of MAPK signaling pathways may result in more long-lasting effects requiring nuclear gene transcription.21, 23 Besides, fear conditioning and long-term potentiation-mediated increase of Arc expression in the lateral amygdala is dependent on activation of the MAPK cascade,74 and hippocampal cAMP/MAPK signaling is involved in long-term consolidation of contextual fear conditioning.75 Collectively, these findings emphasize a crucial involvement of the MEK/MAPK cascade as a nodal point in 5-HT1AR/5-HT4R-mediated effects on emotional memory and expression of Arc mRNA (Figure 5d).
In conclusion, emotional memory impairments and baseline reductions of Arc transcription were found in the FSL rat line of depression. Memory functions and enhancement of Arc transcription were specifically induced by escitalopram and pharmacological treatments, increasing postsynaptic 5-HT function via stimulation of cAMP/MAPK signaling cascades. The FSL rat line provides a preclinical model recapitulating reversible deficits of cognitive processing that is disrupted in depression.
Acknowledgments
This work was supported by the Swedish Research Council, Swedish Royal Academy of Sciences, Söderberǵs stiftelse, Swedish Brain Fund and Karolinska Institute Faculty Funds (KID) (TME). We thank Dr Xiaoqun Zhang, Dr Hongshi Qi, Serena Balasso and Eleonora Tandoi for excellent experimental support.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Agid Y, Buzsáki G, Diamond DM, Frackowiak R, Giedd J, Girault JA, et al. How can drug discovery for psychiatric disorders be improved. Nat Rev Drug Discov. 2007;3:189–201. doi: 10.1038/nrd2217. [DOI] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive mechanisms in depression: implications for treatment. Annu Rev Neurosci. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- Montgomery SA. Why do we need new and better antidepressants. Int Clin Psychopharmacol. 2006;21 (Suppl 1:S1–S10. doi: 10.1097/01.yic.0000199455.39552.1c. [DOI] [PubMed] [Google Scholar]
- Harmer CJ. Serotonin and emotional processing: does it help explain antidepressant drug action. Neuropharmacology. 2008;55:1023–1028. doi: 10.1016/j.neuropharm.2008.06.036. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Chattarji S, Diamond DM, Jay TM, Reagan LP, Svenningsson P, et al. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry. 2010;15:237–249. doi: 10.1038/mp.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, et al. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry. 2005;57:935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Levy J, Fromm SJ, Nugent AC, Talagala SL, Hasler G, et al. The effects of tryptophan depletion on neural responses to emotional words in remitted depression. Biol Psychiatry. 2009;66:441–450. doi: 10.1016/j.biopsych.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren SO, Eriksson TM, Elvander-Tottie E, D'Addario C, Ekström JC, Svenningsson P, et al. The role of 5-HT(1A) receptors in learning and memory. Behav Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, et al. Serotonin 1Areceptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with WAY-100635. Mol Psychiatry. 2004;9:386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- Borg J. Molecular imaging of the 5-HT(1A) receptor in relation to human cognition. Behav Brain Res. 2008;195:103–111. doi: 10.1016/j.bbr.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H, et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int J Neuropsychopharmacol. 2008;11:465–476. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Howley E, Shi X, Sobanska A, Clarke G, Friedman L, et al. Antagonist but not agonist labeling of serotonin-1A receptors is decreased in major depressive disorder. J Psychiatr Res. 2009;43:887–894. doi: 10.1016/j.jpsychires.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, et al. Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron. 2007;55:712–725. doi: 10.1016/j.neuron.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Fontana DJ, Daniels SE, Wong EH, Clark RD, Eglen RM. The effects of novel, selective 5-hydroxytryptamine (5-HT)4 receptor ligands in rat spatial navigation. Neuropharmacology. 1997;36:689–696. doi: 10.1016/s0028-3908(97)00055-5. [DOI] [PubMed] [Google Scholar]
- Rantamäki T, Hendolin P, Kankaanpää A, Mijatovic J, Piepponen P, Domenici E, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32:2152–2162. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Stork PJ, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002;12:258–266. doi: 10.1016/s0962-8924(02)02294-8. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panja D, Dagyte G, Bidinosti M, Wibrand K, Kristiansen AM, Sonenberg N, et al. Novel translational control in Arc-dependent long term potentiation consolidation in vivo. J Biol Chem. 2009;284:31498–31511. doi: 10.1074/jbc.M109.056077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VR, Pintchovski SA, Chin J, Peebles CL, Mitra S, Finkbeiner S. AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat Neurosci. 2006;7:887–895. doi: 10.1038/nn1708. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDAR activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M, Montag D. Learning-induced arg 3.1/arc mRNA expression in the mouse brain. Learn Mem. 2003;10:99–107. doi: 10.1101/lm.53403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Waltereit R, Dammermann B, Wulff P, Scafidi J, Staubli U, Kauselmann G, et al. Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J Neurosci. 2001;21:5484–5493. doi: 10.1523/JNEUROSCI.21-15-05484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordera R, Pei Q, Newson M, Gray K, Sprakes M, Sharp T. Effect of different 5-HT1A receptor antagonists in combination with paroxetine on expression of the immediate-early gene Arc in rat brain. Neuropharmacology. 2003;44:893–902. doi: 10.1016/s0028-3908(03)00096-0. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Friedman E, Mathé AA, Yadid G. The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29:739–759. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Tfilin M, Sudai E, Merenlender A, Gispan I, Yadid G, Turgeman G. Mesenchymal stem cells increase hippocampal neurogenesis and counteract depressive-like behavior. Mol Psychiatry. 2010;15:1164–1175. doi: 10.1038/mp.2009.110. [DOI] [PubMed] [Google Scholar]
- Ryan B, Musazzi L, Mallei A, Tardito D, Gruber SH, El Khoury A, et al. Remodelling by early-life stress of NMDA receptor-dependent synaptic plasticity in a gene-environment rat model of depression. Int J Neuropsychopharmacol. 2009;12:553–559. doi: 10.1017/S1461145708009607. [DOI] [PubMed] [Google Scholar]
- Chen F, Madsen TM, Wegener G, Nyengaard JR. Imipramine treatment increases the number of hippocampal synapses and neurons in a genetic animal model of depression. Hippocampus. 2010;20:1376–1384. doi: 10.1002/hipo.20718. [DOI] [PubMed] [Google Scholar]
- El Khoury A, Gruber SH, Mørk A, Mathé AA. Adult life behavioral consequences of early maternal separation are alleviated by escitalopram treatment in a rat model of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:535–540. doi: 10.1016/j.pnpbp.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Petersén A, Wörtwein G, Gruber SH, El-Khoury A, Mathé AA. Nortriptyline mediates behavioral effects without affecting hippocampal cytogenesis in a genetic rat depression model. Neurosci Lett. 2009;451:148–151. doi: 10.1016/j.neulet.2008.12.046. [DOI] [PubMed] [Google Scholar]
- Eglen RM, Bonhaus DW, Johnson LG, Leung E, Clark RD. Pharmacological characterization of two novel and potent 5-HT4R agonists, RS 67333 and RS 67506, in vitro and in vivo. Br J Pharmacol. 1995;115:1387–1392. doi: 10.1111/j.1476-5381.1995.tb16628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgen M, Elvander E, Madjid N, Ogren SO. Analysis of the role of 5-HT1A receptors in spatial and aversive learning in the rat. Neuropharmacology. 2005;48:830–852. doi: 10.1016/j.neuropharm.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Aubert I, Burbaud P, Fredholm BB, Bloch B. Cellular distribution of adenosine A2A receptor mRNA in the primate striatum. J Comp Neurol. 1998;399:229–240. doi: 10.1002/(sici)1096-9861(19980921)399:2<229::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, et al. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, et al. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–784. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Misane I, Ogren SO. Effects of Hypericum perforatum (St. John's wort) on passive avoidance in the rat: evaluation of potential neurochemical mechanisms underlying its antidepressant activity. Pharmacopsychiatry. 2001;34:S89–S97. doi: 10.1055/s-2001-15449. [DOI] [PubMed] [Google Scholar]
- Garnovskaya MN, van Biesen T, Hawe B, Casanas Ramos S, Lefkowitz RJ, Raymond JR. Ras-dependent activation of fibroblast mitogen-activated protein kinase by 5-HT1AR via a G protein beta gamma-subunit-initiated pathway. Biochemistry. 1996;35:13716–13722. doi: 10.1021/bi961764n. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. 5-Hydroxytryptamine induces a protein kinase A/mitogen-activated protein kinase-mediated and macromolecular synthesis-dependent late phase of long-term potentiation in the amygdala. J Neurosci. 2007;27:3111–3119. doi: 10.1523/JNEUROSCI.3908-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norum JH, Hart K, Levy FO. Ras-dependent ERK activation by the human G(s)-coupled serotonin receptors 5-HT4(b) and 5-HT7(a) J Biol Chem. 2003;278:3098–3104. doi: 10.1074/jbc.M206237200. [DOI] [PubMed] [Google Scholar]
- Frodl T, Schaub A, Banac S, Charypar M, Jäger M, Kümmler P, et al. Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci. 2006;31:316–323. [PMC free article] [PubMed] [Google Scholar]
- Pfennig A, Littmann E, Bauer M. Neurocognitive impairment and dementia in mood disorders. J Neuropsychiatry Clin Neurosci. 2007;4:373–382. doi: 10.1176/jnp.2007.19.4.373. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Kubik S, Haghighi N, Steward O, Guzowski JF. Rapid activation of plasticity-associated gene transcription in hippocampal neurons provides a mechanism for encoding of one-trial experience. J Neurosci. 2009;29:898–906. doi: 10.1523/JNEUROSCI.4588-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61:105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. Subtype-specific alterations of γ-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and γ-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Auer DP, Pütz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- Nudmamud-Thanoi S, Reynolds GP. The NR1 subunit of the glutamate/NMDA receptor in the superior temporal cortex in schizophrenia and affective disorders. Neurosci Lett. 2004;372:173–177. doi: 10.1016/j.neulet.2004.09.035. [DOI] [PubMed] [Google Scholar]
- Nowak G, Ordway GA, Paul IA. Alterations in the N-methyl-d-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–164. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology. 2008;33:2175–2186. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Nishi K, Kanemaru K, Hasegawa S, Watanabe A, Diksic M. Both acute and chronic buspirone treatments have different effects on regional 5-HT synthesis in Flinders Sensitive Line rats (a rat model of depression) than in control rats. Neurochem Int. 2009;54:205–214. doi: 10.1016/j.neuint.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarch I. Cognitive toxicity of pharmacotherapeutic agents used in social anxiety disorder. Int J Clin Pract. 2009;63:1085–1094. doi: 10.1111/j.1742-1241.2009.02085.x. [DOI] [PubMed] [Google Scholar]
- Uher R, Maier W, Hauser J, Marusic A, Schmael C, Mors O, et al. Differential efficacy of escitalopram and nortriptyline on dimensional measures of depression. Br J Psychiatry. 2009;194:252–259. doi: 10.1192/bjp.bp.108.057554. [DOI] [PubMed] [Google Scholar]
- Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Chao MV. Promoting neurotrophic effects by GPCR ligands. Novartis Found Symp. 2006;276:181–189. [PubMed] [Google Scholar]
- Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–137. doi: 10.1016/s0896-6273(02)00942-x. [DOI] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Chen J, Shen C, Meller E. 5-HT1A receptor-mediated regulation of mitogen-activated protein kinase phosphorylation in rat brain. Eur J Pharmacol. 2002;452:155–162. doi: 10.1016/s0014-2999(02)02297-5. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, et al. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, Scheiner ZS, et al. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nat Neurosci. 2008;11:1074–1082. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.