Abstract
3-Iodothyronamine (T1AM) is a biogenic amine derivative of thyroid hormone present in tissue and blood of vertebrates. Approximately 99% of the circulating thyroid hormones are bound to plasma proteins, including three major thyroid hormone-binding proteins, and the question arises as to whether circulating T1AM is also bound to serum factors. We report here that T1AM is largely bound to a single protein component of human serum. Using T1AM-affinity chromatography, we isolated this protein, and sequence analysis identified it as apolipoprotein B-100 (apoB-100), the protein component of several low density lipoprotein particles. Consistent with this finding, we demonstrate that >90% of specifically bound T1AM in human serum resides in the apoB-100-containing low density lipoprotein fraction. T1AM reversibly binds to apoB-100-containing lipoprotein particles with an equilibrium dissociation constant (KD) of 17 nm and a T1AM/apoB-100 stoichiometry of 1:1. Competition binding assays demonstrate that this binding site is highly selective for T1AM. Intracellular T1AM uptake is significantly enhanced by apoB-100-containing lipoprotein particles. Modest enhancements to apoB-100 cellular uptake and secretion by T1AM were observed; however, multidose T1AM treatment did not affect lipid or lipoprotein inventory in vivo. Thus, it appears that apoB-100 serves as a carrier of circulating T1AM and affords a novel mechanism by which T1AM gains entry to cells.
Keywords: Lipid Transport, Lipoprotein, Serum, Thyroid, Thyroid Hormone
Introduction
3-Iodothyronamine (T1AM)2 is a naturally occurring derivative of thyroid hormone that has biological actions distinct from those of the predominant secreted form of thyroid hormone, thyroxine (T4), and its peripherally derived deiodination product 3,5,3′-triiodothyronine (T3), the endogenous ligand for thyroid hormone receptors (1). T1AM has rapid short term effects, including induction of hypothermia, bradycardia, and hyperglycemia in mice (2–5). In addition, T1AM administration rapidly triggers a shift in fuel usage toward lipids and away from carbohydrates in both mice and Siberian hamsters (6). It has been suggested that T1AM may be generated in vivo by enzymatic deiodination and decarboxylation of T4, but this has not yet been demonstrated experimentally (7). T1AM has been detected in rodent brain, heart, and liver tissues and also in circulation of mice, guinea pigs, and Siberian hamsters (2, 6, 8). Quantitative analysis of T1AM levels in rat using liquid chromatography coupled to tandem mass spectrometry (LC/MS/MS) revealed that tissue concentrations of T1AM are substantially higher than serum concentrations, and in certain tissues, such as the liver, T1AM is present at significantly higher levels than T4 and T3 (9). However, human sera analyzed with a recently developed highly selective T1AM immunoassay demonstrated T1AM levels comparable with those of total circulating T4 (10).
Thyroid hormones are present in circulation largely bound to carrier proteins. More than 99% of circulating T4 is bound to serum proteins, including thyroxine-binding protein, and transthyretin (11–13). T3 binds to the same proteins, although with lower affinity. Because of the chemical similarities and potential biosynthetic origins of T1AM from thyroid hormones, and because of the presence of T1AM in both serum and tissues, we investigated whether T1AM, like thyroid hormones, was also bound to carrier proteins in serum.
EXPERIMENTAL PROCEDURES
Materials
l-Thyroxine (T4), 3,3′,5-triiodo-l-thyronine (T3), 3,3′,5′- triiodo-l-thyronine (rT3), 3,5-diiodo-l-thyronine (3,5-T2), and l-thyronine (T0) were obtained from Sigma, and 3-iodo-l-thyronine (T1) was from Toronto Research Chemicals Inc. (Canada). T1AM and other thyronamines such as 3,3′,5,5′-tetraiodothyronamine (T4AM), 3,3′,5′-triiodothyronamine (rT3AM), 3,5,3′-triiodothyronamine (T3AM), 3,5-diiodothyronamine (3,5-T2AM), 3,3′-diiodothyronamine (3,3′-T2AM), and thyronamine (T0AM) were synthesized according to the literature (14). Anhydrous dimethylformamide (DMF) was obtained by passing through two columns of activated molecular sieves. Final compounds were characterized by 1H NMR and mass spectrometry.
Determination of T1AM Binding to Serum Protein
500 μl of normal pooled serum (human and rat serum from Innovative Research, Novi, MI; mouse serum from Millipore, Billerica, MA) was incubated with tracer quantities of [125I]T1AM for 24 h at 4 °C in the presence or absence of excess unlabeled T1AM (50 μm). Bound and free [125I]T1AM was separated by filtering through 3K Amicon ultracentrifugal filters (Fisher) at 3000 rpm on a table top centrifuge (Beckman, GS-6R centrifuge) followed by repeated washing with Tris-HCl buffer (0.1 m (pH 7.4)) at 4 °C. After excessive washing, the bound [125I]T1AM was measured by gamma counting (Packard Gamma Cobra II D5005). A control experiment was done in a similar manner by using 500 μl of buffer instead of sera. For the concentration-dependent binding experiment, 80 μl of normal pooled human serum was incubated with different concentrations of [125I]T1AM (from 0.1 nm to 10 μm), in 0.1 m Tris-HCl buffer (pH 7.4) for 24 h at 4 °C in the presence or absence of excess unlabeled T1AM (50 μm). Bound and free T1AM were separated by incubation with 0.5 ml of a charcoal (0.5%)/dextran (0.05%) suspension in Tris-HCl buffer for 15 min at 4 °C with gentle shaking. After centrifugation for 15 min at 4000 × g the supernatant was used for the measurement of radioactivity by gamma counting. Specific binding of [125I]T1AM in this experiment, and all other experiments reported in this paper, was determined by subtracting nonspecific binding from total binding.
Preparation of Affinity Chromatography Supports
All solvents (distilled water, buffer) used in this synthesis were de-gassed with argon. Synthesis of T1AM containing activated disulfide (compound 3) and tyramine containing activated disulfide (compound 7, supplemental Fig. S1) are described in the supplemental material. Compounds 3 and 7 were both immobilized at thiol-Sepharose 4B (Sigma) according to the following procedure: 1 g of freeze-dried activated thiol-Sepharose 4B was suspended in distilled water, and the slurry was poured into a 25 × 1.2-cm column and washed for 15 min with distilled water. The free thiol form of Sepharose 4B was prepared according to the manufacturer's protocol. The thiol-Sepharose 4B column was then equilibrated with immobilization buffer (0.5 m NaCl, 1 mm EDTA, 10 mm sodium acetate (pH 5.0)). The coupling reaction was performed by incubating activated disulfide containing either T1AM (compound 3) or tyramine with the thiol-Sepharose matrix in buffer (DMF/buffer = 1:1, 10 mm sodium acetate, 0.5 m NaCl, and 1 mm EDTA) by gentle swirling for 6 h at 4 °C. The support was then washed with 10 mm sodium acetate (pH 6.0), and the amount of immobilized compound 4 was determined by UV-spectroscopic quantification of the released 2-thiopyridone at 343 nm (15). An alternative procedure was also found to be successful, and this is described in the supplemental material, method B.
Affinity Chromatography
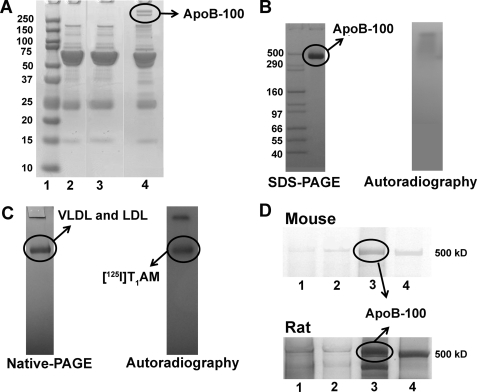
Normal pooled human serum (1 ml) was incubated with the T1AM-attached thiol-Sepharose matrix (4 ml) in 0.01 m PBS buffer containing 1 mm EDTA, overnight at 4 °C. After incubation, the column was washed exhaustively with PBS buffer (8 times, 50 ml) containing different concentrations of salt (NaCl, 137–500 mm) until A280 <0.02. Bound proteins were eluted by cleavage of the disulfide bond with 1% DTT in 4 ml of PBS buffer (1 h at room temperature), and the resulting eluates were collected separately. Identification of T1AM-binding protein was determined by gel electrophoresis (4–20% Tris-HCl gel) of the DTT eluates obtained from three affinity columns, namely T1AM-attached Sepharose, tyramine-attached Sepharose, and activated thio-Sepharose. Coomassie stain was used to visualize the gel for both human and mouse sera, and silver stain was used for rat. Extra protein bands (circled in Fig. 3A), observed in lane 4, correspond to the eluate from T1AM-attached thiol-Sepharose matrix and were analyzed by LC/MS (supplemental material) using an Agilent 1100 series capillary LC system (Agilent Technologies Inc, Santa Clara, CA) and an LTQ linear ion trap mass spectrometer (ThermoFisher, San Jose, CA).
FIGURE 3.
Gel electrophoresis and autoradiography. A, SDS-polyacrylamide gel of eluted fractions from T1AM affinity chromatography of human serum. SDS-polyacrylamide gel (4–20% Tris-HCl) of standard protein marker (lane 1), DTT eluent from activated thiol-Sepharose (control) column (lane 2), tyramine-attached Sepharose (control) column (lane 3), and T1AM-attached Sepharose column (lane 4) are shown. Protein sequencing revealed that both high molecular weight bands in lane 4 correspond to apoB-100. B, SDS-polyacrylamide gel (3–8% Tris acetate) of isolated lipoprotein showing major protein band corresponding to apoB-100 and the corresponding autoradiography that indicates T1AM does not covalently bind with apoB-100 protein. C, native-polyacrylamide gel (4–16% BisTris with 4% stacking gel) of isolated apoB-100-containing lipoproteins (VLDL and LDL) and the corresponding autoradiography showing [125I]T1AM binding to apoB-100-containing lipoproteins obtained by exposing dried native-polyacrylamide gel to film for 10 days at −80 °C. D, SDS-polyacrylamide gel showing T1AM- specific binding protein in mouse and rat serum; DTT eluent from activated thio-Sepharose (control) column (lane 1), tyramine-attached Sepharose (control) column (lane 2), and from T1AM-attached Sepharose column (lane 3), and standard protein marker (lane 4).
Serum Fractionation by Density Centrifugation
Very low density lipoprotein (VLDL; <1.006 kg/liter), low density lipoprotein (LDL; 1.019–1.063 kg/liter), and high density lipoprotein (HDL; 1.063–1.21 kg/liter) were separated from normal pooled human serum by a standard micro-ultracentrifugation technique, using KBr for density adjustment (16). Purified lipoprotein fractions were analyzed by running gel electrophoresis, native-PAGE, SDS-PAGE, and agarose gel (supplemental material) (17–20). Coomassie stain was used after running gel electrophoresis to visualize the protein in all cases.
Gel Electrophoresis and Autoradiography
Isolated apoB-100-containing lipoprotein (in 0.1 m Tris-HCl buffer (pH 7.4)) was incubated with tracer quantity of [125I]T1AM for 24 h at 4 °C. Separation of bound from free [125I]T1AM was achieved by Sephadex G-25 chromatography. The reaction mixture was then analyzed by running gel electrophoresis (4–16% BisTris native-PAGE with 4% stacking gel) (21–23) followed by staining with Coomassie Blue. Gels were dried and subjected to autoradiography at −80 °C for 7 days.
ApoB-100 Ligand Binding Experiments
For the saturable binding experiment, LDL was incubated with different concentrations of [125I]T1AM (from 0.25 to 200 nm), in 0.1 m Tris-HCl buffer (pH 7.4) for 24 h at 4 °C in the presence or absence of excess unlabeled T1AM (50 μm). Nonspecific binding was determined by including 50 μm unlabeled T1AM at each concentration of labeled T1AM, [125I]T1AM. Bound [125I]T1AM was separated from free [125I]T1AM by using charcoal/dextran solution (as mentioned above), and the radioactivity of bound [125I]T1AM was measured with a gamma counter. All samples were run in triplicate. The dissociation constant (KD) and the maximum number of binding sites (Bmax) were determined by nonlinear least squares curve fit utilizing the ligand depletion method to determine free concentration (Graphpad Prism Software, San Diego). For the competition binding assays, apoB-100-containing lipoprotein was incubated with tracer quantity of [125I]T1AM in the presence or absence of excess (0.1 pm to 100 μm) of unlabeled different structural analogues (thyronines: T0, T1, 3,3′-T2, 3,5-T2, T3, rT3, and T4; thyronamines: T0AM, T1AM, 3,3′-T2AM, 3,5-T2AM, T3AM, rT3AM, and T4AM; serotonin and tyramine) in buffer for 24 h at 4 °C. Free [125I]T1AM was removed from the bound by using charcoal/dextran solution as described above, and the radioactivity in the supernatant was counted.
Cell Cultures
HepG2 cells and normal human skin fibroblasts were obtained from ATCC (Manassas, VA). Cells were cultured in flasks with Dulbecco's modified Eagle's media (DMEM) supplemented with 10% (v/v) fetal calf serum (culture medium, DMEM/FBS) and 1% mg/ml penicillin/streptomycin at 37 °C with 5% CO2. Cells were used for experiments at 80% confluency.
Effect of LDL on the Entry of T1AM into HepG2 Cells
HepG2 cells were washed with Dulbecco's phosphate-buffered saline (supplemented with CaCl2 and MgCl2 (pH 7.2)), harvested by trypsinization (0.05% trypsin and 0.5 mm EDTA), and then diluted with cell culture medium (DMEM/FBS) so as to seed them at about 5 × 104 cells/well (2 ml) in 6-well plates (3.5 × 1.0 cm; Labware). To induce maximal expression of LDL receptors, the usual culture medium (DMEM/FBS) was changed 48–60 h before the experiment with DMEM supplemented with 10% (v/v) human lipoprotein-deficient serum (LDS; Sigma; product number S5519) (24).
Incubation Procedure
A detailed procedure is described in the literature (24). In brief, HepG2 cells were washed with 2 ml of Hanks' balanced salt solution (Thermo Scientific) buffered with 15 mm HEPES and preincubated with 2 ml of Hanks' balanced salt solution for 45 min at 37 °C under 5% CO2. For analysis of saturable uptake of T1AM in cells, 0.5 ml of the incubation mixture was added to each well. Typically, in each 6-well plate, three wells were used for the total cell uptake of T1AM, whereas the other three wells were used for the nonsaturable cell uptake of T1AM. The incubation mixture for test experiments contained 0.5 nm [125I]T1AM plus LDL (10 μg/ml) in the presence and absence of 10 μm unlabeled T1AM. However, the incubation mixture for control experiments contain only 0.5 nm [125I]T1AM in the presence and absence of 10 μm unlabeled T1AM (24). After incubation for the specified time at 37 °C under 5% CO2, the plates were washed three times in rapid succession with 2 ml of ice-cold Dulbecco's phosphate-buffered saline containing 0.1% BSA. Cells were solubilized in 1 ml of 0.1 n NaOH with gentle shaking, and a 750-μl aliquot was used for measurement of radioactivity. Protein concentration was determined with a BCA protein assay (Thermo Scientific). Results (means ± S.D.) are expressed in terms of specific (saturable) uptake of T1AM per mg of cell protein. For determination of the amount of intracellular and cell surface T1AM uptake, a similar procedure was used with an incubation time of 60 min. After incubation, fractionation was performed using a subcellular protein fractionation kit (Thermo Scientific), and the saturable T1AM uptake in each fraction was determined by gamma counting.
Uptake of 125I-LDL to Fibroblasts
To induce the maximal expression of LDL receptors, the usual culture medium (DMEM/FBS) was changed 48–60 h before the experiment with DMEM supplemented with 10% (v/v) human lipoprotein-deficient serum (DMEM/LDS) (see above). In a separate experiment, 10 μg/ml of 125I-LDL (Biomedical Technologies Inc., MA) was preincubated with different concentrations of T1AM (0, 10, 40, 80, 160, and 320 pm) for 24 h at 4 °C. Cells were washed with Dulbecco's phosphate-buffered saline, harvested by trypsinization, and resuspended in incubation medium (DMEM supplemented with 10% LDS and 24 mm bicarbonate at pH 7.4). To determine binding and uptake of 125I-LDL, fibroblasts were incubated with 125I-LDL in the presence and absence of a 50-fold excess of unlabeled LDL in DMEM supplemented with 24 mm bicarbonate and 10% human LDS at pH 7.4 for 2 h (25). Intracellular uptake and surface binding of 125I-LDL to the fibroblasts at 37 °C were determined as described previously (25).
Effect of T1AM on Secretion of ApoB Protein in HepG2 Cells
HepG2 cells were grown in 75-cm2 flasks in culture medium for 24 h at 37 °C with 5% CO2. The medium was then removed, and the cells were washed with Hanks' balanced salt solution and incubated with DMEM supplemented with 10% LDS. Cells were then treated with different doses of T1AM (dissolved in DMSO), and the same volume of DMSO was used for the control experiment. ApoB secretion into the media after 48 h was measured as described previously (26–29). ApoB secretion into media was determined by ELISA (“CardioCHEK,” ALerCHEK) according to the manufacturer's instructions. ApoB secretion was normalized to total cell protein as determined by BCA protein assay.
Animals
The experimental protocol was in compliance with the Federal guidelines for care and handling of small rodents and approved by the Institutional Animal Care and Use Committee (IACUC) of Oregon Health and Science University. Animals were housed in a temperature-controlled room with alternating 12-h periods of light and dark and had free access to food and water. All animals were allowed to adapt to the environment for at least 2 weeks prior to treatment. Wild type mice (C57BL/6J; male; 8–10 weeks old; The Jackson Laboratory, Bar Harbor, ME) were given high fat diet (Rodent Diet 60% kcal % fat, Research Diet Inc; Item D12492) for 15 days prior to the administration of T1AM and continued the same high fat diet during 15 days administration of T1AM. Conversely, apoB-100 transgenic mice (B6.SJL-Tg(APOB)1102Sgy N20+?; female; 9–11 weeks old; Taconic Laboratories, Hudson, NY) were given only normal rodent chow diet throughout the experiment. Animals were injected intraperitoneally once daily with different doses of T1AM (0.4, 0.25, and 0.01 mg/kg), and the control mice were treated with same volume of saline. Each group contained five individual mice. Weight gain of all mice was monitored every day during the period of administration. After 15 days administration of T1AM, mice were euthanized with CO2 and blood was collected via cardiac puncture into tubes containing EDTA (BD Microtainer). Food was removed from the mice 4 h before the collection of blood. Lipid profile and the amounts of apoB of all mice were examined from serum. The amounts of apoB protein in the transgenic mice were determined by using an ELISA kit (see above).
Statistical Analysis
Values are reported as mean ± S.D. Statistical analysis was performed with Student's t test. p > 0.05 was considered not to be significant.
RESULTS
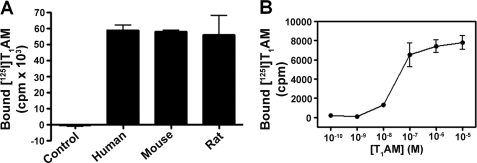
To test whether T1AM was protein-bound in serum, we incubated a tracer quantity [125I]T1AM with serum from mouse, rat, and human, separated bound T1AM from free by centrifugal dialysis filtration, and measured the amount of bound T1AM by gamma counting compared with a buffer control. For each sample (and all subsequent binding experiments) specific binding of T1AM was assessed by adding a large excess of unlabeled T1AM to determine the component of nonspecific T1AM binding. A comparable amount of specifically bound T1AM was observed in serum from all three species (Fig. 1A). Using human serum, we next assessed the concentration dependence of T1AM-specific binding by incubating a range of T1AM concentrations (0.1 nm to 10 μm) supplemented with tracer [125I]T1AM (Fig. 1B). In this case, free T1AM was separated from bound by charcoal filtration. Analysis of the specifically bound radioactivity revealed saturation binding of T1AM to a macromolecular serum component.
FIGURE 1.
A, T1AM-specific binding to macromolecular components in serum from human, mouse, and rat. B, concentration dependence of specific T1AM binding in human serum.
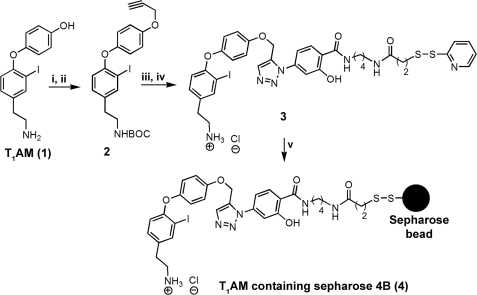
We devised an affinity chromatography strategy for isolating the putative T1AM serum-binding protein(s) that involved the chemical synthesis of T1AM-immobilized to a solid support (Fig. 2). The amine group of T1AM was first protected with a tert-butyloxycarbonyl (BOC) group, and then the BOC-protected T1AM was reacted with propargyl bromide in DMF under basic conditions to afford alkyne 2. Activated disulfide N-[4-(p-azidosalicylamido)butyl]-3′-(2′-pyridyldithio) propionamide was used as a cross-linker, which contains the azide functional group. The azide was then reacted with the alkyne group of 2 in the presence of 5 mol % of sodium ascorbate and 1 mol % of copper(II) sulfate in DMF providing a white solid fluorescent compound. Deprotection of the BOC group with dry HCl/ethyl acetate gave T1AM-containing activated disulfide 3. For a control column, we attached tyramine to Sepharose beads through its phenolic-OH group using similar chemistry. Both T1AM and tyramine containing activated disulfides were covalently attached to Sepharose 4B beads via a disulfide exchange reaction, the progress of which was monitored by following the generation of 2-mercaptopyridine by UV-visible spectrophotometry.
FIGURE 2.
Preparation of affinity chromatography support. Reagents and conditions are as follows: (i) Boc2O, NaHCO3, THF, and H2O (95%); (ii) propargyl bromide, K2CO3, and N,N-dimethylformamide (85%); (iii) cross-linker N-[4-(p-azidosalicylamido) butyl]-3′-(2′-pyridyldithio) propionamide, CuSO4, 5H2O, sodium ascorbate, and N,N-dimethylformamide (80%); (iv) HCl/ethyl acetate (90%); (v) gentle shaking of thiol-Sepharose 4B beads with T1AM containing activated disulfide in 10 mm sodium acetate (pH 5.0) for 6 h.
Pooled normal human serum was incubated with T1AM-derivatized Sepharose beads (compound 4), washed extensively with a PBS/NaCl gradient, and the flow-through (eluate) obtained during washing was collected until an A280 <0.02 in the eluent was observed. Bound protein was eluted by cleaving the disulfide bond connecting T1AM to the Sepharose bead with 1% DTT. The DTT eluate was analyzed by SDS-PAGE. SDS-PAGE results show that the DTT eluate obtained from the T1AM-derivatized column contained two unique high molecular weight bands compared with the thiol-Sepharose and tyramine control columns (Fig. 3A). The protein contained in these two bands (Fig. 3A, lane 4) was extracted from the gel and sequenced using standard proteomic mass spectrometry techniques. Sequence analysis showed that both bands corresponded to the same protein-apolipoprotein B-100 (apoB-100), the major protein component of very low density lipoprotein (VLDL), and the sole protein component of low density lipoprotein (LDL) particles (30). Mass spectrometry analysis of these two bands (Fig. 3A, lane 4) showed 25% protein sequence coverage for apoB-100 (supplemental Tables S1–S3).
We next examined whether T1AM could bind to intact lipoprotein particles containing apoB-100 such as VLDL and LDL. A mixture of VLDL and LDL was isolated from pooled normal human serum by sequential density ultracentrifugation (16, 31). SDS-PAGE of this preparation revealed the presence of apoB-100 (∼500 kDa) as the major protein present in this lipoprotein fraction (Fig. 3B and supplemental Fig. S6). This apoB-100-enriched fraction was incubated with [125I]T1AM (32), and free [125I]T1AM was separated from bound [125I]T1AM by gel filtration. Native-PAGE of the bound fraction revealed that T1AM co-migrates with apoB-100-containing particles (Fig. 3C and supplemental Fig. S7), whereas no radioactivity was observed in the SDS-PAGE (Fig. 3B). Furthermore, mass spectrometry analysis of this particular band in the native-polyacrylamide gel (circled in Fig. 3C) revealed that apoB-100 is the major protein present (supplemental Tables S4–S6). These results indicate that T1AM binds reversibly to VLDL and LDL particles, most likely through association with apoB-100. We also used a similar affinity chromatography strategy to identify T1AM-specific binding protein(s) from mouse and rat serum. SDS-PAGE results show that the DTT eluate obtained from the T1AM-derivatized columns incubated with rodent sera also contained apoB-100 (Fig. 3D).
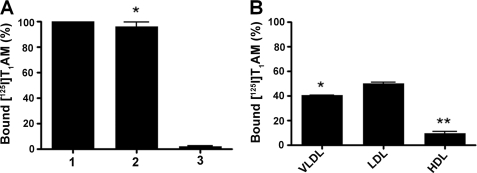
We next examined the distribution of bound T1AM in human serum to determine what fraction of bound T1AM was associated with apoB-100-containing lipoprotein particles. [125I]T1AM was incubated with human serum followed by sequential fractionation of the serum by density ultracentrifugation and analysis of bound T1AM by gamma counting. More than 95% of the specifically bound T1AM was found associated with lipoproteins, and the remaining <5% was bound to other unidentified serum components (Fig. 4A). The apoB-100-containing lipoprotein fraction was further fractionated by density ultracentrifugation into VLDL, LDL, and high density lipoprotein (HDL) fractions, and the amount of bound [125I]T1AM was determined by gamma counting. More than 90% of the specifically bound T1AM was found associated with the apoB-100-containing lipoprotein particles such as VLDL and LDL, with roughly equal distribution between VLDL and LDL; less than 10% of the labeled T1AM was contained in the HDL fraction (Fig. 4B).
FIGURE 4.
A, distribution of specifically bound [125I]T1AM in human serum: (column 1) unfractionated human serum; (column 2) lipoprotein fraction from human serum; (column 3) remaining serum minus the lipoprotein fraction. B, distribution of specifically bound [125I]T1AM in lipoprotein VLDL, LDL, and HDL fractions from the lipoprotein fraction (column 2 in (A)) derived from human serum. Normal pooled human serum was incubated with tracer quantity of [125I]T1AM in 0.1 m Tris-HCl buffer (pH 7.4) for 24 h at 4 °C in the presence or absence of excess unlabeled T1AM (50 μm). Free [125I]T1AM was removed using charcoal-dextran solution. Different lipoprotein fractions (VLDL, LDL and HDL) were separated by a standard micro-ultracentrifugation technique. *, p ≤ 0.05; **, p ≤ 0.01.
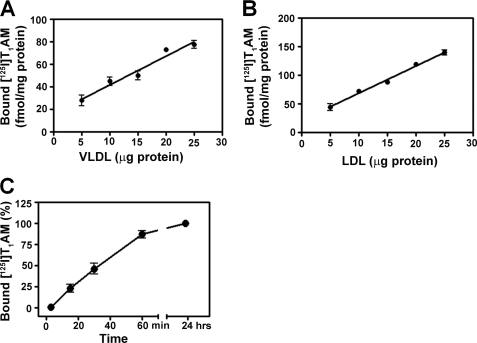
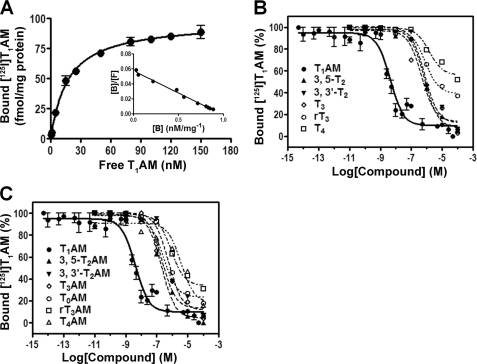
We next examined the relationship between the bound T1AM and the amount of apoB-100 in the context of both VLDL and LDL (Fig. 5, A and B). Different amounts of purified VLDL and LDL (5–25 μg) were incubated with [125I]T1AM, and the bound [125I]T1AM was quantified. The amount of [125I]T1AM specifically bound was found to be proportional to the amount of apoB-100-containing lipoprotein particle added to the incubation mixture. The time dependence of [125I]T1AM binding to LDL was also examined with the finding that 90% of the specific binding occurred within 60 min at 4 °C (pH 7.4) (Fig. 5C). We next studied the equilibrium binding properties of T1AM with apoB-100 in the context of a human serum fraction enriched in VLDL and LDL such that apoB-100 was the major protein component present as discussed previously (Fig. 3B and supplemental material). T1AM binding to this preparation was found to be concentration-dependent with saturation of specific binding at high T1AM concentrations. The data showed best fit to a one-site model, suggesting a single apoB-100-binding site for T1AM (Fig. 6A). Scatchard analysis of these data indicated single site binding with an equilibrium dissociation constant (KD) of 17 nm and a ligand/protein stoichiometry of 1:1(Fig. 6A, inset). We also examined equilibrium binding of T1AM to a highly purified LDL fraction (i.e. no VLDL such as that used in Fig. 4B) and observed a similar saturation binding curve with a similar KD of 48 nm (supplemental Fig. S8). To evaluate the specificity of T1AM binding to apoB-100, a competition binding assay was performed between [125I]T1AM and similar analogues, including iodothyronines (T0, T1, 3,3′-T2, 3,5-T2, T3, rT3, and T4), iodothyronamines (T0AM, T1AM, 3,3′-T2AM, 3,5-T2AM, T3AM, rT3AM, and T4AM), and other biogenic amines (tyramine and serotonin). The apoB-100-containing lipoprotein was incubated with a tracer quantity of [125I]T1AM in the presence of different concentrations of unlabeled competitor compounds for 24 h at 4 °C. Free [125I]T1AM was separated from bound by charcoal absorption, and bound [125I]T1AM was quantified. As expected, unlabeled T1AM competed successfully with [125I]T1AM for the binding site of apoB-100, and the corresponding IC50 value was 5 nm (Fig. 6, B and C, and Table 1). However, all other competitor compounds were at least 50–1000 times less effective in competition binding against [125I]T1AM (Fig. 6, B and C). IC50 and % inhibition values from these competition experiments are provided in Table 1. These results indicate that the apoB-100 binding of T1AM is highly selective.
FIGURE 5.
Specific binding of [125I]T1AM as a function of VLDL concentration (A) and LDL concentration (B) is shown. Purified VLDL and LDL fractions were incubated with a tracer quantity of [125I]T1AM in the presence or absence of excess T1AM (50 μm) in 0.1 m Tris-HCl buffer for 24 h at 4 °C. C, time course of specific binding of T1AM to LDL. Each data point is the mean ± S.D. of three experiments.
FIGURE 6.
Equilibrium binding of [125I]T1AM to apoB-100-containing lipoprotein particles. An equilibrium dissociation constant (KD) of 17 nm was determined from the fit (A). Scatchard analysis reveals a single and saturable binding site with a 1:1 stoichiometry between ligand and protein (inset of A). Competition of [125I]T1AM binding to apoB-100-containing lipoprotein with unlabeled thyronines (B) and thyronamines (C) is shown. For competition binding assays, apoB-100-containing lipoprotein was incubated with tracer quantity of [125I]T1AM in the presence or absence of excess T1AM and the indicated concentrations of competitor ligand for 24 h at 4 °C. Plotted data are the mean ± S.D. of three experiments.
TABLE 1.
IC50 values and % inhibition from competition binding to apoB-100 with [125I]T1AM
| Compounds | IC50 | % of inhibition at 1 μm concentration of inhibitorsa |
|---|---|---|
| nm | ||
| T1AM | 5 | 91 |
| 3′-T1AM | 259 | 48 |
| T0AM | 268 | 54 |
| 3,5-T2AM | 209 | 72 |
| 3,3′-T2AM | 570 | 62 |
| T3AM | 224 | 62 |
| rT3AM | 865 | 37 |
| T4AM | 3834 | 32 |
| T0 | 14 | |
| T1 | 24 | |
| 3,5-T2 | 738 | 56 |
| 3,3′-T2 | 776 | 54 |
| T3 | 479 | 53 |
| rT3 | 818 | 34 |
| T4 | 1518 | 21 |
| Tyramine | 3 | |
| Serotonin | 3 |
a Percent inhibition at 1 μm concentration of inhibitors was determined in a separate experiment.
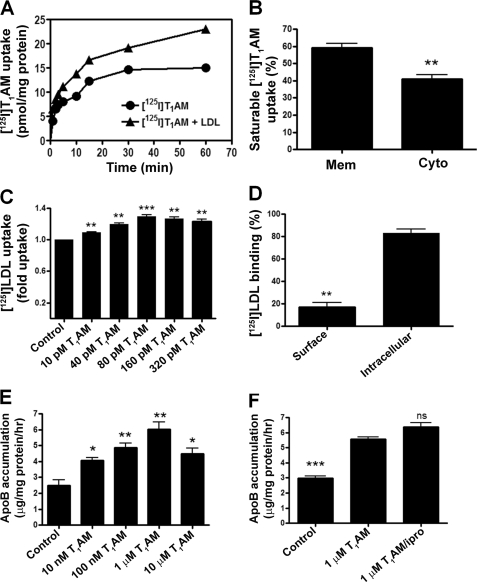
We studied the effect of LDL on the time course of T1AM uptake in HepG2 cells in which LDL receptors are expressed maximally (33). Equilibrium uptake of T1AM increased by ∼50% over 60 min in the presence of exogenously added LDL (10 μg/ml) compared with that in LDL-free media (Fig. 7A). When we examined where the T1AM taken up by HepG2 resided, we found that 60% was membrane-associated, whereas 40% resided in the cytoplasm (Fig. 7B). We next evaluated whether T1AM affected LDL uptake into fibroblasts in which LDL receptors are overexpressed (24, 33). A dose-dependent 20% increase in the saturable uptake of LDL in fibroblasts was observed (Fig. 7C). About 20% of this was surface-associated and could be released with heparin treatment, whereas about 80% was intracellular and heparin-resistant (Fig. 7D). Motivated by reports of T3 stimulation of apoB synthesis and secretion in HepG2 cells (28, 34), we next examined whether T1AM had any effect on this process in vitro. We found that T1AM stimulated apoB secretion in a dose-dependent manner with a maximal increase in apoB secretion of 2.4-fold (Fig. 7E). We have shown previously that T1AM is oxidatively deaminated to 3-iodothyroacetic acid (TA1) in HepG2 cells, and the question arises whether T1AM or the metabolite TA1 is mediating the observed increase in apoB secretion (35). As such, HepG2 cells were treated with T1AM and iproniazid, a broad spectrum amine oxidase inhibitor that blocks the T1AM to TA1 oxidative deamination, and apoB secretion was assessed. We found that T1AM increased the secretion of apoB equally in the presence and absence of iproniazid indicating that this effect is specific to T1AM and not TA1 (Fig. 7F).
FIGURE 7.
In vitro functional effects of T1AM. A, saturable uptake of [125I]T1AM by LDL receptor-competent HepG2 cells in the absence or presence of LDL (10 μg/ml). Plotted data are the mean ± S.D. of three experiments, each performed in triplicate. B, subcellular localization (membrane and cytoplasm) of [125I]T1AM taken up by HepG2 cells in an LDL-dependent manner after 60 min of incubation. C, saturable uptake of 125I-labeled LDL (10 μg/ml) by LDLR-competent fibroblasts cells at different concentrations of T1AM (0, 10, 40, 80, 160, and 320 pm). The data are plotted as fold uptake of 125I-labeled LDL relative to the control (T1AM untreated). D, subcellular localization (surface and intracellular) of 125I-labeled LDL in the presence of T1AM. Human fibroblasts were incubated with 125I-labeled LDL at 37 °C and preincubated with 80 pm T1AM, in the presence and absence of a 50-fold excess of unlabeled LDL in DMEM supplemented with 24 mm bicarbonate and 10% human LDS at pH 7.4 for 2 h (25). E, effect of T1AM on apoB production by HepG2 cells. HepG2 cells were treated with T1AM or vehicle for 2 days in DMEM supplemented with 10% human LDS (28). F, effect of iproniazid (100 μm) on the production of apoB stimulated by T1AM in HepG2 cells. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ns, not significant.
Given these in vitro results, we were next interested to find out whether T1AM elicited similar effects in vivo. For these studies, we used wild type mice (C57BL/6J) fed a high cholesterol diet as well as human apoB-100 transgenic mice that have substantially elevated levels of circulating human apoB-100 (36–38). Mice were treated once daily (intraperitoneally) with 0, 0.025, 0.1, or 0.4 mg/kg T1AM for 15 days; doses of T1AM higher than ∼0.5 mg/kg induce anorexia, which would interfere with the circulating lipid levels.3 In addition, these T1AM doses do not induce hypothermia, hyperglycemia, or any of the other previously reported actions of T1AM. After 15 days of dosing, serum was collected and evaluated for VLDL, LDL, HDL, apoB (in the transgenic mice only), triglyceride, and total cholesterol content (supplemental Fig. S3, A–E). As expected, the human apoB-100 transgenic mice had elevated levels of apoB-100-containing LDL and VLDL (supplemental Fig. S3, A and B) but reduced levels of HDL (supplemental Fig. S3C) compared with WT/high cholesterol-fed mice. This also corresponded to elevated triglycerides (supplemental Fig. S3D) and unchanged total cholesterol (supplemental Fig. S3E). T1AM treatment at all doses showed no effect on any of these serum markers and also did not change circulating apoB levels in the apoB-100 transgenic mice relative to vehicle control.
DISCUSSION
We demonstrate here that T1AM, like the thyroid hormones T4 and T3, is largely protein bound in circulation. Using affinity chromatography, we isolated the predominant T1AM-binding protein from human and rodent serum, which turned out to be apoB-100, the unique protein component of low density lipoprotein particles. More than 95% of specifically bound T1AM was found to be associated with the lipoprotein fraction in human serum, and more than 90% of the lipoprotein-bound T1AM was distributed approximately equally between apoB-100-containing LDL and VLDL particles. T1AM binds reversibly to apoB-100 with a KD of 17 nm and a T1AM/apoB-100 stoichiometry of 1:1. This binding site is highly selective for T1AM as none of the iodothyronamines or iodothyronines competed effectively with T1AM in ligand competition binding assays and had IC50 values 50–1000 times higher than that of T1AM.
ApoB-100 is a >500-kDa polypeptide that functions as the major protein component of LDL, VLDL, IDL, and Lp(a) lipoprotein particles (30). Circulating apoB-100 polypeptide is never free but is part of a large particle composed of lipids and protein. Depending on the circulating lipid inventory, which is a function of life style and genetics in humans, the concentration of circulating apoB-100 in humans is 1.5–3.0 μm (77–153 mg/dl) (39).
Although the majority of circulating thyronine-based thyroid hormones is bound by nonlipoprotein carriers, small amounts of T4 and T3 (3% of T4 and 6% of T3) are bound to VLDL, LDL, and HDL lipoprotein particles (40). Thyronine binding to LDL and VLDL involves apoB-100, whereas binding to HDL occurs at the HDL-specific apolipoprotein A-I (apoA-I) (41, 42). ApoB-100 reportedly contains a binding site for T4 with a KD of 1 μm, and apoB-100-bound T4 may facilitate entry of T4 into cells via LDL/LDL receptor-mediated endocytosis. We found a higher affinity interaction between T1AM and apoB-100, and T4 was unable to compete against T1AM for binding to apoB-100. We conclude from this that the T1AM-binding site on apoB-100 is distinct from the previously reported T4-binding site and that the T4-binding site does not allosterically influence the T1AM-binding site.
The finding that circulating T1AM is largely bound to apoB-100 as part of a lipoprotein particle may explain the discrepancy in the reported serum levels of T1AM using different bioanalytical methods (43). Using an LC/MS/MS approach, Saba et al. (9) reported high tissue concentrations of T1AM but very low serum concentrations, on the order of 0.3 nm. In contrast, Hoefig et al. (10) measured substantially higher serum T1AM concentrations averaging ∼66 nm using an immunoassay based on a monoclonal antibody that selectively binds T1AM. This immunoassay did not involve the use of an extraction procedure for isolating biogenic amines from the serum matrix, whereas all LC/MS/MS-based approaches involve some kind of a chemical extraction step. It is therefore possible that the typical biogenic amine extraction procedures do not quantitatively liberate T1AM from its unique binding site residing within a lipoprotein particle and that extraction-based approaches quantify free and not total circulating T1AM. Additionally, if total T1AM plasma concentration in humans is on the order of 70 nm, and as discussed previously circulating apoB-100 concentrations range from 1.5–3.0 μm, then the T1AM serum-binding sites are normally in excess compared with the total T1AM serum concentration suggesting that is largely bound and not free in circulation.
The obvious question that arises concerns the functional role of specific, high affinity binding of T1AM to apoB-100-containing lipoprotein particles. In experiments aimed to address this, we observed modest effects by T1AM in vitro on LDL uptake and apoB-100 secretion; however, none of these effects were apparent in vivo. For this we used multiple dose T1AM treatment on diet-induced hypercholesterolemic wild type or transgenic human apoB-100 mice and observed no change in circulating lipid or lipoprotein inventory, suggesting that T1AM had no measurable effect on either synthesis or clearance of apoB-100. One caveat to these in vivo studies is that the maximum dose of T1AM was limited to 0.4 mg/kg due to a dose-limiting side effect of appetite suppression at doses higher than 0.5 mg/kg, which would indirectly result in lipid lowering; nevertheless, T1AM did not induce a significant dose-dependent change to VLDL, LDL, HDL, apoB-100, triglyceride, or total cholesterol levels.
However, the addition of exogenous LDL to cultured cells incubated with radiolabeled T1AM resulted in a 50% enhancement in the cellular uptake of T1AM, suggesting that the physiological role of T1AM association with apoB-100 may be to provide a mechanism for transportation and entry of T1AM into target cells via the LDL receptor (LDLR)-mediated endocytosis or some other LDLR-independent pathway. Essentially all cell types express LDLRs for the purpose of accessing cholesterol and other lipids, and LDLRs are especially prevalent in hepatocytes where they mediate the first step in cholesterol clearance (33). Consistent with this is the fact that endogenous T1AM is also most abundant in the liver (9).
The robust, specific uptake of T1AM into a variety of cell types has been previously reported with the mechanism of this transport process remaining unclear (44). Transport by a biogenic amine plasma membrane transporter related to dopamine, serotonin, or norepinephrine reuptake transporters was ruled out based on the lack of an observed ion or pH dependence. In addition, a systematic functional screen of most members of the solute carrier (SLC) transporter family did not reveal any orphan family members specific for T1AM transport. It has been further demonstrated that T1AM is not a substrate for monocarboxylate transporter (MCT)-8 or MCT-10, both established specific transporters of T4 and T3 (45). A T1AM uptake mechanism based on receptor-mediated endocytosis could not be ruled out, and the prospect of this as the uptake route of T1AM is clearly strengthened by this study. Indeed, receptor-mediated endocytosis involving apoB-100 is a well established cellular uptake mechanism for small molecule lipids such as cholesterol and triglycerides. If this proves also to be the case for T1AM, then it suggests that a target of the biological action of T1AM resides within the cell and not on the plasma membrane. This situation further suggests that the standard theories regarding free and bound hormone fractions where only the free fraction is considered to be biologically active may not be relevant for T1AM.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant DK-52798 (to T. S. S.) and Center Grants 5P30CA-069533 and 5P30EY-010572. This work was also supported by the Proteomics Shared Resource that is generously funded by Oregon Opportunity.

This article contains supplemental Figs. S1–S8, Tables S1–S6, and additional references.
B. Hettinger and T. S. Scanlan, unpublished data.
- T1AM
- 3-iodothyronamine
- apoB-100
- apolipoprotein B-100
- T4
- l-thyroxine
- T3
- 3,5,3′-triiodo-l-thyronine
- rT3
- 3,3′,5′-triiodo-l-thyronine
- 3,5-T2
- 3,5-diiodo-l-thyronine
- T1
- 3-iodo-l-thyronine
- T0
- l-thyronine
- BOC
- tert-butyloxycarbonyl
- LDS
- lipoprotein-deficient serum
- DMF
- dimethylformamide
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- LDLR
- LDL receptor.
REFERENCES
- 1. Piehl S., Hoefig C. S., Scanlan T. S., Köhrle J. (2011) Thyronamines. Past, present, and future. Endocr. Rev. 32, 64–80 [DOI] [PubMed] [Google Scholar]
- 2. Scanlan T. S., Suchland K. L., Hart M. E., Chiellini G., Huang Y., Kruzich P. J., Frascarelli S., Crossley D. A., Bunzow J. R., Ronca-Testoni S., Lin E. T., Hatton D., Zucchi R., Grandy D. K. (2004) 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat. Med. 10, 638–642 [DOI] [PubMed] [Google Scholar]
- 3. Doyle K. P., Suchland K. L., Ciesielski T. M., Lessov N. S., Grandy D. K., Scanlan T. S., Stenzel-Poore M. P. (2007) Novel thyroxine derivatives, thyronamine and 3-iodothyronamine, induce transient hypothermia and marked neuroprotection against stroke injury. Stroke 38, 2569–2576 [DOI] [PubMed] [Google Scholar]
- 4. Klieverik L. P., Foppen E., Ackermans M. T., Serlie M. J., Sauerwein H. P., Scanlan T. S., Grandy D. K., Fliers E., Kalsbeek A. (2009) Central effects of thyronamines on glucose metabolism in rats. J. Endocrinol. 201, 377–386 [DOI] [PubMed] [Google Scholar]
- 5. Regard J. B., Kataoka H., Cano D. A., Camerer E., Yin L., Zheng Y. W., Scanlan T. S., Hebrok M., Coughlin S. R. (2007) Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J. Clin. Invest. 117, 4034–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braulke L. J., Klingenspor M., DeBarber A., Tobias S. C., Grandy D. K., Scanlan T. S., Heldmaier G. (2008) 3-Iodothyronamine: a novel hormone controlling the balance between glucose and lipid utilization. J. Comp. Physiol. B 178, 167–177 [DOI] [PubMed] [Google Scholar]
- 7. Scanlan T. S. (2009) Minireview. 3-Iodothyronamine (T1AM). A new player on the thyroid endocrine team? Endocrinology 150, 1108–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chiellini G., Frascarelli S., Ghelardoni S., Carnicelli V., Tobias S. C., DeBarber A., Brogioni S., Ronca-Testoni S., Cerbai E., Grandy D. K., Scanlan T. S., Zucchi R. (2007) Cardiac effects of 3-iodothyronamine. A new aminergic system modulating cardiac function. FASEB J. 21, 1597–1608 [DOI] [PubMed] [Google Scholar]
- 9. Saba A., Chiellini G., Frascarelli S., Marchini M., Ghelardoni S., Raffaelli A., Tonacchera M., Vitti P., Scanlan T. S., Zucchi R. (2010) Tissue distribution and cardiac metabolism of 3-iodothyronamine. Endocrinology 151, 5063–5073 [DOI] [PubMed] [Google Scholar]
- 10. Hoefig C. S., Köhrle J., Brabant G., Dixit K., Yap B., Strasburger C. J., Wu Z. (2011) Evidence for extrathyroidal formation of 3-iodothyronamine in humans as provided by a novel monoclonal antibody-based chemiluminescent serum immunoassay. J. Clin. Endocrinol. Metab. 96, 1864–1872 [DOI] [PubMed] [Google Scholar]
- 11. Robbins J. (1976) Thyroxine-binding proteins. Prog. Clin. Biol. Res. 5, 331–355 [PubMed] [Google Scholar]
- 12. Schussler G. C. (1990) Thyroxine-binding proteins. Thyroid 1, 25–34 [DOI] [PubMed] [Google Scholar]
- 13. Schussler G. C. (2000) The thyroxine-binding proteins. Thyroid 10, 141–149 [DOI] [PubMed] [Google Scholar]
- 14. Hart M. E., Suchland K. L., Miyakawa M., Bunzow J. R., Grandy D. K., Scanlan T. S. (2006) Trace amine-associated receptor agonists. Synthesis and evaluation of thyronamines and related analogues. J. Med. Chem. 49, 1101–1112 [DOI] [PubMed] [Google Scholar]
- 15. Schürer H., Stembera K., Knoll D., Mayer G., Blind M., Förster H. H., Famulok M., Welzel P., Hahn U. (2001) Aptamers that bind to the antibiotic moenomycin A. Bioorg. Med. Chem. 9, 2557–2563 [DOI] [PubMed] [Google Scholar]
- 16. Brousseau T., Clavey V., Bard J. M., Fruchart J. C. (1993) Sequential ultracentrifugation micromethod for separation of serum lipoproteins and assays of lipids, apolipoproteins, and lipoprotein particles. Clin. Chem. 39, 960–964 [PubMed] [Google Scholar]
- 17. Ståhlman M., Davidsson P., Kanmert I., Rosengren B., Borén J., Fagerberg B., Camejo G. (2008) Proteomics and lipids of lipoproteins isolated at low salt concentrations in D2O/sucrose or in KBr. J. Lipid Res. 49, 481–490 [DOI] [PubMed] [Google Scholar]
- 18. Fu T., Mukhopadhyay D., Davidson N. O., Borensztajn J. (2004) The peroxisome proliferator-activated receptor α (PPARα) agonist ciprofibrate inhibits apolipoprotein B mRNA editing in low density lipoprotein receptor-deficient mice: effects on plasma lipoproteins and the development of atherosclerotic lesions. J. Biol. Chem. 279, 28662–28669 [DOI] [PubMed] [Google Scholar]
- 19. Wiesner P., Leidl K., Boettcher A., Schmitz G., Liebisch G. (2009) Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 50, 574–585 [DOI] [PubMed] [Google Scholar]
- 20. Lynn E. G., Siow Y. L., Frohlich J., Cheung G. T., O K. (2001) Lipoprotein-X stimulates monocyte chemoattractant protein-1 expression in mesangial cells via nuclear factor-κB. Kidney Int. 60, 520–532 [DOI] [PubMed] [Google Scholar]
- 21. Bietrix F., Yan D., Nauze M., Rolland C., Bertrand-Michel J., Coméra C., Schaak S., Barbaras R., Groen A. K., Perret B., Tercé F., Collet X. (2006) Accelerated lipid absorption in mice overexpressing intestinal SR-BI. J. Biol. Chem. 281, 7214–7219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aviram M., Lund-Katz S., Phillips M. C., Chait A. (1988) The influence of the triglyceride content of low density lipoprotein on the interaction of apolipoprotein B-100 with cells. J. Biol. Chem. 263, 16842–16848 [PubMed] [Google Scholar]
- 23. Aviram M., Bierman E. L., Chait A. (1988) Modification of low density lipoprotein by lipoprotein lipase or hepatic lipase induces enhanced uptake and cholesterol accumulation in cells. J. Biol. Chem. 263, 15416–15422 [PubMed] [Google Scholar]
- 24. Benvenga S., Robbins J. (1990) Enhancement of thyroxine entry into low density lipoprotein (LDL) receptor-competent fibroblasts by LDL. An additional mode of entry of thyroxine into cells. Endocrinology 126, 933–941 [DOI] [PubMed] [Google Scholar]
- 25. Goldstein J. L., Basu S. K., Brown M. S. (1983) Receptor-mediated endocytosis of low density lipoprotein in cultured cells. Methods Enzymol. 98, 241–260 [DOI] [PubMed] [Google Scholar]
- 26. Manthey K. C., Chew Y. C., Zempleni J. (2005) Riboflavin deficiency impairs oxidative folding and secretion of apolipoprotein B-100 in HepG2 cells, triggering stress response systems. J. Nutr. 135, 978–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stewart B. J., Roede J. R., Doorn J. A., Petersen D. R. (2009) Lipid aldehyde-mediated cross-linking of apolipoprotein B-100 inhibits secretion from HepG2 cells. Biochim. Biophys. Acta 1791, 772–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adeli K., Sinkevitch C. (1990) Secretion of apolipoprotein B in serum-free cultures of human hepatoma cell line, HepG2. FEBS Lett. 263, 345–348 [DOI] [PubMed] [Google Scholar]
- 29. Maiyoh G. K., Kuh J. E., Casaschi A., Theriault A. G. (2007) Cruciferous indole-3-carbinol inhibits apolipoprotein B secretion in HepG2 cells. J. Nutr. 137, 2185–2189 [DOI] [PubMed] [Google Scholar]
- 30. Jonas A., Phillips M. C.. (2008) in Biochemistry of Lipids, Lipoproteins and Membranes (Vance D. E., Vance J. E., eds), 5th Ed, pp. 485–506, Elsevier, New York [Google Scholar]
- 31. Havel R. J., Eder H. A., Bragdon J. H. (1955) The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34, 1345–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miyakawa M., Scanlan T. S. (2006) Synth. Commun. 36, 891–902 [Google Scholar]
- 33. Benvenga S., Robbins J. (1998) Thyroid hormone efflux from monolayer cultures of human fibroblasts and hepatocytes. Effect of lipoproteins and other thyroxine transport proteins. Endocrinology 139, 4311–4318 [DOI] [PubMed] [Google Scholar]
- 34. Theriault A., Ogbonna G., Adeli K. (1992) Thyroid hormone modulates apolipoprotein B gene expression in HepG2 cells. Biochem. Biophys. Res. Commun. 186, 617–623 [DOI] [PubMed] [Google Scholar]
- 35. Wood W. J., Geraci T., Nilsen A., DeBarber A. E., Scanlan T. S. (2009) Iodothyronamines are oxidatively deaminated to iodothyroacetic acids in vivo. ChemBioChem 10, 361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Callow M. J., Stoltzfus L. J., Lawn R. M., Rubin E. M. (1994) Expression of human apolipoprotein B and assembly of lipoprotein(a) in transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 91, 2130–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Purcell-Huynh D. A., Farese R. V., Jr., Johnson D. F., Flynn L. M., Pierotti V., Newland D. L., Linton M. F., Sanan D. A., Young S. G. (1995) Transgenic mice expressing high levels of human apolipoprotein B develop severe atherosclerotic lesions in response to a high fat diet. J. Clin. Invest. 95, 2246–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qiu S., Bergeron N., Kotite L., Krauss R. M., Bensadoun A., Havel R. J. (1998) Metabolism of lipoproteins containing apolipoprotein B in hepatic lipase-deficient mice. J. Lipid Res. 39, 1661–1668 [PubMed] [Google Scholar]
- 39. Beghin L., Duhal N., Poulain P., Hauw P., Lacroix B., Lecerf J. M., Bonte J. P., Fruchart J. C., Luc G. (2000) Measurement of apolipoprotein B concentration in plasma lipoproteins by combining selective precipitation and mass spectrometry. J. Lipid Res. 41, 1172–1176 [PubMed] [Google Scholar]
- 40. Benvenga S., Gregg R. E., Robbins J. (1988) Binding of thyroid hormones to human plasma lipoproteins. J. Clin. Endocrinol. Metab. 67, 6–16 [DOI] [PubMed] [Google Scholar]
- 41. Benvenga S., Cahnmann H. J., Robbins J. (1990) Localization of the thyroxine-binding sites in apolipoprotein B-100 of human low density lipoproteins. Endocrinology 127, 2241–2246 [DOI] [PubMed] [Google Scholar]
- 42. Benvenga S., Cahnmann H. J., Rader D., Kindt M., Robbins J. (1992) Thyroxine binding to the apolipoproteins of high density lipoproteins HDL2 and HDL3. Endocrinology 131, 2805–2811 [DOI] [PubMed] [Google Scholar]
- 43. Scanlan T. S. (2011) Endogenous 3-iodothyronamine (T1AM). More than we bargained for. J. Clin. Endocrinol. Metab. 96, 1674–1676 [DOI] [PubMed] [Google Scholar]
- 44. Ianculescu A. G., Giacomini K. M., Scanlan T. S. (2009) Identification and characterization of 3-iodothyronamine intracellular transport. Endocrinology 150, 1991–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ianculescu A. G., Friesema E. C., Visser T. J., Giacomini K. M., Scanlan T. S. (2010) Transport of thyroid hormones is selectively inhibited by 3-iodothyronamine. Mol. Biosyst. 6, 1403–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.