Background: We examined the impact of insulin-like growth factor binding protein-4 (IGFBP-4) on growth factor-induced angiogenesis in vivo.
Results: IGFBP-4 inhibited IGF-1 and FGF-2, but not VEGF-induced angiogenesis, and this inhibition depended on p38 MAPK activity.
Conclusion: The anti-angiogenic activity of IGFBP-4 depends in part on p38 MAPK.
Significance: New insight is provided into how blood vessels respond to endogenous inhibitors during growth factor-stimulated angiogenesis.
Keywords: Angiogenesis, Basement membrane, Extracellular matrix proteins, Insulin-like growth factor (IGF), p38 MAPK, FGF-2, VEGF
Abstract
An in-depth understanding of the molecular and cellular complexity of angiogenesis continues to advance as new stimulators and inhibitors of blood vessel formation are uncovered. Gaining a more complete understanding of the response of blood vessels to both stimulatory and inhibitory molecules will likely contribute to more effective strategies to control pathological angiogenesis. Here, we provide evidence that endothelial cell interactions with structurally altered collagen type IV may suppress the expression of insulin-like growth factor binding protein-4 (IGFBP-4), a well documented inhibitor of the IGF-1/IGF-1R signaling axis. We report for the first time that IGFBP-4 differentially inhibits angiogenesis induced by distinct growth factor signaling pathways as IGFBP-4 inhibited FGF-2- and IGF-1-stimulated angiogenesis but failed to inhibit VEGF-induced angiogenesis. The resistance of VEGF-stimulated angiogenesis to IGFBP-4 inhibition appears to depend on sustained activation of p38 MAPK as blocking its activity restored the anti-angiogenic effects of IGFBP-4 on VEGF-induced blood vessel growth in vivo. These novel findings provide new insight into how blood vessels respond to endogenous inhibitors during angiogenesis stimulated by distinct growth factor signaling pathways.
Introduction
An expanding body of evidence indicates that angiogenesis, or the development of new blood vessels from pre-existing vessels, can be controlled by a balance of pro-angiogenic molecules such as growth factors and cytokines, as well as inhibitory factors, including thrombospondins and fragments of extracellular matrix (ECM)2 proteins (1–3). However, the molecular mechanisms that govern the delicate balance that helps to maintain vascular homeostasis within different tissues are not well understood. The molecular mechanisms by which stimulatory and inhibitory factors influence new blood vessel formation may be modulated in part by extracellular cues from the local microenvironment (4–6). The response of vascular cells to angiogenic growth factors may depend on cellular communication with the surrounding ECM, and studies have documented important roles for the integrin family of ECM receptors in modulating growth factor signaling (7–9). In addition, previous reports have documented the biological relevance of structurally altered or denatured ECM molecules such as collagen in regulating angiogenesis in vivo (10–17). To this end, a phase I clinical trial to examine the effects of an anti-denatured collagen antibody TRC093 on advanced metastatic tumors was recently completed with encouraging clinical results (18).
Previous reports have suggested that distinct integrin ECM receptors such as αvβ3 and αvβ5 may differentially regulate angiogenic signaling stimulated by different growth factors, as antagonists of αvβ3 inhibited FGF-2-induced angiogenesis while exhibiting a reduced capacity to impact VEGF-induced angiogenesis (7, 8). Integrin-dependent angiogenic signaling pathways have been shown to involve downstream effector molecules, including members of the mitogen-activated protein kinase (MAPK) family (7, 8). These studies and many others suggest that the local composition and structural integrity of the ECM, along with their respective cell surface receptors, may play critical roles in coordinating and fine-tuning the response of vascular cells to diverse angiogenic stimuli to facilitate productive angiogenesis.
Interestingly, members of the insulin-like growth factor binding protein (IGFBP) family are rapidly gaining attention as critical regulators of a variety of normal physiological and pathological processes, because IGFBPs have been shown to regulate diverse cellular events such as proliferation, migration, apoptosis, and differentiation, all crucial processes thought to govern specific steps within the angiogenic cascade (19, 20). IGFBPs are an important family of secreted proteins that exhibit high affinity binding to the peptide growth factors IGF-1 and IGF-2, thereby functioning to modulate their bioavailability and binding to IGF receptors (19, 20).
Among the six well characterized members of the IGFBP family, IGFBP-4 has been associated predominately with inhibitory functions such as reducing cellular proliferation and DNA synthesis as well as inducing apoptosis in a cell type- and tissue-specific manner (21, 22). Importantly, studies have shown reduced levels of IGFBP-4 in malignant colon cancer as compared with normal colon tissues and, in addition, increasing the expression of IGFBP-4 in prostate and colon cancer cells slowed the growth of these tumors in vivo (23, 24). Recently, we showed reduced levels of IGFBP-4 are expressed in human biopsies of metastatic melanoma as compared with primary melanoma (25). However, opposite findings have been documented in other tumor types, reinforcing the complexity of the cell type- and tissue-specific effects of IGFBP-4 (26, 27). Interestingly, IGFBP-4 has been shown to modulate the behavior of several distinct cell types, including endothelial and vascular smooth muscle cells, and studies have shown that IGFBP-4 can inhibit endothelial tube formation in vitro, and expression of protease-resistant IGFBP-4 in mammary tumor cells is associated with smaller tumors and fewer blood vessels (28–32). Given these interesting studies, it's possible that IGFBP-4 may function in part to help maintain vascular homeostasis and differentiation thereby limiting pathological angiogenesis. However, relatively few studies have examined the impact of IGFBP-4 on angiogenesis in vivo, or how IGFBP-4 may regulate angiogenesis induced by distinct growth factors. Therefore, we sought to examine the expression of IGFBP-4 in endothelial cells interacting with a physiologically relevant ECM molecule expressed in the basement membranes of angiogenic blood vessels and to determine the biological impact of IGFBP-4 on angiogenesis induced by distinct growth factors.
Here we present, for the first time, evidence for a reduction in endothelial cell expression of IGFBP-4 following interactions with structurally altered collagen. Unexpectedly, our studies suggest that, although IGFBP-4 potently inhibited FGF-2- and IGF-1-induced angiogenesis, it failed to significantly inhibit VEGF-induced angiogenesis in vivo. This lack of inhibitory activity was due, in part, to elevated levels of activated p38 MAPK. Collectively, these finding provide new molecular insight into the mechanisms by which endogenously expressed inhibitors, such as IGFBP-4, may differentially regulate distinct growth factor-induced signaling pathways during angiogenesis.
EXPERIMENTAL PROCEDURES
Reagents, Kits, Chemicals, and Antibodies
Ethanol, methanol, phosphate-buffered saline (PBS), bovine serum albumin (BSA), purified human collagen type IV, and cortisone acetate were obtained from Sigma. Human recombinant growth factors FGF-2, IGF-1, and VEGF, TNF-α, and recombinant IGFBP-4 were obtained from R&D Systems (Minneapolis, MN). Antibodies directed to p38 MAPK, phosphorylated (Thr-180/Try-182) p38 MAPK, and phosphorylated (Thr-202/Tyr-204) p44/42 ERK were obtained from Cell Signaling Technology (Danvers, MA). Anti-PCNA and anti-IGFBP-1, -3, -4, -6, and -7 antibodies were from Abcam (Cambridge, MA), and anti-vWF antibody was from Dako (Denmark). Peroxidase-labeled secondary antibody and Alexa 594- and 488-labeled antibodies were obtained from Invitrogen. p38 MAPK inhibitor (SB202190) and inactive control (SB202474) were obtained from EMD Chemicals (Gibbstown, NJ). A BrdU cell proliferation assay kit was from Millipore (Bedford, MA).
Cells and Cell Culture
Human umbilical vein endothelial cells (HUVECs) were obtained from Lonza (Walkersville, MD) and cultured in EBM-2 medium in the presence of 20% FBS and supplements (Lonza). HUVECs were maintained as subconfluent cultures and used between passages 4 and 6.
Cell Proliferation Assays
Microtiter (96-well) culture plates were either uncoated or coated with native or denatured (boiled 15 min) collagen type IV (1.0 μg/ml) for 18 h washed and blocked for 1 h with 1.0% BSA in PBS. HUVECs (2500 cells per well) were resuspended in proliferation buffer (EBM-2 medium containing 1.0 mm MgCl2, 0.2 mm MnCl2, and 0.01% BSA). Cells were either un-stimulated or stimulated with VEGF (20 ng/ml) or FGF-2 (100 ng/ml) in the absence or presence (500 ng/ml to 2.0 μg/ml) of IGFBP-4 and seeded (in triplicate) on the coated wells and allowed to proliferate for either 24 or 48 h. HUVEC proliferation was monitored using the BrdU proliferation assay kit according to the manufacturer's instructions. All assays were completed four times.
Real-time Quantitative RT-PCR
Real-time quantitative RT-PCR was carried out essentially as described previously (12). Briefly, wells from non-tissue culture plates were coated (0.5 μg/ml) with either native triple helical or thermally denatured (boiled 15 min) collagen type IV. Equal numbers of endothelial cells (1.0 × 106) were harvested, washed, and resuspended in RPMI buffer containing 1.0 mm MgCl2, 0.2 mm MnCl2, and 1.0% BSA and added to the coated plates and allowed to incubated for 1 h. Cells were harvested and washed, and total RNA was isolated using an RNeasy plus kit Qiagen (Valencia, CA). Real-time RT-PCR reactions were run in duplicate on a Bio-Rad iQ5 system. Human IGFBP-4 primer sets included 5′-GAGCTGGGTGACACTGCTTG-3′ and 5′-CCCACGAGGACCTCTACATCA-3′. β2-Macroglobulin was used for normalization for all experiments, and primer sets for β2-macroglobulin included 5′-AAAGATGAGTATGCCTGCCG-3′ and 5′-CCTCCATGATGATGCTGCTTACA-3′. Experiments were completed at least four times.
Chick CAM Angiogenesis Assay
The chick chorioallantoic membrane (CAM) angiogenesis assay was carried out essentially as described with some modifications (33). Briefly, CAMs of 10-day-old chick embryos obtained from Charles River, (North Franklin, CT) were separated from the shell membrane. Filter discs pre-treated with cortisone acetate (3.0 mg/ml) containing RPMI only or growth factors FGF-2, IGF-1, or VEGF or TNF-α (40 ng) were place on the CAMs to induce angiogenesis. Twenty-four hours later, dose-response studies were carried out to estimate optimal concentrations of IGFBP-4 by adding IGFBP-4 over a range of 0, 50, 100, or 200 ng per egg per day for a total of 2 days. Maximal inhibition was achieved at 100 ng/egg/day. Therefore, the embryos were treated with IGFBP-4 or control BSA (100 ng/egg/day) topically for 2 days. In experiments where p38 MAPK inhibitors were examined, active (SB202190) p38 inhibitor and control inactive p38 compound (SB202474) were used at a concentration of 10 μm. At the end of the incubation period, the embryos were sacrificed, and the CAM tissues were analyzed. Angiogenesis was quantified by counting the number of angiogenic branching blood vessels within the area of the filter disc. The angiogenic index was determined by subtracting the mean number of blood vessel branch points from untreated CAM from each experimental condition as we described previously (33). Seven to ten embryos were used per condition, and experiments were repeated two to four times.
Immunofluorescence Analysis and Quantification of Blood Vessel Proliferation
CAM tissues from each experimental condition were harvested washed and embedded in OCT and snap frozen. Frozen sections (4.0 μm) were fixed in 50% methanol/50% acetone, air-dried, and blocked with 2.0% BSA in TBS. Tissue sections were co-stained by incubating tissues with anti-PCNA and anti-vWF antibody (1:500 dilution) for 18 h at 4 °C. Tissues were next incubated with Alexa 594- and Alexa 488-labeled secondary antibodies, at 1:500 dilution, for 1 h at room temperature. Tissue sections were washed and examined by immunofluorescence microscopy. Representative images were taken at either 200× or 400× magnification with a Zeiss AX10 compound microscope. To quantify vessel proliferation, CAM tissues (n = 4) from each experimental condition were co-stained for the endothelial cell marker vWF and PCNA. The number of PCNA-positive blood vessels within 5 200× microscopic fields from each of four different CAMs per experiment condition was determined.
Western Blot
Equal numbers of endothelial cells or chick CAM tissues from each experimental condition were harvested, washed, and lysed in RIPA lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA) with 1× protease inhibitor mixture containing phosphatase inhibitors (Sigma). Equal amounts of cell or tissue lysate were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were probed with antibodies directed to IGFBPs-1, -3, -4, -6, and -7 and p38 MAPK, phosphorylated p38 MAPK, ERK, phosphorylated ERK, or control β-actin. Western blots were carried out at least three times with similar results and visualized by chemiluminescence detection. Western blots were quantified by using ImageJ (National Institutes of Health (NIH)) analysis.
Statistical Analysis
Statistical analysis was performed using the InStat statistical program for Macintosh computers. Data were analyzed for statistical significance using Student's t test. p values < 0.05 were considered significant.
RESULTS
Endothelial Cell Interactions with Denatured Collagen Type IV Suppresses Expression of IGFBP-4 in Vitro
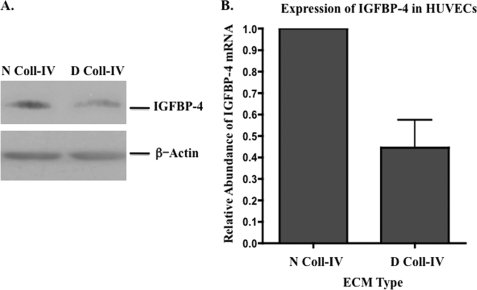
Among the earliest events associated with angiogenesis is the structural remodeling of the vascular basement membrane leading to the formation of denatured collagen (10–17). Remodeling of the non-cellular stroma surrounding blood vessels is thought to promote the creation of a local environment that facilitates angiogenesis. However, the cellular and molecular mechanisms by which biomechanical changes in ECM structure control new vessel development are poorly understood. Previous studies have shown that denatured collagen type IV is often associated with angiogenic vessels in vivo, and cellular interactions with denatured collagen have been shown to regulate angiogenesis in multiple models (10, 11, 13–17, 34). Our previous observations suggested that disruption of tumor cell interactions with denatured collagen might enhance the expression of IGFBP-4, a member of the insulin-like growth factor-binding protein family. Given that IGFBP-4 has been implicated in regulating the behavior of vascular cells, such as vascular smooth muscle and endothelial cells, we sought to determine whether endothelial cell interaction with denatured collagen might alter expression of IGFBP-4. To examine this possibility, HUVECs were seeded on triple helical or denatured collagen-IV and the relative level of IGFBP-4 was assessed. As shown in Fig. 1A, the levels of IGFBP-4 from HUVECs seeded on denatured collagen was reduced by ∼40% as indicated by scanning and quantification by ImageJ (NIH) analysis of the blots from three independent experiments as compared with that observed from cells seeded on triple helical collagen. In similar experiments, the relative levels of other members of the IGFBP family, including IGFBP-1, -3, and -6, exhibited little if any change in expression following HUVEC interactions with native or denatured collagen type IV (supplemental Fig. S1). IGFBP-7, however, could not be detected consistently in lysates of HUVECs. Moreover, endothelial cell interactions with denatured collagen resulted in an ∼50% reduction of IGFBP-4 mRNA as compared with triple-helical collagen. These findings suggest that endothelial cell interactions with denatured collagen-IV, which is known to be present within the vascular basement membrane of angiogenic blood vessels in vivo (10, 11, 13–17, 34), may suppress the expression of IGFBP-4.
FIGURE 1.
Suppression of IGFBP-4 following endothelial cell interactions with denatured collagen. The relative expression of IGFBP-4 was examined in HUVECS following binding to either native collagen type IV (N Coll-IV) or denatured collagen type IV (D Coll-IV). HUVEC were seeded on collagen-coated wells and allowed to incubate, and total cell lysates (A) or mRNA (B) were isolated. A, Western blot of IGFBP-4 expression in HUVECs following 4-h interactions with either native or denatured collagen type IV. B, quantitative RT-PCR analysis of the relative abundance of IGFBP-4 mRNA following 1-h interaction with either native or denatured collagen type IV.
IGFBP-4 Inhibits IGF-1 and FGF-2, but Not VEGF-induced Angiogenesis
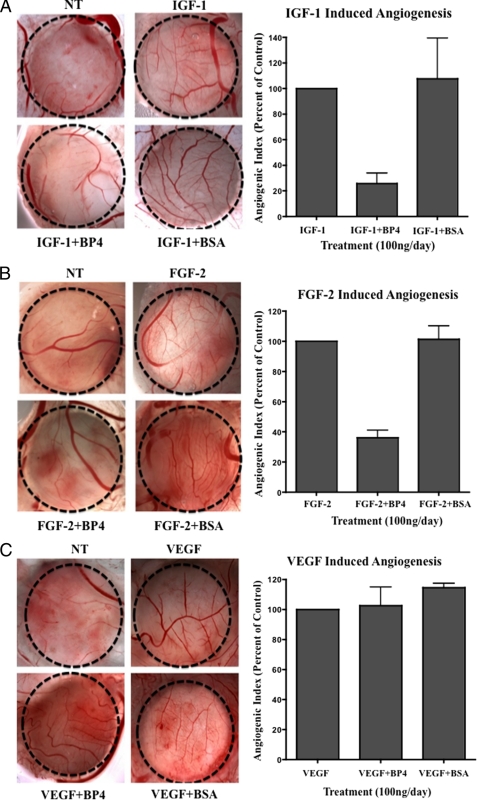
Prior studies have implicated IGFBP-4 in regulating endothelial cell behavior, as IGFBP-4 was shown to inhibit endothelial cord formation in vitro (32). Moreover, studies have demonstrated that different growth factors can induce angiogenesis by distinct molecular mechanisms. Therefore, we sought to examine the inhibitory activity of IGFBP-4 on angiogenesis induced by distinct growth factors in vivo. To facilitate these studies, chick embryos were treated with various concentrations of IGFBP-4 (50–200 ng/CAM/day) or control to establish an optimal inhibitory dose of IGFBP-4, which was determined to be ∼100 ng/CAM/day. As shown in Fig. 2A, IGFBP-4 significantly (p < 0.05) inhibited IGF-1-induced angiogenesis by >70% as compared with controls. Surprisingly, IGFBP-4 also significantly (p < 0.05) inhibited FGF-2-induced angiogenesis by ∼60% as compared with controls (Fig. 2B); however, IGFBP-4 failed to inhibit VEGF-induced angiogenesis (Fig. 2C). To the best of our knowledge, these findings suggest for the first time that IGFBP-4 exhibits differential inhibitory effects on angiogenesis stimulated by distinct growth factors.
FIGURE 2.
IGFBP-4 differentially inhibits growth factor-induced angiogenesis. Angiogenesis was induced within the CAMs of 10-day-old chick embryos with 40 ng of IGF-1 (A), FGF-2 (B), and VEGF (C), and 24 h later the CAMs were either untreated or treated with IGFBP-4 or BSA (100 ng/egg/day) for 2 days. Angiogenesis was quantified by counting the number of branched blood vessels within the confined area of the filter disc. Left panels, representative example of CAM tissue from each experimental condition. Right panel, mean angiogenic index for each condition from four independent experiments expressed as a percentage of control.
Reduced Proliferation Detected within IGF-1- and FGF-2-induced Angiogenic Blood Vessels Treated with IGFBP-4
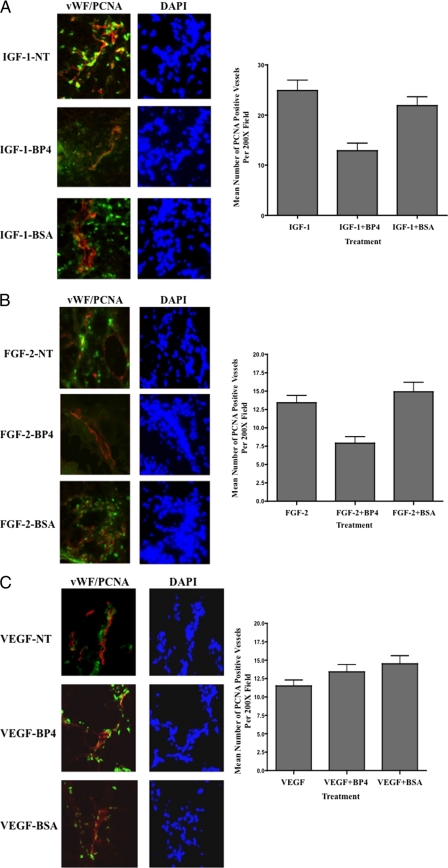
IGFBPs are known to regulate IGF signaling by binding to IGF-1, thereby modulating the ability of this growth factor to bind and activate its cognate receptors (19, 20). Although certain members of the IGFBP family may have both stimulatory and inhibitory activity, IGFBP-4 has been predominately associated with inhibitory activity by reducing IGF-1R signaling (19, 20, 35, 36). IGF-1R signaling has been shown to regulate cellular behavior by modulating processes such as proliferation, survival, and differentiation (36, 37). Therefore, we sough to examine whether the ability of IGFBP-4 to inhibit IGF-1- and FGF-2-induced angiogenesis was associated with alterations in blood vessel proliferation. Frozen sections of CAMs were analyzed by staining for expression of von Willebrand Factor (vWF), a marker of endothelial cells previously used to mark blood vessels in the chick CAM (38) and proliferating cell nuclear antigen (PCNA). As shown in Fig. 3, PCNA staining could be detected within growth factor-stimulated blood vessels from untreated (NT) and BSA-treated CAMs. Importantly, the relative levels of PCNA-positive blood vessels were significantly (p < 0.05) reduced by ∼40% within IGF-1- and FGF-2-stimulated CAMs treated with IGFBP-4 (Fig. 3, A and B). In contrast, no significant (p > 0.05) reduction in the levels of PCNA-positive blood vessels were observed within VEGF-stimulated CAM tissues treated with IGFBP-4. To assess whether IGFBP-4 could impact endothelial cell proliferation in vitro, we examined the effects of IGFBP-4 on HUVEC proliferation. Interaction with denatured collagen type IV failed to significantly alter HUVEC proliferation in the absence of growth factors as compared with native collagen (supplemental Fig. S2, A and B). Interestingly, IGFBP-4 also failed to significantly alter FGF-2- and VEGF-induced proliferation on either native or denatured collagen type IV at concentrations up to 2.0 μg.ml (supplemental Fig. S2, C and D). Although IGFBP-4 failed to inhibit growth factor-induced proliferation of HUVEC in vitro, IGFBP-4 did significantly (p < 0.05) inhibit CAM blood vessel proliferation induced by FGF-2 and IGF-1 by ∼40% but failed to inhibit proliferation of VEGF-stimulated blood vessels in vivo.
FIGURE 3.
Reduced blood vessel proliferation associated with IGFBP-4-mediated inhibition of angiogenesis. CAM tissues from each experimental condition were harvested and snap frozen. 4-μm tissue sections were analyzed by immunofluorescence co-staining. CAM tissues stimulated with IGF-1 (A), FGF-2 (B), and VEGF (C) were co-stained for expression of vWF to mark blood vessels (red) and PCNA (green) to mark proliferating cells. Left panels, representative examples of CAMs co-stained for vWF and PCNA from each experimental condition. Right panels, quantification of the number of PCNA-positive proliferating blood vessels per 200× microscopic field for each experimental condition. Data bars represent the mean number of proliferating blood vessels (± S.E.) from five 200× fields from each of four different CAMs per condition.
Elevated Levels of Phosphorylated p38 MAPK Detected in VEGF-stimulated Angiogenic CAM Tissues
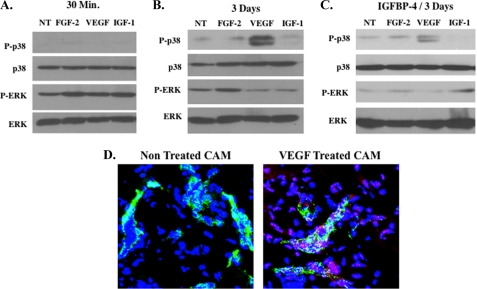
Given the surprising lack of anti-angiogenic effects of IGFBP-4 following VEGF stimulation in vivo, we sought to study potential mechanisms to account for this differential inhibitory activity. CAM lysates were prepared from unstimulated tissues or following 30-min and 3-day stimulation with FGF-2, VEGF, and IGF-1. Alterations in downstream signaling molecules within the MAPK cascade were examined, as MAPK signaling is thought to play an important role in angiogenesis in the CAM model (39, 40). Specifically, pooled CAM tissues (n = 4) from each experimental condition were examined for the relative levels of phosphorylated ERK and p38 MAPKs (p38). As shown in Fig. 4A, 30-min stimulation with FGF-2 and IGF-1 resulted in a slight increase in phosphorylation of ERK as compared with unstimulated CAMs, whereas little change in phosphorylated p38 was detected among any of the growth factors tested. Interestingly, by day 3, which represents the end of the angiogenesis assay, the levels of phosphorylated p38 within VEGF-stimulated angiogenic CAM tissues were increased by ∼4-fold as estimated by scanning and quantification by ImageJ (National Institutes of Health) analysis of the blots (Fig. 4B). Importantly, phosphorylated p38 within these VEGF-stimulated angiogenic CAM tissues remained elevated (∼3-fold) even after treatment with IGFBP-4 at the end of the 3-day angiogenesis assay (Fig. 4C). To examine whether blood vessels within VEGF-stimulated CAM tissues express phosphorylated p38, VEGF-stimulated CAMs were co-stained with anti-phosphorylated p38 (red) and anti-vWF (green) to mark endothelial cells within the blood vessels. As shown in Fig. 4D, although little phosphorylated p38 was detected in unstimulated blood vessels, VEGF-stimulated vessels expressed phosphorylated p38 (red). Phosphorylated p38 was also detected in other cell types within the CAMs, including macrophages (supplemental Fig. S3) as indicated by co-staining for phosphorylated p38 (red) and the monocyte/macrophage marker (green) (41). These data indicate that, although phosphorylated p38 is expressed in multiple cell types in VEGF-stimulated CAMs, elevated levels of phosphorylated p38 is present in VEGF-stimulated endothelial cells within the blood vessels as compared with non-stimulated vessels. Although it is clear that alterations in phosphorylation of ERK occurred over the time course examined, it is also likely that additional time-dependent changes occur in other downstream effectors following differential growth factor stimulation. Our findings provide evidence that, during VEGF-stimulated angiogenesis, elevated levels of phosphorylated p38 persist following IGFBP-4 treatment and that part of the increased phosphorylated p38 observed in VEGF-stimulated CAMs is expressed within the endothelial cells of the blood vessels.
FIGURE 4.
Elevated levels of phosphorylated p38 MAPK detected in VEGF-stimulated angiogenic CAM tissue. To examine changes in expression of MAPKs within angiogenic CAM tissues, lysates were prepared from pooled CAM tissues (n = 4) from each experimental condition. Equal amounts of CAM lysates from each experimental condition were analyzed for the relative levels of p38 and ERK. A, Western blot of untreated CAMs (NT) and CAMs stimulated for 30 min with FGF-2, VEGF, and IGF-1 and analyzed for the expression of total and phosphorylated p38 and ERK. B, Western blot of untreated CAMs (NT) and CAMs stimulated for 3 days with FGF-2, VEGF, and IGF-1 and analyzed for the expression of total and phosphorylated p38 and ERK. C, Western blot of untreated CAMs (NT) and CAMs stimulated for 3 days with FGF-2, VEGF, and IGF-1 in the presence of IGFBP-4 and analyzed for the expression of total and phosphorylated p38 and ERK. D, representative examples of CAMs (n = 4) co-stained for vWF (green) and phosphorylated p38 (red) from untreated and VEGF-treated CAMs. Photos were taken at 400× magnification.
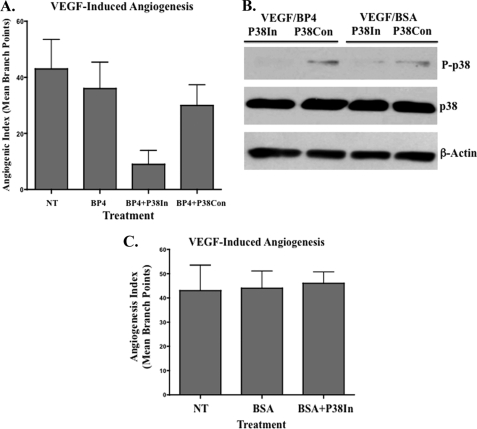
Blocking p38 MAPK Activation Restores the Anti-angiogenic Activity of IGFBP-4 during VEGF-induced Angiogenesis
Given the enhanced levels of phosphorylated p38 in VEGF-stimulated angiogenic CAM vessels, we sought to examine whether elevated levels of phosphorylated p38 play a role in the failure of IGFBP-4 to inhibit VEGF-induced angiogenesis. To this end, angiogenesis was induced with VEGF within the CAMs of 10-day-old chick embryos. Twenty-four hours later, embryos were either untreated or treated with IGFBP-4 in the presence or absence of an active (p38IN) or inactive (p38Con) inhibitor of p38. As shown in Fig. 5A, IGFBP-4 failed to significantly (p > 0.05) inhibit VEGF-induced angiogenesis. In contrast, IGFBP-4 in the presence of the p38 inhibitor (p38IN) significantly (p < 0.05) reduced VEGF-induced angiogenesis by >70%, whereas a combination of IGFBP-4 with the control compound (p38Con) failed to significantly (p > 0.05) inhibit angiogenesis. To confirm that the p38 inhibitor was blocking phosphorylation of p38, lysates from pooled VEGF-stimulated CAM tissues (n = 4) were examined. As shown in Fig. 5B, the levels of phosphorylated p38 were sharply reduced in IGFBP-4-treated angiogenic CAMs in the presence of the active inhibitor (p38IN) but not the inactive control compound (p38Con). Importantly, BSA alone or in combination with the active inhibitor of p38 failed to significantly reduce VEGF-induced angiogenesis (Fig. 5C) suggesting that blocking p38 alone was not sufficient to inhibit VEGF-induced angiogenesis under these conditions. Previous studies have suggested that p38 may play different roles in angiogenesis induced by distinct growth factors, and inhibiting p38 during FGF-2 stimulation was shown to increase angiogenesis in the chick CAM (40). Consistent with these previous finding, inhibiting p38 in our CAM experiments also increased angiogenesis induced with FGF-2 (supplemental Fig. S4). Given previously published evidence suggesting that p38 plays a role in TNF-α-induced angiogenesis (42), we also examined the effects of IGFBP-4 on TNF-α-induced angiogenesis in the chick CAM. As expected, TNF-α induced high levels of phosphorylated p38 in the CAM vessels (supplement Fig. 5A). Consistent with our observations with VEGF-induced angiogenesis in which elevated levels of phosphorylated p38 were induced, IGFBP-4 failed to significantly (p < 0.05) inhibit TNF-α-induced angiogenesis (supplemental Fig. S5B). However, in contrast, the p38 inhibitor alone completely inhibited TNF-α-induced angiogenesis (supplemental Fig. S5B), reinforcing the concept that p38 may play distinct roles in regulating angiogenesis induced by different growth factors. Taken together, our studies suggest for the first time, that the ability of IGFBP-4 to impact VEGF-driven angiogenesis may depend in part on relative levels of phosphorylated p38.
FIGURE 5.
Blocking p38 MAPK activation restores the anti-angiogenic activity of IGFBP-4 during VEGF-induced angiogenesis. Angiogenesis was induced in the CAMs with VEGF and subsequently treated with IGFBP-4 alone (BP4), IGFBP-4, and the active p38 inhibitor (BP4+P38In) or IGFBP-4 and the inactive control (BP4+P38Con). A, data bars represent mean (± S.E.) angiogenic index from each experimental condition (n = 8–10 CAM per condition). Experiments were completed twice with similar results. B, angiogenesis was induced in the CAMs with VEGF and subsequently treated with IGFBP-4 or BSA in the presence of the active p38 inhibitor (p38In) or in active control compound (P38Con). Equal amounts of pooled CAM lysate (n = 4) from each experimental condition were analyzed for the relative levels of total and phosphorylated p38 by Western blot. C, angiogenesis was induced in the CAMs with VEGF and subsequently treated BSA alone or BSA in the presence or the active p38 inhibitor (p38In). Data bars represent mean (± S.E.) angiogenic index from each experimental condition (n = 8–10 CAMs per condition). Experiments were completed twice with similar results.
DISCUSSION
Angiogenesis or the formation of new blood vessels from pre-existing vessels involves a highly coordinated and interconnected set of molecular and cellular events (1–3). The complexity of the angiogenic cascade is further increased as multiple cells types such as smooth muscle cells, pericytes, fibroblasts, tumor cells, bone marrow-derived progenitor cells, inflammatory cells, and endothelial cells contribute to new blood vessel formation (1–3). The stimulatory and inhibitory factors within a particular microenvironment are likely derived from multiple sources and may impact multiple cell types that contribute to blood vessel formation. In addition to the numerous cell types that govern angiogenesis, a complex network of ECM proteins that surround pre-existing blood vessels has been shown to regulate neovascularization (7–17). The majority of quiescent vessels are surrounded by intact basement membranes. These observations are consistent with the notion that cellular interactions with intact vascular basement membranes may function in part, as a physiological breaking system that helps maintain vessels in a differentiated and quiescent state. During processes such as angiogenesis, physical alterations can occur within the three-dimensional structure of ECM proteins, and this is often associated with a transition of cells from quiescence to a proliferative phenotype (10–17). To this end, evidence suggests that cellular interactions with denatured basement membrane components such as collagen type IV and laminin can facilitate new vessel formation, because specific antagonists of these denatured ECM proteins inhibit angiogenesis in vivo (10–17). These studies are consistent with the possibility that structural alterations within the ECM may trigger local biomechanical ECM switches that may release complex breaking mechanisms that normally helps keep blood vessel growth in check.
Our studies suggest that endothelial cell expression of IGFBP-4 is suppressed following binding to denatured as compared with triple helical collagen-IV. Given the known susceptibility of IGFBP-4 to proteolytic cleavage by PAPP-A as well as other matrix metalloproteinase (43, 44), we cannot completely rule out the possibility that proteolytic cleavage contributes to a reduction in IGFBP-4, however, the relative levels of IGFBP-4 mRNA were also reduced in endothelial cells. Our findings are consistent with previously published reports demonstrating the ability of distinct ECM proteins such as fibronectin and collagen type IV to differentially alter expression of IGFBPs such as IGFBP-5 (28). Given the important role of integrins in mediating endothelial cell interactions with ECM proteins, studies are currently underway to examine the potential roles of specific integrin-mediated interactions with denatured collagen in the suppression of endothelial cell IGFBP-4.
Members of the IGFBP family are critical regulators of IGF-1/IGF-1R signaling, and this important pathway is known to modulate the behavior of numerous cell types that contribute to angiogenesis, including tumor cells, vascular smooth muscle cells, and endothelial cells (19, 20). Although IGFBP-4 has been shown to inhibit endothelial tube formation in vitro (32), to the best of our knowledge, its impact on angiogenesis induced by distinct growth factors has not been specifically addressed. In this regard, we examined the effects of IGFBP-4 on angiogenesis induced by several growth factors, including IGF-1, FGF-2, TNF-α, and VEGF. IGFBP-4 inhibited IGF-1-induced angiogenesis by ∼70% as compared with controls. Although these findings were not surprising given the ability of IGFBP-4 to inhibit IGF-1-mediated signaling through the IGF-1R, IGFBP-4 also inhibited FGF-2-induced angiogenesis by ∼60%. The precise molecular mechanisms by which IGFBP-4 inhibited IGF-1- and FGF-2-induced angiogenesis are presently unclear, but this inhibitory activity was associated with a significant reduction in blood vessel proliferation in vivo. Interestingly, IGFBP-4 failed to directly inhibit growth factor-induced HUVEC proliferation in vitro. The lack of the ability of IGFBP-4 to inhibit endothelial cell proliferation in vitro, yet inhibit blood vessel proliferation in vivo might be do the distinct growth conditions used in the in vitro setting as compared with the complex stromal microenvironment in vivo. Alternatively, this lack of activity might be associated with the use of HUVECs. Moreover, IGFBP-4 may indirectly inhibit blood vessel proliferation in vivo by altering recruitment and/or behavior of stromal cells important for blood vessel proliferation such as pericytes, macrophages, or fibroblasts. Given the numerous cell types that contribute to angiogenesis in the CAM and the potential impact of IGFBP-4 might have on distinct cell types, it is possible that the anti-angiogenic activity of IGFBP-4 on IGF-1- and FGF-2-induced angiogenesis in vivo may be associated with both direct and indirect effects of IGFBP-4 on endothelial cells as significantly reduced blood vessel proliferation was observed.
Our studies indicate that IGFBP-4 has a markedly different capacity to inhibit angiogenesis induced by distinct growth factors. Similar to these findings, IGFBP-4 exhibited differential inhibitory effects on histologically distinct tumor types as reports have documented the ability of IGFBP-4 to slow the growth of prostate and colon carcinomas, but showed no inhibitory activity in other tumor types such as renal cell carcinoma (23, 24, 27). Our findings may provide insight to explain in part, the variation in IGFBP-4 inhibitory activity on tumor growth, as angiogenesis within these different tumor types may be driven in part, by distinct growth factor signaling pathways. To this end, angiogenesis associated with renal cell carcinoma has been suggested to be dependent in large part on VEGF-mediated signaling (45). In addition, it is interesting to note that antagonists of integrin αvβ3, which can bind denatured collagen, exhibit reduced capacity to inhibit VEGF-induced angiogenesis while potently inhibiting FGF-2-induced angiogenesis (7).
To begin to examine potential mechanisms to account for the differential inhibitory effects of IGFBP-4, we examined the relative levels of signaling molecules within the MAPK pathway, as this signaling cascade is known to play a role in regulating angiogenesis within this model (39, 40). A small increase in the levels of activated ERK was detected within 30 min of IGF-1 and FGF-2 stimulation, whereas little change in activation of p38 was observed at this time point. However, by 72 h following stimulation with VEGF, CAM tissues exhibited increased levels of activated p38, and although phosphorylated p38 was detected within multiple cell types, including macrophages, it was also detected in endothelial cells. In addition, activated p38 remained high 72 h following VEGF stimulation even in the presence of IGFBP-4. These observations are consistent with a possible role for p38 in regulating the resistance of VEGF-induced angiogenesis to IGFBP-4 treatment. Consistent with this possibility, IGFBP-4 failed to inhibit TNF-α-induced angiogenesis, which also up-regulates phosphorylated p38 in multiple cell types, including endothelial cells, within the blood vessels.
Interestingly, previous reports have documented that p38 can play a role in angiogenesis, because blocking its activation enhances FGF-2-induced endothelial tube formation and enhances FGF-2-induced angiogenesis in the chick CAM (40). In other studies, angiogenesis induced with TNF-α depended, in part, on p38 activation, suggesting a positive role for p38 in new vessel formation (46). Moreover, VEGF-induced activation of p38 is associated with enhanced endothelial cell motility and plays a positive role in VEGFR-2-associated shear stress-induced angiogenesis (46). Moreover, p38 knockout mice exhibit embryonic lethality due in part to defects in placental vascularization (47). Interestingly, the relative levels of activated p38 were shown to be higher in FGF-2-stimulated endothelial cells plated on native as compared with denatured collagen, suggesting that the ability of specific growth factors to regulate p38 may be controlled in part by the structural integrity of ECM proteins (40). Taken together, these and other studies indicate that the contribution of p38 to angiogenesis is complex and likely depends on the particular cell types and tissue context where angiogenesis is occurring. In this regard, our findings suggest that IGFBP-4 can inhibit IGF-1- and FGF-2-induced angiogenesis but not VEGF-induced angiogenesis. This lack of inhibitory activity was due in part to activated p38, because inhibiting p38 in VEGF-stimulated CAM tissues in combination with IGFBP-4 inhibited VEGF-induced angiogenesis. In contrast, the p38 inhibitor alone or in combination with BSA failed to inhibit VEGF- or TNF-α induced angiogenesis.
Given our experimental findings it would be interesting to speculate that structural remodeling of the vascular basement membrane leading to the formation of denatured collagen may lead to the suppression of an endogenous angiogenesis regulator (IGFBP-4) that may function in part to keep blood vessel growth in check. Collectively, our studies provide addition mechanistic insight into the differential inhibitory activity of IGFBP-4 has on angiogenesis induced by distinct growth factors in vivo.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant CA91645 (to P. C. B.), Grant HL65301 (to R. F.), Grants HL083151 and P20RR15555 (Protein, Nucleic Acid Analysis, and Cell Imaging Core) (to C. P. H. V.), and Grant P20 RR181789 (Bioinformatic Core) (to D. M. Wojchowski). This work was also supported by National Centre for Research Resources Grant P20RR15555 and sub-project to PCB (to R. F.) and by institutional support from the Maine Medical Center.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- ECM
- extracellular matrix
- IGFBP-4
- insulin-like growth factor binding protein-4
- FGF-2
- fibroblast growth factor-2
- VEGF
- vascular endothelial growth factor
- CAM
- chorioallantoic membrane
- HUVEC
- human umbilical vein epithelial cell
- vWF
- von Willebrand factor
- PCNA
- proliferating cell nuclear antigen.
REFERENCES
- 1. Pozzi A., Zent R. (2009) Regulation of endothelial cell functions by basement membrane- and arachidonic acid-derived products. Wiley Interdiscip. Rev. Syst. Biol. Med. 1, 254–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen M., Louise Jones J. (2011) Jekyll and Hyde: the role of the microenvironment on the progression of cancer. J. Pathol. 223, 162–176 [DOI] [PubMed] [Google Scholar]
- 3. Petitclerc E., Boutaud A., Prestayko A., Xu J., Sado Y., Ninomiya Y., Sarras M. P., Jr., Hudson B. G., Brooks P. C. (2000) New functions for non-collagenous domains of human collagen type IV: novel integrin ligands inhibiting angiogenesis and tumor growth in vivo. J. Biol. Chem. 275, 8051–8061 [DOI] [PubMed] [Google Scholar]
- 4. Noonan D. M., De Lerma Barbaro A., Vannini N., Mortara L., Albini A. (2008) Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev. 27, 31–40 [DOI] [PubMed] [Google Scholar]
- 5. Sanz L., Alvarez-Vallina L. (2003) The extracellular matrix: a new turn-of-the-screw for anti-angiogenic strategies. Trends Mol. Med. 9, 256–262 [DOI] [PubMed] [Google Scholar]
- 6. Cheresh D. A., Stupack D. G. (2008) Regulation of angiogenesis: apoptotic cues from the ECM. Oncogene 27, 6285–6298 [DOI] [PubMed] [Google Scholar]
- 7. Friedlander M., Brooks P. C., Shaffer R. W., Kincaid C. M., Varner J. A., Cheresh D. A. (1995) Definition of two angiogenic pathways by distinct αv integrins. Science 270, 1500–1502 [DOI] [PubMed] [Google Scholar]
- 8. Hood J. D., Frausto R., Kiosses W. B., Schwartz M. A., Cheresh D. A. (2003) Differential αv integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J. Cell Biol. 162, 933–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brooks P. C., Montgomery A. M., Rosenfeld M., Reisfeld R. A., Hu T., Klier G., Cheresh D. A. (1994) Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79, 1157–1164 [DOI] [PubMed] [Google Scholar]
- 10. Xu J., Rodriguez D., Petitclerc E., Kim J. J., Hangai M., Moon Y. S., Davis G. E., Brooks P. C., Yuen S. M. (2001) Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J. Cell Biol. 154, 1069–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cretu A., Roth J. M., Caunt M., Akalu A., Policarpio D., Formenti S., Gagne P., Liebes L., Brooks P. C. (2007) Disruption of endothelial cell interactions with the novel HU177 cryptic collagen epitope inhibits angiogenesis. Clin. Cancer Res. 13, 3068–3078 [DOI] [PubMed] [Google Scholar]
- 12. Akalu A., Roth J. M., Caunt M., Policarpio D., Liebes L., Brooks P. C. (2007) Inhibition of angiogenesis and tumor metastasis by targeting a matrix immobilized cryptic extracellular matrix epitope in laminin. Cancer Res. 67, 4353–4363 [DOI] [PubMed] [Google Scholar]
- 13. Hangai M., Kitaya N., Xu J., Chan C. K., Kim J. J., Werb Z., Ryan S. J., Brooks P. C. (2002) Matrix metalloproteinase-9-dependent exposure of a cryptic migratory control site in collagen is required before retinal angiogenesis. Am. J. Pathol. 161, 1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Odaka C., Tanioka M., Itoh T. (2005) Matrix metalloproteinase-9 in macrophages induces thymic neovascularization following thymocyte apoptosis. J. Immunol. 174, 846–853 [DOI] [PubMed] [Google Scholar]
- 15. Jo N., Ju M., Nishijima K., Robinson G. S., Adamis A. P., Shima D. T., Mailos C. (2006) Inhibitory effect of an antibody to cryptic collagen type IV epitopes on choroidal neovascularization. Mol. Vis. 12, 1243–1249 [PubMed] [Google Scholar]
- 16. Freimark B., Clark D., Pernasetti F., Nickel J., Myszka D., Baeuerle P. A., Van Epps D. (2007) Targeting of humanized antibody D93 to sites of angiogenesis and tumor growth by binding to multiple epitopes on denatured collagens. Mol. Immunol. 44, 3741–3750 [DOI] [PubMed] [Google Scholar]
- 17. Pernasetti F., Nickel J., Clark D., Baeuerle P. A., Van Epps D., Freimark B. (2006) Novel anti-denatured collagen humanized antibody D93 inhibits angiogenesis and tumor growth: an extracellular matrix-based therapeutic approach. Int. J. Oncol. 29, 1371–1379 [PubMed] [Google Scholar]
- 18. Robert F., Gordon M. S., Rosen L. S., Mendelson D. S., Mulay M., Adams B. J., Alvarez D., Theuer C. P., Leigh B. R. (2010) ASCO Annual Meeting Abst., Abstr. 3038 [Google Scholar]
- 19. Duan C. (2002) Specifying the cellular responses to IGF signals: roles of IGF-binding proteins. J. Endocrinol. 175, 41–54 [DOI] [PubMed] [Google Scholar]
- 20. Hwa V., Oh Y., Rosenfeld R. G. (1999) Endocrine Rev. 20, 761–787 [DOI] [PubMed] [Google Scholar]
- 21. Durai R., Davies M., Yang W., Yang S. Y., Seifalian A. (2006) Biology of insulin-like growth factor binding protein-4 and its role in cancer (review). Int. J. Oncol. 28, 1317–1325 [PubMed] [Google Scholar]
- 22. Zhou R., Flaswinkel H., Schneider M. R., Lahm H., Hoeflich A., Wanke R., Wolf E. (2004) Insulin-like growth factor-binding protein-4 inhibits growth of the thymus in transgenic mice. J. Mol. Endocrinol. 32, 349–364 [DOI] [PubMed] [Google Scholar]
- 23. Damon S. E., Maddison L., Ware J. L., Plymate S. R. (1998) Overexpression of an inhibitory insulin-like growth factor binding protein (IGFBP), IGFBP-4, delays onset of prostate tumor formation. Endocrinology 139, 3456–3464 [DOI] [PubMed] [Google Scholar]
- 24. Durai R., Yang S. Y., Sales K. M., Seifalian A. M., Goldspink G., Winslet M. C. (2007) Insulin-like growth factor binding protein-4 gene therapy increases apoptosis by altering Bcl-2 and Bax proteins and decreases angiogenesis in colorectal cancer. Int. J. Oncol. 30, 883–888 [PubMed] [Google Scholar]
- 25. Yu J. Z., Warycha M. A., Christos P. J., Darvishian F., Yee H., Kaminio H., Berman R. S., Shapiro R. L., Buckley M. T., Liebes L. F., Pavlick A. C., Polsky D., Brooks P. C., Osman I. (2008) Assessing the clinical utility of measuring insulin-like growth factor binding proteins in tissues and sera of melanoma patients. J. Transl. Med. 6, 70–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xie H. L., Li Z. Y., Gan R. L., Li X. J., Zhang Q. L., Hui M., Zhou X. T. (2010) Differential gene and protein expression in primary gastric carcinomas and their lymph node metastases as revealed by combined cDNA microarray and tissue microarray analysis. J. Dig. Dis. 11, 167–175 [DOI] [PubMed] [Google Scholar]
- 27. Ueno K., Hirata H., Majid S., Tabatabai Z. L., Hinoda Y., Dahiya R. (2011) IGFBP-4 activates the Wnt/β-catenin signaling pathway and induces M-CAM expression in human renal cell carcinoma. Int. J. Cancer 129, 2360–2369 [DOI] [PubMed] [Google Scholar]
- 28. Zheng B., Duan C., Clemmons D. R. (1998) The effect of extracellular matrix proteins on porcine smooth muscle cell insulin-like growth factor (IGF) binding protein-5 synthesis and responsiveness to IGF-I. J. Biol. Chem. 273, 8994–9000 [DOI] [PubMed] [Google Scholar]
- 29. Duan C., Clemmons D. R. (1998) Differential expression and biological effects of insulin-like growth factor-binding protein-4 and -5 in vascular smooth muscle cells. J. Biol. Chem. 273, 16836–16842 [DOI] [PubMed] [Google Scholar]
- 30. Wang J., Niu W., Witte D. P., Chernausek S. D., Nikiforov Y. E., Clemens T. L., Sharifi B., Strauch A. R., Fagin J. A. (1998) Overexpression of insulin-like growth factor-binding protein-4 (IGFBP-4) in smooth muscle cells of transgenic mice through a smooth muscle α-actin-IGFBP-4 fusion gene induces smooth muscle hypoplasia. Endocrinology 139, 2605–2614 [DOI] [PubMed] [Google Scholar]
- 31. Ryan A. J., Napoletano S., Fitzpatrick P. A., Currid C. A., O'Sullivan N. C., Harmey J. H. (2009) Expression of a protease-resistant insulin-like growth factor-binding protein-4 inhibits tumour growth in a murine model of breast cancer. Br. J. Cancer 101, 278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moreno M. J., Ball M., Andrade M. F., McDermid A., Stanimirovic D. B. (2006) Insulin-like growth factor binding protein-4 (IGFBP-4) is a novel anti-angiogenic and anti-tumorigenic mediator secreted by dibutyryl cyclic AMP (dB-cAMP)-differentiated glioblastoma cells. GLIA 53, 845–857 [DOI] [PubMed] [Google Scholar]
- 33. Brooks P. C., Montgomery A. M., Cheresh D. A. (1999) Use of the 10-day-old chick embryo model for studying angiogenesis. Methods Mol. Biol. 129, 257–269 [DOI] [PubMed] [Google Scholar]
- 34. Lobov I. B., Brooks P. C., Lang R. A. (2002) Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. U.S.A. 99, 11205–11210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chitnis M. M., Yuen J. S., Protheroe A. S., Pollak M., Macaulay V. M. (2008) The type 1 insulin-like growth factor receptor pathway. Clin. Cancer Res. 14, 6364–6370 [DOI] [PubMed] [Google Scholar]
- 36. Delafontaine P., Song Y. H., Li Y. (2004) Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler. Thromb. Vasc. Biol. 24, 435–444 [DOI] [PubMed] [Google Scholar]
- 37. Clemmons D. R., Maile L. A. (2005) Interaction between insulin-like growth factor-I receptor and αVβ3 integrin linked signaling pathways: cellular responses to changes in multiple signaling inputs. Mol. Endocrinol. 19, 1–11 [DOI] [PubMed] [Google Scholar]
- 38. Leong K. G., Hu X., Li L., Noseda M., Larrivée B., Hull C., Hood L., Wong F., Karsan A. (2002) Activated Notch4 inhibits angiogenesis: role of β1-integrin activation. Mol. Cell. Biol. 22, 2830–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eliceiri B. P., Klemke R., Strömblad S., Cheresh D. A. (1998) Integrin αvβ3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J. Cell Biol. 140, 1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsumoto T., Turesson I., Book M., Gerwins P., Claesson-Welsh L. (2002) p38 MAP kinase negatively regulates endothelial cell survival, proliferation, and differentiation in FGF-2-stimulated angiogenesis. J. Cell Biol. 156, 149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mast J., Goddeeris B. M., Peeters K., Vandesande F., Berghman L. R. (1998) Characterisation of chicken monocytes, macrophages and interdigitating cells by the monoclonal antibody KUL01. Vet. Immunol. Immunopathol. 61, 343–357 [DOI] [PubMed] [Google Scholar]
- 42. Rajashekhar G., Kamocka M., Marin A., Suckow M. A., Wolter W. R., Badve S., Sanjeevaiah A. R., Pumiglia K., Rosen E., Clauss M. (2011) Pro-inflammatory angiogenesis is mediated by p38 MAP kinase. J. Cell. Physiol. 226, 800–808 [DOI] [PubMed] [Google Scholar]
- 43. Laursen L. S., Kjaer-Sorensen K., Andersen M. H., Oxvig C. (2007) Regulation of insulin-like growth factor (IGF) bioactivity by sequential proteolytic cleavage of IGF binding protein-4 and -5. Mol. Endocrinol. 21, 1246–1257 [DOI] [PubMed] [Google Scholar]
- 44. Nagarajan R., Stivers J. T. (2007) Unmasking Anticooperative DNA-binding interactions of vaccinia DNA topoisomerase I. Biochemistry 46, 192–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rini B. I., Small E. J. (2005) Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J. Clin. Oncol. 23, 1028–1043 [DOI] [PubMed] [Google Scholar]
- 46. Gee E., Milkiewicz M., Haas T. L. (2010) p38 MAPK activity is stimulated by vascular endothelial growth factor receptor 2 activation and is essential for shear stress-induced angiogenesis. J. Cell. Physiol. 222, 120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mudgett J. S., Ding J., Guh-Siesel L., Chartrain N. A., Yang L., Gopal S., Shen M. M. (2000) Essential role for p38α mitogen-activated protein kinase in placental angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 97, 10454–10459 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.