Abstract
Activation of the visual pigment by light in rod and cone photoreceptors initiates our visual perception. As a result, the signaling properties of visual pigments, consisting of a protein, opsin, and a chromophore, 11-cis-retinal, play a key role in shaping the light responses of photoreceptors. The combination of pharmacological, physiological, and genetic tools has been a powerful approach advancing our understanding of the interactions between opsin and chromophore and how they affect the function of visual pigments. The signaling properties of the visual pigments modulate many aspects of the function of rods and cones, producing their unique physiological properties.
Keywords: G Protein-coupled Receptors (GPCR), Photoreceptors, Phototransduction, Rhodopsin, Vision, 11-cis-Retinal, Cones, Rods, Visual Cycle, Visual Pigment
Introduction
Our visual experience is initiated in the photoreceptors of our retina when a photon is absorbed by a molecule of visual pigment. The pigment is highly expressed in specialized ciliary structures of the vertebrate rod and cone photoreceptors, called outer segments (see the Prologue of this minireview series by Krzysztof Palczewski). The visual pigment is a G protein-coupled receptor that consists of a protein, opsin, covalently attached to a vitamin A-derived chromophore, 11-cis-retinal (1). Upon the absorption of a photon, the chromophore is isomerized from 11-cis to all-trans, which, in turn, induces changes in the pigment to its physiologically active metarhodopsin II (Meta II)2 state. The activated visual pigment molecule triggers a transduction cascade that ultimately results in the closure of nonselective cation cGMP-gated channels in the outer segment of photoreceptors, hyperpolarization of the photoreceptor, and a reduction in the release of the neurotransmitter glutamate from its synapse (2–4).
The human retina has one type of rod for dim light vision and three types of cone cells that allow color discrimination. Rods and cones share the same principles of phototransduction, the cellular mechanism of light detection. In addition, rods and cones employ homologous or sometimes even identical proteins in their phototransduction cascades. Despite these similarities, rods and cones exhibit important functional differences that can be demonstrated physiologically (Fig. 1, A and B) (5–8). First, rods are so sensitive that they can detect a single photon of light (9), which makes them perfectly suited for dim light vision. On the other hand, cones are up to 100-fold less sensitive than rods (Fig. 1C) (10, 11). As a result, they cannot signal in dim light conditions, depriving us of color vision at night. Second, rods saturate in even moderately bright light and remain nonfunctional during most of the day (12). In contrast, cones have a remarkable ability to adjust their sensitivity and remain photosensitive even in extremely bright light (13). This process, known as light adaptation, prevents cones from saturating in bright light and allows us to see throughout the day. With rods saturated, cones are responsible for most of the visual information reaching our brain during the day. Third, rods experience a long refractory time following exposure to bright light and can take up to 1 h to completely recover their sensitivity (14). In contrast, cones quickly recover their sensitivity within a few minutes. This process, known as dark adaptation (15), prevents cones from becoming refractory and allows us to retain visual perception in a quickly changing light environment.
FIGURE 1.
Difference in kinetics and sensitivity between rod and cone light responses. A, suction electrode recording from a salamander cone. The inner segment of a dissociated cone is drawn in the electrode to collect and measure the membrane current flowing through the cell. The reduction in the current produced by the light stimulation represents the light response of the cell. B, family of flash responses from a salamander rod and red cone to flashes of increasing intensity. Note the difference in rod and cone response kinetics. C, intensity-response functions of salamander rod and red cone based on the responses in B. Note the right shift of the cone intensity-response curve, demonstrating the lower sensitivity of cones compared with rods. This figure was reprinted from Ref. 97 with permission. PIGM, pigment.
The functional differences between rods and cones have been well documented. However, the mechanisms producing these differences are still not well understood and are a subject of active investigation (16). Considering that the activation of multiple G proteins by a single photoactivated pigment molecule represents the first amplification step in the phototransduction cascade, it is likely that changes in the activity or lifetime of the photoactivated pigment will have substantial effects on the light responses of photoreceptors. In addition, considering that there are ∼107–108 pigment molecules in a mammalian rod (17–19), the basal activity of the pigment and its spontaneous activation will also be expected to have substantial effects on the physiological properties of rods and cones. The interaction between opsin and the retina and its effect on the signaling properties of vertebrate pigments can be studied with biochemical or physiological tools by substituting the native chromophore with various chromophore analogs (20, 21). Although removed from the pigment epithelium, photoreceptors retain their functional properties long enough to allow careful physiological studies. The recent development of transgenic tools has added a second powerful approach to studying the opsin-chromophore interaction by substituting the endogenous opsin with various mutant forms. This method has also proven useful in understanding how opsin mutations affect the function of the pigment, potentially leading to visual disorders (22–24). In this minireview, we summarize some of the findings on how opsin-chromophore interactions affect the function of photoreceptors and contribute to the distinct physiological properties of rods and cones.
Rod Versus Cone Pigment Signaling
One way of investigating how visual pigments determine the function of rod and cone photoreceptors is to compare their signaling properties directly. The efficiencies with which rod and cone visual pigments activate the phototransduction cascade and are subsequently inactivated by it can be studied in transgenic photoreceptors coexpressing the two pigment types. Red cone pigment expressed in transgenic Xenopus rods induces a red shift in the spectral sensitivity of the cells, demonstrating the ability of the transgenic cone pigment to couple to the rod phototransduction cascade (25). The spectral separation between the two pigments allows a comparison of the photoresponses generated preferentially by the rod or cone pigment in the same rod. Notably, the two responses have identical amplitude and kinetics, indicating that red cone pigment produces rod-like responses when expressed in rods. Conversely, Xenopus red cones expressing transgenic human rod pigment demonstrate that rod pigment produces cone-like responses when expressed in a cone (25).
More recently, such studies have been extended to transgenic mouse rod photoreceptors. This approach has the great advantage that it enables the use of rhodopsin knock-out animals to generate and functionally characterize mice with rod photoreceptors expressing exclusively cone opsins (26–29). Such a pigment substitution produces a dramatic shift in the spectral sensitivity of these transgenic rods, rendering them most sensitive to ultraviolet light (29), consistent with the peak of the absorption spectrum of mouse S-opsin, or to red light (28), consistent with the peak of the absorption spectrum of human L-opsin. However, the light responses produced by the cone pigment in these transgenic rods still have rod-like amplitude and kinetics. Taken together, these results demonstrate that rod and cone pigments are equivalent with respect to signaling downstream in phototransduction: first, the active lifetimes of both are dictated by shutoff regulated by phosphorylation and arrestin binding rather than by the decay of their physiologically active intermediate (Meta II); second, the rhodopsin kinase and arrestin in a rod or cone act identically on rod and cone pigments; and third, rod and cone pigments have identical efficacies when coupled to a given (rod or cone) phototransduction cascade.
Spontaneous Thermal Activation of Visual Pigment
The visual pigment can undergo spontaneous thermal activation when 11-cis-retinal is spontaneously isomerized to all-trans-retinal in the absence of light. As this event produces a cellular response that is identical to the one produced by a photon of light (30), a high rate of spontaneous activation would interfere with the function of rods as photon counters. However, the mouse rod visual pigment has an astonishingly low rate of spontaneous activation, ∼10−11 s−1 per molecule, corresponding on average to an isomerization every 2000 years (2)! As a result, despite the very high expression level of pigment in the outer segments of mouse rods, their rate of spontaneous thermal pigment activation is only 0.012 s−1 per cell (31).
It had been previously suggested that, due to their low activation energy, cone pigments will undergo spontaneous activation in darkness at a high rate (32). This hypothesis was supported by studies showing that salamander red cones have a high level of activity, or noise, in darkness (33, 34). Considering that cones are 30–100-fold less sensitive than rods (Fig. 1C), a higher rate of cone pigment thermal activation represents a possible mechanism contributing to their lower sensitivity. However, the single photon response in cones is too small to be physiologically observed (33), making the direct measurement of the rate of cone pigment activation impossible by currently available methods.
As discussed above, when cone pigments are expressed in rods, where their activation is amplified by the rod phototransduction cascade, they produce detectable single photon responses. As a result, the thermal activation of cone pigment in transgenic rods produces observable cellular responses that allow the measurement of the molecular rate of cone pigment thermal activation. Using this approach, the rate of spontaneous thermal activation of L-cone opsin expressed in Xenopus rods was found to be some 10,000-fold higher than the corresponding rod pigment thermal activation rate (25). This high rate of spontaneous cone pigment activation results in constitutive activity of the cone phototransduction cascade even in darkness. Thus, amphibian red cones are constantly exposed to “dark light,” which induces adaptation and therefore contributes to their lower sensitivity and faster response kinetics compared with rods. In mouse rods, the rate of spontaneous thermal activation of L-cone pigment was found to be 1.35 × 10−7 s−1 (28), still substantially higher than the corresponding rod pigment rate but ∼40-fold lower than that estimated from Xenopus rods after taking into account the difference in temperature of the mouse and Xenopus tissues. The discrepancy in the thermal rates of cone pigment activation in Xenopus and mouse is likely due to the different chromophores that these two species use (11-cis-3-dehydroretinal, or A2, in Xenopus versus 11-cis-retinal, or A1, in mouse). In addition, the rate of thermal activation of the pigment is directly related to its spectral sensitivity, with longer wavelength pigment having a higher rate of spontaneous activation (35, 36).
Spontaneous Cone Pigment Dissociation
Biochemical studies from the 1960s and 1970s had suggested that, in addition to a higher rate of spontaneous thermal activation, cone pigments might also be prone to spontaneous dissociation into free opsin and 11-cis-retinal (37, 38). In contrast, the covalent bond between opsin and 11-cis-retinal in rod pigments appears to be very stable (39). The stability of the covalent bond between opsin and 11-cis-retinal can be examined physiologically using pharmacological manipulations in amphibian photoreceptors (40). Incubation of dark-adapted salamander cones with exogenous 9-cis-retinal produces a gradual blue spectral shift in the cells' action spectrum, consistent with the gradual exchange of the endogenous 11-cis-chromophore with 9-cis-retinal. In addition, treatment of dark-adapted salamander cones with the chromophore-binding protein CRALBP, which binds specifically 11-cis-retinoids, results in gradual desensitization of the cones, consistent with the gradual removal of chromophore from their pigment. Notably, the subsequent addition of exogenous 11-cis-retinal not only reverses the effect of CRALBP but results in 2-fold higher sensitivity in cones compared with their initial dark-adapted state. Such evidence for the presence of free opsin can be observed in dark-adapted salamander red cones and blue cone pigment-containing blue cones and green rods (41) but not in red rods. These results demonstrate that the formation of the covalent bond between opsin and 11-cis-retinal is reversible in darkness in amphibian red and blue cones but is irreversible in rods. This dissociation results in a surprisingly large amount of free opsin (∼10%) in fully dark-adapted red cones. Notably, with its constitutive transducin-stimulating activity (42), the free cone opsin produces desensitization in salamander red cones equivalent to that of a steady light causing 500 photoisomerizations/s (40), quite similar to the level of constitutive activity produced by thermal activation of their pigment (25, 33, 34). It is not known whether mammalian cone pigments are also prone to spontaneous dissociation similar to their amphibian counterparts.
Lifetime of Photoactivated Pigment
Normally, the decay of the physiologically active Meta II state of the visual pigment to its inactive Meta III form and then to free opsin is slower than its inactivation by the photoreceptor cell. Following absorption of a photon, the pigment is rapidly inactivated in a two-step process that involves partial initial inactivation by rhodopsin kinase (43), followed by the binding of a capping protein, arrestin, which blocks completely the ability of the visual pigment to activate the phototransduction cascade (44). This pigment inactivation takes place within tens of milliseconds after photoactivation (45, 46). In contrast, the decay of the photoactivated pigment to opsin and all-trans-retinal takes seconds in cones and minutes in rods (47). Contrary to early expectations, the experiments with transgenic animals discussed above revealed that the characteristics of the light response are not controlled by the visual pigment and instead depend on the properties of the phototransduction cascade triggered by the pigment. Indeed, recent studies have demonstrated notable differences in the efficiency of activating phototransduction by visual pigment (48) and in the rates of pigment inactivation by phosphorylation (49) in fish rods and cones.
Under dim light conditions, Meta II is inactivated rapidly enough by the cell that its decay does not affect the light response. However, it is possible that, under brighter light conditions, the capacity of the photoreceptor to turn off the photoactivated pigment by phosphorylation and arrestin binding becomes exhausted. This scenario would render the decay of Meta II rate-limiting for the termination of the light response. Indeed, experiments with the chromophore analog 9-demethylretinal support this notion. This retinoid can form functional pigment; however, its Meta II decays slower than that of 11-cis-retinal pigment (50). Salamander cones with pigment regenerated with 9-demethylretinal produce dim flash responses with normal kinetics. However, for flashes activating >0.2% of the 9-demethyl cone pigment, the response inactivation becomes progressively slower with increased light intensity (51), suggesting that, under brighter light, the decay of Meta II might dominate the response shutoff. The validity of this hypothesis remains to be tested in mammalian photoreceptors. It would be particularly significant in cones, where rapid response inactivation is critical for their function in bright light.
The lifetime of the physiologically active Meta II state of rod visual pigments can be estimated from biochemical experiments with purified or heterologously expressed opsins (47). This approach is challenging in the case of cone pigments, as their low abundance and instability make their purification challenging. However, the recent development of transgenic mouse models has rendered possible the estimation of Meta II lifetime in intact photoreceptors. The light response shutoff in wild-type mouse rods reflects the inactivation of the phototransduction cascade. In the absence of arrestin, the rod light response shutoff becomes biphasic, with a rapid initial shutoff driven by the phosphorylation of the pigment, followed by a substantially slower second phase, driven by the decay of the phosphorylated Meta II pigment (Fig. 2A) (44). This has allowed the measurement of the Meta II decay rate of various pigments under physiological conditions. For instance, mouse rod Meta II decays with a time constant of ∼50 s (26, 44). However, the E122Q mutation of rod opsin accelerates Meta II decay to 4.9 s (26). These values are comparable with the numbers derived from biochemical experiments (52). Using the same approach in transgenic mouse rods expressing cone pigments, the rate of mouse S-cone pigment Meta II decay was found to be ∼1.2 s (Fig. 2B) (29), whereas human L-cone pigment Meta II decayed with a time constant of 0.6 s (Fig. 2C) (28), both significantly faster than the corresponding rate of rod pigment Meta II decay. Notably, the difference in decay of S- and L-cone pigment Meta II might be the mechanism producing the difference in response kinetics from mouse S- and M-cones lacking arrestin (53).
FIGURE 2.
Measurements of Meta II decay time constant from rod and cone pigments. The deletion of arrestin in rods renders the decay of Meta II rate-limiting for the response shutoff. As a result, the rate of rod pigment Meta II decay can be measured from the decay rate of the dim flash light response in mouse rods lacking arrestin (A). Using the same approach, the Meta II decay rate of mouse S- and L-cone pigments can be estimated from transgenic mouse rods expressing S-opsin (B) or L-opsin (C) and lacking arrestin (arr−/−) and the endogenous rod pigment (rho−/−). This figure was reprinted from Refs. 28 and 29 with permission.
Pigment Decay and Regeneration
In addition to activating the phototransduction cascade and producing a cellular response, light also renders the pigment molecule unable to detect a subsequent photon. In invertebrate photoreceptors, the pigment is reset to its ground state when it absorbs a second photon that reisomerizes the chromophore from all-trans- back to 11-cis-retinal (54). In contrast, in the vertebrate retina, resetting the visual pigment molecule to its ground state is a complex process called the visual cycle (Fig. 3), which involves multiple enzymatic reactions in and out of photoreceptors. Following photoisomerization of the chromophore, its covalent Schiff base bond to opsin is hydrolyzed, and all-trans-retinal is released from the pigment, leaving it in an apo-opsin form. The chromophore is then reduced to all-trans-retinol by a set of retinal dehydrogenases (55). Photoreceptors express a number of retinal dehydrogenases in their outer segment, and the individual role of each one is still not well understood (56). In the next step of the visual cycle, all-trans-retinol is exported from the outer segments of rods and cones to the adjacent layer of the retinal pigment epithelium (RPE), where, through a series of reactions, it is converted back to 11-cis-retinal. Notably, both the reduction of bleached chromophore and its removal from the outer segment occur much faster in cones than in rods (57). Several recent excellent reviews describe in detail the reactions involved in the recycling of chromophore by the RPE (58–61). Upon returning to the outer segment, 11-cis-retinal binds to opsin and then forms a covalent bond with it to regenerate the visual pigment (62).
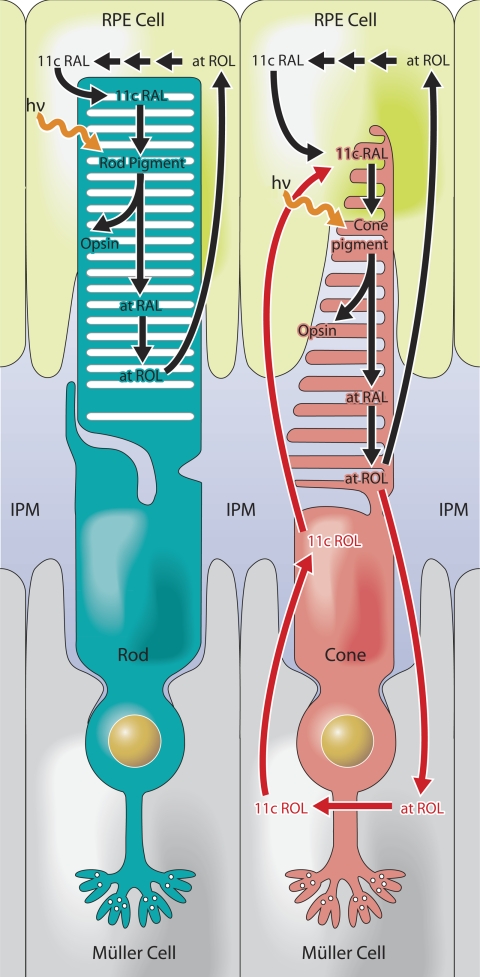
FIGURE 3.
Schematics of two visual cycles in vertebrate eye. The canonical RPE visual cycle (left) recycles all-trans-retinol (at ROL) released from rods and cones following a bleach to 11-cis-retinal (11c ROL), which can be used by both rods and cones for pigment regeneration. The retina visual cycle (right) relies on the Müller cells to recycle all-trans-retinol released from cones to 11-cis-retinol, which only cones can move to their outer segments and oxidize to 11-cis-retinal for regeneration of the pigment. IPM, interphotoreceptor matrix. hν, photon of light.
It has been suggested that the rate-limiting step for rod dark adaptation resides outside photoreceptors and is associated with the delivery of recycled chromophore from the RPE (60). This notion is supported by experiments with knock-in mice expressing mutant E122Q rhodopsin in their rods. This mutation of rod opsin accelerates dramatically both the decay of photoactivated pigment and the regeneration of pigment from opsin and 11-cis-retinal (52), yet the kinetics of pigment regeneration in vivo are not affected by the E122Q mutation (26), indicating that rod pigment regeneration is not rate-limited by the decay of photoactivated pigment or by the formation of a covalent bond between opsin and 11-cis-retinal.
Dark Adaptation
Exposure of photoreceptors to bright light photoactivates (bleaches) a large fraction of their visual pigment, leading to its eventual decay to free opsin. As a result, photoreceptor light sensitivity is reduced. This state of bleach adaptation is produced by two mechanisms. First, the level of visual pigment remaining in photoreceptors and available for subsequent light activation is reduced, and this lowered quantum catch produces a proportional decline in light sensitivity. Second, the photoactivated pigment remains capable of activating the phototransduction cascade either by spontaneous reversal from Meta III to the physiologically active Meta II (64) or by the activation by apo-opsin (65). Although the activity of an opsin molecule is 104–106 times lower than that of Meta II (66, 67), the accumulation of large amounts of opsin following a bright bleach can produce a significant desensitization. The constitutive activation of the phototransduction cascade by opsin produces adaptation similar to that induced by exposing the photoreceptors to steady background light (68). The relative contribution of quantum catch loss and opsin activation varies with bleach fraction (68). For instance, bleaching 50% of the pigment in salamander red cones reduces their sensitivity by 2-fold due to reduced quantum catch and by another 5-fold due to opsin activation (40).
Dark adaptation of rod and cone photoreceptors after exposure to bright light requires regeneration of the visual pigment from opsin and 11-cis-retinal to restore the quantum catch and to remove the constitutive activation produced by opsin. As a result, the process of pigment regeneration can be studied physiologically by monitoring the rate of dark adaptation of photoreceptors. This method has proven particularly useful for studying cone pigment regeneration in the intact eye (69) or in an isolated eyecup (70).
Opsin-Chromophore Interaction during Pigment Regeneration
The regeneration of visual pigment is a two-step process in which 11-cis-retinal first binds noncovalently in the chromophore pocket (71) and then forms a covalent Schiff base bond with opsin (72). The truncated chromophore analog β-ionone, which can bind in the chromophore pocket of opsin without forming a covalent bond with it, has proven very useful for studying the noncovalent interactions between opsin and chromophore (73, 74). In salamander rods, the occupancy of the chromophore pocket by β-ionone activates opsin (75), which produces desensitization of the rods and acceleration of their flash responses. The activation of rod opsin by noncovalently bound chromophore analogs has been demonstrated also in biochemical experiments (74, 76). In contrast, the noncovalent binding of β-ionone to cone opsin in salamander red cones quenches the free opsin activity and reduces their desensitization (77, 78). Based on these findings, a model of pigment regeneration has been proposed in which the binding of 11-cis-retinal to opsin causes transient activation of the complex in rods and a decrease in light sensitivity. In contrast, in cones, the noncovalent binding of 11-cis-retinal to opsin quenches the residual activity of free opsin and promotes an increase in light sensitivity even before the regeneration of the pigment (79, 80). As a result, the noncovalent binding of 11-cis-retinal to rod opsin produces a delay in dark adaptation in rods but accelerates dark adaptation in red cones, contributing in this way to the large difference in time required for the scotopic (rod) and photopic (cone) phases of dark adaptation.
Chromophore Traffic
Timely and efficient pigment regeneration is critical for the proper function of photoreceptors. The mechanism by which the chromophore moves between the photoreceptors and RPE cells is still unclear. A chromophore-binding protein, interphotoreceptor retinoid-binding protein (IRBP), is abundantly expressed by photoreceptors and released in the space around their outer segments (81). IRBP can bind both all-trans- and 11-cis-chromophores, suggesting that it could be a carrier protein transporting the retinoid back and forth between photoreceptors and RPE cells. However, this issue has remained controversial, with contradicting results (82–86) even after the generation of IRBP-deficient mice. Recent physiological studies demonstrate that the deletion of IRBP does not affect the kinetics of dark adaptation in mouse rods or cones, suggesting that it does not play a role in accelerating the traffic of chromophore in and out of photoreceptors (86). Nevertheless, IRBP affects photoreceptor development (87), and its deletion leads to gradual retinal degeneration and possible chromophore deficiency (84, 85), indicating that IRBP plays an important role in photoreceptor function and can regulate chromophore homeostasis.
Chromophore Recycling
The fast turnover of cone visual pigment required for cones to rapidly dark-adapt and to remain functional in bright light imposes the need for rapid recycling of their chromophore from all-trans-retinol back to 11-cis-retinal. The canonical pathway for chromophore recycling (58) involves the RPE, where all-trans-retinol is converted into 11-cis-retinal via a series of enzymatic reactions and then transported back to the photoreceptors for incorporation into opsin. Mounting biochemical evidence indicates that this pathway may not be sufficient for rapidly supplying cones with recycled chromophore (88–90), and it is now clear that a second visual cycle exists in the neural retina (91, 92). This pathway promotes pigment regeneration and dark adaptation specifically in cones and not in rods (Fig. 3). This visual cycle is independent of the RPE and instead involves the Müller cells, which convert all-trans-retinol released from bleached cone pigment into 11-cis-retinol (93, 94). As only cones can oxidize 11-cis-retinol and use it for pigment regeneration (92, 95), this likely represents one of the mechanisms restricting the retina visual cycle to cones. In addition, chromophore can traffic from the inner segment, contacting the Müller cells, to the outer segment, where opsin is located, only in cones but not in rods (96), providing a second possible mechanism for the cone selectivity of this visual cycle. The function of the retina visual cycle has been demonstrated in a variety of species, from salamander and zebrafish to mouse, primates, and humans (63, 91, 92), demonstrating its functional significance. Studies in the mouse indicate that the retina visual cycle promotes cone adaptation 8-fold faster than the RPE (70). However, complete cone recovery requires both visual cycles, indicating that the retina visual cycle is critical for the initial rapid regeneration of mouse M/L-cone pigment during dark adaptation, whereas the slower RPE visual cycle is required to complete the process. The recent demonstration of a functional retina visual cycle in the mouse retina opens exciting new areas of research. The combination of physiological, genetic, and pharmacological tools described here will be a powerful approach for understanding the molecular mechanisms of this pathway and its functional role in healthy as well as in diseased retina.
This work was supported, in whole or in part, by National Institutes of Health Grants EY019312 and EY021126 (to V. J. K.) and by Grant EY002687 (to the Department of Ophthalmology and Visual Sciences, Washington University). This work was also supported by a Research to Prevent Blindness career development award (to V. J. K.). This is the fourth article in the Thematic Minireview Series on Focus on Vision.
- Meta II
- metarhodopsin II
- RPE
- retinal pigment epithelium
- IRBP
- interphotoreceptor retinoid-binding protein.
REFERENCES
- 1. Hargrave P. A. (2001) Invest. Ophthalmol. Vis. Sci. 42, 3–9 [PubMed] [Google Scholar]
- 2. Burns M. E., Baylor D. A. (2001) Annu. Rev. Neurosci. 24, 779–805 [DOI] [PubMed] [Google Scholar]
- 3. Arshavsky V. Y., Lamb T. D., Pugh E. N., Jr. (2002) Annu. Rev. Physiol. 64, 153–187 [DOI] [PubMed] [Google Scholar]
- 4. Ebrey T., Koutalos Y. (2001) Prog. Retin. Eye Res. 20, 49–94 [DOI] [PubMed] [Google Scholar]
- 5. Fu Y., Yau K. W. (2007) Pflugers Arch. 454, 805–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yau K. W. (1994) Invest. Ophthalmol. Vis. Sci. 35, 9–32 [PubMed] [Google Scholar]
- 7. Luo D. G., Kefalov V., Yau K. W. (2008) in The Senses: A Comprehensive Reference (Allan I. B., Akimichi K., Gordon M. S., Gerald W., Thomas D. A., Richard H. M., Peter D., Donata O., Stuart F., Gary K. B., Bushnell M. C., Jon H. K., Esther G., eds) pp. 269–301, Academic Press, New York [Google Scholar]
- 8. Luo D. G., Xue T., Yau K. W. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 9855–9862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baylor D. A., Lamb T. D., Yau K. W. (1979) J. Physiol. 288, 613–634 [PMC free article] [PubMed] [Google Scholar]
- 10. Rushton W. A. (1965) J. Physiol. 181, 641–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baylor D. A. (1987) Invest. Ophthalmol. Vis. Sci. 28, 34–49 [PubMed] [Google Scholar]
- 12. Green D. G. (1971) Science 174, 598–600 [DOI] [PubMed] [Google Scholar]
- 13. Boynton R. M., Whitten D. N. (1970) Science 170, 1423–1426 [DOI] [PubMed] [Google Scholar]
- 14. Rushton W. A. (1965) Proc. R. Soc. Lond. B Biol. Sci. 162, 20–46 [DOI] [PubMed] [Google Scholar]
- 15. Fain G. L., Matthews H. R., Cornwall M. C., Koutalos Y. (2001) Physiol. Rev. 81, 117–151 [DOI] [PubMed] [Google Scholar]
- 16. Kawamura S., Tachibanaki S. (2008) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 150, 369–377 [DOI] [PubMed] [Google Scholar]
- 17. Lyubarsky A. L., Daniele L. L., Pugh E. N., Jr. (2004) Vision Res. 44, 3235–3251 [DOI] [PubMed] [Google Scholar]
- 18. Nickell S., Park P. S., Baumeister W., Palczewski K. (2007) J. Cell Biol. 177, 917–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liang Y., Fotiadis D., Filipek S., Saperstein D. A., Palczewski K., Engel A. (2003) J. Biol. Chem. 278, 21655–21662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cornwall M. C., Jones G. J., Kefalov V. J., Fain G. L., Matthews H. R. (2000) Methods Enzymol. 316, 224–252 [DOI] [PubMed] [Google Scholar]
- 21. Corson D. W., Crouch R. K. (1996) Photochem. Photobiol. 63, 595–600 [DOI] [PubMed] [Google Scholar]
- 22. Jin S., Cornwall M. C., Oprian D. D. (2003) Nat. Neurosci. 6, 731–735 [DOI] [PubMed] [Google Scholar]
- 23. Dizhoor A. M., Woodruff M. L., Olshevskaya E. V., Cilluffo M. C., Cornwall M. C., Sieving P. A., Fain G. L. (2008) J. Neurosci. 28, 11662–11672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kefalov V. J., Cornwall M. C., Fain G. L. (2010) Methods Mol. Biol. 652, 95–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kefalov V., Fu Y., Marsh-Armstrong N., Yau K. W. (2003) Nature 425, 526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Imai H., Kefalov V., Sakurai K., Chisaka O., Ueda Y., Onishi A., Morizumi T., Fu Y., Ichikawa K., Nakatani K., Honda Y., Chen J., Yau K. W., Shichida Y. (2007) J. Biol. Chem. 282, 6677–6684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sakurai K., Onishi A., Imai H., Chisaka O., Ueda Y., Usukura J., Nakatani K., Shichida Y. (2007) J. Gen. Physiol. 130, 21–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fu Y., Kefalov V., Luo D. G., Xue T., Yau K. W. (2008) Nat. Neurosci. 11, 565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shi G., Yau K. W., Chen J., Kefalov V. J. (2007) J. Neurosci. 27, 10084–10093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baylor D. A., Matthews G., Yau K. W. (1980) J. Physiol. 309, 591–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burns M. E., Mendez A., Chen J., Baylor D. A. (2002) Neuron 36, 81–91 [DOI] [PubMed] [Google Scholar]
- 32. Barlow H. B. (1957) Nature 179, 255–256 [DOI] [PubMed] [Google Scholar]
- 33. Rieke F., Baylor D. A. (2000) Neuron 26, 181–186 [DOI] [PubMed] [Google Scholar]
- 34. Sampath A. P., Baylor D. A. (2002) Biophys. J. 83, 184–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ala-Laurila P., Donner K., Koskelainen A. (2004) Biophys. J. 86, 3653–3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luo D. G., Yue W. W., Ala-Laurila P., Yau K. W. (2011) Science 332, 1307–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Crescitelli F. (1984) Vision Res. 24, 1551–1553 [DOI] [PubMed] [Google Scholar]
- 38. Matsumoto H., Tokunaga F., Yoshizawa T. (1975) Biochim. Biophys. Acta 404, 300–308 [DOI] [PubMed] [Google Scholar]
- 39. Defoe D. M., Bok D. (1983) Invest. Ophthalmol. Vis. Sci. 24, 1211–1226 [PubMed] [Google Scholar]
- 40. Kefalov V. J., Estevez M. E., Kono M., Goletz P. W., Crouch R. K., Cornwall M. C., Yau K. W. (2005) Neuron 46, 879–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma J., Znoiko S., Othersen K. L., Ryan J. C., Das J., Isayama T., Kono M., Oprian D. D., Corson D. W., Cornwall M. C., Cameron D. A., Harosi F. I., Makino C. L., Crouch R. K. (2001) Neuron 32, 451–461 [DOI] [PubMed] [Google Scholar]
- 42. Cornwall M. C., Matthews H. R., Crouch R. K., Fain G. L. (1995) J. Gen. Physiol. 106, 543–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen C. K., Burns M. E., Spencer M., Niemi G. A., Chen J., Hurley J. B., Baylor D. A., Simon M. I. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 3718–3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu J., Dodd R. L., Makino C. L., Simon M. I., Baylor D. A., Chen J. (1997) Nature 389, 505–509 [DOI] [PubMed] [Google Scholar]
- 45. Makino C. L., Wen X. H., Lem J. (2003) Curr. Opin. Neurobiol. 13, 404–412 [DOI] [PubMed] [Google Scholar]
- 46. Burns M. E., Pugh E. N., Jr. (2010) Physiology 25, 72–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shichida Y., Imai H. (1998) Cell. Mol. Life Sci. 54, 1299–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tachibanaki S., Tsushima S., Kawamura S. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 14044–14049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tachibanaki S., Arinobu D., Shimauchi-Matsukawa Y., Tsushima S., Kawamura S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 9329–9334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Das J., Crouch R. K., Ma J. X., Oprian D. D., Kono M. (2004) Biochemistry 43, 5532–5538 [DOI] [PubMed] [Google Scholar]
- 51. Estevez M. E., Kolesnikov A. V., Ala-Laurila P., Crouch R. K., Govardovskii V. I., Cornwall M. C. (2009) J. Gen. Physiol. 134, 137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Imai H., Kojima D., Oura T., Tachibanaki S., Terakita A., Shichida Y. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 2322–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nikonov S. S., Brown B. M., Davis J. A., Zuniga F. I., Bragin A., Pugh E. N., Jr., Craft C. M. (2008) Neuron 59, 462–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kusakabe T. G., Takimoto N., Jin M., Tsuda M. (2009) Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2897–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Parker R. O., Crouch R. K. (2010) Exp. Eye Res. 91, 788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maeda A., Maeda T., Sun W., Zhang H., Baehr W., Palczewski K. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19565–19570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Koutalos Y., Cornwall M. C. (2010) Methods Mol. Biol. 652, 129–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saari J. C. (2000) Invest. Ophthalmol. Vis. Sci. 41, 337–348 [PubMed] [Google Scholar]
- 59. McBee J. K., Palczewski K., Baehr W., Pepperberg D. R. (2001) Prog. Retin. Eye Res. 20, 469–529 [DOI] [PubMed] [Google Scholar]
- 60. Lamb T. D., Pugh E. N., Jr. (2004) Prog. Retin. Eye Res. 23, 307–380 [DOI] [PubMed] [Google Scholar]
- 61. Kiser P. D., Golczak M., Maeda A., Palczewski K. (2012) Biochim. Biophys Acta, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pepperberg D. R., Brown P. K., Lurie M., Dowling J. E. (1978) J. Gen. Physiol. 71, 369–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fleisch V. C., Schonthaler H. B., von Lintig J., Neuhauss S. C. (2008) J. Neurosci. 28, 8208–8216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Leibrock C. S., Lamb T. D. (1997) J. Physiol. 501, 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fain G. L., Matthews H. R., Cornwall M. C. (1996) Trends Neurosci. 19, 502–507 [DOI] [PubMed] [Google Scholar]
- 66. Cornwall M. C., Fain G. L. (1994) J. Physiol. 480, 261–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fan J., Woodruff M. L., Cilluffo M. C., Crouch R. K., Fain G. L. (2005) J. Physiol. 568, 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jones G. J., Cornwall M. C., Fain G. L. (1996) J. Gen. Physiol. 108, 333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mahroo O. A., Lamb T. D. (2004) J. Physiol. 554, 417–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kolesnikov A. V., Tang P. H., Parker R. O., Crouch R. K., Kefalov V. J. (2011) J. Neurosci. 31, 7900–7909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Matsumoto H., Yoshizawa T. (1975) Nature 258, 523–526 [DOI] [PubMed] [Google Scholar]
- 72. Bownds D. (1967) Nature 216, 1178–1181 [DOI] [PubMed] [Google Scholar]
- 73. Crouch R. K., Veronee C. D., Lacy M. E. (1982) Vision Res. 22, 1451–1456 [DOI] [PubMed] [Google Scholar]
- 74. Buczyłko J., Saari J. C., Crouch R. K., Palczewski K. (1996) J. Biol. Chem. 271, 20621–20630 [DOI] [PubMed] [Google Scholar]
- 75. Kefalov V. J., Carter Cornwall M., Crouch R. K. (1999) J. Gen. Physiol. 113, 491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kono M., Crouch R. K. (2010) Methods Mol. Biol. 652, 85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jin J., Crouch R. K., Corson D. W., Katz B. M., MacNichol E. F., Cornwall M. C. (1993) Neuron 11, 513–522 [DOI] [PubMed] [Google Scholar]
- 78. Isayama T., Chen Y., Kono M., Degrip W. J., Ma J. X., Crouch R. K., Makino C. L. (2006) Vis. Neurosci. 23, 899–908 [DOI] [PubMed] [Google Scholar]
- 79. Corson D. W., Kefalov V. J., Cornwall M. C., Crouch R. K. (2000) J. Gen. Physiol. 116, 283–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kefalov V. J., Crouch R. K., Cornwall M. C. (2001) Neuron 29, 749–755 [DOI] [PubMed] [Google Scholar]
- 81. Gonzalez-Fernandez F. (2003) Vision Res. 43, 3021–3036 [DOI] [PubMed] [Google Scholar]
- 82. Ripps H., Peachey N. S., Xu X., Nozell S. E., Smith S. B., Liou G. I. (2000) Vis. Neurosci. 17, 97–105 [DOI] [PubMed] [Google Scholar]
- 83. Palczewski K., Van Hooser J. P., Garwin G. G., Chen J., Liou G. I., Saari J. C. (1999) Biochemistry 38, 12012–12019 [DOI] [PubMed] [Google Scholar]
- 84. Parker R. O., Fan J., Nickerson J. M., Liou G. I., Crouch R. K. (2009) J. Neurosci. 29, 4616–4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jin M., Li S., Nusinowitz S., Lloyd M., Hu J., Radu R. A., Bok D., Travis G. H. (2009) J. Neurosci. 29, 1486–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Parker R., Wang J. S., Kefalov V. J., Crouch R. K. (2011) J. Neurosci. 31, 4714–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wisard J., Faulkner A., Chrenek M. A., Waxweiler T., Waxweiler W., Donmoyer C., Liou G. I., Craft C. M., Schmid G. F., Boatright J. H., Pardue M. T., Nickerson J. M. (2011) Invest. Ophthalmol. Vis. Sci. 52, 5804–5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bustamante J. J., Ziari S., Ramirez R. D., Tsin A. T. (1995) Am. J. Physiol. 269, R1346–R1350 [DOI] [PubMed] [Google Scholar]
- 89. Das S. R., Bhardwaj N., Kjeldbye H., Gouras P. (1992) Biochem. J. 285, 907–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mata N. L., Radu R. A., Clemmons R. C., Travis G. H. (2002) Neuron 36, 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang J. S., Estevez M. E., Cornwall M. C., Kefalov V. J. (2009) Nat. Neurosci. 12, 295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang J. S., Kefalov V. J. (2009) Curr. Biol. 19, 1665–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang J. S., Kefalov V. J. (2011) Prog. Retin. Eye Res. 30, 115–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fleisch V. C., Neuhauss S. C. (2010) Prog. Retin. Eye Res. 29, 476–486 [DOI] [PubMed] [Google Scholar]
- 95. Jones G. J., Crouch R. K., Wiggert B., Cornwall M. C., Chader G. J. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 9606–9610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jin J., Jones G. J., Cornwall M. C. (1994) Vis. Neurosci. 11, 389–399 [DOI] [PubMed] [Google Scholar]
- 97. Kefalov V. J. (2010) in Encyclopedia of the Eye (Dartt D. A., Besharse J. C., Dana R., eds) Vol. 3, pp. 389–396, Academic Press, New York [Google Scholar]