Background: Sialidase activity is a key feature of bacterial vaginosis (BV), but possible substrates have not undergone in-depth investigation.
Results: We show that sialidase activity in BV clinical specimens removes sialic acids from secretory immunoglobulin A (SIgA) and other sialoglycans.
Conclusion: Desialylation of SIgA promotes further exodeglycosylation and proteolysis.
Significance: Hydrolysis of SIgA and other sialoglycans may contribute to the etiology of BV.
Keywords: Glycobiology, Glycoprotein, Glycosidases, Hydrolases, Sialic Acid, Bacterial Vaginosis, Immunoglobulin A, Sialidase
Abstract
Bacterial vaginosis (BV) is a common polymicrobial imbalance of the vaginal flora associated with a wide variety of obstetric and gynecologic complications including serious infections and preterm birth. As evidenced by high recurrence rates following treatment, interventions for BV are still lacking. Several hydrolytic activities, including glycosidases and proteases, have been previously correlated with BV and have been hypothesized to degrade host sialoglycoproteins that participate in mucosal immune functions. Sialidase activity is most predictive of BV status and correlates strongly with adverse health outcomes. Here we combine clinical specimens with biochemical approaches to investigate secretory immunoglobulin A (SIgA) as a substrate of BV-associated glycosidases and proteases. We show that BV clinical specimens hydrolyze sialic acid from SIgA, but not in the presence of the sialidase inhibitor dehydro-deoxy-sialic acid. The collective action of BV-associated glycosidases exposes underlying mannose residues of SIgA, most apparent on the heavily N-glycosylated secretory component of the antibody. Terminal sialic acid residues on SIgA protect underlying carbohydrate residues from exposure and hydrolysis by exoglycosidases (galactosidase and hexosaminidase). It is known that both IgG and SIgA are present in the human reproductive tract. We show that the IgG heavy chain is more susceptible to proteolysis than its IgA counterpart. Gentle partial deglycosylation of the SIgA secretory component enhanced susceptibility to proteolysis. Together, these data support a model of BV in which SIgA is subject to stepwise exodeglycosylation and enhanced proteolysis, likely compromising the ability of the reproductive mucosa to neutralize and eliminate pathogens.
Introduction
Bacterial vaginosis (BV)2 is the most common of all vaginal conditions that bring women to healthcare providers (1). It is characterized by a loss of beneficial normal flora (Lactobacilli) and an overgrowth of various anaerobic bacteria such as species of Gardnerella, Mobiluncus, Prevotella, Bacteroides, Atopobium, Ureaplasma, and many others (2, 3). Reproductive age women with BV are more likely to experience infections of the placenta and amniotic fluid, preterm labor with delivery of premature low birth weight infants, endometritis, and increased susceptibility to other infections such as HIV, urinary tract infection, and pelvic inflammatory disease (4–12). Importantly, a number of BV-associated bacterial species have been detected in amniotic fluid and other sites, consistent with invasive infection (13).
Unfortunately, a basic understanding of BV and effective therapeutic interventions is lacking. Moreover, possible routes of transmission and mechanisms of recurrence are not well defined. Within months of antibiotic treatment, BV recurs in up to 30% of women (14), and most women experience recurrence within a year (15). As might be expected from the high recurrence rate of BV, there is considerable disagreement in the clinical literature about whether antibiotics may benefit certain women at risk for preterm birth (16–18). New approaches are clearly needed to unravel the causal factors in BV and harness this information to develop improved prevention, diagnostic, and treatment strategies.
BV is a heterogeneous polymicrobial condition; however, both symptomatic and asymptomatic individuals share certain clinical and microbiological features. Two major sets of diagnostic criteria have been developed for BV. Amsel criteria are based on clinical findings, including thin discharge, fishy odor upon potassium hydroxide treatment, elevated vaginal pH (>4.5) attributed to reduced lactic acid bacteria, and microscopic examination of the discharge demonstrating more than 20% of the exfoliated epithelial cells that are studded with attached bacteria (“clue cells”) (19). The Nugent scoring system is based on qualitative evaluation of bacterial morphotypes on Gram-stained slides where higher scores indicate a loss of Gram-positive lactobacilli, the overgrowth of Gram-negative and Gram-variable bacteria, and the presence of Mobiluncus-like curved rods (20).
Most recently, culture-independent approaches have confirmed and extended our understanding of BV, revealing the diversity and longitudinal variability of the vaginal microflora in BV in exquisite detail (3, 21). Despite these important advances in our understanding of BV, the polymicrobial nature of this condition continues to confound traditional approaches that have focused on single-organism etiologies. For example, genetic systems do not yet exist for most of the BV-associated bacteria, and animal models of vaginal monomicrobial inoculation have so far been unsuccessful in reproducing BV-like phenotypes. These limitations highlight the need for alternative approaches that employ combinations of ex vivo (clinical specimen-based) and in vitro (biochemistry-based) BV model systems.
The reproductive tract is rich in heavily glycosylated proteins that contribute to the characteristic mucus that coats mucosal surfaces. Carbohydrate chains that modify mucosal proteins are often capped with sialic acid residues (22). Galactose and N-acetyl glucosamine or N-acetyl galactosamine most often underlie sialic acids. These moieties are found on all cell surfaces and many secreted proteins and are involved in numerous examples of host-microbe interactions (23, 24). Previous investigations have shown an association between Amsel criteria or high Nugent score (a score of 7–10) and the presence of vaginal sialidase, β-galactosidase, and N-acetylhexosaminidase activities (25, 26). These activities also correlated with thin (low viscosity) vaginal fluids, suggesting a role in mucus degradation (26), possibly similar to the interactions between some gastrointestinal bacteria and the gastrointestinal mucous layer (27, 28). Vaginal sialidase has since been independently correlated with risk of chorioamnionitis, preterm birth, and other infectious complications of BV (5, 29) and is rarely ever found in women with low Nugent scores (scores of 0–3). Production of sialidase by isolated BV-associated bacteria grown in culture suggests that BV-associated sialidases are bacterial in origin (25, 30). Furthermore, sialidase activity has also been associated with certain protease activities in vaginal fluids of women with BV (26, 29). However, specific mucosal substrates of BV-associated glycosidases and proteases are not well characterized, and their possible mechanistic roles in the underlying etiology of BV remain elusive.
IgA is the most abundant class of antibodies in the body (31). At mucosal surfaces, secretory IgA (SIgA) recognizes and neutralizes foreign antigens, facilitates antigen transport, interacts with phagocytes, and participates in the exclusion of pathogens and their toxins from mucosal surfaces (32–35). Mucosal deficiency in secretory IgA increases host susceptibility to mucosal infections, as well as allergic and autoimmune conditions (36, 37). The secreted form of IgA (secretory IgA or SIgA) consists of a dimer of Y-shaped antibody units supported by accessory proteins: the small J-chain and the large, heavily N-glycosylated secretory component (SC) (31). Both SC and the heavy chain (HC) display carbohydrate chains terminating in sialic acid residues (38–41). The glycosylation state of IgA is reported to modulate interaction with Fcα receptors of phagocytic cells and to participate in anchoring of SIgA and bound antigen in the mucus layer (42, 43). Many different glycoforms of SIgA have been documented and are sometimes associated with specific disease states (44). However, in comparison with other mucosal surfaces, the roles of SIgA in the reproductive tract are not well understood. Some studies suggest that women with BV may experience hydrolysis of vaginal IgA (45, 46). However, processes of SIgA hydrolysis in the reproductive tract are incompletely characterized.
Here we investigate SIgA hydrolysis in clinical BV specimens and normal matched controls using a combination of biochemical assays, electrophoresis analysis, and lectin blotting. These studies demonstrate SIgA as a substrate of BV-associated hydrolases and suggest that BV-associated glycosidases cooperate in stepwise deglycosylation of SIgA that may enhance its susceptibility to proteolysis.
EXPERIMENTAL PROCEDURES
Collection and Handling of Vaginal Swabs
Vaginal swabs (Starplex) were self-collected in accordance with institutional review board-approved protocols as a part of the Contraceptive CHOICE Study. The CHOICE study is a prospective cohort study of thousands of women in the St. Louis region evaluating the acceptability, continuation, and satisfaction of modern contraceptive methods (47). Swabs were rolled across glass slides for Gram staining and Nugent scoring as previously described (20), recapped, and stored at −80 °C for future elution and biochemical analysis. BV specimens used in electrophoresis and blotting experiments were paired with healthy controls matched based on race, age, and number of lifetime sexual partners. To elute, vaginal swabs were immersed in 1 ml of 100 mm sodium acetate buffer at pH 5.5 in a 2-ml deep 96-well block (Eppendorf), and incubated for approximately 1 h at room temperature in a biosafety cabinet with gentle periodic agitation. Eluted samples were then stored at −80 °C enclosed with a foil seal.
Kinetic Analysis of Sialidase Activity Using Synthetic Fluorogenic Substrate
Fluorogenic sialidase substrate 2–4-methylumbelliferyl-α-d-N-acetylneuraminic acid sodium salt (4MUSia) from Gold BioTechnology was dissolved at 10 mm in water, aliquotted, and frozen at −80 °C. Immediately before use, thawed 4MUSia aliquots were diluted in sterile 100 mm sodium acetate buffer at pH 5.5. Eluted vaginal swab material was suspended on a plate shaker, and 50 μl/well was arrayed in a black polypropylene assay plate (Eppendorf). 100 μl of 300 μm 4MUSia was then added to each well, the plate was sealed with an optically clear seal, and fluorescence was measured at excitation of 365 nm (bandwidth, 9 nm) emission of 440 nm (bandwidth, 20 nm) every 3 min on a Tecan Infinite M200 at 30 °C, with orbital shaking before each read. Relative sialidase activity was measured as the slope of the change in fluorescence over the initial approximately linear increase in fluorescence.
Generation of Fluorescent-Labeled Secretory IgA Tracer for Gel Electrophoresis
Secretory IgA from human colostrum (Sigma) 0.6 mg in 2 ml of PBS was supplemented with 1.6 ml of 100 mm sodium bicarbonate solution and 0.4 ml of freshly prepared 4 mg/ml FITC in sodium bicarbonate. The reaction was protected from light and mixed by rotating at 23 °C for 80 min. Removal of excess dye was performed using a PD Miditrap G-25 desalting column (GE Healthcare) equilibrated with water containing 10 mm NaCl, giving a stock concentration of 0.2 mg/ml.
Processing of IgA by Vaginal Swab Elutions
Equal 40-μl volumes of 0.2 mg/ml SIgA-fluorescein (SIgA-F) and vaginal swabs in acetate buffer were combined and incubated at 37 °C for 16 h. The reaction was quenched by the addition of 3× Laemmli loading buffer with 10% fresh β-mercaptoethanol and heating for 5 min at 96 °C to prepare for SDS-PAGE.
Gel Electrophoresis
PAGE was performed using precast gels (Bio-Rad) in Tris buffer with SDS. Fluorescein-labeled proteins were imaged in gels using long wave UV excitation in a Bio-Rad GelDoc XR+. For gel quantitation, the best examples of PAGE gels were analyzed in ImageJ by integrating across bands using Profile Plot and integrating across equal band widths. The data were plotted in GraphPad Prism. Both parametric (t test) and nonparametric statistical tests for significance, set at p < 0.05, were applied to clinical sample analysis.
Mobility Scoring Procedure
Relative mobility of secretory component and heavy chain of SIgA was examined after incubation with clinical specimens and scored qualitatively by four blinded observers. Observers were asked to compare specimen-treated SIgA to mobility of bands in sialidase-treated and untreated control lanes. Scores were determined as follows: bands equivalent to untreated SC or HC (0 points), bands equivalent to sialidase-treated SC or HC (1 point), or bands that migrated further than sialidase-treated SIgA (2 points). Scores of all observers were averaged, and the Mann Whitney U test was used to determine statistical significance. An example of gels rated by observers is shown in Fig. 3D.
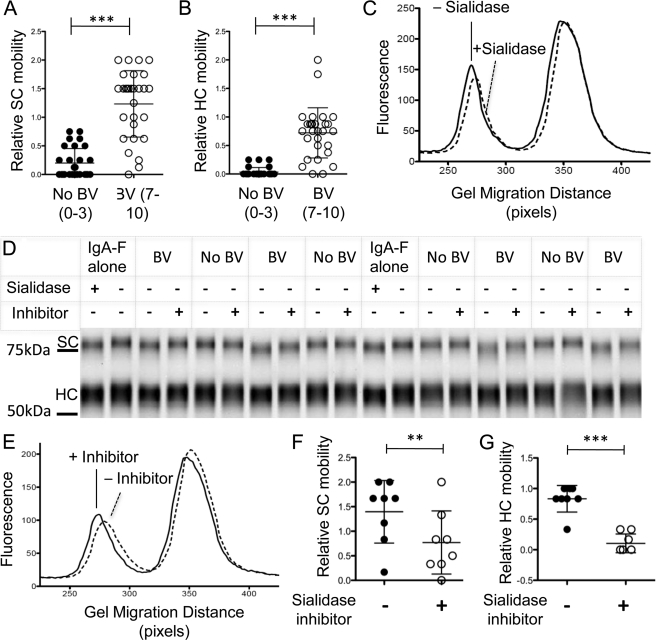
FIGURE 3.
Electrophoretic mobility changes of SIgA caused by BV clinical samples can be prevented using the sialidase inhibitor dehydro-deoxy-sialic acid. SIgA-F was mixed with material eluted from vaginal swabs from women with or without BV (numbers in parentheses indicate Nugent scores) to give 50 mm sodium acetate, pH 5.5, and 0.1 mg/ml SIgA-F. The mixture was incubated for 16 h at 37 °C, then denatured, and loaded onto a denaturing polyacrylamide gel. **, p < 0.005; ***, p < 0.0001. A and B, exposure to BV samples frequently resulted in faster migration of SIgA SC and HC. The graphs are metadata from 20 independently scored BV-positive and 20 BV-negative samples. C, histogram of IgA fluorescence versus migration distance following incubation in a representative BV-positive and matched BV-negative specimen. D, representative gel showing controls and specimens with and without the addition of 500 μm sialidase inhibitor (DDSia). E, a representative histogram of SIgA fluorescence versus migration distance in the presence or absence of DDSia. F and G, processing of IgA secretory component (F) and heavy chain (G) in BV specimens is reduced by inhibitors. In contrast, parallel incubation of SIgA-F with normal vaginal samples yields no apparent change in mobility, and the addition of sialidase inhibitor had no effect.
Determination of Neu5Ac Sialic Acid Released by BV Samples and Test of Linkages Cleaved
Human serum IgA or bovine submaxillary mucin (Sigma) was diluted to 1 mg/ml in 100 mm sodium acetate, pH 5.5. A lyophilized pellet of mutanolysin-released cell wall from COH1 expressing NeuA plasmid (54) was resuspended in 1 ml of Milli-Q H2O and centrifuged to remove insoluble material. To remove any free sialic acid and other small molecules, 500 μl of each substrate was applied to a centrifugal 10,000 molecular weight cutoff filter (Sartorius-Stedim), thrice concentrated 10×, and reconstituted to the original volume with 100 mm sodium acetate buffer. These samples were then used as stocks of 1 mg/ml for the assay below. 10 μl of 1 mg/ml substrate (or 10 μl of buffer for BV samples alone) was mixed with 10 μl of BV sample and incubated at 37 °C for 18 h. For controls, 10 μl of substrate was mixed with 10 μl of acetate buffer (mock) or 10 μl of substrate with 9 μl of acetate buffer and 1 μl (5 milliunits) of AUS in sodium acetate. After 18 h, 80 μl of acetate buffer was added to make 100 μl total and then spun for 3 min at 20,000 × g to remove solids. The supernatants were then applied to 10-kDa cutoff columns and spun at 11,000 × g for 8 min. The flowthrough (35 μl) was then mixed with 35 μl of 2× 1,2-diamino-4,5-methylenedioxybenzene solution and derivatized for 2 h at 50 °C, followed by HPLC with fluorescence detection as previously described (24). The Neu5Ac sialic acid peak was quantified by integration. The released proportion of Neu5Ac was calculated to be (recovered amount − Neu5Ac in clinical sample alone)/(quantity of Neu5Ac released from the substrate by AUS). Neu5Ac present in the clinical samples prior to IgA addition represented ∼5% of the total sialic acid in the reactions.
Sialidase Inhibition
Deoxy-dehydro-sialic acid (DDSia) (Toronto Research Chemicals) was dissolved at 50 mm in water, aliquotted, frozen, and thawed just before use. 0.8 μl of DDSia was added to 40 μl of vaginal swab eluate, then mixed with 40 μl of 0.2 mg/ml SIgA-F, sealed, and incubated for 8 h at 37 °C. As a control, 80 μl of 0.1 mg/ml SIgA-F in 50 mm acetate buffer was incubated with 0.8 μl of 5 milliunits/μl AUS (EY labs). Fine resolution was obtained using Bio-Rad TGX Criterion Any kDTM gels.
Proteolysis of IgG and IgA
Human colostrum secretory IgA (Sigma), human serum IgA (Kent Laboratories), and human serum IgG (EMD) at 0.8 mg/ml in Dulbecco's phosphate buffered saline were incubated overnight at 37 °C with subtilisin at the indicated concentrations from ∼0.5 to 33 μg/ml. Following PAGE, the gels were Coomassie-stained, and band intensity was quantitated in ImageJ.
Kinetic Proteolysis Fluorescence Assay and Synthesis of Quenched IgG
Human serum IgG (7.5 mg/ml) exchanged into 250 μl of DPBS was combined with 300 μl of 4 mg/ml FITC in sodium bicarbonate 0.1 m and protected from light while being rotated to mix for 18 h at room temperature (23 °C). Excess dye was removed on a desalting column, giving a 1.5 mg/ml stock solution of quenched FITC-labeled IgG (IgG-F) in 10 mm NaCl with 0.02% sodium azide. Enhancement of fluorescence upon proteolysis was tested using the protease subtilisin as a positive control. To measure IgGase activity in clinical samples, 20 μl of BV sample in 100 mm sodium acetate was combined with 100 μl of IgG-F at a concentration of 50 μg/ml in Dulbecco's phosphate-buffered saline without calcium and magnesium, supplemented with sodium azide to 0.02%. The plates were sealed with optically clear film, the reaction mixtures were incubated at 37 °C, and fluorescence was measured every 5 min for several hours on a Tecan F200 plate reader using an excitation filter at 485 nm and an emission filter at 535 nm.
IgA Exodeglycosylation and Lectin Blotting
20 μl of 1.25 mg/ml SIgA (Sigma) was mixed with 20 μl of elution from vaginal swabs. The mock control consisted of 20 μl of 1.25 mg/ml SIgA and 20 μl of 100 mm sodium acetate, pH 5.5 (hereafter referred to as acetate buffer). The AUS-treated control contained 20 μl of 1.25 mg/ml SIgA, 18 μl of acetate buffer, and 2 μl of AUS. The AUS, β1–4 galactosidase (New England Biolabs), and β-N-acetylhexosaminidase-treated (Prozyme) control contained 20 μL of 1.25 mg/ml SIgA, 14 μl of acetate buffer, and 2 μl of each enzyme. Following overnight incubation at 37 °C, 20 μl of 3× Laemmli sample buffer was added to each reaction. The samples were then heated to 95 °C for 3 min, and 17 μl of each was then loaded into three separate Any kDTM polyacrylamide gels (Bio-Rad). One separate gel was stained with Coomassie to visualize total protein. Proteins were transferred to 0.45-μm pore nitrocellulose (Bio-Rad) for Western blotting. The blots were blocked for 2 h with 5% BSA (Sigma A3059) in TBST at room temperature. Terminal sialic acid was probed with Sambucus nigra agglutinin (SNA)-HRP direct conjugate (EY labs) at 1:4000. Terminal mannose was probed with ConA-HRP (EY labs) at 1:5000. The blots were incubated with the specified lectin for 1.5 h in 5% BSA TBST at room temperature. The blots were then washed six times for 2 min each with TBST. Detection was performed with SuperSignal West Pico (Thermo Scientific) for ConA-HRP and SuperSignal West Femto for SNA-HRP according to the manufacturer's instructions. We note a relatively high level of exogenous SIgA addition in these experiments (see above). This optimization reduced the background level of lectin reactivity in the specimens (very low signal for specimen alone) and emphasizes the high level of glycosidase activity present.
Partial Deglycosylation and Proteolysis of SIgA
75 μl of 0.2 mg/ml SIgAF was mixed with 20 μl of 5× N-glycanase reaction buffer, 5 μl of 15% Nonidet P-40 (final concentration, 0.75%), and 2 μl of N-glycanase (Prozyme). The mock control was the same, except 2 μl of distilled water was used in place of the N-glycanase. SDS was not used. 0.75% Nonidet P-40 in the absence of N-glycanase did not sensitize SIgA-F to subtilisin, suggesting that the detergent did not disrupt the native fold of the antibody (data not shown). The reactions were incubated overnight at 37 °C. The partially deglycosylated SIgA-F was then mixed with subtilisin at 0.05–2 μg/ml overnight at 37 °C, and proteolysis was determined by the intensity of the fluorescent bands on SDS-PAGE.
RESULTS
Secretory IgA Is Mucosal Sialoglycoprotein Substrate of BV Sialidases
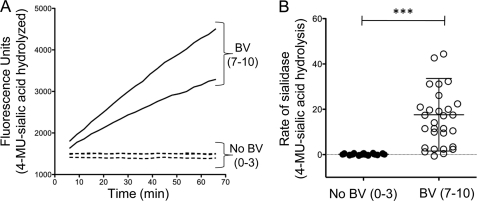
The fluorogenic substrate N-acetyl neuraminic acid-4-methyl umbelliferone (4MUSia) was used in kinetic assays with vaginal fluids eluted from self-collected swabs to confirm earlier reports that sialidase enzyme activity correlates strongly with BV status by Nugent score (48). The kinetics of sialidase activity were measured (Fig. 1A) using 60 buffered vaginal swab elutions (30 with Nugent scores of 0–3 and 30 with Nugent scores of 7–10; matched by race, age, and sexual experience). Consistent with previous reports, nearly all of the BV samples (Nugent scores of 7–10) and none of the matched healthy controls (Nugent scores of 0–3) demonstrated detectable sialidase activity (p < 0.0001) (Fig. 1B).
FIGURE 1.
Sialidase enzyme activity reflects microbiological status. BV status was assessed by Nugent scoring of Gram-stained vaginal smears (scores shown in parentheses). A, kinetics of sialidase activity was measured in vaginal swab elutions using 4MUSia. Briefly, sialidase activity was measured by combining one volume of BV sample with two volumes of substrate at 300 μm to give a final concentration of 200 μm in 100 mm sodium acetate buffer, pH 5.5. Representative data are shown. B, rates of 4MUSia hydrolysis are elevated in BV, whereas normal controls were sialidase negative. ***, p < 0.0001.
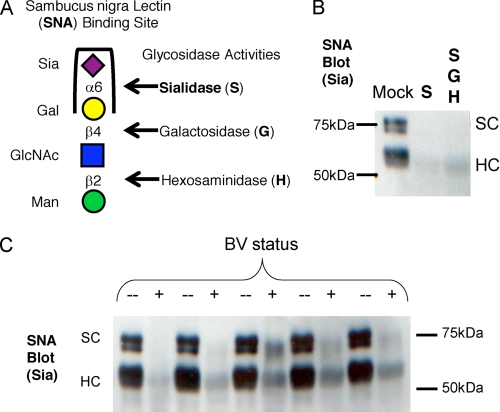
To investigate SIgA as a substrate of sialidases present in BV clinical specimens, we used SNA, a sialic acid-binding lectin, in blotting experiments as described under “Experimental Procedures.” SNA has a well described selectivity for α2–6-linked sialic acids, which is the dominant reported linkage for sialic acid on SIgA (Fig. 2A). These experiments demonstrate a loss of SNA lectin reactivity after incubation of SIgA with control AUS (Fig. 2B) or with BV specimens (Fig. 2C).
FIGURE 2.
Removal of α2–6-linked sialic acid on secretory component and heavy chain of SIgA by BV vaginal specimens but not normal controls. Vaginal samples from BV-positive women with high levels of sialidase activity, alongside matched healthy controls, were used to process SIgA in overnight incubations in 50 mm sodium acetate buffer at 37 °C. A, the treatment of human colostrum SIgA with sialidase from A. ureafaciens results in a loss of SNA lectin binding, indicating removal of α2–6-linked sialic acids. B, control treatment of SIgA with purified AUS abolishes SNA reactivity. C, SNA reactivity was lost in treatments of SIgA with BV samples but maintained with normal controls.
To further investigate SIgA as a target of BV sialidases and other BV-associated hydrolases, we developed a novel assay to measure SIgA hydrolysis activities in clinical BV specimens (Nugent score of 7–10) and healthy controls (Nugent score of 0–3). The assay uses purified, fluorescein-labeled SIgA (hereafter referred to as SIgA-F) as an exogenous sialoglycoprotein substrate and vaginal swab elutions as a source of hydrolytic activities (Fig. 3). The use of labeled exogenously added substrates allows estimation of enzymatic activities within each clinical sample, while avoiding confounding factors such as individual differences in endogenous SIgA induction, stability, and glycosylation state. SIgA-F was added directly to biological material eluted from vaginal swabs as described under “Experimental Procedures.” Following overnight incubation, polyacrylamide gel electrophoresis and fluorescent visualization allowed analysis of SC and HC mobility. We did not observe evidence of large proteolytic fragments of labeled IgA under these conditions. Rather, electrophoretic mobility was consistent with SIgA desialylation in most BV samples (Fig. 3, A–C). In some cases, mobility shifts suggested hydrolysis of other exposed or underlying carbohydrate residues.
SIgA Desialylation in BV Is Sialidase-dependent and Can Be Inhibited
To further investigate SIgA as a substrate of BV sialidases, a specific sialidase inhibitor, DDSia, which structurally resembles the acidic sugar substrate and the putative transition state of sialidase enzymes, was used during incubations with BV samples, followed by electrophoretic analysis (Fig. 3D). The addition of DDSia significantly reduced the faster migration of secretory component and heavy chain (Fig. 3, E and G) when incubated for 8 h with BV specimens. These data provide additional evidence that BV sialidases hydrolyze SIgA sialic acids and provide proof of principle that diverse sialidases from BV-associated bacteria can be inhibited by the available molecule DDSia.
Loss of Sialic Acid and Further Deglycosylation of SIgA
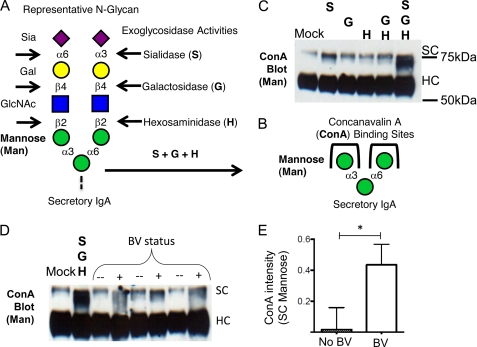
Several glycosidase activities have been associated with BV, including sialidase, β-galactosidase, and N-acetylhexosaminidase activities (26). In general, these enzyme activities have been described as exoglycosidases that require a specific terminal carbohydrate residue for activity. The exquisite specificity of commercially available microbial exoglycosidase enzymes has been elegantly harnessed in glycan structural determination studies of SIgA and other glycoproteins (40). Here we utilize available enzymes or vaginal specimens in combination with lectin blotting techniques to investigate the hydrolysis of a common terminal trisaccharide motif of SIgA and the exposure of underlying, otherwise cryptic, mannose residues on SC (Fig. 4, A–C).
FIGURE 4.
Deglycosylation of SIgA reveals underlying sugars. A and B, a common trisaccharide motif on N-glycans of SC (sialic acid (Sia)–galactose (Gal)–N-acetyl glucosamine (GlcNAc)) can be digested by successive exoglycosidase activities, revealing otherwise cryptic α-linked mannose (Man) residues, which are recognized by the lectin ConA. C, ConA shows little binding to secretory component until Sia, Gal, and N-acetyl hexose have been removed by combined treatment with sialidase, galactosidase, and hexosaminidase. C, ConA reactivity is revealed upon treatment with commercial exoglycosidase enzymes. D, representative ConA blot following SIgA treatment with BV specimens and normal controls (n = 3 each). E, quantitation of ConA reactivity with secretory component (gel in four dimensions) revealed statistically significant differences between BV samples and normal controls. Gel bands were integrated using ImageJ with rectangles of equal size extending from the top of mock treated secretory component to just above heavy chain without background subtraction. The data were normalized relative to mock treated SC (set at 1). The data shown are representative of three experiments. Statistical significance was examined by unpaired t test.
We show that exposure of SIgA SC to three commercial enzymes: sialidase, β-galactosidase, and N-acetylhexosaminidase, under nondenaturing conditions, produces a mobility shift greater than β-galactosidase/N-acetylhexosaminidase alone and greater than sialidase alone (data not shown). This result is a reflection of known exoglycosidase activities on terminal sialyllactosamine substrates. Sialic acid hydrolysis by sialidase is required before β-galactosidase or N-acetylhexosaminidase enzymes can act (Fig. 4, A and B). As shown above, the treatment of SIgA with commercial sialidase or BV specimens resulted in a loss of reactivity with SNA, a lectin that recognizes terminal α2–6-linked sialic acids (Fig. 2, B and C). In the additional presence of commercial β-galactosidase and N-acetylhexosaminidase, ConA-reactive SC bands were present at increased intensity, indicating the presence of exposed terminal α-mannose residues (Fig. 4, B and C).
We reasoned that successive deglycosylation of SIgA in BV may also result in exposure of underlying mannose residues. To test this hypothesis, we used clinical BV specimens and normal matched controls as sources of enzyme activity. Incubation with SIgA with BV specimens resulted in increased ConA reactivity (Fig. 4, D and E) of SC compared with normal controls, indicating the loss of sialic acid and increased exposure of mannose. This deglycosylation of SC may explain why SIgA-F incubated in some BV samples resulted in greater apparent hydrolysis of SC bands compared with the sialidase-treated control (Fig. 3D). The ConA blot confirms that the lower molecular mass SC material bears terminal mannose residues, consistent with the removal of sialic acid, galactose, and N-acetylglucosamine in the BV samples but not in healthy controls (Fig. 4, D and E).
IgG Is More Sensitive to Proteolysis than SIgA and Is Proteolyzed at Greater Rate in BV
A few studies have correlated protease activity with BV status (26, 29, 45). Correlation of BV with proteases such as proline-directed peptidases has been shown using chemically synthesized small molecule substrates. Other proteolysis assays have used complex mixtures of biotinylated substrates derived from human sources such as cervical mucous (49). However, investigations of purified mucosal immune glycoproteins relevant to BV have been limited. In particular, IgG and SIgA require further investigation as relevant substrates of protease-mediated hydrolysis in BV.
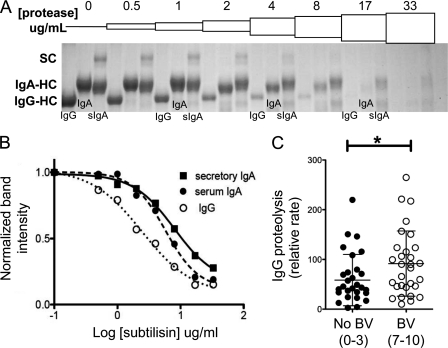
The female reproductive tract has substantial levels of both SIgA and IgG. We hypothesized that IgA might exhibit greater resistance to proteolysis compared with IgG and that women with BV (Nugent score of 7–10) may have higher proteolytic activity against IgG compared with women without BV (Nugent score of 0–3). To test the first hypothesis, purified unlabeled serum IgG, serum IgA (lacking secretory component), and SIgA were incubated with increasing concentrations of the bacterial protease subtilisin, which can cleave human antibodies resulting in the disappearance of protein by gel electrophoresis. After treating human secretory IgA, serum IgA, and IgG with a range of protease concentrations, we found that IgG was proteolyzed more readily, with less protease, than the more stable IgA (Fig. 5, A and B). Secretory IgA also appeared to be slightly more resistant than serum IgA at high protease concentrations, consistent with previous reports of IgA stabilization by secretory component (50).
FIGURE 5.
Secretory IgA is more protease resistant than IgG. Human antibodies were tested for sensitivity for bacterial protease. A, human secretory IgA, serum IgA, and serum IgG at 0.8 mg/ml were combined with subtilisin protease solutions in PBS and incubated overnight at 37 °C. To observe differences in proteolytic cleavage, we used a dilution series of protease. As protease concentration is increased from 0 to 17 μg/ml, the differential sensitivity of HC bands can be observed. Although the majority of IgG is cleaved at 4 μg/ml [subtilisin], much of the IgA remains, and serum IgA is proteolyzed before secretory IgA. The data shown are representative of five independent experiments. B, antibody HC cleavage was quantified, showing the expected dependence on protease concentration. HC band intensities for the representative gel shown were integrated using ImageJ (National Institutes of Health) and normalized to the band intensity at 0 [subtilisin] using GraphPad Prism. C, vaginal proteases can cleave IgG and are found at significantly higher levels in BV specimens compared with normal controls, as determined by unpaired t test. *, p < 0.05.
To test the second hypothesis, that women with BV have higher IgG protease activity compared with women without BV, we developed a novel and sensitive strategy to measure proteolysis of human IgG in clinical BV specimens and compare them with matched healthy controls. The approach is based on fluorescent labeling of candidate glycoprotein substrates such as IgG, the internal quenching of fluorescein-labeled proteins, and the proteolytic release of unquenched fluorescent peptides. Using human IgG-F as a quenched substrate in clinical specimens and healthy controls, kinetic assays sensitively quantified proteolytic activity (Fig. 5C). These data clearly show that some degree of IgG protease activity is commonly present in the vagina. The assay detected a mild but significant elevation of IgG-F protease activity in BV-positive samples as compared with BV-negative samples (p < 0.02; Fig. 5C). Gel electrophoresis confirmed that samples with the most protease activity cleaved IgG-F to generate fluorescent low molecular mass fragments (data not shown).
A Link between SIgA Deglycosylation and Proteolysis
Glycosylation is commonly held to protect proteins from protease cleavage. Supporting the protective effect of glycans, proteomics researchers consistently observe “missed cleavages” surrounding glycosylation sites—a lack of protease cleavage at expected substrate recognition sites. Indeed, the heavy glycosylation of SIgA is hypothesized to stabilize the protein against proteases found in polymicrobial mucosal environments (51). To test whether glycosidases contribute to the proteolytic stability of SIgA, we examined the effects of deglycosylation on proteolysis in a biochemical model system.
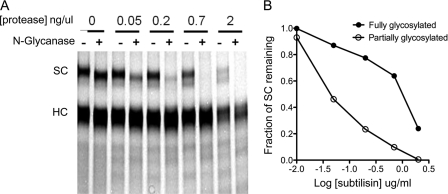
We performed a gentle and partial N-deglycosylation of SIgA-F under native conditions with N-glycanase (Peptide:N-Glycosidase F) from E. meningoseptica. This treatment resulted in the appearance of a lower molecular mass band corresponding to partially deglycosylated SC. Sensitivity of the fully glycosylated or partially glycosylated SC bands within each lane was compared. These experiments show that partial N-deglycosylation makes IgA SC more sensitive to proteolytic cleavage (Fig. 6). Complete N-deglycosylation of secretory component, which reduces the apparent molecular mass of secretory component from 75 to ∼55 kDa, was only achieved using harsh denaturing conditions. Harsh detergent (SDS) and heating enhanced proteolysis regardless of the presence of N-glycanase (data not shown). In contrast, we used neutral buffer conditions lacking SDS, which are likely to better represent native physiological conditions under which deglycosylation might occur in vivo.
FIGURE 6.
Partial deglycosylation sensitizes SIgA to proteolysis. Human SIgA was gently N-deglycosylated with N-glycanase without detergent or reducing agent or mock treated with buffer without glycosidase enzyme, followed by exposure to a range of protease concentrations. A, subtilisin more readily cleaves a partially N-glycosylated SIgA substrate within the secretory component. Fluorescein-labeled SIgA (0.2 mg/ml) was treated with and without N-glycanase in the presence of Nonidet P-40. The partially N-deglycosylated SIgA secretory component runs at a lower molecular mass. Deglycosylation results in acceleration of proteolysis of the secretory component by subtilisin at 0.05–2 μg/ml. B, quantification of SC bands shows that partially deglycosylated secretory component is proteolyzed with about 10 times less protease.
BV Sialidases Release Sialic Acids in Many Contexts
The data presented here clearly indicate that BV sialidases can act on sialic acids in α2–6-linkages (Fig. 2). The exact proportions of α2–3- and α2–6-linked sialic acids on serum or secretory IgA have not been reported and may vary depending on the donor or cell line. Attempts to perform lectin blotting experiments using the α2–3-selective lectin from Maackia amurensis did not show specific binding under similar conditions used in SNA experiments, possibly because of the low proportion of α2–3-linked sialic acid on IgA. In our hands, reactions of secretory and serum IgA with Newcastle disease virus sialidase, reported to be selective for α2–3-linked sialic acid, cleaved only about 10% of sialic acids from IgA (data not shown). In contrast, we note that BV clinical specimens exhibiting a mean release of sialic acids from IgA had a 95% confidence interval of 81.6–104%, indicating nearly complete desialylation compared with IgA treated with AUS, which is known to release sialic acid without regard to linkage position (Fig. 7).
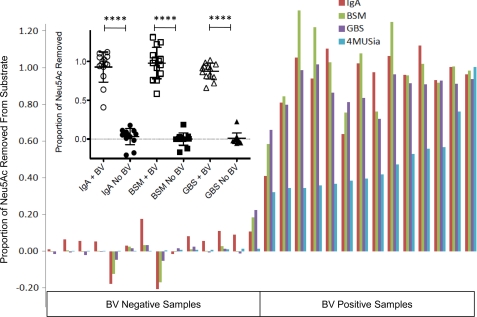
FIGURE 7.
BV sialidases act on sialic acids presented in multiple contexts relevant to the reproductive mucosa. BV specimens containing high levels of sialidase were incubated alongside matched controls (n = 14 each) with macromolecules bearing terminal sialic acids in different contexts. Released sialic acid was quantified by derivatization and HPLC as described in “Experimental Procedures” to assess the ability of BV sialidases to cleave different types of sialic acid-containing substrates. All of the data were normalized to total sialic acid (Neu5Ac) released by digestion with A. ureafaciens sialidase, shown in separate experiments to result in complete release as compared with acid hydrolysis. All of the substrates tested, including 2,3-linked sialic acids from the group B Streptococcus (GBS) capsule and primarily O-linked sialo-glycans from bovine submaxillary mucin (BSM), were desialylated to near completion by BV samples, whereas control specimens had little evidence of sialic acid hydrolysis. The Mann-Whitney U test was used to examine statistical significance (p < 0.0001 for all substrates; inset). The levels of sialidase activity (4MUSia) indicated for each sample are initial rates normalized relative to the sample with highest activity level.
To definitively investigate whether BV sialidases were active toward sialic acids present in α2–3-linkages and on O-glycans, we tested other substrates with defined linkages to determine the selectivity of sialidases present in BV clinical samples. First, we used an exclusively α2–3-linked capsular polysaccharide preparation from group B Streptococcus (serotype III strain COH1). Incubation of BV samples with group B Streptococcus polysaccharide resulted in release of most of the sialic acids (95% confidence interval of mean hydrolysis, 81.7%-93.4%), demonstrating that BV enzymes are capable of cleaving α2–3-linked sialic acid from the common α2–3-sialyl N-acetyl lactosamine moiety present in on serotype III GBS and at the termini of many common mammalian N-glycans, including known terminal glycans on IgA.
The data presented here clearly indicate that BV sialidases can act on sialic acids present on N-glycans (Fig. 4). To determine whether BV sialidases cleave sialic acids from O-glycans, we used an O-glycan-rich substrate-bovine submaxillary mucin. Again, most sialic acids were cleaved from the bovine submaxillary mucin substrate (95% confidence interval of mean, 86–109% hydrolysis). These data indicate that BV sialidases can act on O-glycans. Together, these results show that sialidases in BV are capable of cleaving the glycosidic linkage of α2–3 and α2–6 Neu5Ac present on both N- and O-glycans.
DISCUSSION
Here we used combinations of clinical specimens and defined biochemical systems to measure BV-associated glycosidase and protease activities and investigate cooperation and synergy between these enzymes. We demonstrate that SIgA is a relevant substrate of BV-associated sialidases in clinical samples, a process that can be inhibited using deoxy-dehydro-sialic acid. BV-associated β-galactosidases and N-acetylhexosaminidases also acted on SIgA in clinical samples, exposing otherwise cryptic terminal mannose residues on SC. Together, these experiments implicate sialic acids as a first line of biochemical defense against deglycosylation of SIgA in the reproductive tract. Biochemical models illustrate processive exodeglycosylation of SC and show that gentle partial deglycosylation of the heavily glycosylated SC enhances its susceptibility toward proteolysis, likely by removal of steric hindrance provided by the glycan. The evidence supports a model of BV in which elevated protease activity together with multiple exoglycosidase enzymes may produce a state of hydrolysis and depletion of important mucosal sialoglycoproteins such as SIgA.
The data show that sialic acids of multiple potential mucosal sialoglycoprotein substrates are processed by BV sialidases. In addition to hydrolysis of prominent N-glycans of secretory IgA with mostly α2–6-linked sialic acids, BV-associated sialidases also hydrolyzed sialic acids from O-glycans of mammalian mucin and accessed α2–3-linked sialic acids from the highly sialylated capsular polysaccharide of group B Streptococcus. Thus, BV sialidases have broad substrate flexibility, and SIgA is likely one of many mucosal sialoglycan substrates that undergo deglycosylation in BV.
Mucosal functions of SIgA in innate and adaptive immunity may be impaired in BV through combined actions of glycosidase and protease activities produced by BV-associated bacteria. The glycosylation state of SIgA has been shown to modulate macrophage and neutrophil functions through interaction with Fcα receptors (42). In an alternative mechanism, immune exclusion is a process whereby mucous, SIgA, and bound antigens or bacteria are eliminated by mucociliary and peristaltic movements (52). SIgA is believed to be anchored in the mucous layer through glycans on SC (43). Thus, deglycosylation of SC may reduce the ability of the host to exclude BV-associated bacteria.
Other mucosal pathogens have also evolved strategies to enzymatically attack IgA antibodies. For example, IgA1 proteases expressed by the pathogens Neisseria gonorrhoeae, Neisseria meningitidis, and certain oral Streptococci are site-specific and proline-directed (i.e. prolidases) (53). These IgA proteases cleave at the hinge region of the IgA1 heavy chain, separating the Fc constant region from the Fab containing the antigen-binding site. Prolidase activity has been correlated with BV in several studies (29, 45, 46). BV prolidase activity has been measured using small molecule proline amidase substrates with the minimal requirement of proline cleavage. To our knowledge, a distinct protein substrate has not been defined for the prolidase activity associated with BV. More general protease activities have also been correlated with BV using mucosal glycoprotein substrates derived from clinical specimens (49) or exogenous IgG as shown here. Clearly, further studies are needed to better characterize vaginal proteases and their native substrates in BV.
We emphasize that the data presented here are consistent with the BV literature. The exogenous substrate strategy used here has the advantages of sensitive in-gel detection of fluorescein-labeled SIgA, no cleavage of epitopes needed for detection by Western, and the ability to detect hydrolytic activity without the confounding factors of endogenous glycoprotein induction or hydrolysis. The data are consistent with previous measurements of endogenous IgAs using ELISA, Western blotting, and other immunochemical methods. For example, studies of endogenous IgA by Western blot of vaginal specimens reported lower molecular mass bands and smears consistent with IgA degradation in BV (46). Other studies report reduced titers of anti-Gardnerella vaginalis IgA antibodies in women with BV, titers inversely correlated with sialidase, protease, and IL-8 levels (45).
Taken together, the data strongly suggest that combinations of enzymes in BV can promote synergistic hydrolysis of protective mucosal glycoproteins. Although sialidase activity is nearly universally associated with BV, levels of sialidase activity can vary considerably between individuals. Other glycosidase and protease activities correlated with BV may also be present at widely different levels in different individuals. Thus, distinct BV-associated bacterial communities in different individuals may contribute different combinations and concentrations of glycosidases and proteases to the hydrolytic milieu. These studies provide a strong foundation for further efforts aimed at understanding how combinations and levels of activities in different women may predispose them to adverse health outcomes, particularly during pregnancy.
Acknowledgment
We thank Jacques Baenziger for providing Newcastle disease virus sialidase.
This work was supported in part by an anonymous donation to the Choice project (to J. F. P.), and laboratory analysis was funded (in part) by a Basil O'Connor Award from the March of Dimes (to A. L. L.).
- BV
- bacterial vaginosis
- AUS
- Arthrobacter ureafaciens sialidase
- DDSia
- dehydro-deoxy sialic acid
- HC
- heavy chain
- IgG-F
- fluorescein labeled IgG
- SIgA
- secretory immunoglobulin A
- SIgA-F
- fluorescein labeled SIgA
- SC
- secretory component
- 4MUSia
- 2-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid sodium salt
- SNA
- S. nigra agglutinin
- ConA
- concanavalin A.
REFERENCES
- 1. Allsworth J. E., Peipert J. F. (2007) Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet. Gynecol. 109, 114–120 [DOI] [PubMed] [Google Scholar]
- 2. Oakley B. B., Fiedler T. L., Marrazzo J. M., Fredricks D. N. (2008) Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Appl. Environ Microbiol. 74, 4898–4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Srinivasan S., Liu C., Mitchell C. M., Fiedler T. L., Thomas K. K., Agnew K. J., Marrazzo J. M., Fredricks D. N. (2010) Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One 5, e10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hillier S. L., Nugent R. P., Eschenbach D. A., Krohn M. A., Gibbs R. S., Martin D. H., Cotch M. F., Edelman R., Pastorek J. G., 2nd, Rao A. V. (1995) Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N. Engl. J. Med. 333, 1737–1742 [DOI] [PubMed] [Google Scholar]
- 5. Zhang X., Xu X., Li J., Li N., Yan T., Ju X. (2002) [Relationship between vaginal sialidase bacteria vaginosis and chorioammionitis]. Zhonghua fu chan ke za zhi 37, 588–590 [PubMed] [Google Scholar]
- 6. Rezeberga D., Lazdane G., Kroica J., Sokolova L., Donders G. G. (2008) Placental histological inflammation and reproductive tract infections in a low risk pregnant population in Latvia. Acta Obstet. Gynecol. Scand. 87, 360–365 [DOI] [PubMed] [Google Scholar]
- 7. Hitti J., Hillier S. L., Agnew K. J., Krohn M. A., Reisner D. P., Eschenbach D. A. (2001) Vaginal indicators of amniotic fluid infection in preterm labor. Obstet. Gynecol. 97, 211–219 [DOI] [PubMed] [Google Scholar]
- 8. Wiesenfeld H. C., Hillier S. L., Krohn M. A., Amortegui A. J., Heine R. P., Landers D. V., Sweet R. L. (2002) Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet. Gynecol. 100, 456–463 [DOI] [PubMed] [Google Scholar]
- 9. Wiesenfeld H. C., Hillier S. L., Krohn M. A., Landers D. V., Sweet R. L. (2003) Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin. Infect Dis. 36, 663–668 [DOI] [PubMed] [Google Scholar]
- 10. Watts D. H., Krohn M. A., Hillier S. L., Eschenbach D. A. (1990) Bacterial vaginosis as a risk factor for post-cesarean endometritis. Obstet. Gynecol. 75, 52–58 [PubMed] [Google Scholar]
- 11. Donders G. G., Van Calsteren K., Bellen G., Reybrouck R., Van den Bosch T., Riphagen I., Van Lierde S. (2009) Predictive value for Preterm birth of abnormal vaginal flora, bacterial vaginosis, and aerobic vaginitis during the first trimester of pregnancy. Br. J. Obstet. Gynaecol. 116, 1315–1324 [DOI] [PubMed] [Google Scholar]
- 12. Svare J. A., Schmidt H., Hansen B. B., Lose G. (2006) Bacterial vaginosis in a cohort of Danish pregnant women, prevalence and relationship with preterm delivery, low birthweight and perinatal infections. Br. J. Obstet. Gynaecol. 113, 1419–1425 [DOI] [PubMed] [Google Scholar]
- 13. DiGiulio D. B., Romero R., Kusanovic J. P., Gómez R., Kim C. J., Seok K. S., Gotsch F., Mazaki-Tovi S., Vaisbuch E., Sanders K., Bik E. M., Chaiworapongsa T., Oyarzún E., Relman D. A. (2010) Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am. J. Reprod Immunol. 64, 38–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larsson P. G. (1992) Treatment of bacterial vaginosis. Int. J. STD AIDS 3, 239–247 [DOI] [PubMed] [Google Scholar]
- 15. Bradshaw C. S., Morton A. N., Hocking J., Garland S. M., Morris M. B., Moss L. M., Horvath L. B., Kuzevska I., Fairley C. K. (2006) High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 193, 1478–1486 [DOI] [PubMed] [Google Scholar]
- 16. Carey J. C., Klebanoff M. A., Hauth J. C., Hillier S. L., Thom E. A., Ernest J. M., Heine R. P., Nugent R. P., Fischer M. L., Leveno K. J., Wapner R., Varner M. (2000) Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N. Engl. J. Med. 342, 534–540 [DOI] [PubMed] [Google Scholar]
- 17. Simcox R., Sin W. T., Seed P. T., Briley A., Shennan A. H. (2007) Prophylactic antibiotics for the prevention of preterm birth in women at risk: a meta-analysis. Aust. N. Z. J. Obstet. Gynaecol. 47, 368–377 [DOI] [PubMed] [Google Scholar]
- 18. McDonald H. M., Brocklehurst P., Gordon A. (2007) Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst. Rev. January 24; (1): CD000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Amsel R., Totten P. A., Spiegel C. A., Chen K. C., Eschenbach D., Holmes K. K. (1983) Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 74, 14–22 [DOI] [PubMed] [Google Scholar]
- 20. Nugent R. P., Krohn M. A., Hillier S. L. (1991) Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 29, 297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ravel J., Gajer P., Abdo Z., Schneider G. M., Koenig S. S., McCulle S. L., Karlebach S., Gorle R., Russell J., Tacket C. O., Brotman R. M., Davis C. C., Ault K., Peralta L., Forney L. J. (2011) Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U.S.A. 108, 4680–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Varki A., Schauer R. (2009) Sialic Acids in Essentials of Glycobiology, 2nd Ed., Ch. 14, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- 23. Angata T., Varki A. (2002) Chemical diversity in the sialic acids and related α-keto acids: an evolutionary perspective. Chem. Rev. 102, 439–469 [DOI] [PubMed] [Google Scholar]
- 24. Lewis A. L., Desa N., Hansen E. E., Knirel Y. A., Gordon J. I., Gagneux P., Nizet V., Varki A. (2009) Innovations in host and microbial sialic acid biosynthesis revealed by phylogenomic prediction of nonulosonic acid structure. Proc. Natl. Acad. Sci. U.S.A. 106, 13552–13557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Briselden A. M., Moncla B. J., Stevens C. E., Hillier S. L. (1992) Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. J. Clin. Microbiol. 30, 663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olmsted S. S., Meyn L. A., Rohan L. C., Hillier S. L. (2003) Sex. Trans. Dis. 30, 257–261 [DOI] [PubMed] [Google Scholar]
- 27. Linden S. K., Sutton P., Karlsson N. G., Korolik V., McGuckin M. A. (2008) Mucins in the mucosal barrier to infection. Mucosal Immunol. 1, 183–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McGuckin M. A., Lindén S. K., Sutton P., Florin T. H. (2011) Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 9, 265–278 [DOI] [PubMed] [Google Scholar]
- 29. Cauci S., McGregor J., Thorsen P., Grove J., Guaschino S. (2005) Combination of vaginal pH with vaginal sialidase and prolidase activities for prediction of low birth mass and preterm birth. Am. J. Obstet. Gynecol. 192, 489–496 [DOI] [PubMed] [Google Scholar]
- 30. Moncla B. J., Braham P., Hillier S. L. (1990) Sialidase (neuraminidase) activity among gram-negative anaerobic and capnophilic bacteria. J. Clin. Microbiol. 28, 422–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kerr M. A. (1990) The structure and function of human IgA. Biochem. J. 271, 285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stokes C. R., Soothill J. F., Turner M. W. (1975) Immune exclusion is a function of IgA. Nature 255, 745–746 [DOI] [PubMed] [Google Scholar]
- 33. Perrier C., Sprenger N., Corthésy B. (2006) Glycans on secretory component participate in innate protection against mucosal pathogens. J. Biol. Chem. 281, 14280–14287 [DOI] [PubMed] [Google Scholar]
- 34. Cerutti A., Chen K., Chorny A. (2011) Immunoglobulin responses at the mucosal interface. Annu. Rev. Immunol. 29, 273–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Egmond M., Damen C. A., van Spriel A. B., Vidarsson G., van Garderen E., van de Winkel J. G. (2001) IgA and the IgA Fc receptor. Trends Immunol. 22, 205–211 [DOI] [PubMed] [Google Scholar]
- 36. Daele J., Zicot A. F. (2000) Humoral immunodeficiency in recurrent upper respiratory tract infections: some basic, clinical and therapeutic features. Acta Otorhinolaryngol. Belg. 54, 373–390 [PubMed] [Google Scholar]
- 37. Yel L. (2010) Selective IgA deficiency. J. Clin. Immunol. 30, 10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baenziger J., Kornfeld S. (1974) Structure of the carbohydrate units of IgA1 immunoglobulin: I. Composition, glycopeptide isolation, and structure of the asparagine-linked oligosaccharide units. J. Biol. Chem. 249, 7260–7269 [PubMed] [Google Scholar]
- 39. Royle L., Mattu T. S., Hart E., Langridge J. I., Merry A. H., Murphy N., Harvey D. J., Dwek R. A., Rudd P. M. (2002) An analytical and structural database provides a strategy for sequencing O-glycans from microgram quantities of glycoproteins. Anal. Biochem. 304, 70–90 [DOI] [PubMed] [Google Scholar]
- 40. Royle L., Roos A., Harvey D. J., Wormald M. R., van Gijlswijk-Janssen D., Redwan el-R. M., Wilson I. A., Daha M. R., Dwek R. A., Rudd P. M. (2003) Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J. Biol. Chem. 278, 20140–20153 [DOI] [PubMed] [Google Scholar]
- 41. Deshpande N., Jensen P. H., Packer N. H., Kolarich D. (2010) GlycoSpectrumScan: fishing glycopeptides from MS spectra of protease digests of human colostrum sIgA. J. Proteome Res. 9, 1063–1075 [DOI] [PubMed] [Google Scholar]
- 42. Mattu T. S., Pleass R. J., Willis A. C., Kilian M., Wormald M. R., Lellouch A. C., Rudd P. M., Woof J. M., Dwek R. A. (1998) The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J. Biol. Chem. 273, 2260–2272 [DOI] [PubMed] [Google Scholar]
- 43. Phalipon A., Cardona A., Kraehenbuhl J. P., Edelman L., Sansonetti P. J., Corthésy B. (2002) Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17, 107–115 [DOI] [PubMed] [Google Scholar]
- 44. Tanaka M., Seki G., Someya T., Nagata M., Fujita T. (2011) Aberrantly glycosylated IgA1 as a factor in the pathogenesis of IgA nephropathy. Clin. Dev. Immunol. 470803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cauci S., Guaschino S., Driussi S., De Santo D., Lanzafame P., Quadrifoglio F. (2002) Correlation of local interleukin-8 with immunoglobulin A against Gardnerella vaginalis hemolysin and with prolidase and sialidase levels in women with bacterial vaginosis. J. Infect. Dis. 185, 1614–1620 [DOI] [PubMed] [Google Scholar]
- 46. Cauci S., Monte R., Driussi S., Lanzafame P., Quadrifoglio F. (1998) Impairment of the mucosal immune system: IgA and IgM cleavage detected in vaginal washings of a subgroup of patients with bacterial vaginosis. J. Infect Dis. 178, 1698–1706 [DOI] [PubMed] [Google Scholar]
- 47. Secura G. M., Allsworth J. E., Madden T., Mullersman J. L., Peipert J. F. (2010) The contraceptive CHOICE project: reducing barriers to long acting reversible contraception. Am. J. Obstet Gynecol. 203, e111–e117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bradshaw C. S., Morton A. N., Garland S. M., Horvath L. B., Kuzevska I., Fairley C. K. (2005) Evaluation of a point-of-care test, BVBlue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J. Clin. Microbiol. 43, 1304–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wiggins R., Millar M. R., Soothill P. W., Hicks S. J., Corfield A. P. (2002) Application of a novel human cervical mucin-based assay demonstrates the absence of increased mucinase activity in bacterial vaginosis. Int. J. STD AIDS 13, 755–760 [DOI] [PubMed] [Google Scholar]
- 50. Lindh E. (1975) Increased risistance of immunoglobulin A dimers to proteolytic degradation after binding of secretory component. J. Immunol. 114, 284–286 [PubMed] [Google Scholar]
- 51. Bonner A., Almogren A., Furtado P. B., Kerr M. A., Perkins S. J. (2009) The nonplanar secretory IgA2 and near planar secretory IgA1 solution structures rationalize their different mucosal immune responses. J. Biol. Chem. 284, 5077–5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boullier S., Tanguy M., Kadaoui K. A., Caubet C., Sansonetti P., Corthésy B., Phalipon A. (2009) Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J. Immunol. 183, 5879–5885 [DOI] [PubMed] [Google Scholar]
- 53. Kilian M., Reinholdt J., Lomholt H., Poulsen K., Frandsen E. V. (1996) Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS 104, 321–338 [DOI] [PubMed] [Google Scholar]