Abstract
In addition to rods and cones, the mammalian eye contains a third class of photoreceptor, the intrinsically photosensitive retinal ganglion cell (ipRGC). ipRGCs are heterogeneous irradiance-encoding neurons that primarily project to non-visual areas of the brain. Characteristics of ipRGC light responses differ significantly from those of rod and cone responses, including depolarization to light, slow on- and off-latencies, and relatively low light sensitivity. All ipRGCs use melanopsin (Opn4) as their photopigment. Melanopsin resembles invertebrate rhabdomeric photopigments more than vertebrate ciliary pigments and uses a Gq signaling pathway, in contrast to the Gt pathway used by rods and cones. ipRGCs can recycle chromophore in the absence of the retinal pigment epithelium and are highly resistant to vitamin A depletion. This suggests that melanopsin employs a bistable sequential photon absorption mechanism typical of rhabdomeric opsins.
Keywords: Circadian Rhythms, Eye, Photoreceptors, Rhodopsin, Vision, Melanopsin
Introduction
In 1927, Clyde Keeler noted that the blind rodless mouse retained pupillary light responses (PLRs)2 despite apparent degradation of all rod and cone photoreceptors (1). Over 50 years later, Ebihara and Tsuji (2) similarly noted that mice with the same outer retinal degeneration mutation (now called rd1) continue to synchronize their circadian rhythms to light-dark cycles despite apparent visual blindness. Extensive experiments by Foster and co-workers (3) in the 1990s demonstrated that preservation of light entrainment of the circadian clock was not a unique phenomenon to the rd1 mutation but was observed after the introduction of synthetic outer retinal degeneration gene constructs. However, circadian entrainment to light was lost in both anophthalmic mice lacking eyes (4) as well as those blind from optic nerve hypoplasia (Math5−/−) (5). These results demonstrated that a photoreceptive cell must be preserved in eyes of animals with complete outer retinal degeneration.
In 2002, Berson et al. (6) used a retrograde tracing technique to identify retinal ganglion cells (RGCs) projecting specifically to the master circadian pacemaker, the suprachiasmatic nucleus (SCN) of the hypothalamus. Patch-clamp recording of these cells revealed that they were intrinsically photosensitive; these cells have subsequently been called intrinsically photosensitive RGCs (ipRGCs) or, alternatively, photosensitive RGCs (we will use the former term in this minireview). ipRGCs constitute 2–3% of RGCs and project in the mouse brain primarily to non-visual centers, including the SCN, the olivary pretectal nucleus (OPN; locus of the PLR), and the intergeniculate leaflets (IGLs) of the geniculate nuclei (7, 8).
The identity of the photopigment subserving the ipRGCs became a critical question. Two candidates were postulated: melanopsin and cryptochrome. Melanopsin (Opn4) was discovered in 1998 by Provencio et al. (9), who identified this novel opsin from the dermal melanophores of Xenopus. The mammalian homolog was found to be expressed in a small subset of RGCs (10, 11). Cryptochrome is a flavin-based photopigment that subserves circadian entrainment in Arabidopsis and Drosophila (12). There are two mammalian cryptochrome homologs, Cry1 and Cry2, which are also expressed in the inner retina (13). Interestingly, knock-outs of either Opn4 or Cry1;Cry2 in mice do not prevent circadian or diurnal entrainment to light-dark cycles or eliminate the PLR (14–16). However, when Opn4 knock-out mice are combined with the homozygous rd1 mutation (17) (or equivalently compounded with Gnat1 and Cng3a mutations to eliminate outer retinal photoreceptive function (18)), mice lose all circadian entrainment and PLRs, whereas these phenomena are at least partially preserved in cryptochrome null mice with retinal degeneration (19). These results demonstrated that melanopsin is necessary and sufficient for inner retinal photoreception.
Subtypes of ipRGCs and Neural Connections
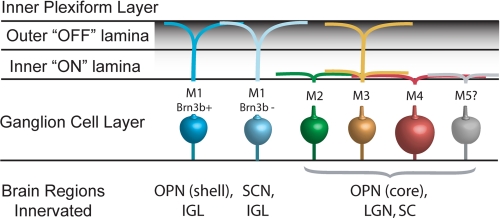
Subsequent work has confirmed that the ipRGC population and the melanopsin-expressing cell population are identical (7, 20). Initially, the melanopsin-containing ganglion cells were believed to be a homogeneous cell population carrying photic information to the SCN, OPN, and IGLs. However, further analysis of the anatomic and functional properties of genetically labeled ipRGCs has revealed deeper complexity, with the identification of distinct subtypes of ipRGCs (Fig. 1) (21, 22). The SCN receives input primarily from “M1” ipRGCs, which are characterized anatomically by dendritic arborization in the outer sublamina of the inner plexiform layer (IPL) of the retina (22). The OPN receives innervation from both M1 cells and “M2” cells, whose dendritic fields are found in the inner sublamina of the IPL (22). M1 cells primarily utilize melanopsin-dependent intrinsic photosensitivity, whereas M2 cells appear to rely more on conveyance of light information from upstream rods and cones (24).
FIGURE 1.
ipRGC subtypes. Shown is an illustration of the morphologic and molecularly defined subtypes of ipRGCs (M1–M5 and M1 Brn3b transcription factor-positive and -negative) with neural projections, including the SCN, OPN, lateral geniculate nucleus (LGN), the IGLs of the geniculate nucleus, and the superior colliculus (SC).
More sophisticated, genetically based anatomic tracing tools have recently uncovered a more diverse assortment of ipRGC subtypes. By combining mice expressing Cre recombinase under the control of the Opn4 promoter and Cre-activated reporters such as GFP or alkaline phosphatase, Ecker et al. (25) were able to identify cells that express melanopsin at low levels or transiently (both of which are undetectable by immunostaining or β-galactosidase). This revealed ∼2000 melanopsin-labeled cells in each mouse retina compared with the ∼600–800 M1 cells detected by analysis of β-galactosidase in Opn4tau-lacZ mice (7, 25). The family of ipRGCs has now been expanded to include the previously described M1 and M2 cells, as well as bistratified M3 cells, which are functionally similar to M2 cells (26, 27), and M4 cells, which are characterized by their large soma size and dendritic field (25) (as well as perhaps a rare M5 classification). Using this distinction, Ecker et al. showed that M2–M4 cells send processes to diverse brain regions, including areas involved in visual processing such as the dorsal and ventral subregions of the lateral geniculate nucleus (in addition to the aforementioned IGLs) and the superior colliculus (25). Intriguingly, these melanopsin RGCs function in visual circuits and cause cellular activity in the visual cortex of two different types of “melanopsin-only” mice lacking conventional photoreceptors (25, 28). M2–M5 cells show much weaker intrinsic photoresponses than M1 cells and may function in vivo primarily as conduits for outer retina-derived light signals (see below) (25).
Using additional molecular genetic labeling tools, Chen et al. (29) found a further subdivision in projections among M1 cells. Brn3b is a marker for RGCs but is not expressed in all ipRGCs (29, 30). Brn3b-positive M1 ipRGCs make up the majority of projections to the OPN, whereas Brn3b-negative M1 cells project mainly to the SCN. In keeping with this anatomical distinction, mice in which Brn3b-positive ipRGCs have been ablated using a targeted diphtheria toxin show severely reduced PLRs but normal photo-entrainment and masking in light-dark cycles (29). The full diversity of ipRGC types continues to expand (Fig. 1).
Redundant and Unique ipRGC Photoreceptor Functions in Behavior and Physiology
As noted above, mice lacking melanopsin continue to show PLRs and circadian entrainment, as do mice lacking rods and cones (14–16). Two models could explain this redundancy. First, ipRGCs could receive outer retinal input from rod- and cone-based pathways and pass this signaling on to downstream brain nuclei such as the SCN and OPN, signaling in series. Alternatively, non-ipRGC ganglion cells could transduce outer retinal signals to these brain regions in parallel with ipRGCs. Analysis of mice in which ipRGCs were ablated using diphtheria toxin (31, 32) or using toxic antibodies (33) showed complete loss of circadian entrainment and PLRs, consistent with the serial model in which all non-visual signaling is transduced by ipRGCs. Because rods and cones provide input to ipRGCs, the contributions of these outer photoreceptors and the intrinsic photosensitivity of melanopsin combine under normal conditions to affect behavior. Mice carrying null alleles of melanopsin still entrain to light-dark cycles but show reduced behavioral phase shifts in response to brief light pulses and reduced circadian period lengthening in response to constant light (15). Conversely, mice with melanopsin as their only photoreceptors entrain to light-dark cycles and phase-shift to bright light pulses indistinguishably from wild-type mice (3, 17). Melanopsin appears to exert its unique influence on behavior at bright light intensities or exposures of long duration. For example, the PLR of mice without melanopsin appears normal at low light intensities and for short durations; however, at high light intensities, the response is reduced, and at long durations of high intensity, the pupil begins to escape constriction (14, 35). Similarly, negative masking, or the suppression of behavioral activity by light exposure, is also lost over several hour-long light durations in Opn4−/− mice (36). Being nocturnal, mice and rats naturally confine the majority of their sleep during the light portion of a light-dark cycle. However, short bouts of sleep are induced during the night if light is presented at an inappropriate phase (37). In mice lacking melanopsin, this acute light-mediated sleep induction is lost (38–40). These effects on circadian entrainment and sleep in melanopsin-deficient animals may give insight into the higher incidence of seasonal affective disorder (SAD) among humans who carry a particular allele of the melanopsin gene (41).
Although a lack of melanopsin is associated with the deficiencies discussed above, the influence of rod and cone photoreception on ipRGCs should not be understated. Photo-entrainment, PLRs, and other non-visual light-mediated behaviors function normally at low light intensities without melanopsin (17, 18, 32). Distinct responses from outer photoreceptors and melanopsin can be observed at the level of the SCN (42). At scotopic light intensities, rods alone are sufficient for photo-entrainment, and even at photopic light levels at which rods cannot support pattern vision, they signal through cones to support entrainment (43). Cones can also mediate circadian phase shifting, but with short light exposures. Mice lacking cones sensitive to middle wavelength light exhibit reduced phase shifting in response to pulses of green light for 15 min or less but behave similarly to wild-type animals when exposed to light of shorter wavelength or longer pulses of middle wavelength light (44). To spectrally separate cone input from other photoreceptors, Lall et al. (45) utilized mice in which the mouse middle wavelength cones were replaced with human long wavelength-sensitive cones. In these mice, long durations of long wavelength light yield weak phase shifts, but the same number of photons delivered as discontinuous 15-min pulses produces phase shifts similar in magnitude to those observed in response to melanopsin/rod-stimulating wavelengths of light. It is likely that in an animal with functional rods, cones, and melanopsin, all three photoreceptors play a role in photic entrainment, PLRs, and masking but exert their strongest influence at specific wavelengths, intensities, and stimulus durations.
Role of Melanopsin during Retinal Development
Melanopsin is expressed early in retinal development, at about embryonic day 10. Melanopsin-containing ganglion cells are light-responsive from birth (21, 46–48), making them the first light-sensitive cells functional in the newborn retina. At birth, the projections from the ipRGCs sit poised at the ventral surface of the SCN and begin sending projections into the SCN between postnatal day (P) 1 and P4 (47, 50, 51). Additionally, the number of melanopsin-containing ganglion cells decreases dramatically between P0 and P19 (21).
Rod and cone circuitry to RGCs is not functional until at least P10 in mice (52–54). Might the early expression of melanopsin in ipRGCs have functional significance during development? Negative phototaxis, or an avoidance of bright light, is observed in mouse pups as early as P6 and is dependent on the presence of melanopsin (55). Melanopsin also contributes to the light-mediated segregation of ipsi- and contralateral retinogeniculate projections during visual development (56). This contribution to segregation of retinal efferents coincides with a melanopsin-dependent enhancement of waves of retinal activity observed in early postnatal development (56).
Physiology of ipRGC Photosensitivity
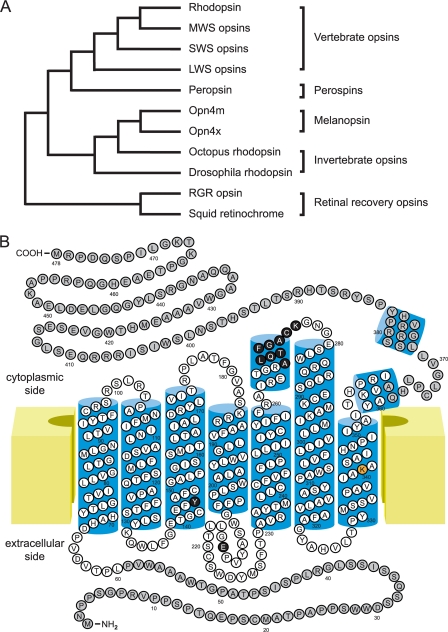
Currently, two melanopsin gene families are recognized (57). The originally identified gene from Xenopus represents one family found in all vertebrates except mammals and is designated Opn4x. The second gene family, Opn4m, occurs in all vertebrates surveyed but is the only melanopsin gene found in mammals. Comparison of the phylogenetic position of the two families indicates that they split at a very early point in vertebrate evolution. Both melanopsin families share greater sequence similarity with the opsins found in invertebrate rhabdomeric photoreceptors compared with vertebrate ciliary photoreceptors, displaying 39 and 30% sequence similarities, respectively (Fig. 2A) (9). Specific similarities to rhabdomeric opsins include the presence of an aromatic versus acidic counterion and the insertion of a rhabdomere-like sequence in a portion of the third cytoplasmic loop associated with G-protein specificity (Fig. 2B) (9).
FIGURE 2.
Structural relationship of melanopsin to other opsins. A, dendrogram of the relatedness of melanopsin (Opn4m and Opn4x) to other members of the opsin family demonstrating the close relationship of melanopsin to rhapdomeric opsins. MWS, middle wavelength-sensitive; SWS, short wavelength-sensitive; LWS, long wavelength-sensitive; RGR, retinal G-protein-coupled receptor. B, two-dimensional diagram of human melanopsin based on the structures of bovine rhodopsin (34, 49) and squid rhodopsin (83). Amino acids in white are commonly used in sequence comparisons within and between species. Highlighted amino acids include the retinal attachment site in orange (Lys-300), the two proposed Schiff base counterions in black (Tyr-106 and Glu-175), and a rhabdomere-like G-protein specificity sequence in black (Ala-268–Lys-276). C- and N-terminal structures of rhodopsins are not understood as well as the core transmembrane domain.
As the phylogenetic position suggests, melanopsin and ipRGC function is different from ciliary photoreceptor function. Whereas light causes a graded hyperpolarization in rods and cones, light leads to depolarization and the production of action potentials in ipRGCs. The exact mechanism by which this is accomplished is still not well understood but likely resembles the steps occurring in rhabdomeric photoreceptors, where opsins, such as those found in Drosophila photoreceptors, are coupled to a Gq/phospholipase C (PLC) second messenger cascade that induces depolarizing currents in the cell by activation of transient receptor potential (TRP) and TRP-like channels (58, 59). This contrasts with the mechanism of ciliary opsin, such as in mammalian rhodopsin, which couples to a Gt/phosphodiesterase cascade that leads to hyperpolarizing currents via a cyclic nucleotide-gated ion channel (60).
The definitive second messenger cascade of melanopsin has not been established but does appear to utilize Gq family signaling. When melanopsin is expressed in heterologous expression systems, general disruption of G-protein signaling interferes with melanopsin-dependent light responses (61, 62). Additionally, Gq inhibitors and antibodies as well as PLC inhibitors reduce light responses (61, 63), but Gi/o inhibitors do not (62). In ipRGCs, the light response has been shown to occur in isolated ipRGC membranes, where Gq and PLC inhibitors (but not other G-protein inhibitors) alter the light response (64), suggesting that melanopsin uses a Gq family (Gq/11/14) pathway.
The channel(s) activated to produce the membrane depolarization remain to be determined. Canonical TRP channels have been suggested as the effector channels, as they are the mammalian homolog of invertebrate TRP channels activated by rhabdomeric opsins (65). In particular, TRPC6 and TRPC7 are of interest, as they are localized to ipRGCs (66, 67). However, light-dependent ipRGC activity is preserved in mice lacking TRPC3, TRPC6, or TRPC7 (68), suggesting either functional redundancy or the presence of an as yet unidentified effector channel.
Compared with rhodopsin, melanopsin appears to be expressed at low levels in ipRGCs, estimated to be 1/10,000 rhodopsin density (69). The sum effect of the low pigment density, long-lasting meta-state, and Gq/PLC signaling is light responses that, compared with rods and cones, are very slow in onset, relatively insensitive, and very slow to inactivate (21). Latencies from lights-on to ipRGC firing can be several seconds, whereas off-latencies can be up to tens of seconds from lights-off. Latency is directly related to the light intensity, as are the cells' maximal and steady-state firing rates, with high intensity lights leading to shorter on-times, longer off-times, and higher firing rates (21, 70). Irradiance-response relationships recorded with multielectrode array techniques show threshold sensitivities of 1 × 1013 to 1 × 1014 photons/cm2/s for individual ipRGCs, with peak sensitivity at 480 nm and an action spectrum consistent with opsin photoreception (21). The proportionality of response times and firing rates to light intensity is consistent with the concept that ipRGCs are irradiance detectors rather than pigments subserving vision.
Mechanisms of Melanopsin Pigment Regeneration
Ciliary opsins require regeneration of chromophore in a second cell type (Fig. 3A). The dominant retinal recycling mechanism in the mammalian retina is located in the retinal pigment epithelium (RPE). There, all-trans-retinal is converted back to 11-cis-retinal by a series of enzymes, including lecithin:retinol acyltransferase (LRAT) and the rate-limiting enzyme RPE65 (71). The 11-cis-retinal is then returned to photoreceptors. Initial reports indicated that ipRGC function was dependent upon the RPE-based photocycle (72, 73). Knock-outs of the genes for those two critical enzymes in the RPE photocycle mentioned, Rpe65 and LRAT, yield a 100-fold less sensitive PLR compared with the wild type. However, ipRGC sensitivity, measured in isolated retinas on multielectrode arrays, is unchanged in Rpe65 and LRAT knock-out mice (74). In addition, acute poisoning of the RPE photocycle with all-trans-retinylamine does not alter PLR sensitivity in mice in which ipRGCs are the only functioning photoreceptors (74). Retinoid-depleted outer retina seems to inhibit inner retina activity, but the RPE photocycle itself is not necessary for ipRGC function.
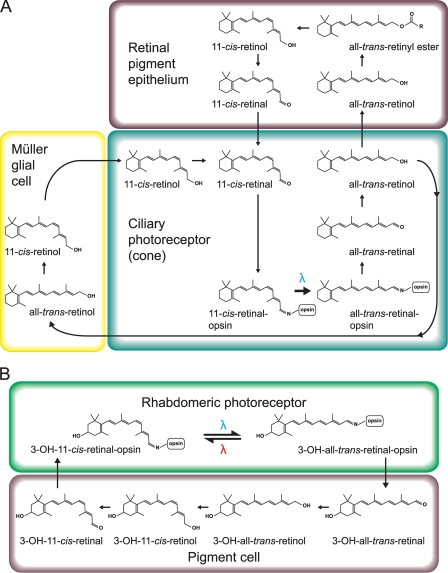
FIGURE 3.
Summary of chromophore recycling steps. Illustrated are steps for ciliary (A) and rhabdomeric (B) photoreceptor photocycles. Differences between a sequential bistable mechanism of chromophore regeneration seen in rhabdomeric photoreceptors and the thermally unstable activation of opsins in ciliary photoreceptors are illustrated. A blue lambda indicates light activation of opsin from the ground state to meta-state and conversion of the retinoid from the cis- to trans-conformation. A red lambda indicates the sequential absorption of a photon at a second wavelength that converts the meta-state to the ground state and re-isomerizes the retinoid back to the cis-conformation. Note that rhabdomeric photoreceptors often use alternative retinoids such as 11-cis-3-hydroxyretinal (3-OH-11-cis-retinal) in Drosophila used in the example above.
A major biochemical difference between ciliary and rhapdomeric opsins is the mechanism of chromophore regeneration. After a ciliary opsin absorbs a photon of light, 11-cis-retinal is isomerized into all-trans-retinal and released from the opsin protein (60). Regeneration of the chromophore then requires its transport to a second cell type with the enzymatic machinery to re-isomerize the trans- to 11-cis-retinal (Fig. 3A) (71). In rhabdomeric opsins, all-trans-retinal is not released from the opsin. Instead, the opsin absorbs a second photon at a specific reversing wavelength and re-isomerizes all-trans- to 11-cis-retinal (Fig. 3B). These opsins are thus “bistable” and feature an activated form, or meta-state, of the opsin that is thermally stable for seconds to minutes (whereas the meta-state of vertebrate ciliary opsins is extremely labile, lasting only milliseconds (75, 76)). Bistable pigments still may have a secondary method of chromophore regeneration that relies upon other isomerases that are light-dependent (77, 78) and located within the cell or light-independent and that take place in a second cell type (Fig. 3B) (79). If melanopsin is indeed rhabdomere-like, such a bistable mechanism could help explain the remarkable resistance of ipRGC function to systemic vitamin A depletion (80). The question of melanopsin bistability has been most thoroughly investigated in heterologous cellular expression systems. In these systems, light responses are dependent upon the addition of retinoid, with 11-cis- and 9-cis-retinal producing the most robust responses. Use of all-trans-retinal produces a response one-tenth to one-quarter that found with cis-retinal, but this response increases with long exposure to high levels of light at wavelengths >540 nm or with the coexpression of arrestin with melanopsin and the use of white light (62, 63). However, it is unclear if light exposure is directly isomerizing all-trans-retinal bound to melanopsin or is photoconverting all-trans-retinal free in solution into cis-conformations that then bind to apo-melanopsin. Attempts to demonstrate long wavelength photoreversal in vivo have had mixed and conflicting results. Mure et al. (81) demonstrated that pre-exposure of mice to long wavelength light (630 nm) potentiated cell firing responses in the SCN in response to blue light (480 nm), suggesting in vivo photoreversal. Presumably, this was through increased ipRGC activity, as no potentiation in SCN activity in melanopsin null animals was observed. However, attempts to replicate this result studying ipRGC firing in isolated retina by multielectrode array and an identical light protocol found no changes in ipRGC activity (82).
However, even a mechanism of bistability does not fully explain the behavior of melanopsin. Routinely, after a 1-min 480-nm light exposure in vitro, full ipRGC activity is seen after 5 min of dark incubation (21). Restoration of activity in the dark is not consistent with a purely bistable pigment and requires a mechanism whereby all-trans-retinal in the dark is converted back to 11-cis-retinal. Such a mechanism is suggested by the work of Terakita et al. (84). Using purified recombinant Amphioxus melanopsin, this group showed restoration of the activated melanopsin absorption spectrum toward the pre-exposure spectrum after a 5-min dark incubation. With subsequent exposure to orange light, the absorbance spectrum fully returned to its pre-exposure appearance. Therefore, multiple mechanisms of chromophore regeneration are possible (Fig. 4A).
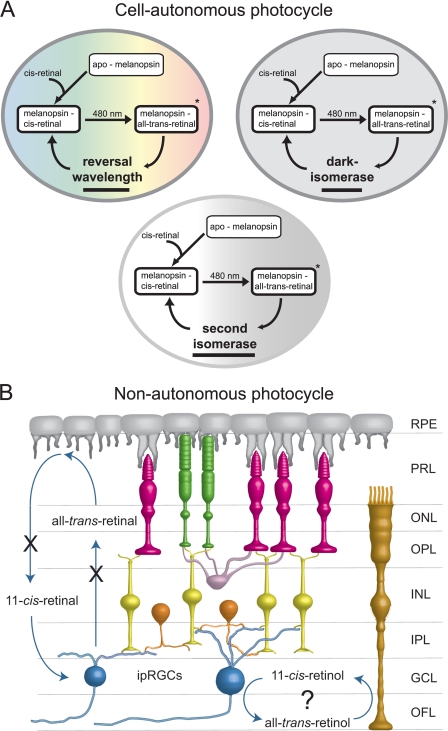
FIGURE 4.
Potential models for cell-autonomous and non-autonomous melanopsin chromophore recycling. A, three potential models for cell-autonomous chromophore recycling, including photoreversal, intrinsic dark isomerase, and second isomerase mechanisms. These mechanisms are not mutually exclusive. B, model for non-autonomous pigment recycling using Müller glial-based recycling. X indicates block to LRAT or RPE65, neither of which disrupts ipRGC function. Anatomic structures shown include the RPE, photoreceptor layer (PRL), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), IPL, ganglion cell layer (GCL), and optic fiber layer (OFL).
A second retinal photocycle has been described for cone photopigment recycling. Characterized initially in the cone-dominant chicken retina and localized to the Müller glial cells, this cycle has been found recently in mouse and primate retinas (85, 86). This pathway works in parallel to the RPE photocycle to supply cones with chromophore. This mechanism provides cones with the alcohol 11-cis-retinol, which cones (but not rods) are capable of converting to 11-cis-retinal (87). Müller glial cells have closer contacts with ipRGCs than with other RGCs (88). Therefore, it is possible that ipRGCs make use of the Müller glial photocycle, although this has not been demonstrated to date (Fig. 4B).
Melanopsin Purification
A major impediment to the study of mammalian melanopsin has been its scarcity. Although Amphioxus melanopsin has been purified and studied in vitro (84, 89, 90), the paucity of melanopsin-expressing cells in the mammal (i.e. 2000 cells in the murine retina) makes purification from in vivo sources unfeasible. Heterologous expression systems for opsins are fraught with issues of proper protein folding and secondary modifications that could alter characteristics such as absorption spectra (62, 91). For example, heterologously expressed and purified mammalian melanopsin in one study had a peak absorption spectrum between 420 and 440 nm, ∼50 nm blue-shifted compared with in vivo sensitivity (91). Only one study (92) has been able to partially characterize native mammalian melanopsin in situ by a process of immunomagnetic enrichment of ipRGCs from multiple retinas. HPLC analysis of the melanopsin purified from dark-adapted retinas showed only 11-cis-retinal present, whereas ipRGCs exposed to a brief flash of 480-nm light contained predominately all-trans-retinal with small amounts of 11-cis-retinal. In addition, some evidence was obtained for the production of two photoproducts in the difference spectrum of illuminated solubilized melanopsin and the subsequent acid trapping of the illuminated pigment. However, changes in the difference spectrum were very small, presumably because of limited starting material and the scarcity of ipRGCs.
Frontiers of Melanopsin Research
Understanding the molecular mechanisms of non-visual ocular photoreception assume s increased importance as melanopsin begins to be used as a means to confer light sensitivity on cells. Lin et al. (93) were able to confer widespread photosensitivity to blind retinas by virally mediated transduction of melanopsin, restoring some visual function to blind mice with this process. Ye et al. (23) have recently utilized melanopsin to create an optogenetic system capable of secreting glucagon-like peptide 1 in response to light in synthetic implants for the treatment of diabetes.
Given that ipRGCs were definitively identified less than 10 years ago, tremendous progress has occurred in understanding this important pathway in a relatively short time. However, several important questions await answers in the years ahead. The functional redundancy of rod/cone “through-signaling” and intrinsic photosensitivity still remains incompletely characterized. What selective pressures have retained melanopsin in this system when its function can be largely subsumed by outer retinal photoreceptors? The exact signal transduction mechanism for melanopsin also remains unclear, in particular the identities of effector cation channels in ipRGCs. The photocycle of melanopsin remains poorly characterized. It is clearly independent of retinal pigment epithelial function, but whether it is cell-autonomous (as heterologous expression experiments would suggest) or relies on a second cell type for chromophore recycling remains to be determined. Of course, the ultimate understanding of mechanisms of melanopsin-based photoreception awaits purification of the pigment and determination of its three-dimensional structure and native chromophore dynamics, which await development of scalable heterologous expression systems that faithfully produce functional pigment.
This work supported, in whole or in part, by National Institutes of Health Grants T32EY00703 (to T. S.) and P30EY1730 (to R. N. V. G.). This work was also supported by an unrestricted grant from Research to Prevent Blindness and the Burroughs-Wellcome Translational Scientist Award (to R. N. V. G.). This is the sixth article in the Thematic Minireview Series on Focus on Vision.
- PLR
- pupillary light response
- RGC
- retinal ganglion cell
- SCN
- suprachiasmatic nucleus
- ipRGC
- intrinsically photosensitive RGC
- OPN
- olivary pretectal nucleus
- IGL
- intergeniculate leaflet
- IPL
- inner plexiform layer
- P
- postnatal day
- PLC
- phospholipase C
- TRP
- transient receptor potential
- RPE
- retinal pigment epithelium
- LRAT
- lecithin:retinol acyltransferase.
REFERENCES
- 1. Keeler C. E. (1927) Am. J. Physiol. 81, 107–112 [Google Scholar]
- 2. Ebihara S., Tsuji K. (1980) Physiol. Behav. 24, 523–527 [DOI] [PubMed] [Google Scholar]
- 3. Freedman M. S., Lucas R. J., Soni B., von Schantz M., Muñoz M., David-Gray Z., Foster R. (1999) Science 284, 502–504 [DOI] [PubMed] [Google Scholar]
- 4. Laemle L. K., Ottenweller J. E. (1998) Physiol. Behav. 64, 165–171 [DOI] [PubMed] [Google Scholar]
- 5. Wee R., Castrucci A. M., Provencio I., Gan L., Van Gelder R. N. (2002) J. Neurosci. 22, 10427–10433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berson D. M., Dunn F. A., Takao M. (2002) Science 295, 1070–1073 [DOI] [PubMed] [Google Scholar]
- 7. Hattar S., Liao H. W., Takao M., Berson D. M., Yau K. W. (2002) Science 295, 1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hattar S., Kumar M., Park A., Tong P., Tung J., Yau K. W., Berson D. M. (2006) J. Comp Neurol. 497, 326–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Provencio I., Jiang G., De Grip W. J., Hayes W. P., Rollag M. D. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Provencio I., Rodriguez I. R., Jiang G., Hayes W. P., Moreira E. F., Rollag M. D. (2000) J. Neurosci. 20, 600–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Provencio I., Rollag M. D., Castrucci A. M. (2002) Nature 415, 493. [DOI] [PubMed] [Google Scholar]
- 12. Oztürk N., Song S. H., Ozgür S., Selby C. P., Morrison L., Partch C., Zhong D., Sancar A. (2007) Cold Spring Harb. Symp. Quant. Biol. 72, 119–131 [DOI] [PubMed] [Google Scholar]
- 13. Thompson C. L., Selby C. P., Partch C. L., Plante D. T., Thresher R. J., Araujo F., Sancar A. (2004) Brain Res. Mol. Brain Res. 122, 158–166 [DOI] [PubMed] [Google Scholar]
- 14. Lucas R. J., Hattar S., Takao M., Berson D. M., Foster R. G., Yau K. W. (2003) Science 299, 245–247 [DOI] [PubMed] [Google Scholar]
- 15. Panda S., Sato T. K., Castrucci A. M., Rollag M. D., DeGrip W. J., Hogenesch J. B., Provencio I., Kay S. A. (2002) Science 298, 2213–2216 [DOI] [PubMed] [Google Scholar]
- 16. Ruby N. F., Brennan T. J., Xie X., Cao V., Franken P., Heller H. C., O'Hara B. F. (2002) Science 298, 2211–2213 [DOI] [PubMed] [Google Scholar]
- 17. Panda S., Provencio I., Tu D. C., Pires S. S., Rollag M. D., Castrucci A. M., Pletcher M. T., Sato T. K., Wiltshire T., Andahazy M., Kay S. A., Van Gelder R. N., Hogenesch J. B. (2003) Science 301, 525–527 [DOI] [PubMed] [Google Scholar]
- 18. Hattar S., Lucas R. J., Mrosovsky N., Thompson S., Douglas R. H., Hankins M. W., Lem J., Biel M., Hofmann F., Foster R. G., Yau K. W. (2003) Nature 424, 76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Gelder R. N., Wee R., Lee J. A., Tu D. C. (2003) Science 299, 222. [DOI] [PubMed] [Google Scholar]
- 20. Gooley J. J., Lu J., Chou T. C., Scammell T. E., Saper C. B. (2001) Nat. Neurosci. 4, 1165. [DOI] [PubMed] [Google Scholar]
- 21. Tu D. C., Zhang D., Demas J., Slutsky E. B., Provencio I., Holy T. E., Van Gelder R. N. (2005) Neuron 48, 987–999 [DOI] [PubMed] [Google Scholar]
- 22. Baver S. B., Pickard G. E., Sollars P. J., Pickard G. E. (2008) Eur. J. Neurosci. 27, 1763–1770 [DOI] [PubMed] [Google Scholar]
- 23. Ye H., Daoud-El Baba M., Peng R. W., Fussenegger M. (2011) Science 332, 1565–1568 [DOI] [PubMed] [Google Scholar]
- 24. Schmidt T. M., Kofuji P. (2009) J. Neurosci. 29, 476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ecker J. L., Dumitrescu O. N., Wong K. Y., Alam N. M., Chen S. K., LeGates T., Renna J. M., Prusky G. T., Berson D. M., Hattar S. (2010) Neuron 67, 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berson D. M., Castrucci A. M., Provencio I. (2010) J. Comp. Neurol. 518, 2405–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmidt T. M., Kofuji P. (2011) J. Comp. Neurol. 519, 1492–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown T. M., Gias C., Hatori M., Keding S. R., Semo M., Coffey P. J., Gigg J., Piggins H. D., Panda S., Lucas R. J. (2010) PLoS Biol. 8, e1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen S. K., Badea T. C., Hattar S. (2011) Nature 476, 92–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Badea T. C., Cahill H., Ecker J., Hattar S., Nathans J. (2009) Neuron 61, 852–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hatori M., Le H., Vollmers C., Keding S. R., Tanaka N., Buch T., Waisman A., Schmedt C., Jegla T., Panda S. (2008) PLoS ONE 3, e2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Güler A. D., Ecker J. L., Lall G. S., Haq S., Altimus C. M., Liao H. W., Barnard A. R., Cahill H., Badea T. C., Zhao H., Hankins M. W., Berson D. M., Lucas R. J., Yau K. W., Hattar S. (2008) Nature 453, 102–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Göz D., Studholme K., Lappi D. A., Rollag M. D., Provencio I., Morin L. P. (2008) PLoS ONE 3, e3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., Yamamoto M., Miyano M. (2000) Science 289, 739–745 [DOI] [PubMed] [Google Scholar]
- 35. Zhu Y., Tu D. C., Denner D., Shane T., Fitzgerald C. M., Van Gelder R. N. (2007) Invest. Ophthalmol. Vis. Sci. 48, 1268–1275 [DOI] [PubMed] [Google Scholar]
- 36. Mrosovsky N., Hattar S. (2003) Chronobiol. Int. 20, 989–999 [DOI] [PubMed] [Google Scholar]
- 37. Benca R. M., Gilliland M. A., Obermeyer W. H. (1998) Sleep 21, 451–460 [DOI] [PubMed] [Google Scholar]
- 38. Altimus C. M., Güler A. D., Villa K. L., McNeill D. S., Legates T. A., Hattar S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 19998–20003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lupi D., Oster H., Thompson S., Foster R. G. (2008) Nat. Neurosci. 11, 1068–1073 [DOI] [PubMed] [Google Scholar]
- 40. Tsai J. W., Hannibal J., Hagiwara G., Colas D., Ruppert E., Ruby N. F., Heller H. C., Franken P., Bourgin P. (2009) PLoS Biol. 7, e1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roecklein K. A., Rohan K. J., Duncan W. C., Rollag M. D., Rosenthal N. E., Lipsky R. H., Provencio I. (2009) J. Affect. Disord. 114, 279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brown T. M., Wynne J., Piggins H. D., Lucas R. J. (2011) J. Physiol. 589, 1173–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Altimus C. M., Güler A. D., Alam N. M., Arman A. C., Prusky G. T., Sampath A. P., Hattar S. (2010) Nat. Neurosci. 13, 1107–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dollet A., Albrecht U., Cooper H. M., Dkhissi-Benyahya O. (2010) Chronobiol. Int. 27, 768–781 [DOI] [PubMed] [Google Scholar]
- 45. Lall G. S., Revell V. L., Momiji H., Al Enezi J., Altimus C. M., Güler A. D., Aguilar C., Cameron M. A., Allender S., Hankins M. W., Lucas R. J. (2010) Neuron 66, 417–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tarttelin E. E., Bellingham J., Bibb L. C., Foster R. G., Hankins M. W., Gregory-Evans K., Gregory-Evans C. Y., Wells D. J., Lucas R. J. (2003) Exp. Eye Res. 76, 393–396 [DOI] [PubMed] [Google Scholar]
- 47. Sekaran S., Lupi D., Jones S. L., Sheely C. J., Hattar S., Yau K. W., Lucas R. J., Foster R. G., Hankins M. W. (2005) Curr. Biol. 15, 1099–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hannibal J., Fahrenkrug J. (2004) Neuroreport 15, 2317–2320 [DOI] [PubMed] [Google Scholar]
- 49. Hargrave P. A., McDowell J. H. (1992) FASEB J. 6, 2323–2331 [DOI] [PubMed] [Google Scholar]
- 50. McNeill D. S., Sheely C. J., Ecker J. L., Badea T. C., Morhardt D., Guido W., Hattar S. (2011) Neural Dev. 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Muñoz Llamosas M., Huerta J. J., Cernuda-Cernuda R., García-Fernández J. M. (2000) Brain Res. Dev. Brain Res. 120, 1–6 [DOI] [PubMed] [Google Scholar]
- 52. Bakall B., Marmorstein L. Y., Hoppe G., Peachey N. S., Wadelius C., Marmorstein A. D. (2003) Invest. Ophthalmol. Vis. Sci. 44, 3622–3628 [DOI] [PubMed] [Google Scholar]
- 53. Tian N., Copenhagen D. R. (2003) Neuron 39, 85–96 [DOI] [PubMed] [Google Scholar]
- 54. Ratto G. M., Robinson D. W., Yan B., McNaughton P. A. (1991) Nature 351, 654–657 [DOI] [PubMed] [Google Scholar]
- 55. Johnson J., Wu V., Donovan M., Majumdar S., Rentería R. C., Porco T., Van Gelder R. N., Copenhagen D. R. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 17374–17378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Renna J. M., Weng S., Berson D. M. (2011) Nat. Neurosci. 14, 827–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bellingham J., Chaurasia S. S., Melyan Z., Liu C., Cameron M. A., Tarttelin E. E., Iuvone P. M., Hankins M. W., Tosini G., Lucas R. J. (2006) PLoS Biol. 4, e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hardie R. C. (2011) Pflugers Arch. 461, 493–498 [DOI] [PubMed] [Google Scholar]
- 59. Hardie R. C. (2001) J. Exp. Biol. 204, 3403–3409 [DOI] [PubMed] [Google Scholar]
- 60. Palczewski K. (2006) Annu. Rev. Biochem. 75, 743–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Qiu X., Kumbalasiri T., Carlson S. M., Wong K. Y., Krishna V., Provencio I., Berson D. M. (2005) Nature 433, 745–749 [DOI] [PubMed] [Google Scholar]
- 62. Melyan Z., Tarttelin E. E., Bellingham J., Lucas R. J., Hankins M. W. (2005) Nature 433, 741–745 [DOI] [PubMed] [Google Scholar]
- 63. Panda S., Nayak S. K., Campo B., Walker J. R., Hogenesch J. B., Jegla T. (2005) Science 307, 600–604 [DOI] [PubMed] [Google Scholar]
- 64. Graham D. M., Wong K. Y., Shapiro P., Frederick C., Pattabiraman K., Berson D. M. (2008) J. Neurophysiol. 99, 2522–2532 [DOI] [PubMed] [Google Scholar]
- 65. Hardie R. C., Postma M. (2008) in The Senses: A Comprehensive Reference (Masland R., Albright T., eds) Vol. 1, pp. 77–130, Academic Press, San Diego, CA [Google Scholar]
- 66. Hartwick A. T., Bramley J. R., Yu J., Stevens K. T., Allen C. N., Baldridge W. H., Sollars P. J., Pickard G. E. (2007) J. Neurosci. 27, 13468–13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Warren E. J., Allen C. N., Brown R. L., Robinson D. W. (2006) Eur. J. Neurosci. 23, 2477–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Perez-Leighton C. E., Schmidt T. M., Abramowitz J., Birnbaumer L., Kofuji P. (2011) Eur. J. Neurosci. 33, 856–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Do M. T., Kang S. H., Xue T., Zhong H., Liao H. W., Bergles D. E., Yau K. W. (2009) Nature 457, 281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Berson D. M. (2003) Trends Neurosci. 26, 314–320 [DOI] [PubMed] [Google Scholar]
- 71. Kiser P. D., Golczak M., Maeda A., Palczewski K. (2011) Biochim. Biophys. Acta, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fu Y., Zhong H., Wang M. H., Luo D. G., Liao H. W., Maeda H., Hattar S., Frishman L. J., Yau K. W. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 10339–10344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Doyle S. E., Castrucci A. M., McCall M., Provencio I., Menaker M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 10432–10437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tu D. C., Owens L. A., Anderson L., Golczak M., Doyle S. E., McCall M., Menaker M., Palczewski K., Van Gelder R. N. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 10426–10431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hardie R. C., Raghu P. (2001) Nature 413, 186–193 [DOI] [PubMed] [Google Scholar]
- 76. Hillman P., Hochstein S., Minke B. (1983) Physiol. Rev. 63, 668–772 [DOI] [PubMed] [Google Scholar]
- 77. Seki T. (1984) J. Gen. Physiol. 84, 49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Robles L. J., Camacho J. L., Torres S. C., Flores A., Fariss R. N., Matsumoto B. (1995) J. Comp. Neurol. 358, 605–614 [DOI] [PubMed] [Google Scholar]
- 79. Wang X., Wang T., Jiao Y., von Lintig J., Montell C. (2010) Curr. Biol. 20, 93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Thompson C. L., Blaner W. S., Van Gelder R. N., Lai K., Quadro L., Colantuoni V., Gottesman M. E., Sancar A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 11708–11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mure L. S., Rieux C., Hattar S., Cooper H. M. (2007) J. Biol. Rhythms 22, 411–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mawad K., Van Gelder R. N. (2008) J. Biol. Rhythms 23, 387–391 [DOI] [PubMed] [Google Scholar]
- 83. Murakami M., Kouyama T. (2008) Nature 453, 363–367 [DOI] [PubMed] [Google Scholar]
- 84. Terakita A., Tsukamoto H., Koyanagi M., Sugahara M., Yamashita T., Shichida Y. (2008) J. Neurochem. 105, 883–890 [DOI] [PubMed] [Google Scholar]
- 85. Wang J. S., Estevez M. E., Cornwall M. C., Kefalov V. J. (2009) Nat. Neurosci. 12, 295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang J. S., Kefalov V. J. (2009) Curr. Biol. 19, 1665–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang J. S., Kefalov V. J. (2011) Prog. Retin. Eye Res. 30, 115–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Viney T. J., Balint K., Hillier D., Siegert S., Boldogkoi Z., Enquist L. W., Meister M., Cepko C. L., Roska B. (2007) Curr. Biol. 17, 981–988 [DOI] [PubMed] [Google Scholar]
- 89. Gomez Mdel P., Angueyra J. M., Nasi E. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 9081–9086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Koyanagi M., Terakita A. (2008) Photochem. Photobiol. 84, 1024–1030 [DOI] [PubMed] [Google Scholar]
- 91. Newman L. A., Walker M. T., Brown R. L., Cronin T. W., Robinson P. R. (2003) Biochemistry 42, 12734–12738 [DOI] [PubMed] [Google Scholar]
- 92. Walker M. T., Brown R. L., Cronin T. W., Robinson P. R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8861–8865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lin B., Koizumi A., Tanaka N., Panda S., Masland R. H. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 16009–16014 [DOI] [PMC free article] [PubMed] [Google Scholar]