Background: Intracellular Aβ degradation is enhanced in the presence of apoE.
Results: Lowering cellular cholesterol levels facilitates intracellular trafficking of Aβ to lysosomes and enhances its degradation. Conversely, increasing cholesterol levels retards the delivery and inhibits degradation of Aβ.
Conclusion: The cholesterol efflux function of apoE mediates its ability to promote Aβ degradation.
Significance: We demonstrate a direct role for cholesterol in AD.
Keywords: Alzheimer Disease, Amyloid, ApoE, Cholesterol, Microglia, Rab7, Endosome Trafficking
Abstract
Allelic variation in the apolipoprotein E (APOE) gene is the major risk factor of sporadic Alzheimer disease. ApoE is the primary cholesterol carrier in the brain. Previously, we demonstrated that intracellular degradation of β-amyloid (Aβ) peptides by microglia is dramatically enhanced in the presence of apoE. However, the molecular mechanisms subserving this effect remain unknown. This study reports a mechanistic link between apoE-regulated cholesterol homeostasis and Aβ degradation. We demonstrate that promoting intracellular Aβ degradation by microglia is a common feature of HDL apolipoproteins, including apoE and apoA-I. This effect was not dependent on the direct interaction of apoE and Aβ. Regulation of Aβ degradation was achieved by solely manipulating cellular cholesterol levels. The expression and the activity of Aβ degrading enzymes, however, were not regulated by cholesterol. We observed that reducing cellular cholesterol levels by apoE resulted in faster delivery of Aβ to lysosomes and enhanced degradation. Moreover, apoE facilitated the recycling of Rab7, a small GTPase responsible for recruiting the motor complex to late endosomes/lysosomes. These data indicate that faster endocytic trafficking of Aβ-containing vesicles in the presence of apoE resulted from efficient recycling of Rab7 from lysosomes to early endosomes. Thus, apoE-induced intracellular Aβ degradation is mediated by the cholesterol efflux function of apoE, which lowers cellular cholesterol levels and subsequently facilitates the intracellular trafficking of Aβ to lysosomes for degradation. These findings demonstrate a direct role of cholesterol in the intracellular Aβ degradation.
Introduction
The polymorphism of the apolipoprotein E (APOE) gene is the major genetic risk factor for sporadic, late onset Alzheimer disease (AD)2 (1, 2). There are three common apoE isoforms in human, apoE2, -E3, and -E4, which differ in only two amino acid residues (3). Possession of APOE4 alleles confers increased AD risk and an earlier age of onset in a gene dose-dependent manner, whereas inheriting the apoE2 allele is protective (1, 4). Recently, Bateman and co-workers demonstrated that Aβ clearance is impaired in human late onset AD patients (5). Importantly, the APOE4 allele is associated with decreased Aβ clearance (6, 7). We have demonstrated that apoE plays a direct role in the normal, physiological clearance of Aβ from the brain. ApoE was shown to facilitate both intracellular and extracellular proteolytic degradation of Aβ in an isoform- and lipidation status-dependent manner (8). However, the molecular mechanisms by which apoE influences proteolysis of Aβ remain unclear. In this study, we focus on the mechanism of apoE-facilitated intracellular Aβ degradation by microglia.
ApoE is the predominant apolipoprotein in the central nervous system (CNS) and acts to scaffold the formation of high density lipoprotein (HDL) particles. ApoE regulates the redistribution and homeostasis of cholesterol within the CNS, which is necessary for the maintenance of the structural and functional integrity of synapses and membranes (9, 10). In the brain apoE is mainly produced by astrocytes and to a much lesser extent by microglia. Cholesterol is transferred to apoE through the activity of ATP-binding cassette transporter A1 (ABCA1) and related transporters, including ABCG1 and ABCG4, to form HDL particles, resulting in the reduction of cellular cholesterol levels (11–13). Conversely, cells can use HDL as an exogenous source of cholesterol through a receptor-mediated endocytosis pathway (14).
A growing body of evidence suggests that dysregulated cholesterol metabolism may be involved in the pathogenesis of AD. Epidemiological studies also suggested a positive correlation of hypercholesterolemia and a high incidence of coronary artery disease to the increased risk of AD (15). Inhibition of cholesterol synthesis by statins was shown to decrease the Aβ levels and AD pathology in several animal models of AD (16, 17). Moreover, high fat diets dramatically exacerbate AD-related pathology in mouse models (18–20). Although cholesterol has been widely investigated in the production of Aβ, there is limited knowledge about its involvement in the clearance of Aβ. Deficiency of Abca1 results in the accumulation of cholesterol and exacerbates the formation of amyloid plaques, whereas overexpression of Abca1 reduces brain Aβ levels and amyloid deposition (13, 21–23). Amyloid precursor protein processing and Aβ production were not affected by these genetic manipulations, indicating their influence on AD pathology was a result of alternations in Aβ clearance. Increasing apoE expression through liver X receptor activation promotes Aβ degradation both in vitro and in vivo (8). Importantly, the ability of apoE to facilitate Aβ clearance is dependent upon the lipidation status of apoE, as highly lipidated species were more effective in promoting Aβ degradation. Indeed, microglia deficient in Abca1, which have poorly lipidated apoE-HDLs, exhibited impaired cholesterol efflux function and were unable to efficiently degrade soluble Aβ (sAβ). These reports combined suggest the importance of the cholesterol efflux function of apoE in its effects on intracellular sAβ degradation.
In this study we report that apoE facilitates intracellular Aβ degradation by microglia through modulation of cellular cholesterol levels. This activity does not require direct interaction between apoE and Aβ. Depleting cellular cholesterol through its transfer to apoE-containing HDL particles alone was sufficient to promote Aβ degradation. Furthermore, we observed that the intracellular transport of Aβ through the endocytic system was accelerated in the presence of apoE. The cholesterol lowering effect of apoE enhanced the recycling of Rab7, a small GTPase protein in the motor complex on late endocytic vesicles, which in turn facilitated the trafficking of Aβ-containing vesicles to lysosomes for degradation. These findings provide a mechanistic explanation of apoE-facilitated intracellular Aβ degradation and demonstrate a direct role for cholesterol in AD.
EXPERIMENTAL PROCEDURES
Reagents
Human apoE and apoA-I were purchased from rPeptide (Bogart, GA) and Sigma (St. Louis, MO), respectively. The 4F peptide (Ac-DWFKAFYDKVAEKFKEAF-NH2) (24) was custom-made by American Peptide (Sunnyvale, CA). Lovastatin, methyl-β-cyclodextrin (MβCD), water-soluble cholesterol (cholesterol-loaded MβCD), and U18666A were purchased from Sigma.
Primary Microglial Culture
Primary microglia were derived from the brains of C57BL/6 or Apoe−/− mice at postnatal days 1–3 using a mild trypsinization protocol as previously described (25, 26). Purified microglia were then maintained in DMEM/F-12 (Invitrogen) containing 2% heat-inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin for 3–5 days before further experiments.
Preparation of Aβ Peptides
Lyophilized unlabeled (American Peptide) or Alexa 555-labeled Aβ1–42 (AnaSpec, Fremont, CA) and Aβ12–28 (American Peptide) were dissolved to a final concentration of 1 mg/ml in DMSO and stored at −80 °C until use. For flow cytometry, the Aβ1–42 was then labeled with Alexa 488 fluorophores (Invitrogen) using the manufacturer's protocol as previously described (8, 27). In brief, the Aβ reaction mixture was allowed to fibrilize at 37 °C overnight. Unincorporated fluorophores were then removed by ultracentrifugation at 100,000 × g at 4 °C. The pellet was resuspended in DMSO, sonicated, and ultracentrifuged. The steps were subsequently repeated until most of the pellet fraction was solubilized in DMSO. The supernatant contains the operationally defined “soluble Aβ,” which consists of primarily monomeric Aβ, with only low levels of small oligomeric species (28).
Intracellular Aβ Degradation Assay
Primary microglia were plated overnight in 12-well plates at a density of 2.2 × 105 cells/well in DMEM/F-12 (Invitrogen) containing 2% FBS (Invitrogen). On the day before pretreatment, cells were switched to serum-free DMEM/F-12 overnight. Microglia were then treated with apoE, apoA-I, 4F peptide, U18666A, or cholesterol-loaded MβCD at the indicated concentrations for 24 h. For experiments in which MβCD or Aβ12–28 were used, microglia were incubated with these reagents for 30 min before the addition of Aβ. For lovastatin-treated groups, cells were incubated with lovastatin supplemented with 250 μm mevalonate and 2% lipoprotein-deficient fetal bovine serum (Sigma) in DMEM/F-12 for 48 h (29). After pretreatments, cells were then incubated with 2 μg/ml Aβ1–42 for 18 h. After washing extensively with PBS to remove residual extracellular Aβ, cells were lysed in 1% SDS containing protease inhibitor mixture, including 2 μg/ml leupeptin, 2 μg/ml aprotinin, and 250 μm PMSF. Remaining intracellular Aβ was quantified by ELISA using 6E10 as the capture antibody and 4G8-HRP as the detection antibody (Covance, Princeton, NJ). Synthetic Aβ1–42 was used to generate a standard curve. Plates were developed using a TMB substrate kit (Pierce), and the reaction was stopped by the addition of an equal volume of 1 m HCl. The results were read using a Spectramax colorimetric plate reader (Molecular Devices, Sunnyvale, CA). The amount of remaining intracellular Aβ was normalized to the total protein in the lysates.
Flow Cytometry
Primary murine microglia were plated at a density of 7 × 105 cells/well in a six-well plate in DMEM/F-12 containing 2% FBS. On the day before pretreatment, culture media were replaced with serum-free DMEM/F-12 overnight. Cells were then pretreated with the drugs of interest for 24 h followed by administrating Alexa 488-labeled Aβ for 6 h. For experiments in which MβCD or Aβ12–28 were used, microglia were incubated with these reagents for 30 min before the addition of Alexa 488-labeled Aβ. Cells were then washed with PBS and fixed with 4% paraformaldehyde. After fixation, cells were washed with PBS and collected for analysis by flow cytometry using the EPICS-XL MCL (Beckman-Coulter, Brea, CA).
Cellular Cholesterol Quantification
Cellular cholesterol levels were measured by an Amplex Red cholesterol assay kit (Invitrogen) according to the manufacturer's instruction and normalized to protein levels measured with a BCA assay kit (Pierce).
Competitive Inhibition Assay
Inhibition of Aβ1–42 binding to natural apoE3 particles in the presence of Aβ12–28 was performed as described (30). In brief, 100 μl of Aβ1–42 (2 μg/ml in 0.5 m sodium bicarbonate buffer, pH 9.6) was incubated on polystyrene 96-well plates at 4 °C overnight to immobilize Aβ1–42. Plates were then washed with PBS plus 0.01% Tween20 (PBST) and blocked with 1% skim milk in PBST for 1 h at 37 °C. Immortalized E3-DSE astrocytes (a gift from Dr. Holtzman, Washington University, St. Louis, MO) were cultured in serum-free DMEM/F-12 with 1 mm sodium pyruvate for 3 days to obtain condition media containing lipidated apoE3 particles. Phosphoramidon (10 μm) was added to the conditioned media to block insulin degrading enzyme and neprilysin activity (8). The conditioned media was preincubated with an increasing concentration of Aβ12–28 (0–10 μg/ml) for 1 h at 37 °C and added to the 96-well plate containing immobilized Aβ for an additional 3 h. In parallel, a series of apoE dilutions was incubated on the wells coated with an anti-apoE antibody (WUE-4, a gift from Dr. Holtzman) to generate a standard curve for calibration. The plate was then washed with PBS, and apoE bound to Aβ1–42 was detected using a HRP-conjugated anti-apoE antibody (Abcam, Cambridge, MA). The chromogenic reaction was developed with a TMB substrate kit (Pierce), stopped by the addition of 1 m HCL, and measured using a Spectramax colorimetric plate reader (Molecular Devices). OD values were first calibrated to the standard curve and then converted to percentages, considering the binding of apoE3 to Aβ1–42 in the absence of Aβ12–28 as 100%. Nonspecific binding was determined by replacing coated Aβ1–42 with bovine serum albumin and/or omitting the conditioned media in the assay.
Colocalization Assay and Immunohistochemistry
Primary microglia were plated on coverslips in 6-well plates at a density of 3 × 105 cells/well plate in DMEM/F-12 containing 2% FBS. Cells were serum-starved overnight and pretreated with the drugs of choice for 24 h. To label the late endosomes and lysosomes, Alexa 488-labeled microspheres (20 nm, Invitrogen) were diluted (1:10) and incubated in PBS with 1 mg/ml bovine serum albumin (BSA) for 10 min at room temperature, washed with serum-free DMEM/F-12, and applied to the cells at 10 μl/ml for 30 min. After being washed extensively to remove non-internalized microspheres, cells were incubated for 4 h in 37 °C to allow microspheres to accumulate in late endosomes/lysosomes. Colocalization of Aβ and late endosomes/lysosomes was measured by a pulse-chase experiment. Cells were incubated with 2 μg/ml Alexa 555-labeled Aβ, washed thoroughly, and further incubated in 37 °C for 15 min. Cells were then washed with PBS and fixed in 4% paraformaldehyde. The nuclei were visualized by DAPI staining. For measuring the colocalization of Aβ and Rab7, the cells were treated as stated above but omitted the step of applying microspheres. Rab7 was detected using an anti-Rab7 antibody (Cell Signaling, Danvers, MA) followed by a corresponding Alexa 488-conjugated secondary antibody (Invitrogen). Coverslips were mounted on glass slides and observed using a Zeiss LSM 510 confocal microscope. The microscopic images were subjected to the colocalization assay using NIH ImageJ software (Bethesda, MD) with both Intensity Correlation Analysis (31) and Colocalization Test plugins (32). The algorithm of these two methods takes a specific approach to automatically estimate the threshold for both channels and to exclude the overlaps from randomly distributed fluorescent signals. In our samples, colocalization coefficients calculated using both methods were very close. Thus, only the Pearson correlation coefficients obtained from Intensity Correlation Analysis plugin were plotted.
Time-lapse Live Cell Imaging
Primary microglial cells were plated on Delta T tissue culture plates at a density of 3 × 105 cells/plate. Cells were incubated overnight in serum-free DMEM/F-12 and treated with 1 μg/ml apoE or 10 μm U18666A for 24 h as indicated. Cells were stained with DiD (Invitrogen) for 5 min to visualize membrane and vesicles. Alexa 488-labeled Aβ1–42 (1 μg/ml) was applied to the cell for 5 min. After a brief wash, live cell imaging was performed using a Zeiss LSM 510 confocal microscope. A heated stage and objectives were used to maintain the temperature of culture media at 37 °C. Immediately after washing off extracellular Aβ, time-lapse images were taken every 10 s for 10 min. Images of 512 × 512 pixels were acquired at 7.5 s/frame (29). The paths of movement were tracked using MetaMorph software (Molecular Devices) to calculate the velocity of these vesicles. 12–20 vesicles per cell were analyzed. It normally took 15- 20 min for Aβ-containing vesicles to traffic from the plasma membrane to the perinuclear compartments. The fluorescently labeled vesicles then stayed in that region for a long period of time with minimal movement, indicating the fluorophores reached lysosomes and stayed. Since we were focusing on the trafficking of endolytic vesicles, these immobile vesicles were excluded in the statistic analysis. To avoid bias exclusion of certain vesicles, only four most motile Aβ-containing vesicles per cell were selected to represent the directed movement of endolytic vesicles. To eliminate the perturbations from photo or mechanical drifting while recording, four most stationary vesicles were set as the base-line motility (VS) and subtracted that from the speed of motile vesicles (VM), V = VM − VS. The average trafficking speed in the first 6 min of recording of the four most motile vesicles was plotted as one data point in the figure.
Subcellular Fractionation
Primary microglia plated in 1 × 106 cells/well in 6-well plates were serum-starved overnight and treated with drug of interest for 24 h. Cells were washed with PBS and lysed by incubation in relaxation buffer (100 mm KCl, 3 mm NaCl, 3.5 mm MgCl2, 1.25 mm EGTA, and 10 mm PIPES, pH 7.3) on ice for 15 min followed by 10 s of sonication. Cell debris was cleared by centrifugation at 500 × g for 5 min at 4 °C. The supernatant was then centrifuged for 1 h at 110,000 × g at 4 °C in a SW50.1 rotor (Beckman-Coulter, Fullerton, CA). The resulting supernatant was collected and saved as the “cytosolic” fractions, and the pellet was resuspended in relaxation buffer as the “membrane” fractions. These subcellular fractions were subjected to Western blot analysis to determine the relative amount of Rab7 in each fraction. The membrane marker flotillin was used to assess the efficacy of the fractionation procedure.
Rab-Guanine Nucleotide Dissociation Inhibitor (GDI) Mediated Rab7 Extraction
The in vitro extraction of Rab7 by GDI was evaluated as described (33–35). Cells were harvested by scraping in homogenization buffer (20 mm HEPES, pH 7.4, 250 mm sucrose, 1 mm EDTA, 1 mm dithiothreitol plus protease inhibitor mixture) and homogenized with 5 passes through a 22-gauge needle. The homogenates were centrifuged at 3000 × g at 4 °C for 5 min. The resulting supernatants were further centrifuged at 110,000 × g for 1 h at 4 °C. The pellet was resuspended in GDI extraction buffer (20 mm HEPES, 100 mm KCl, 1 mm MgCl2, pH 7.4, plus protease inhibitor mixture) and saved as “crude membrane fractions.” Protein concentration of the fractions was determined by the BCA assay using BSA as the standard. Crude membranes containing 20–25 μg of proteins were diluted in GDI extraction buffer plus 1 mm GDP and 0.5 mg/ml BSA. Recombinant GDI (GenWay, San Diego, CA) in the indicated amount was added to each sample for 15 min at 37 °C. Samples were then immediately centrifuged at 110,000 × g for 30 min. The supernatant containing extracted Rab7 was collected and subjected to Western blot analysis. Separate non-GDI extracted aliquots from the same crude membrane fractions were used as the loading control of Rab7.
Western Blot
Lysates or subcellular fractions were resolved in 12% Tris-glycine SDS-polyacrylamide gels and transferred to PVDF membranes. Blots were probed with anti-Rab7 (Cell Signaling), anti-flotillin (BD Biosciences) or anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies overnight at 4 °C. Chemiluminescent signals (Millipore, Billerica, MA) were visualized by exposure to Kodak Biomax x-ray film. Band intensities were quantified using NIH ImageJ software.
Statistics
All statistical analyses were performed using Prism software (GraphPad, San Diego, CA). Graphs are shown as the means ± S.E. Values statistically different from controls were calculated using a two-tailed t test or one-way analysis of variance with the Tukey-Kramer multiple comparisons test to determine p values. In the Aβ12–28 competitive inhibition assay, data were analyzed by a one-site competition nonlinear regression fit algorithm.
RESULTS
Facilitation of Aβ Degradation Is a Common Feature of HDL Apolipoproteins
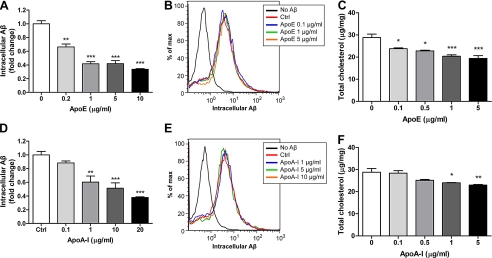
Microglia are able to internalize sAβ from extracellular milieu and degrade it intracellularly without resecretion (8). The clearance of Aβ was assessed by measuring the remaining Aβ in the cells after an 18-h period that allows for extensive degradation of internalized sAβ. Consistent with our previous findings, preincubation of microglia with apoE for 24 h resulted in the enhanced intracellular degradation of Aβ in a dose-dependent manner (Fig. 1A) (8). Similarly, another apolipoprotein, apoA-I, was also capable of promoting Aβ degradation in a dose-dependent manner, demonstrating that facilitation of Aβ degradation is a common feature of HDL apolipoproteins (Fig. 1D). To avoid the possibility of treatment-induced variation in Aβ uptake, the cumulative internalization of Aβ was monitored using flow cytometry with fluorescently labeled Aβ. Although the internalized Aβ was degraded over time, the fluorophore was retained in the cells; thus, accumulated fluorescence reflected the total Aβ internalized (27). No significant change in Aβ uptake after the treatment of apoE and apoA-I indicated that the difference in remaining intracellular Aβ resulted from the change in efficiency of Aβ degradation (Fig. 1, B and E).
FIGURE 1.
Facilitation of Aβ degradation is a common feature of apolipoproteins. A and D, wild type primary microglia were preincubated with increasing concentrations of purified apoE (A) or apoA-I (D) for 24 h followed by the application of 2 mg/ml Aβ1–42. After 18 h of incubation, intracellular Aβ levels were quantified by ELISA and normalized to total protein (n = 6). B and E, uptake of Aβ was assessed by applying Alexa 488-labeled Aβ1–42 to microglia after pretreatment with apoE (B) or apoA-I (E). The accumulation of the fluorophore was analyzed by flow cytometry. C and F, total cellular cholesterol levels were quantified and normalized to total protein (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with the vehicle treated control.
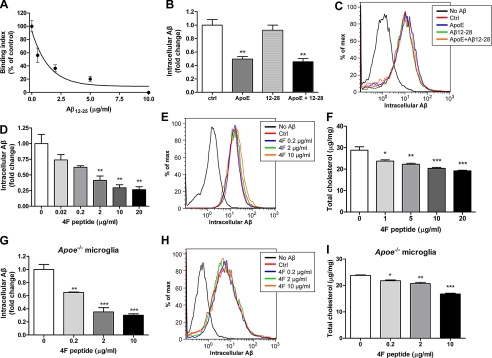
The direct association of apoE with Aβ has been extensively investigated (36–38). ApoA-I is also shown to bind to Aβ (39). However, it is unknown if the facilitation of degradation requires direct interaction between apolipoproteins and Aβ or rather that this effect is due to the common lipid transport function of the apolipoproteins. The residues 12–28 of Aβ were identified as the binding site of apoE (37). It has been shown that using this small fragment of Aβ (Aβ12–28) was able to competitively block the binding of full-length Aβ to apoE (30), suppressing fibril formation and enhancing the survival of cultured neurons (40). However, we found that Aβ12–28 was not able to inhibit apoE-induced Aβ degradation by microglia (Fig. 2, B and C), although at the same concentration (5 μg/ml), Aβ12–28 was able to largely block the binding of Aβ1–42 and apoE (Fig. 2A). These data indicate that the direct apoE-Aβ interaction is not required for apoE to promote intracellular Aβ degradation by microglia.
FIGURE 2.
Facilitation of Aβ degradation relies on the cholesterol efflux function of apolipoproteins. A, in a competitive inhibition assay conditioned media containing native apoE3 particles from E3-DSE astrocyte were incubated with Aβ12–28 at the indicated concentrations for 30 min and transferred to Aβ1–42-coated plates for 3 h. ApoE bound to the immobilized Aβ was detected using an apoE-specific antibody. Results are expressed as a percentage of remaining apoE bound, considering binding of apoE to Aβ in the absence of Aβ12–28 as 100%. The data represent the pooled outcome of five independent experiments and were fitted into a one-site competition curve. B, D, and G, wild type (B and D) and Apoe−/− (G) primary microglia were preincubated with apoE 1 μg/ml (B) or the 4F peptide at various concentrations, as indicated (D and G) for 24 h followed by applying 2 μg/ml Aβ1–42. 5 μg/ml Aβ12–28 was administered simultaneously with Aβ1–42 as indicated in B. After 18 h incubation, cells were lysed, and the intracellular Aβ levels were analyzed by ELISA (n = 6 for B and D; n = 3 for G). C, E, and H, uptake of Aβ was monitored by applying Alexa 488-labeled Aβ1–42 to the wild type (C and E) and Apoe−/− (H) primary microglia after pretreatment with the indicated reagents for 24 h. The accumulation of fluorophore was then analyzed by flow cytometry. The histogram is representative of the outcome from three independent experiments. F and I, total cellular cholesterol levels of wild type (F) or Apoe−/− (I) primary microglia treated with the 4F peptide were analyzed and normalized to total protein (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with the vehicle treated control.
Facilitation of Aβ Degradation Relies on the Cholesterol Efflux Function of Apolipoproteins
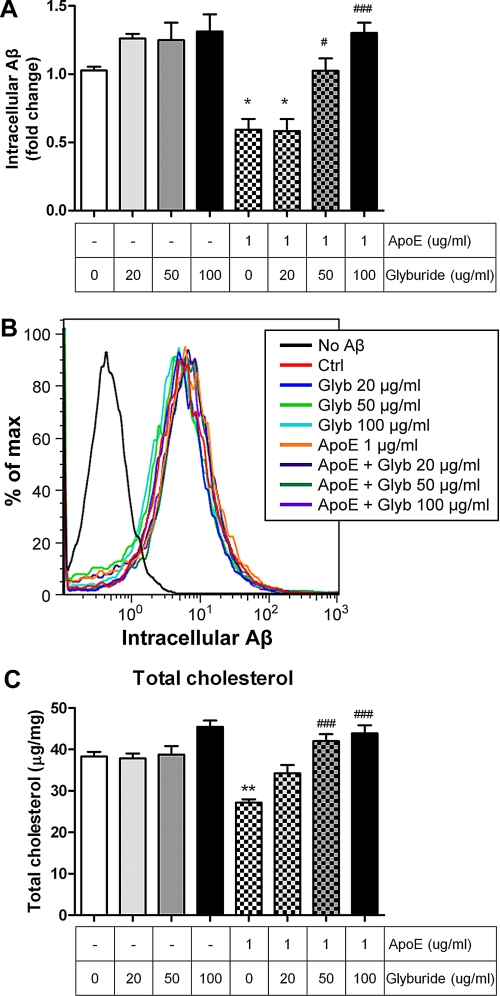
To ascertain whether the cholesterol efflux property of apolipoproteins is required for the enhanced Aβ degradation, we employed an apoA-I mimetic peptide, 4F, that possesses the structure of the essential lipid binding domain but lacks the receptor binding domain. Importantly, it has no sequence homology with apoA-I (41, 42). The mimetic peptide has been shown to form HDL-like particles and is capable of mediating cholesterol efflux similar to apoA-I-HDL (43, 44). The 4F peptide reduced cellular cholesterol levels when supplied to the cultured primary microglia, similar to apoE and apoA-I (Figs. 1, C, and F, and 2, F and I), whereas no Aβ binding activity to the 4F peptide was observed (data not shown). The 4F peptide, therefore, provides an excellent tool to parse out which of the two highly connected properties of apoE promotes Aβ degradation. Application of the 4F peptide facilitated Aβ degradation in a dose-dependent manner (Fig. 2D). Control studies demonstrated that Aβ uptake was not influenced by the treatment with the 4F peptide (Fig. 2E). However, it remains possible that the mimetic peptides interact with the endogenous apoE-HDL particles to prevent their turnover and eventually increase their overall amounts (43, 45) and in this way promote Aβ degradation rather than by the direct cholesterol lowering effect of the 4F peptide. To evaluate this possibility, we performed the same assay in apoE-deficient microglia. The ability to enhance Aβ degradation by the 4F peptide was not changed in the Apoe−/− background (Fig. 2, G and H). To further confirm that the cholesterol efflux function mediates apoE-induced Aβ degradation, we blocked the ABCA1 activity with glyburide (46, 47). ABCA1 mediates cholesterol efflux onto apolipoproteins to form HDL particles, and blocking the activity of ABCA1 by treatment of glyburide was able to inhibit apoE-induced Aβ degradation without influencing the uptake of Aβ (Fig. 3). These results demonstrated that lowering cellular cholesterol levels by cholesterol efflux was sufficient to promote microglial Aβ degradation. Importantly, the data demonstrated that the direct interaction with Aβ is not necessarily required for the enhancement of intracellular Aβ degradation and that apolipoproteins act through their ability to promote cholesterol efflux from cells to enhance Aβ degradation.
FIGURE 3.
Blocking cholesterol efflux impairs the ability of apoE to enhance Aβ degradation. A, wild type primary microglia were preincubated with glyburide at the indicated concentrations in the presence or absence of 1 μg/ml apoE as indicated for 24 h followed by the administration of 2 μg/ml Aβ42 for 18 h. The remaining intracellular Aβ levels were quantified by ELISA, and data were normalized to total protein (n = 3). B, wild type primary microglia were pretreated for 24 h. Alexa 488-labeled Aβ1–42 were then applied for 6 h. Aβ uptake was analyzed by flow cytometry. The histogram plots are the representative graphs of the outcome from three independent experiments. C, total cellular cholesterol levels were analyzed and normalized to total protein (n = 3). *, p < 0.05, **, p < 0.01 compared with the vehicle treated control; #, p < 0.05, ###, p < 0.001 compared with the 1 μg/ml apoE-treated group.
Degradation of Aβ Is Influenced by Cellular Cholesterol Levels
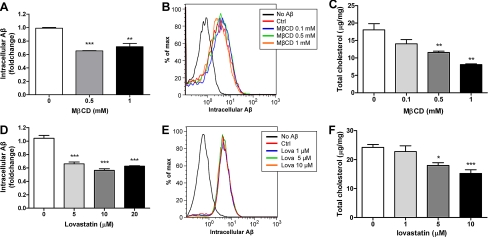
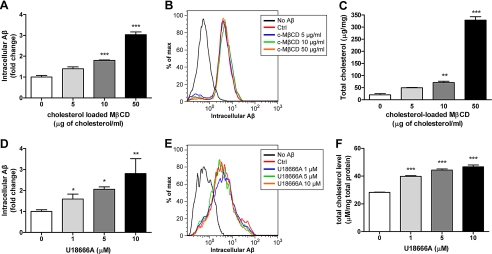
If cellular cholesterol levels have critical regulatory roles of Aβ degradation, decreasing cholesterol should enhance Aβ degradation. To understand whether cellular cholesterol levels could directly regulate Aβ degradation, we used a variety of pharmacological reagents to directly manipulate cellular cholesterol levels. We first utilized MβCD, a cholesterol chelator, to decrease cellular cholesterol by direct extraction of cholesterol from the plasma membrane (48). A low dosage of MβCD was able to promote Aβ degradation without interfering with its uptake (Fig. 4, A and B). Decreasing endogenous cholesterol synthesis by inhibiting HMG-CoA reductase activity with lovastatin also resulted in the facilitation of Aβ degradation (Fig. 4, D and E). Conversely, increasing cellular cholesterol levels by incubating microglia with cholesterol-loaded MβCD resulted in impaired Aβ degradation (Fig. 5, A and B). Since Aβ is degraded mainly by neprilysin in the late endosomes/lysosomes (49), we tested if cholesterol accumulation preferentially in the endocytic system would also impair Aβ degradation. Cells take up HDL or LDL particles through LDL receptor-mediated endocytosis. Lipoproteins are then transported to late endosomes where cholesterol ester carried is hydrolyzed to free cholesterol (50, 51). The transport of cholesterol from late endosomes and lysosomes to other cellular compartments is regulated by Niemann-Pick C-1 protein (NPC1), which is located on the membrane of late endosomes and lysosomes (52). Cells carrying defective NPC1 exhibit cholesterol accumulation in late endocytic vesicles. U18666A is a widely used NPC1 inhibitor that acts to stimulate the accumulation of cholesterol within the endocytic system (53). Treatment of primary microglia with U18666A resulted in the increase of cellular cholesterol levels and an inhibition of Aβ degradation (Fig. 5, D and E). Control incubations monitored total cellular cholesterol levels and verified that all drugs elicited their expected actions (Fig. 4, C and F, and 5, C and F). Since the cholesterol homeostasis is tightly regulated in the cells, it is possible that manipulating cellular cholesterol levels feeds back to regulate apoE expression, which in turn affects Aβ degradation. To verify this possibility, we performed the same treatments in Apoe−/− microglia to rule out the possibility of the involvement of apoE-HDL particles in previous experiments. We found that Aβ degradation regulated by directly manipulating cellular cholesterol levels did not require the presence of apoE (supplemental Fig. S1). These data strongly demonstrate that modulating cellular cholesterol levels alone is sufficient to regulate intracellular Aβ degradation. Thus, intracellular Aβ degradation is likely to be facilitated by apoE's ability to reduce cellular cholesterol levels by stimulating cholesterol efflux.
FIGURE 4.
Reducing cellular cholesterol levels promotes Aβ degradation. A and D, to lower cellular cholesterol levels, wild type primary microglia were preincubated with MβCD (A) or lovastatin (D) at the indicated concentrations for 30 min or 48 h followed by administration of 2 μg/ml Aβ42 for 6 or 18 h, respectively. Remaining intracellular Aβ was analyzed using ELISA (n = 3). B and E, uptake of fluorescently labeled Aβ was monitored after pretreatment with MβCD (B) or lovastatin (E) for 30 min or 48 h, respectively, using flow cytometry. The accumulation of the fluorophore was then analyzed by flow cytometry. The histogram is representative of the outcome from three independent experiments. C and F, total cellular cholesterol levels were quantified and normalized to total protein (n = 3). *, p < 0.05, **, p < 0.01, ***, p < 0.001 compared with the vehicle-treated control.
FIGURE 5.
Increasing cellular cholesterol levels impairs Aβ degradation. A and D, to increase cellular cholesterol levels, wild type primary microglia were preincubated with cholesterol-loaded MβCD (A) or U18666A (D) at the indicated concentrations for 24 h followed by administration of 2 μg/ml Aβ1–42 for 18 h. The remaining intracellular Aβ was analyzed using ELISA (n = 3). B and E, uptake of fluorescently labeled Aβ was monitored after pretreated with cholesterol-loaded MβCD (B) or U18666A (E) for 24 h using flow cytometry. The histogram is representative of the outcome from three independent experiments. C and F, total cellular cholesterol levels were quantified and normalized to total protein (n = 3). *, p < 0.05; **, p < 0.01, ***, p < 0.001 compared with the vehicle-treated control.
The Transcription of Aβ Degrading Enzymes Is Not Regulated by Cholesterol Levels
We further investigated whether the regulation of Aβ degradation by modulating cellular cholesterol levels was a result of changes in the levels of Aβ degrading enzymes. Proteases that degrade Aβ include neprilysin, insulin degrading enzyme, matrix metalloproteinase 9, angiotensin-converting enzyme, endothelin converting enzyme 1 and 2, and plasmin (54–56). Quantitative real-time PCR was performed to quantify their transcripts levels. We found no correlation linking cholesterol modulation and the levels of the transcripts analyzed (data not shown). Although the expression of these enzymes was not changed, their functionality could be affected by their distribution or microenvironments, such as pH and lipid rafts. Thus, we utilized fluorogenic substrates that are specific to these enzymes and act as independent indicators to analyze their enzyme activity. The results showed that the enzyme activities were not influenced by apoE treatment (supplemental Fig. S2). Thus, the apoE- and cholesterol-mediated regulation of Aβ degradation is not due to altered levels or activity of Aβ degrading enzymes.
Cholesterol Levels Regulate Aβ Transport
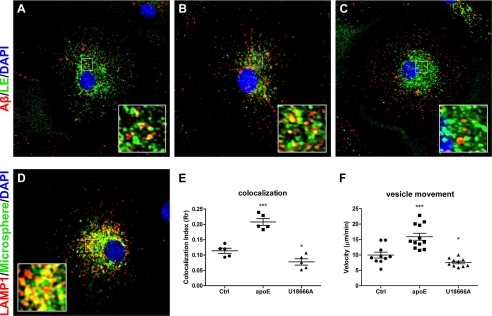
Since uptake of Aβ and the levels and activity of Aβ degrading enzymes were not influenced by apoE, the step that apoE induces to facilitate Aβ degradation must take place between the plasma membrane and lysosome. Previous studies demonstrated that the motility of endosomes depends on the membrane cholesterol composition. Lowering cholesterol was reported to enhance the motility of folate receptor-containing endosomes, whereas increasing cholesterol paralyzes them (29). We thus hypothesized that the trafficking of Aβ to lysosomes might be the rate-limiting step for Aβ degradation and regulated by the levels of cellular cholesterol. The cholesterol lowering effect of apoE might facilitate the trafficking of Aβ to lysosomes and ultimately results in enhanced Aβ degradation. We first evaluated the delivery of Aβ to late endosomes/lysosomes by a pulse-chase experiment. Fluorescently labeled Aβ was incubated with microglia for 7 min followed by a 15-min chase. The localization of Aβ to late endosomes/lysosomes was assessed by labeling this compartment with fluorescence-labeled microspheres, which are internalized via pinocytosis, trafficked through the endocytic pathway, and accumulated in late endosomes and lysosomes (27, 57). The fluorescent microspheres faithfully labeled the LAMP1-positive endosomes/lysosomes (Fig. 6D). With this method, we demonstrated that in the presence of apoE, Aβ was delivered faster to the late endosomes/lysosomes, reflected by its higher colocalization index compared with vehicle-treated controls (Fig. 6, A, B, and E). Conversely, when microglia were pretreated with U18666A to increase endosomal cholesterol levels, Aβ transport was slower, indicated by the lower colocalization index (Fig. 6, C and E). To further confirm the idea of Aβ trafficking under the influence of cellular cholesterol levels, we performed live cell imaging to directly track the motility of Aβ. Fluorescently labeled Aβ was applied to primary microglia, and time-lapse confocal images were taken to calculate the velocity of the vesicular movement (supplemental Videos S1–S3). The Aβ-containing vesicles moved significantly faster when cells were pretreated with apoE compared with the non-treated controls, whereas the vesicles staggered after U18666A pretreatment (Fig. 6F). The results combined suggest that the cholesterol-lowering effect of apoE accelerates the trafficking of Aβ toward late endosomes/lysosomes. Consequently, enhanced Aβ degradation is observed.
FIGURE 6.
Cholesterol levels regulate the trafficking of Aβ-containing endosomes. A–C, a pulse-chase experiment was used to monitor the trafficking of Aβ after manipulating cellular cholesterol levels. Wild type primary microglia were preincubated with vehicle (A), 1 μg/ml apoE (B), or 10 μm U18666A (C) for 24 h. Before the application of Aβ, cells were pulsed with fluorescent microspheres (green) for 30 min followed by incubation for 4 h to label the late endosomes/lysosomes. Alexa 555-labeled Aβ1–42 (1 μg/ml) was applied to cells for 7 min. After an extensive wash with DMEM-F-12, cells were incubated in fresh culture media for another 10 min. Representative images demonstrate the colocalization of Aβ (red) and late endosomes/lysosomes (green). Nuclei were stained with DAPI (blue). D, to verify that fluorescent microspheres efficiently label late endosomes/lysosomes, wild type primary microglia were pulsed with fluorescent microspheres for 30 min and followed by incubation for 4 h. Cells were fixed and immunostained with an anti-LAMP1 antibody. The representative image demonstrates the colocalization of microspheres (green) and the late endosomal/lysosomal marker LAMP1 (red). Nuclei were stained with DAPI (blue). E, images taken from A–C were analyzed with ImageJ for the colocalization of Aβ and late endosomes/lysosomes (n = 5; *. p < 0.05, ***, p < 0.001 compared with the vehicle-treated control). F, wild type primary microglia were preincubated with vehicle, 1 μg/ml apoE, or 10 μm U18666A for 18 h. Alexa 488-labeled Aβ1–42 (2 μg/ml) were applied to cells for 7 min. After extensive washing with PBS, time-lapse images were taken to record the movement of Aβ in the cells. Images were acquired at every 10 s for 10 min and analyzed using Metamorph software. Each data point plotted is the average of the traveled distance of the 4 most motile fluorescent Aβ-containing endosomes in a cell (n = 4, *, p < 0.05, ***, p < 0.001).
Cholesterol Regulates the Trafficking of Aβ-containing Endosomes via the Recruitment of Rab7
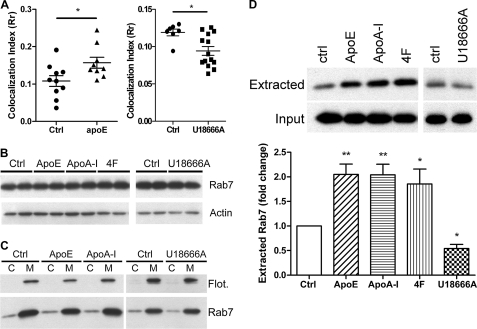
It has been well established that endocytic trafficking is regulated by Rab proteins and their effectors (58, 59). Among these Rab proteins, Rab7 is recruited to endosomes, which are sorted and directed into a lysosomal degradation pathway. Rab7 regulates the trafficking, maturation, and fusion of endosomes with lysosomes (60, 61). The activity of Rab7 was shown to be affected by endosomal cholesterol levels (29, 35). To characterize the possible role of Rab7 in the apoE-enhanced endocytic trafficking, we first performed the pulse-chase experiment as described above to analyze the colocalization of Aβ and Rab7. The amount of Aβ colocalized with Rab7 was increased in the cells pretreated with apoE yet decreased in the cells pretreated with U18666A, suggesting the involvement of Rab7 in the trafficking of Aβ (Fig. 7A). However, no significant changes in Rab7 protein levels were observed in the total cell lysates (Fig. 7B). Detailed analysis also showed no shift in its subcellular distribution between cytosolic and membrane fractions (Fig. 7C). Like other Rab proteins, Rab7 is tightly associated with the membranes by virtue of the geranylgeranylation of cysteine residues at its C terminus (58, 62). Double-prenylated Rab proteins are also present in cytosol where their prenylated cysteines are tightly bound to the GDI. Rab proteins normally cycle between the membrane and cytosol. Generally, after a vesicle fusion event, Rab proteins are retrieved from the membrane by GDI and then re-delivered to its membrane of origin for reutilization (63–65). Previous studies suggested that extraction of Rab7 from lysosomes for its recycling is affected by the cellular cholesterol levels (35). Reducing cellular cholesterol levels enhances the recycling of Rab7, whereas accumulation of cellular cholesterol paralyzed the endocytic system. We thus hypothesized that apoE might enhance the recycling of Rab7, as apoE is able to reduce cellular cholesterol levels (Fig. 1C). The recycling of Rab7 was assessed by its extractability by GDI. We found that Rab7 from membrane fractions was more extractable in microglia pretreated with apolipoproteins or the 4F mimetic peptide, whereas it was less extractable in U18666A-treated cells (Fig. 7D). The data are consistent with the finding on other cell types (35) and suggest that lowering cellular cholesterol levels by apoE facilitates the recycling of Rab7 and subsequently results in accelerated endocytic trafficking of Aβ for lysosomal degradation.
FIGURE 7.
Cholesterol regulates the recycling of Rab7. A, wild type primary microglia were preincubated with vehicle, 1 μg/ml apoE, or 10 μm U18666A for 18 h. Alexa 555-labeled Aβ1–42 (1 μg/ml) was applied to cells for 7 min. After an extensive wash with DMEM-F-12, cells were incubated in fresh culture media for another 30 min. Rab7 was visualized by immunostaining. The colocalization of Aβ and Rab7 were analyzed with ImageJ (n = 9; *, p < 0.05). B–D, wild type primary microglia were treated with vehicle, apoE (1 μg/ml), apoA-I (5 μg/ml), 4F peptide (10 μg/ml), or U18666A (10 μm) for 24 h. B, cell lysates were subjected to Western blot analysis using the appropriate antibodies. Actin served as a protein-loading control. C, membrane (M) and cytosolic (C) fractions were isolated from cell lysates and analyzed by Western blot analysis. Flotillin-1 serves as a control for both fraction efficiency and protein-loading. D, crude membrane fractions (25 μg of total membrane protein) from cell lysates were incubated with GDI 0.2 μm for 15 min and pelleted with ultracentrifugation. GDI-extracted Rab7 in the supernatants was analyzed by WB analysis. Total membrane fractions without the treatment of GDI served as the protein-loading controls. The band intensity of extracted Rab7 was normalized to total Rab7 input from the membrane fractions and expressed as relative extractability compared with vehicle-treated control (n = 5). *, p < 0.05, **, p < 0.01 compared with the vehicle treated control.
DISCUSSION
In this study we have demonstrated a link between cholesterol homeostasis and Aβ metabolism and provided a mechanistic explanation of how apoE facilitates intracellular Aβ degradation by microglia. We report that promoting microglial Aβ degradation is a common feature of apolipoproteins, including apoE and apoA-I. Interfering with ABCA1 function by glyburide impaired the capacity of apoE to promote Aβ degradation, consistent with the hypothesis that Aβ degradation promoted by apolipoproteins is mediated through reduction of cellular cholesterol levels rather than their affinity for Aβ. The 4F mimetic peptide provided us with another discriminatory tool to dissect the molecular properties of apoE. The 4F peptide is designed to provide similar cholesterol binding affinity as apoA-I and can perform cholesterol efflux through ABCA1 (66). Although the 4F peptide demonstrated no binding to Aβ, it was capable of promoting intracellular Aβ degradation, similar to the actions of apoE and apoA-I. This result strongly supports the hypothesis that apoE facilitates Aβ degradation via reducing cellular cholesterol levels. Indeed, oral administration of 4F peptide to a mouse model of AD for 4 months has been shown to reduce Aβ levels and plaque load and improve memory retention (24). Although the authors concluded that this was the anti-inflammatory effect of the mimetic peptide, they could not exclude the involvement of cholesterol efflux with Aβ degradation. Since direct modulation of cellular cholesterol was capable of influencing the efficiency of intracellular Aβ degradation, our data suggest apoE facilitates intracellular Aβ degradation by means of regulating cellular cholesterol levels.
It has been documented that apoE co-localizes with amyloid deposits in AD patients and can also bind soluble Aβ found in the brain, cerebrospinal fluid, and plasma (67–71). ApoE directly interacts with Aβ, and this binding is both isoform- and lipidation status-dependent (36, 37, 69, 72–74). ApoE2 binds Aβ more efficiently than apoE3 and apoE4. Lipidated native apoE has much higher affinity for Aβ than unlipidated forms of this apolipoprotein (38). The isoform- and lipidation status-dependent binding affinity correspond with the genetic risk for AD imparted by the individual human isoforms, suggesting that apoE may act as a molecular chaperone participating in the metabolism and amyloidogenesis of Aβ. Thus, it is reasonable to hypothesize that apoE may facilitate Aβ degradation through its chaperone activity. To our surprise, however, this interaction is not required for the apoE-stimulated intracellular Aβ degradation. Blocking the apoE-Aβ interaction by Aβ12–28 was not able to inhibit apoE-promoted Aβ degradation. Nevertheless, we could not exclude the possible involvement of apoE-Aβ binding in the degradation of Aβ in vivo. Our previous study demonstrated that apoE-containing HDL particles stimulated the insulin degrading enzyme-dependent proteolysis of sAβ in an ex vivo assay (8). These data provide direct evidence that apoE promotes Aβ degradation possibly through at least two distinct mechanisms in vivo, suggesting that in the extracellular milieu apoE-HDL might act as a chaperone to enhance sAβ clearance, and we are currently exploring this hypothesis.
As the major genetic risk factor for sporadic AD, apoE has been suggested to play an important role in the clearance of Aβ in an isoform-specific manner (75). We previously reported that supplying primary microglia with apoE resulted in isoform-dependent enhancement of Aβ degradation, in which apoE2 had the greatest effect but apoE4 had a minimum effect (8). In this study we demonstrated that cholesterol efflux is the key property of apoE to promote intracellular Aβ degradation. Since the cholesterol efflux activity is apoE isoform-dependent (E2 > E3 > E4) (76, 77), the higher risk of AD observed in the apoE4 carriers may be due to the poorer efficiency of cholesterol efflux by apoE4. Cellular cholesterol levels governed by the activity of apoE isoforms may directly regulate Aβ degradation (8). This hypothesis is further supported by the observation that the colocalization between Aβ and late endosomes/lysosomes was significantly reduced when microglia were pretreated with apoE4 versus apoE2.3 Increasing apoE levels or lipidation status, which reflects cholesterol efflux activities, by using liver X receptor agonists or overexpressing ABCA1 has been shown to ameliorate AD pathology in several mouse models of AD (8, 23, 78–80). Intracellular cholesterol distribution and levels are crucial for cell functions and, thus, are highly regulated in the cells. Cholesterol is highly enriched in the plasma membrane, whereas it is low in the endoplasmic reticulum, where it is synthesized. The level of cholesterol decreases from trans- to cis-Golgi apparatus (81). Noteworthy, in this study, changes in the levels of total cholesterol did not linearly correlate to the efficiency of intracellular Aβ degradation, especially when comparing the effectiveness of different cholesterol modulating reagents to regulate the degradation of Aβ. Apolipoproteins and the mimetic peptide were most effective to elicit heightened Aβ degradation activity with only moderate cholesterol reduction. Moreover, building up cholesterol primarily in the endocytic system by the NPC inhibitor led to impaired Aβ degradation. These results suggest that regulating cholesterol levels in certain subcellular compartments or the composition of cholesterol derivatives might be more important than just manipulating the overall levels of cellular total cholesterol in regulating Aβ degradation. Thus, using apoE to remove excessive cholesterol may be the most effective way to enhance Aβ clearance. However, it remains to be determined whether the beneficial effects would be similar under the genetic background of different Apoe alleles.
A striking observation of this study is that the motility of Aβ-containing vesicles was increased upon apoE-dependent reduction of cellular cholesterol levels. Cholesterol has been shown to play an important role in the intracellular transport. It is not a passive component of endosomal membranes but, rather, is directly involved in the sorting and transport of endocytic vesicles (82, 83). Chen et al. (29) reported that the motility of folate receptor-containing endosomes is regulated by the cholesterol. Cholesterol depletion by MβCD increased motility of folate receptor endosomes, although increasing cellular cholesterol using U18666A retarded it. Our results are entirely consistent with their findings. We extended these observations and documented that the enhanced vesicle motility was not random but was directed toward lysosomes as evidenced by enhanced colocalization of Aβ and lysosomes. The motility analysis of Aβ-containing vesicles showed the direct influence of cholesterol on vesicular trafficking speed, yet it is still possible that cholesterol may affect the sorting of Aβ in the endolytic system (84). Active vesicle transport relies on a variety of motor proteins to transport cargo along cytoskeletal network. The recruitment of specific dynein or kinesin motor complexes onto vesicles is regulated by Rab GTPases. Among these Rab GTPases, Rab7 is shown to play a critical role in the endocytic processes, regulating the sorting of endosomes and biogenesis of lysosomes (60, 61). In Niemann-Pick C disease, dysfunction of NPC1 results in missorting and accumulation of cholesterol in the late endosomes. The cholesterol loading decreases the capacity of GDI to extract membrane-associated Rab7, thus inhibiting these vesicles from switching from minus- to plus-end-directed motility, eventually resulted in a loss of bi-directional motility and paralysis (35, 85). Failure to recycle Rab7 to the membrane interferes with the switch in early-to-late endosome maturation and ultimately results in the paralysis of the endocytic system (29, 35, 60). Conversely, cholesterol depletion has been reported to enhance the motility of folate receptor-containing endosomes. Reducing cellular cholesterol levels significantly decreased the association of Rab7 with folate receptor endosomal membrane, suggesting the increased recycling of Rab7 (29). In our study the capacity of GDI to extract membrane-associated Rab7 was also enhanced in apoE-treated microglia, suggesting that facilitated Rab7 recycling led to accelerated transport of Aβ-containing vesicles. It is notable that this property is related to the cholesterol efflux function of apoE as using the 4F mimetic peptide elicited the same results. Although the underlying reason why GDI extraction of Rab7 is modulated by cholesterol is unknown, results from previous studies suggested that this regulation perhaps involves oxysterol-binding protein-related protein 1L through the Rab7 effector Rab7-interacting lysosomal protein (35, 86, 87). Whether this activity subsequently regulates the extraction of Rab7 by GDI requires further investigation. Our data indicate that the cholesterol content of endocytic vesicles is critical for Rab7 action, which impacts the efficiency of Aβ delivery to the lysosome for subsequent degradation.
In summary, we report that apoE promotes intracellular Aβ degradation through reducing cellular cholesterol levels. This is the first evidence that provides a mechanistic explanation of the role of apoE in facilitating intracellular Aβ degradation. Reducing the cellular cholesterol level by apoE does not change the expression and the activity of Aβ degrading enzymes while promoting the trafficking of Aβ to lysosome for degradation by modulating Rab7 recycling. These findings demonstrated a direct role of cholesterol in Aβ proteolytic degradation and provide a new insight into this aspect of AD pathogenesis.
Supplementary Material
Acknowledgments
We thank Qingguang Jiang, Shweta Mandrekar, and Paige Cramer for insightful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant R01-AG030482 (to G. E. L.).

This article contains supplemental Videos S1–S3 and Figs. S1 and S2.
C. Y. Lee, W. Tse, and G. E. Landreth, unpublished data.
- AD
- Alzheimer disease
- Aβ
- β-amyloid
- sAβ
- soluble Aβ
- MβCD
- methyl-β-cyclodextrin
- NPC1
- Niemann-Pick C-1
- GDI
- guanine nucleotide dissociation inhibitor.
REFERENCES
- 1. Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer disease in late onset families. Science 261, 921–923 [DOI] [PubMed] [Google Scholar]
- 2. Schmechel D. E., Saunders A. M., Strittmatter W. J., Crain B. J., Hulette C. M., Joo S. H., Pericak-Vance M. A., Goldgaber D., Roses A. D. (1993) Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 9649–9653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahley R. W., Rall S. C., Jr. (2000) Apolipoprotein E, far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1, 507–537 [DOI] [PubMed] [Google Scholar]
- 4. Corder E. H., Saunders A. M., Risch N. J., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Jr., Rimmler J. B., Locke P. A., Conneally P. M., Schmader K. E. (1994) Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat. Genet. 7, 180–184 [DOI] [PubMed] [Google Scholar]
- 5. Mawuenyega K. G., Sigurdson W., Ovod V., Munsell L., Kasten T., Morris J. C., Yarasheski K. E., Bateman R. J. (2010) Decreased clearance of CNS β-amyloid in Alzheimer disease. Science 330, 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castellano J. M., Kim J., Stewart F. R., Jiang H., DeMattos R. B., Patterson B. W., Fagan A. M., Morris J. C., Mawuenyega K. G., Cruchaga C., Goate A. M., Bales K. R., Paul S. M., Bateman R. J., Holtzman D. M. (2011) Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 3, 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bales K. R., Liu F., Wu S., Lin S., Koger D., DeLong C., Hansen J. C., Sullivan P. M., Paul S. M. (2009) Human apoE isoform-dependent effects on brain β-amyloid levels in PDAPP transgenic mice. J. Neurosci. 29, 6771–6779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang Q., Lee C. Y., Mandrekar S., Wilkinson B., Cramer P., Zelcer N., Mann K., Lamb B., Willson T. M., Collins J. L., Richardson J. C., Smith J. D., Comery T. A., Riddell D., Holtzman D. M., Tontonoz P., Landreth G. E. (2008) ApoE promotes the proteolytic degradation of Aβ. Neuron 58, 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahley R. W. (1988) Apolipoprotein E, cholesterol transport protein with expanding role in cell biology. Science 240, 622–630 [DOI] [PubMed] [Google Scholar]
- 10. Kim J., Basak J. M., Holtzman D. M. (2009) The role of apolipoprotein E in Alzheimer disease. Neuron 63, 287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lund-Katz S., Phillips M. C. (2010) High density lipoprotein structure-function and role in reverse cholesterol transport. Subcell. Biochem. 51, 183–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grehan S., Tse E., Taylor J. M. (2001) Two distal downstream enhancers direct expression of the human apolipoprotein E gene to astrocytes in the brain. J. Neurosci. 21, 812–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wahrle S. E., Jiang H., Parsadanian M., Legleiter J., Han X., Fryer J. D., Kowalewski T., Holtzman D. M. (2004) ABCA1 is required for normal central nervous system apoE levels and for lipidation of astrocyte-secreted apoE. J. Biol. Chem. 279, 40987–40993 [DOI] [PubMed] [Google Scholar]
- 14. Xu S., Laccotripe M., Huang X., Rigotti A., Zannis V. I., Krieger M. (1997) Apolipoproteins of HDL can directly mediate binding to the scavenger receptor SR-BI, an HDL receptor that mediates selective lipid uptake. J. Lipid Res. 38, 1289–1298 [PubMed] [Google Scholar]
- 15. Martins I. J., Berger T., Sharman M. J., Verdile G., Fuller S. J., Martins R. N. (2009) Cholesterol metabolism and transport in the pathogenesis of Alzheimer disease. J. Neurochem. 111, 1275–1308 [DOI] [PubMed] [Google Scholar]
- 16. Refolo L. M., Pappolla M. A., LaFrancois J., Malester B., Schmidt S. D., Thomas-Bryant T., Tint G. S., Wang R., Mercken M., Petanceska S. S., Duff K. E. (2001) A cholesterol-lowering drug reduces β-amyloid pathology in a transgenic mouse model of Alzheimer disease. Neurobiol. Dis. 8, 890–899 [DOI] [PubMed] [Google Scholar]
- 17. Petanceska S. S., DeRosa S., Olm V., Diaz N., Sharma A., Thomas-Bryant T., Duff K., Pappolla M., Refolo L. M. (2002) Statin therapy for Alzheimer disease. Will it work? J. Mol. Neurosci. 19, 155–161 [DOI] [PubMed] [Google Scholar]
- 18. Fitz N. F., Cronican A., Pham T., Fogg A., Fauq A. H., Chapman R., Lefterov I., Koldamova R. (2010) Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high fat diet in APP23 mice. J. Neurosci. 30, 6862–6872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levin-Allerhand J. A., Lominska C. E., Smith J. D. (2002) Increased amyloid levels in APPSWE transgenic mice treated chronically with a physiological high fat high cholesterol diet. J. Nutr. Health Aging 6, 315–319 [PubMed] [Google Scholar]
- 20. Refolo L. M., Malester B., LaFrancois J., Bryant-Thomas T., Wang R., Tint G. S., Sambamurti K., Duff K., Pappolla M. A. (2000) Hypercholesterolemia accelerates the Alzheimer amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 7, 321–331 [DOI] [PubMed] [Google Scholar]
- 21. Hirsch-Reinshagen V., Maia L. F., Burgess B. L., Blain J. F., Naus K. E., McIsaac S. A., Parkinson P. F., Chan J. Y., Tansley G. H., Hayden M. R., Poirier J., Van Nostrand W., Wellington C. L. (2005) The absence of ABCA1 decreases soluble apoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J. Biol. Chem. 280, 43243–43256 [DOI] [PubMed] [Google Scholar]
- 22. Koldamova R., Staufenbiel M., Lefterov I. (2005) Lack of ABCA1 considerably decreases brain apoE level and increases amyloid deposition in APP23 mice. J. Biol. Chem. 280, 43224–43235 [DOI] [PubMed] [Google Scholar]
- 23. Wahrle S. E., Jiang H., Parsadanian M., Kim J., Li A., Knoten A., Jain S., Hirsch-Reinshagen V., Wellington C. L., Bales K. R., Paul S. M., Holtzman D. M. (2008) Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J. Clin. Invest. 118, 671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Handattu S. P., Garber D. W., Monroe C. E., van Groen T., Kadish I., Nayyar G., Cao D., Palgunachari M. N., Li L., Anantharamaiah G. M. (2009) Oral apolipoprotein A-I mimetic peptide improves cognitive function and reduces amyloid burden in a mouse model of Alzheimer disease. Neurobiol. Dis. 34, 525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saura J., Tusell J. M., Serratosa J. (2003) High yield isolation of murine microglia by mild trypsinization. Glia 44, 183–189 [DOI] [PubMed] [Google Scholar]
- 26. Koenigsknecht J., Landreth G. (2004) Microglial phagocytosis of fibrillar β-amyloid through a β1 integrin-dependent mechanism. J. Neurosci. 24, 9838–9846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mandrekar S., Jiang Q., Lee C. Y., Koenigsknecht-Talboo J., Holtzman D. M., Landreth G. E. (2009) Microglia mediate the clearance of soluble Aβ through fluid phase macropinocytosis. J. Neurosci. 29, 4252–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen C. L., Murphy R. M. (1995) Solvent effects on self-assembly of β-amyloid peptide. Biophys. J. 69, 640–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen H., Yang J., Low P. S., Cheng J. X. (2008) Cholesterol level regulates endosome motility via Rab proteins. Biophys. J. 94, 1508–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sadowski M., Pankiewicz J., Scholtzova H., Ripellino J. A., Li Y., Schmidt S. D., Mathews P. M., Fryer J. D., Holtzman D. M., Sigurdsson E. M., Wisniewski T. (2004) A synthetic peptide blocking the apolipoprotein E/β-amyloid binding mitigates β-amyloid toxicity and fibril formation in vitro and reduces β-amyloid plaques in transgenic mice. Am. J. Pathol. 165, 937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Q., Lau A., Morris T. J., Guo L., Fordyce C. B., Stanley E. F. (2004) A syntaxin 1, Gα(o), and N-type calcium channel complex at a presynaptic nerve terminal. Analysis by quantitative immunocolocalization. J. Neurosci. 24, 4070–4081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Costes S. V., Daelemans D., Cho E. H., Dobbin Z., Pavlakis G., Lockett S. (2004) Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys. J. 86, 3993–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ganley I. G., Pfeffer S. R. (2006) Cholesterol accumulation sequesters Rab9 and disrupts late endosome function in NPC1-deficient cells. J. Biol. Chem. 281, 17890–17899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hutt D. M., Da-Silva L. F., Chang L. H., Prosser D. C., Ngsee J. K. (2000) PRA1 inhibits the extraction of membrane-bound rab GTPase by GDI1. J. Biol. Chem. 275, 18511–18519 [DOI] [PubMed] [Google Scholar]
- 35. Lebrand C., Corti M., Goodson H., Cosson P., Cavalli V., Mayran N., Fauré J., Gruenberg J. (2002) Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 21, 1289–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. LaDu M. J., Falduto M. T., Manelli A. M., Reardon C. A., Getz G. S., Frail D. E. (1994) Isoform-specific binding of apolipoprotein E to β-amyloid. J. Biol. Chem. 269, 23403–23406 [PubMed] [Google Scholar]
- 37. Strittmatter W. J., Weisgraber K. H., Huang D. Y., Dong L. M., Salvesen G. S., Pericak-Vance M., Schmechel D., Saunders A. M., Goldgaber D., Roses A. D. (1993) Binding of human apolipoprotein E to synthetic amyloid β peptide. Isoform-specific effects and implications for late-onset Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 8098–8102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tokuda T., Calero M., Matsubara E., Vidal R., Kumar A., Permanne B., Zlokovic B., Smith J. D., Ladu M. J., Rostagno A., Frangione B., Ghiso J. (2000) Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer amyloid β peptides. Biochem. J. 348, 359–365 [PMC free article] [PubMed] [Google Scholar]
- 39. Koldamova R. P., Lefterov I. M., Lefterova M. I., Lazo J. S. (2001) Apolipoprotein A-I directly interacts with amyloid precursor protein and inhibits A β aggregation and toxicity. Biochemistry 40, 3553–3560 [DOI] [PubMed] [Google Scholar]
- 40. Ma J., Brewer H. B., Jr., Potter H. (1996) Alzheimer A β neurotoxicity; Promotion by antichymotrypsin, apoE4; inhibition by A β-related peptides. Neurobiol. Aging 17, 773–780 [DOI] [PubMed] [Google Scholar]
- 41. Datta G., Chaddha M., Hama S., Navab M., Fogelman A. M., Garber D. W., Mishra V. K., Epand R. M., Epand R. F., Lund-Katz S., Phillips M. C., Segrest J. P., Anantharamaiah G. M. (2001) Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J. Lipid Res. 42, 1096–1104 [PubMed] [Google Scholar]
- 42. Navab M., Anantharamaiah G. M., Reddy S. T., Hama S., Hough G., Grijalva V. R., Yu N., Ansell B. J., Datta G., Garber D. W., Fogelman A. M. (2005) Apolipoprotein A-I mimetic peptides. Arterioscler. Thromb. Vasc. Biol. 25, 1325–1331 [DOI] [PubMed] [Google Scholar]
- 43. Navab M., Anantharamaiah G. M., Fogelman A. M. (2005) An apolipoprotein A-I mimetic works best in the presence of apolipoprotein A-I. Circ. Res. 97, 1085–1086 [DOI] [PubMed] [Google Scholar]
- 44. Navab M., Anantharamaiah G. M., Reddy S. T., Hama S., Hough G., Grijalva V. R., Wagner A. C., Frank J. S., Datta G., Garber D., Fogelman A. M. (2004) Oral D-4F causes formation of pre-β high density lipoprotein and improves high density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation 109, 3215–3220 [DOI] [PubMed] [Google Scholar]
- 45. Ou J., Wang J., Xu H., Ou Z., Sorci-Thomas M. G., Jones D. W., Signorino P., Densmore J. C., Kaul S., Oldham K. T., Pritchard K. A., Jr. (2005) Effects of D-4F on vasodilation and vessel wall thickness in hypercholesterolemic LDL receptor-null and LDL receptor/apolipoprotein A-I double-knockout mice on Western diet. Circ. Res. 97, 1190–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fielding P. E., Nagao K., Hakamata H., Chimini G., Fielding C. J. (2000) A two-step mechanism for free cholesterol and phospholipid efflux from human vascular cells to apolipoprotein A-1. Biochemistry 39, 14113–14120 [DOI] [PubMed] [Google Scholar]
- 47. Wang N., Silver D. L., Thiele C., Tall A. R. (2001) ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J. Biol. Chem. 276, 23742–23747 [DOI] [PubMed] [Google Scholar]
- 48. Yancey P. G., Rodrigueza W. V., Kilsdonk E. P., Stoudt G. W., Johnson W. J., Phillips M. C., Rothblat G. H. (1996) Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J. Biol. Chem. 271, 16026–16034 [DOI] [PubMed] [Google Scholar]
- 49. Iwata N., Tsubuki S., Takaki Y., Watanabe K., Sekiguchi M., Hosoki E., Kawashima-Morishima M., Lee H. J., Hama E., Sekine-Aizawa Y., Saido T. C. (2000) Identification of the major Aβ1–42-degrading catabolic pathway in brain parenchyma. Suppression leads to biochemical and pathological deposition. Nat. Med. 6, 143–150 [DOI] [PubMed] [Google Scholar]
- 50. Maxfield F. R., Mondal M. (2006) Sterol and lipid trafficking in mammalian cells. Biochem. Soc. Trans. 34, 335–339 [DOI] [PubMed] [Google Scholar]
- 51. Maxfield F. R., Wüstner D. (2002) Intracellular cholesterol transport. J. Clin. Invest. 110, 891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mukherjee S., Maxfield F. R. (2004) Lipid and cholesterol trafficking in NPC. Biochim. Biophys. Acta 1685, 28–37 [DOI] [PubMed] [Google Scholar]
- 53. Feng B., Zhang D., Kuriakose G., Devlin C. M., Kockx M., Tabas I. (2003) Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc. Natl. Acad. Sci. U.S.A. 100, 10423–10428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mukherjee A., Hersh L. B. (2002) Regulation of amyloid β-peptide levels by enzymatic degradation. J. Alzheimers Dis. 4, 341–348 [DOI] [PubMed] [Google Scholar]
- 55. Falkevall A., Alikhani N., Bhushan S., Pavlov P. F., Busch K., Johnson K. A., Eneqvist T., Tjernberg L., Ankarcrona M., Glaser E. (2006) Degradation of the amyloid β-protein by the novel mitochondrial peptidasome, PreP. J. Biol. Chem. 281, 29096–29104 [DOI] [PubMed] [Google Scholar]
- 56. Soto C., Castaño E. M. (1996) The conformation of Alzheimer β peptide determines the rate of amyloid formation and its resistance to proteolysis. Biochem. J. 314, 701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Falcone S., Cocucci E., Podini P., Kirchhausen T., Clementi E., Meldolesi J. (2006) Macropinocytosis. Regulated coordination of endocytic and exocytic membrane traffic events. J. Cell Sci. 119, 4758–4769 [DOI] [PubMed] [Google Scholar]
- 58. Novick P., Zerial M. (1997) The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 9, 496–504 [DOI] [PubMed] [Google Scholar]
- 59. Seachrist J. L., Ferguson S. S. (2003) Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci. 74, 225–235 [DOI] [PubMed] [Google Scholar]
- 60. Poteryaev D., Datta S., Ackema K., Zerial M., Spang A. (2010) Identification of the switch in early-to-late endosome transition. Cell 141, 497–508 [DOI] [PubMed] [Google Scholar]
- 61. Rupper A., Lee K., Knecht D., Cardelli J. (2001) Sequential activities of phosphoinositide 3-kinase, PKB/Aakt, and Rab7 during macropinosome formation in Dictyostelium. Mol. Biol. Cell 12, 2813–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pfeffer S., Aivazian D. (2004) Targeting Rab GTPases to distinct membrane compartments. Nat. Rev. Mol. Cell Biol. 5, 886–896 [DOI] [PubMed] [Google Scholar]
- 63. Cavalli V., Vilbois F., Corti M., Marcote M. J., Tamura K., Karin M., Arkinstall S., Gruenberg J. (2001) The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI-Rab5 complex. Mol. Cell 7, 421–432 [DOI] [PubMed] [Google Scholar]
- 64. Pfeffer S. R. (2001) Rab GTPases. Specifying and deciphering organelle identity and function. Trends Cell Biol. 11, 487–491 [DOI] [PubMed] [Google Scholar]
- 65. Seabra M. C., Mules E. H., Hume A. N. (2002) Rab GTPases, intracellular traffic, and disease. Trends Mol. Med. 8, 23–30 [DOI] [PubMed] [Google Scholar]
- 66. Remaley A. T., Thomas F., Stonik J. A., Demosky S. J., Bark S. E., Neufeld E. B., Bocharov A. V., Vishnyakova T. G., Patterson A. P., Eggerman T. L., Santamarina-Fojo S., Brewer H. B. (2003) Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J. Lipid Res. 44, 828–836 [DOI] [PubMed] [Google Scholar]
- 67. Namba Y., Tomonaga M., Kawasaki H., Otomo E., Ikeda K. (1991) Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 541, 163–166 [DOI] [PubMed] [Google Scholar]
- 68. Näslund J., Thyberg J., Tjernberg L. O., Wernstedt C., Karlström A. R., Bogdanovic N., Gandy S. E., Lannfelt L., Terenius L., Nordstedt C. (1995) Characterization of stable complexes involving apolipoprotein E and the amyloid β peptide in Alzheimer disease brain. Neuron 15, 219–228 [DOI] [PubMed] [Google Scholar]
- 69. Wisniewski T., Frangione B. (1992) Apolipoprotein E, a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci. Lett. 135, 235–238 [DOI] [PubMed] [Google Scholar]
- 70. Kuo Y. M., Emmerling M. R., Vigo-Pelfrey C., Kasunic T. C., Kirkpatrick J. B., Murdoch G. H., Ball M. J., Roher A. E. (1996) Water-soluble Aβ (N-40, N-42) oligomers in normal and Alzheimer disease brains. J. Biol. Chem. 271, 4077–4081 [DOI] [PubMed] [Google Scholar]
- 71. Wisniewski T., Golabek A., Matsubara E., Ghiso J., Frangione B. (1993) Apolipoprotein E, binding to soluble Alzheimer β-amyloid. Biochem. Biophys. Res. Commun. 192, 359–365 [DOI] [PubMed] [Google Scholar]
- 72. Aleshkov S., Abraham C. R., Zannis V. I. (1997) Interaction of nascent apoE2, apoE3, and apoE4 isoforms expressed in mammalian cells with amyloid peptide β (1–40). Relevance to Alzheimer disease. Biochemistry 36, 10571–10580 [DOI] [PubMed] [Google Scholar]
- 73. Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. (1993) Apolipoprotein E, high avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 1977–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. LaDu M. J., Pederson T. M., Frail D. E., Reardon C. A., Getz G. S., Falduto M. T. (1995) Purification of apolipoprotein E attenuates isoform-specific binding to β-amyloid. J. Biol. Chem. 270, 9039–9042 [DOI] [PubMed] [Google Scholar]
- 75. Holtzman D. M. (2004) In vivo effects of apoE and clusterin on amyloid-β metabolism and neuropathology. J. Mol. Neurosci. 23, 247–254 [DOI] [PubMed] [Google Scholar]
- 76. Hara M., Matsushima T., Satoh H., Iso-o N., Noto H., Togo M., Kimura S., Hashimoto Y., Tsukamoto K. (2003) Isoform-dependent cholesterol efflux from macrophages by apolipoprotein E is modulated by cell surface proteoglycans. Arterioscler. Thromb. Vasc. Biol. 23, 269–274 [DOI] [PubMed] [Google Scholar]
- 77. Michikawa M., Fan Q. W., Isobe I., Yanagisawa K. (2000) Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J. Neurochem. 74, 1008–1016 [DOI] [PubMed] [Google Scholar]
- 78. Lefterov I., Bookout A., Wang Z., Staufenbiel M., Mangelsdorf D., Koldamova R. (2007) Expression profiling in APP23 mouse brain. Inhibition of Aβ amyloidosis and inflammation in response to LXR agonist treatment. Mol. Neurodegener. 2, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Riddell D. R., Zhou H., Comery T. A., Kouranova E., Lo C. F., Warwick H. K., Ring R. H., Kirksey Y., Aschmies S., Xu J., Kubek K., Hirst W. D., Gonzales C., Chen Y., Murphy E., Leonard S., Vasylyev D., Oganesian A., Martone R. L., Pangalos M. N., Reinhart P. H., Jacobsen J. S. (2007) The LXR agonist TO901317 selectively lowers hippocampal Aβ42 and improves memory in the Tg2576 mouse model of Alzheimer disease. Mol. Cell. Neurosci. 34, 621–628 [DOI] [PubMed] [Google Scholar]
- 80. Koldamova R. P., Lefterov I. M., Staufenbiel M., Wolfe D., Huang S., Glorioso J. C., Walter M., Roth M. G., Lazo J. S. (2005) The liver X receptor ligand T0901317 decreases amyloid β production in vitro and in a mouse model of Alzheimer disease. J. Biol. Chem. 280, 4079–4088 [DOI] [PubMed] [Google Scholar]
- 81. Pfrieger F. W. (2003) Cholesterol homeostasis and function in neurons of the central nervous system. Cell. Mol. Life Sci. 60, 1158–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gruenberg J. (2001) The endocytic pathway. A mosaic of domains. Nat. Rev. Mol. Cell Biol 2, 721–730 [DOI] [PubMed] [Google Scholar]
- 83. Helms J. B., Zurzolo C. (2004) Lipids as targeting signals. Lipid rafts and intracellular trafficking. Traffic 5, 247–254 [DOI] [PubMed] [Google Scholar]
- 84. Umebayashi K. (2003) The roles of ubiquitin and lipids in protein sorting along the endocytic pathway. Cell Struct. Funct. 28, 443–453 [DOI] [PubMed] [Google Scholar]
- 85. Zhang M., Dwyer N. K., Love D. C., Cooney A., Comly M., Neufeld E., Pentchev P. G., Blanchette-Mackie E. J., Hanover J. A. (2001) Cessation of rapid late endosomal tubulovesicular trafficking in Niemann-Pick type C1 disease. Proc. Natl. Acad. Sci. U.S.A. 98, 4466–4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rocha N., Kuijl C., van der Kant R., Janssen L., Houben D., Janssen H., Zwart W., Neefjes J. (2009) Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 glued and late endosome positioning. J. Cell Biol. 185, 1209–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vihervaara T., Uronen R. L., Wohlfahrt G., Björkhem I., Ikonen E., Olkkonen V. M. (2011) Sterol binding by OSBP-related protein 1L regulates late endosome motility and function. Cell. Mol. Life Sci. 68, 537–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.