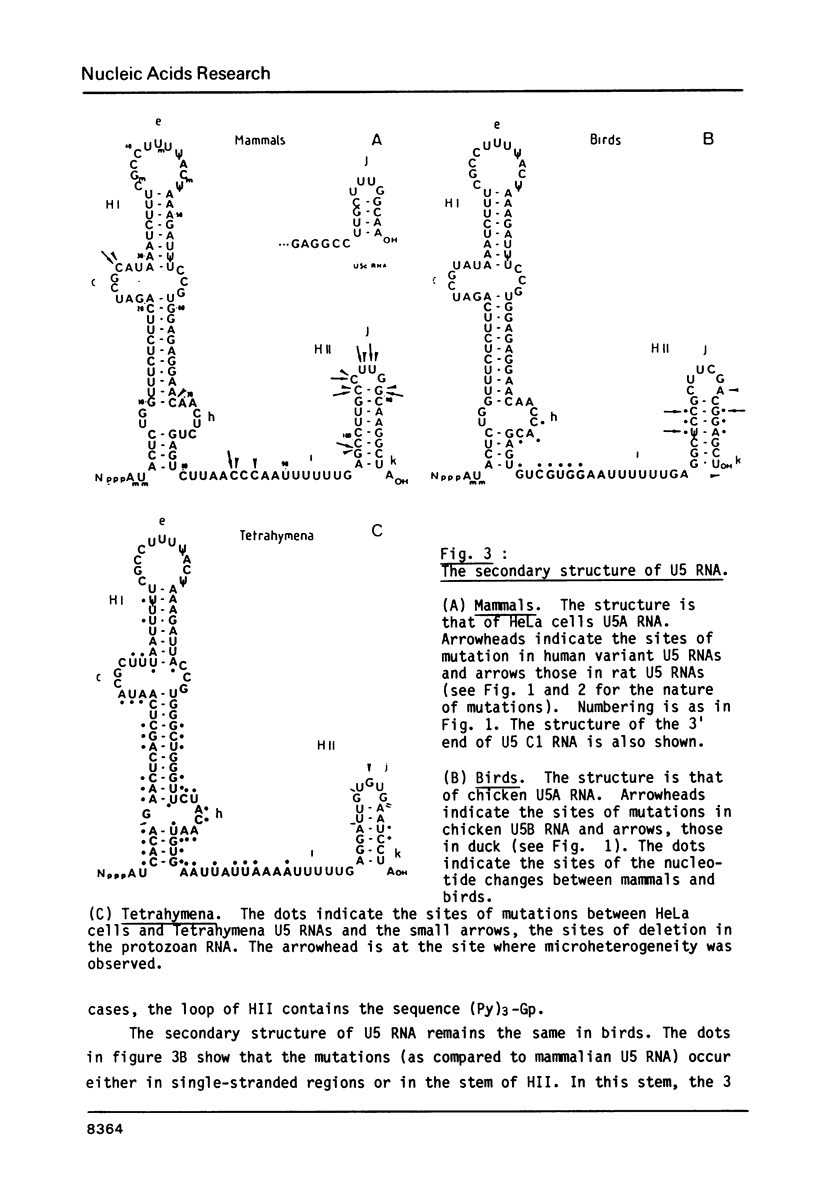
Abstract
The nucleotide sequence of chicken, pheasant, duck and Tetrahymena pyriformis U5 RNAs as well as that of new mammalian variant U5 RNAs was determined and compared to that of rat and HeLa cells U5 RNAs. Primary structure conservation is about 95% between rat and human cells, 82% between mammals and birds and 57% between the Protozoan and mammals. The same model of secondary structure, a free single-stranded region flanked by two hairpins can be constructed from all RNAs and is identical to the model previously proposed for mammalian U5 RNA on an experimental basis (1). Thus, this model is confirmed and is likely to be that of an ancestor U5 RNA. The 3' region of the U5 RNA molecule constitutes domain A, and is common to U1, U2, U4 and U5 RNAs (2). The characteristic nucleotide sequences of domain A are highly conserved throughout the phylogenetic evolution of U5 RNA suggesting that they are important elements in the function of the four small RNAs. Another region of high evolutionary conservation is the top part of the 5' side hairpin whose conserved sequence is specific to U5 RNA. It might participate in the particular function of U5 RNA.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branlant C., Krol A., Ebel J. P., Lazar E., Gallinaro H., Jacob M., Sri-Widada J., Jeanteur P. Nucleotide sequences of nuclear U1A RNAs from chicken, rat and man. Nucleic Acids Res. 1980 Sep 25;8(18):4143–4154. doi: 10.1093/nar/8.18.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Lazar E., Haendler B., Jacob M. U2 RNA shares a structural domain with U1, U4, and U5 RNAs. EMBO J. 1982;1(10):1259–1265. doi: 10.1002/j.1460-2075.1982.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deimel B., Louis C. H., Sekeris C. E. The presence of small molecular weight RNAs in nuclear ribonucleoprotein particles carrying HnRNA. FEBS Lett. 1977 Jan 15;73(1):80–84. [PubMed] [Google Scholar]
- Gallinaro H., Jacob M. An evaluation of small nuclear RNA in hnRNP. FEBS Lett. 1979 Aug 1;104(1):176–182. doi: 10.1016/0014-5793(79)81110-2. [DOI] [PubMed] [Google Scholar]
- Harada F., Kato N., Nishimura S. The nucleotide sequence of nuclear 4.8S RNA of mouse cells. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1332–1340. doi: 10.1016/0006-291x(80)91620-4. [DOI] [PubMed] [Google Scholar]
- Kato N., Harada F. Nucleotide sequence of nuclear 5S RNA of mouse cells. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1468–1476. doi: 10.1016/0006-291x(81)90784-1. [DOI] [PubMed] [Google Scholar]
- Krol A., Branlant C., Lazar E., Gallinaro H., Jacob M. Primary and secondary structures of chicken, rat and man nuclear U4 RNAs. Homologies with U1 and U5 RNAs. Nucleic Acids Res. 1981 Jun 25;9(12):2699–2716. doi: 10.1093/nar/9.12.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A., Gallinaro H., Lazar E., Jacob M., Branlant C. The nuclear 5S RNAs from chicken, rat and man. U5 RNAs are encoded by multiple genes. Nucleic Acids Res. 1981 Feb 25;9(4):769–787. doi: 10.1093/nar/9.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liautard J. P., Sri-Widada J., Brunel C., Jeanteur P. Structural organization of ribonucleoproteins containing small nuclear RNAs from HeLa cells. Proteins interact closely with a similar structural domain of U1, U2, U4 and U5 small nuclear RNAs. J Mol Biol. 1982 Dec 15;162(3):623–643. doi: 10.1016/0022-2836(82)90392-8. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Steitz J. A. Sequence of U1 RNA from Drosophila melanogaster: implications for U1 secondary structure and possible involvement in splicing. Nucleic Acids Res. 1981 Dec 11;9(23):6351–6368. doi: 10.1093/nar/9.23.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northemann W., Scheurlen M., Gross V., Heinrich P. C. Circular dichroism of ribonucleoprotein complexes from rat liver nuclei. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1130–1137. doi: 10.1016/0006-291x(77)90973-1. [DOI] [PubMed] [Google Scholar]
- Okada N., Sakamoto K., Itoh Y., Ohshima Y. Sequence determination of rat U5 RNA using a chemical modification procedure for counteracting sequence compression. J Biochem. 1982 Apr;91(4):1281–1291. doi: 10.1093/oxfordjournals.jbchem.a133813. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pousada C., Hayes D. H. Poly(A)-containing RNA in Tetrahymena pyriformis. Eur J Biochem. 1976 Dec;71(1):117–124. doi: 10.1111/j.1432-1033.1976.tb11096.x. [DOI] [PubMed] [Google Scholar]
- Sri-Widada J., Liautard J. P., Brunel C., Jeanteur P. Interaction of snRNAs with rapidly sedimenting nuclear sub-structures (hnRNPs) from HeLa cells. Nucleic Acids Res. 1983 Oct 11;11(19):6631–6646. doi: 10.1093/nar/11.19.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]



