Background: Androgen stimulates prostate cancer cell proliferation and reduction of the p27 cyclin-dependent kinase inhibitor.
Results: Androgen stimulates TORC2-mediated AKT activation, which in turn increases p27 phosphorylation and proteasomal degradation.
Conclusion: Androgen is a physiological stimulator of the TORC2/AKT/p27 growth pathway.
Significance: TORC2 may function independently of TORC1 in mediating growth and survival signals.
Keywords: Androgen, Phosphorylation, Prostate Cancer, Signaling, TOR Complex (TORC), AKT, SIN1, p27
Abstract
Androgen receptor (AR) plays a central role in prostate cancer (PCa) growth, with androgen deprivation or AR down-regulation causing cell-cycle arrest and accumulation of the p27 cyclin-dependent kinase inhibitor. The molecular basis for this AR regulation of cell-cycle progression remains unclear. Here we demonstrate that androgen can rapidly reduce p27 protein in PCa cells by increasing its proteasome-mediated degradation. This rapid androgen-stimulated p27 degradation was mediated by AKT through the phosphorylation of p27 T157. Significantly, androgen increased TORC2-mediated AKT S473 phosphorylation without affecting the PDK1-mediated AKT T308 phosphorylation or TORC1 activity. The TORC2 activation was further supported by enhanced mTOR/RICTOR association and increased phosphorylation of additional TORC2 substrates, SGK1 and PKCα. The androgen-stimulated nuclear translocation of AR was associated with markedly-increased nuclear SIN1, a critical component of TORC2. Finally, the androgen-mediated TORC2/AKT activation targets a subset of AKT substrates including p27 and FOXO1, but not PRAS40. This study reveals a pathway linking AR to a selective activation of TORC2, the subsequent activation of AKT, and phosphorylation of a discrete set of AKT substrates that regulate cellular proliferation and survival. These findings establish that TORC2 can function as a central regulator of growth in response to signals that are distinct from those regulating TORC1, and support efforts to target TORC2 for cancer therapy.
Introduction
Prostate cancer (PCa)3 is the most common malignancy in men worldwide. There were an estimated 217,730 new PCa diagnoses and 32,050 deaths in the United States during 2010, which makes PCa the second leading cause of cancer death in men. Localized PCa can be cured by radical prostatectomy or radiation therapies, but treatment options are limited for patients who present with locally advanced or metastatic PCa, or in patients who recur after surgery or radiation. PCa is characterized by its exquisite dependence on androgen (testosterone and dihydrotestosterone, DHT), and suppression of androgen receptor (AR) activity through androgen-deprivation therapy (surgical castration, hormonal manipulation using gonadotropin-releasing hormone agonists, or AR antagonists) is the first line of therapeutic intervention for metastatic PCa. Androgen deprivation initially leads to a favorable clinical response in most patients, but does not eradicate disease and it eventually recurs as castration-recurrent/resistant PCa (CRPC), which is almost invariably lethal. Intriguingly, the emergence of CRPC is due, at least in part, to reactivation of the AR despite castrate levels of androgen (1). Recent therapeutic approaches that further suppress AR action in CRPC lead to responses in a substantial subset of patients, but these effects are partial and temporary (2).
Developing more effective means to interfere with AR requires an in-depth understanding of the molecular mechanism(s) by which AR controls tumor growth. Androgen deprivation induces a G0/G1 cell cycle arrest (3), but the precise actions of AR that mediate cell cycle progression and subsequent tumor growth remain unclear. We reported previously that androgens stimulate proliferation of the LNCaP PCa cell line through a transcriptional mechanism leading to activation of mammalian target of rapamycin (mTOR) complex 1 (TORC1) and subsequent increased translation of D cyclins (4). However, additional mechanisms have been implicated in androgen-stimulated proliferation of PCa cells, and the critical AR regulated genes/pathways remain to be firmly established (5).
We previously developed a castration-resistant PCa cell line (CWR22R3) whose growth was not dependent on androgens, but could be enhanced by DHT (6). Significantly, shRNA-mediated AR down-regulation in these cells caused cell cycle arrest with a marked increase in cyclin-dependent kinase inhibitor p27, without changes in D cyclins. Conversely, androgens have been reported to stimulate a rapid decrease in p27 in androgen dependent PCa cells (3), suggesting that AR may directly or indirectly regulate p27 expression. This study set out to define the molecular mechanisms underlying androgen-regulated p27 expression. We found that a rapid down-regulation of p27 in response to DHT was due to AKT-mediated phosphorylation of p27 and subsequent proteasome-mediated degradation. Significantly, while AKT activation in response to growth factors and other stimuli is mainly mediated through phosphoinositide 3-kinase (PI3K) and phosphoinositide-dependent protein kinase 1 (PDK1)-mediated phosphorylation of T308 in the AKT catalytic domain, this site was not significantly affected by androgens. Instead, we found that androgen selectively increased TORC2-mediated phosphorylation of AKT S473. Consistent with this result, we found that TORC2 activity in PCa cells was increased rapidly and selectively by androgen, independently of TORC1. Moreover, the androgen-stimulated nuclear translocation of AR was associated with a marked increase in nuclear SIN1 and rapamycin-insensitive companion of mTOR (RICTOR), two key proteins in TORC2. Finally, in addition to p27, androgen-stimulated AKT also targeted Forkhead box protein O1 (FOXO1), but not PRAS40. Together these findings indicate that AR drives cell cycle progression through activation of TORC2 and AKT, with subsequent selective targeting of AKT substrates including p27.
EXPERIMENTAL PROCEDURES
Transfections and RT-PCR
CWR22R3 cells were maintained in DMEM with 10% charcoal-dextran stripped (CDS) serum (6). CWR22Rv1 cells were in DMEM with 10% FBS and LNCaP cells in RPMI 1640 containing 10% FBS. Cells were switched to medium with 10% CDS serum for 3 days prior to DHT stimulations. Expression vectors for p27 (pcDNA3-Flag-p27 wild type, S10A, S10E and T187A) were generously provided by Masaki Mastsumoto (Kyushu University, Japan). The pcDNA3-Flag-p27T157A plasmid was constructed using the QuikChange Site-directed Mutagenesis kit (Stratagene). Cells were plated into 24-well plates at a density of 75% 1 day before transfection, and transfected for 24 h using Lipofectamine 2000 (Invitrogen).
Rapamycin and AKT inhibitor VIII were from Calbiochem, and IGF-1 was from Sigma. For quantitative RT-PCR, total RNA was prepared with TRIzol (Invitrogen) and cDNA was synthesized using reverse transcriptase (Invitrogen). qRT-PCR was performed with the SYBR Green detection method with a Mx3000p Quantitative PCR system (Stratagene).
Subcellular Fractionation and Immunoblotting
Cell fractionation was performed using the NE-PER Nuclear and Cytoplasmic Extraction kit (Pierce). For whole cell lysates, cells were lysed with RIPA lysis buffer (25 mm TrisHCl, pH 7.6, 150 mm NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS) containing Halt™ protease inhibitor mixture (Pierce) and 100 μm Na3VO4, added immediately before use. Total protein content was quantified via BCA method, and separated on 4–12% precast gels (Invitrogen). After transfer to nitrocellulose membranes, blots were blocked with 5% BSA in TBST (0.05% Tween 20 in TBS) for 1 h. Membrane was incubated in primary antibody at 4 °C overnight, followed by incubation with horseradish peroxidase conjugated anti-mouse or rabbit secondary antibodies at 4 °C for 1 h. The filters were then washed and treated with chemiluminescence detection reagents. The shown figures were representative of at least three independent experiments. Antibodies were from the following sources: anti-phospho-p27 Thr-157 (R&D Systems), anti-phospho-p27 S10 and anti-phospho-p27 T187 (Abgent), anti-tubulin (Sigma), and anti-AR (Upstate Biotechnology and Santa Cruz Biotechnology). Additional antibodies (including rabbit poly- and monoclonal phospho-Akt, Thr-308 antibodies) were from Cell Signaling Technology. Secondary anti-mouse and anti-rabbit antibodies were from Promega.
Immunoprecipitation
Cells were rinsed once with ice-cold PBS and lysed with lysis buffer (40 mm TrisHCl, pH 7.5, 120 mm NaCl and 0.3% CHAPS, and protease inhibitors (Roche complete mini)). The soluble fractions were collected after centrifugation at 12,100 × g for 15 min. For immunoprecipitations, mTOR antibody (Santa Cruz Biotechnology) was added to the lysates and incubated with rotation overnight at 4 °C. Protein G-Sepharose was then added and the incubation was continued for an additional 1 h. Immunoprecipitates were washed extensively with lysis buffer, and immunoprecipitated proteins were recovered by the addition of sample buffer and boiling for 5 min, followed by resolution on 4–12% precast gels (Invitrogen).
Infection and Immunofluorescence Microscopy
pMSCV-mSin1.1-myc was purchased from Addgene, and the viruses were packaged in Phoenix cells. After 2 rounds of infection into LNCaP cells and selection in puromycin, the LNCaP cells were split into appropriate density for immunofluorescence. Cells grown on coverslips were fixed in 2% paraformaldehyde and permeabilized with 0.1% Triton X-100. Cells were stained with primary antibodies in blocking buffer (5% milk in PBST) for 45 min and then rinsed and incubated with secondary antibody for 45 min. Cells were then rinsed with PBS, stained with 4,6-diamidino-2-phenylindole (DAPI). The slides were examined with the Zeiss LSM510 Confocal System.
RESULTS
Androgen Rapidly Stimulates Proteasome-mediated p27 Degradation
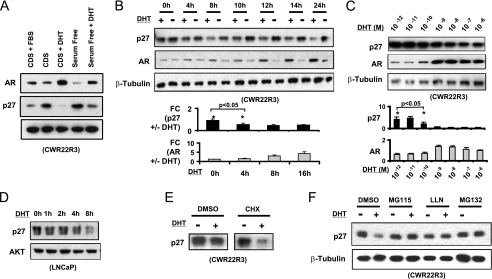
CWR22R3 cells were cultured in medium with steroid hormone-depleted (charcoal-dextran stripped, CDS) serum and then stimulated for 16 h with DHT (10 nm) or FBS. Concomitant with the well-described androgen-mediated AR stabilization and increased protein level (7), p27 protein was markedly decreased (Fig. 1A). This did not appear to simply reflect an increased fraction of cycling cells, as DHT also similarly decreased p27 when cells were cultured in serum free medium, where they remain predominantly in G0/G1 (Fig. 1A). Moreover, a decrease in p27 could be detected as early as 4 h after DHT stimulation (Fig. 1B). We also assessed the dose response to DHT to determine whether effects on p27 occurred at the same concentrations as effects on AR stabilization and proliferation. Over a range of DHT concentrations, p27 protein levels began to decrease at 10−10-10−9 m DHT, consistent with the dose response for increased AR levels (Fig. 1C) and proliferation (data not shown). The rapid DHT-stimulated decrease in p27 was also observed in another independently derived CWR22 cell line, CWR22Rv1 (see Fig. 2A), and in LNCaP cells as previously described (3) (Fig. 1D), where it occurred prior to the increase in cyclin D seen after 8–12 h (4).
FIGURE 1.
DHT increases the proteasome-mediated p27 protein degradation. A, CWR22R3 cells cultured in CDS-serum medium or in serum-free medium were stimulated without/with DHT (10 nm) or FBS for 16 h and immunoblotted for p27, AR, or β-tubulin (protein loading control). B, CWR22R3 cells in CDS serum medium were stimulated with 10 nm DHT (+) or vehicle ethanol control (−) for 0–24 h and then immunoblotted. The optical densities of the immunoblot bands were quantified by ImageJ and normalized against the corresponding internal protein loading controls. The relative fold change (FC) of p27 or AR with versus without DHT treatment of three independent experiments is presented. The error bars represent the mean ± S.E. *, p < 0.05. C, CWR22R3 cells in CDS serum were treated with different concentrations of DHT for 8 h, and cell lysates were immunoblotted. The p27 or AR WB bands at each DHT concentration were quantified, normalized, and shown in the bottom panels (n = 3, *, p < 0.05). D, LNCaP cells cultured in medium with CDS serum for 72 h were treated with DHT (10 nm) for 0 to 8 h and immunoblotted for p27 and total AKT (loading control). E, CWR22R3 cells in CDS serum medium were treated for 8 h with (+) or without (−) 10 nm DHT in the presence of vehicle (DMSO) or cycloheximide (CHX). 20 μg of total protein were loaded and then immunoblotted for p27. F, CWR22R3 cells in CDS serum medium were treated without (−) or with (+) 10 nm DHT in conjunction with proteasome inhibitors (MG115, LLN, MG132) or DMSO vehicle for 8 h, and proteins were immunoblotted.
FIGURE 2.
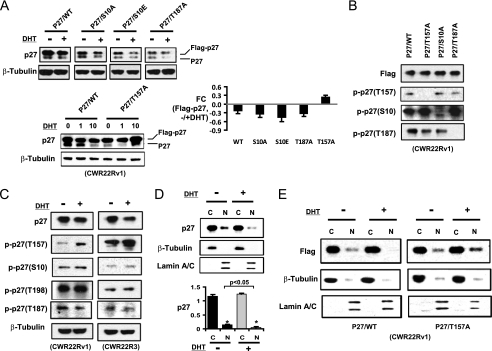
DHT induced p27 degradation and nuclear loss are dependent on p27 T157 phosphorylation. A, Flag-tagged p27 wild type (WT) or point mutants were transfected into CWR22Rv1 cells for 24 h, followed by culture in CDS serum medium for 2 day and then treatment with 1 or 10 nm DHT for 8 h (left panels). Lysates were then immunoblotted for endogenous (lower band, p27) and transfected (upper band, Flag-p27) p27. The Flag-tagged p27 bands were quantified and normalized. The relative fold change (FC) of Flag-p27 after 10 nm DHT treatment versus without DHT treatment is shown in the right panel (n = 3; error bar: mean ± S.E.). B, CWR22Rv1 cells transfected with the indicated wild type or mutant p27 vectors were immunoblotted with anti-Flag or the indicated phosphospecific anti-p27 antibody. C, CWR22Rv1 or CWR22R3 cells were stimulated with 10 nm DHT for 6 h, with the addition of MG132 during the last 2 h before harvesting. Proteins were then immunoblotted. D, CWR22Rv1 cells were stimulated with DHT for 6 h followed by cytoplasmic and nuclear fractionation and immunoblotting. The p27 bands were quantified and normalized against the internal controls (n = 3. Error bar: Mean + S.E. *, p < 0.05). E, Flag-tagged p27 wild type or T157A vectors were transfected into CWR22Rv1 cells for 24 h, followed by culture in medium with 10% CDS serum and then treatment with/without DHT for 6 h. Cytoplasmic and nuclear proteins were then extracted and immunoblotted with anti-Flag. The β-tubulin and Lamin A/C were blotted as internal markers for cytoplasmic and nuclear fractions, respectively.
The predominant mechanism regulating p27 levels is posttranslational modification and degradation (8, 9). We first determined by quantitative real time RT-PCR that DHT did not decrease p27 mRNA (supplemental Fig. S1). We next confirmed that DHT could still markedly decrease p27 proteins in cells treated with cyclohexamide (CHX) to block new protein synthesis, indicating that DHT was accelerating p27 protein degradation (Fig. 1E). Moreover, the DHT-stimulated decrease of p27 could be prevented with proteasome inhibitors (MG115, LLN, or MG132), confirming that DHT was stimulating proteasome-mediated p27 degradation (Fig. 1F).
DHT-induced p27 Degradation Is Mediated by AKT
In cycling cells, p27 is predominantly degraded in late G1/S phase through CDK2-mediated phosphorylation on Thr-187, which is recognized by the SKP2-SCF E3 ubiquitin ligase (8, 10, 11). In contrast, the initial decline in p27 as resting cells transition from G0 to early G1 is mediated by nuclear export, which depends on Ser-10 phosphorylation by KIS and subsequent ubiquitination by the cytoplasmic KPC complex (12, 13). In addition, p27 can be phosphorylated on Thr-157 by AKT, which impairs nuclear import and increases cytoplasmic accumulation and degradation (14–16). Finally, tyrosine phosphorylation of p27 by Src may relieve its CDK2 inhibition and enhance p27 Thr-187 phosphorylation/degradation (17, 18). While AR has been reported to interact with and activate Src (19), we did not detect tyrosine phosphoryation of p27 in response to DHT by anti-pTyr immunoblotting (data not shown). Therefore, to identify Ser/Thr phosphorylation sites responsible for DHT-stimulated p27 degradation, the Ser-10, Thr-157, and Thr-187 sites of p27 were modified by site-directed mutagenesis.
Flag-tagged wild-type and mutant p27 expression vectors were transiently transfected into CWR22Rv1 cells (used in these experiments due to their higher transfection efficiency) for 24 h, followed by 2 days in steroid depleted medium and then stimulation for 8 h with DHT. Immunoblotting with an anti-p27 antibody showed that both the endogenous and transfected Flag-tagged wild-type p27 were decreased in response to DHT (Fig. 2A). Replacement of Ser-10 with alanine or glutamate, which has been reported to enhance p27 nuclear export (20, 21), did not prevent the DHT-stimulated decrease in p27. Similarly, mutation of the CDK2 site (T187A) did not prevent the DHT-stimulated decrease in p27. In contrast, the T157A mutant p27 was not decreased by DHT, supporting a critical role of Thr-157 for DHT stimulated p27 degradation (Fig. 2A). To further investigate DHT-stimulated phosphorylation of these sites on endogenous p27, we used phosphospecific p27 antibodies. The specificity of these antibodies was first confirmed by immunoblotting lysates from cells transfected with the S10A, T157A, and T187A mutants (Fig. 2B). We then treated steroid-depleted cells with DHT and a proteasome inhibitor to block degradation of phosphorylated p27. Using the phosphospecfic antibodies to assess the endogenous p27 in both CWR22Rv1 and CWR22R3 cells, we found that only pT157 was markedly increased after DHT treatment (Fig. 2C).
Nuclear p27 was also decreased after DHT stimulation (Fig. 2D). To determine whether the loss of nuclear p27 was dependent on Thr-157, CWR22Rv1 cells were transfected for 24 h with Flag-tagged wild type or T157A mutant p27, followed by culturing for 2 days in steroid-depleted medium. MG132 (to prevent proteasome mediated p27 degradation) was then added for 30 min prior to the addition of DHT for 6 h, followed by separation into nuclear and cytoplasmic fractions. Significantly, DHT decreased the nuclear distribution of the wild-type p27, but not the T157A mutant (Fig. 2E). Together these findings indicate that DHT stimulates the phosphorylation of p27 at Thr-157, with subsequent loss of nuclear p27.
DHT Selectively Stimulates TORC2 Activation of AKT
Consistent with increased AKT-mediated Thr-157 phosphorylation of p27 in response to DHT, AKT was activated within 2 h after DHT treatment as indicated by the increased phosphorylation of Ser-473 and phosphorylation of AKT substrates as detected using an AKT motif antibody (Fig. 3A). Significantly, during the initial 4 h DHT treatment, we did not observe an increase in phosphorylation of Thr-308 in the AKT catalytic domain (Fig. 3A and quantified in supplemental Fig. S2), which is required for kinase activity and is mediated by PDK1 in response to PI3K activation and PH domain-mediated membrane recruitment. In contrast, phosphorylation of Thr-308 in response to IGF-1 was readily detected (supplemental Fig. S3). While uncoupling of Ser-473 and Thr-308 phosphorylation has not been reported previously in response to physiological stimuli, Thr-308 can be phosphorylated independently of Ser-473 in TORC2-deficient cells (20, 22–25). Therefore, these results indicate that the DHT-stimulated AKT activity reflects enhanced Ser-473 phosphorylation of an AKT pool with pre-existing Thr-308 phosphorylation.
FIGURE 3.
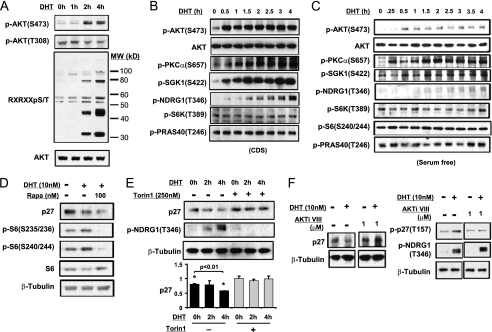
DHT stimulated p27 degradation is mediated by TORC2 activation of AKT. A, CWR22R3 cells cultured in CDS-serum medium were stimulated with DHT (10 nm) for 0–6 h and the cell lysates were blotted for AKT Ser-473 or Thr-308 phosphorylation as well as for phospho-(Ser/Thr) AKT Substrate Motif (RXX(S/T)). B, CWR22R3 cells cultured in CDS-serum medium were stimulated with DHT (10 nm) for 0–4 h, and the cell lysates were blotted for the indicated phospho- and total proteins. C, CWR22R3 cells cultured in serum-free medium were stimulated with DHT (10 nm) for 0–4 h, and the cell lysates were blotted for the indicated phospho- and total proteins. D, CWR22R3 cells cultured in CDS-serum medium were stimulated without/with DHT (10 nm) or with DHT in the presence of 100 nm rapamycin for 8 h, and the cell lysates were blotted. E, CWR22R3 cells cultured in medium with CDS serum were stimulated with DHT (10 nm) for 0–4 h without/with 250 nm Torin1. The quantification of p27 Western blots is shown in the bottom panel (n = 3. Error bar: mean ± S.E. *, p < 0.01). F, CWR22R3 cells cultured in CDS-serum medium were treated with DHT (10 nm) for 4 h without/with 1 μm of AKTi VIII (left panel). CWR22Rv1 cells cultured in medium with CDS serum were transfected with wild type or T157A mutant p27 and treated with DHT (10 nm) for 4 h without/with 1 μm of AKTi VIII (right panel).
The TORC2 complex has been identified as the kinase mediating AKT Ser-473 phosphorylation (24). Therefore, to confirm TORC2 activation, we assessed the phosphorylation of additional TORC2 substrates (22, 26, 27). Significantly, we detected DHT-stimulated rapid phosphorylation of the two other established TORC2 substrates, SGK1 and PKCα, as well as phosphorylation of the SGK1 substrate NDRG1 (28) (Fig. 3B). In contrast, we did not detect increased phosphorylation of the TORC1 substrate p70 S6 kinase or the TORC1-associated AKT substrate, PRAS40.
We also observed a similarly rapid increase in TORC2 substrate phosphorylation when cells cultured in serum-free medium were stimulated with DHT (Fig. 3C). Under these serum-free conditions, we again confirmed that DHT did not stimulate an increase in Thr-308 phosphorylation, while both Thr-308 and Ser-473 phosphorylation were stimulated by IGF-1 (supplemental Fig. S4). Consistent with the DHT-stimulated decrease in p27 being TORC1-independent, it was not prevented by TORC1 inhibition with short-term rapamycin treatment, which effectively blocked S6 phosphorylation (Fig. 3D). Rapamycin by itself had no effect on p27 levels during 8 h of treatment (supplemental Fig. S5). In contrast, the mTOR kinase inhibitor Torin 1, which inhibits both TORC1 and TORC2 (29) and effectively blocked SGK1 activation and subsequent NDRG1 phosphorylation, prevented the DHT stimulated decrease in p27 (Fig. 3E). These findings further supported the conclusion that the DHT-stimulated activation of AKT and increased p27 degradation were mediated through TORC2.
It should be noted that in a previous report we did not detect a markedly-rapid DHT-stimulated increase in AKT Ser-473 phosphorylation in LNCaP cells (4). This may reflect high basal PI3K/AKT activity in PTEN deficiency LNCaP cells versus low basal PI3K/AKT activity in PTEN intact CWR22R3 cells. Consistent with DHT stimulation of TORC2 in these cells, while AKT S473 was moderately increased, PKCα phosphorylation was increased within 1–2 h by DHT in androgen-starved LNCaP cells (supplemental Fig. S6). During this time, p27 was decreased with increasing p27 Thr-157 phosphorylation.
Similar to AKT, SGK1 is an AGC kinase with overlapping substrate specificity, and it has been reported to phosphorylate p27 on Thr-157 (30, 31). To address whether DHT-stimulated p27 phosphorylation is mediated by AKT and/or SGK1, we used an AKT specific inhibitor (AKTi VIII) that prevents AKT activation by blocking its PH domain (which is not present in SGK1) (32). At a concentration that suppressed IGF-1 stimulated phosphorylation of AKT substrates (supplemental Fig. S7), AKTi VIII blocked the DHT-stimulated down-regulation of p27 (Fig. 3F). Moreover, we confirmed that the drug did not block the SGK1-mediated phosphorylation of NDRG1, while it effectively blocked AKT-mediated p27 phosphorylation (Fig. 3F). These results established that AKT was mediating the DHT-stimulated p27 Thr-157 phosphorylation and subsequent degradation.
DHT Stimulation of TORC2 Is Associated with SIN1 Stabilization and Nuclear Accumulation
We next focused on the mechanisms by which DHT may increase TORC2 activity. Components that distinguish the TORC1 and TORC2 complexes include Raptor (associated with TORC1) and Rictor (associated with TORC2). DHT did not alter levels of mTOR, Raptor or Rictor, but mTOR co-immunoprecipitations showed that DHT caused an increase in the mTOR association with Rictor versus Raptor (Fig. 4A), consistent with that DHT selectively activates TORC2.
FIGURE 4.
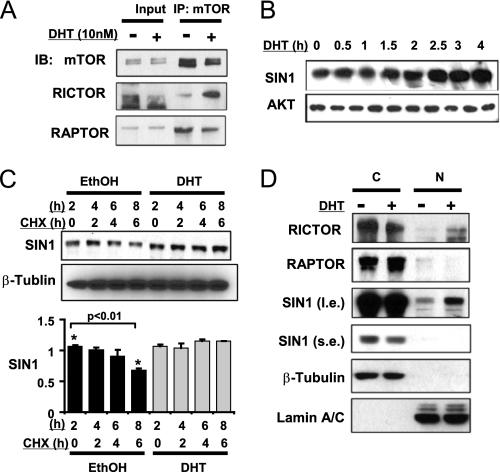
DHT increases RICTOR and SIN1 nuclear accumulation and SIN1 protein stability. A, CWR22R3 cells cultured in medium with CDS serum were treated without/with DHT (10 nm) for 3 h. The cell lysates were immunoprecipitated with anti-mTOR (IP) antibody and immunoblotted (IB) as indicated. B, CWR22R3 cells cultured in medium with CDS serum were treated with DHT (10 nm) for 0–4 h and immunoblotted for SIN1 and total AKT (protein loading control). C, CWR22R3 cells cultured in medium with CDS serum were treated without/with DHT (10 nm) for 8 h while CHX was added during the last 6 h. The quantification of SIN1 is shown in the bottom panel (n = 3. Error bar: mean ± S.E. *, p < 0.01). D, CWR22R3 cells cultured in medium with CDS serum were treated without/with DHT (10 nm) for 4 h and then fractionated into cytoplasmic (C) or nuclear (N) portions. Each fraction was immunoblotted. β-tubulin, and Lamin A/C were blotted as markers of the cytoplasmic or nuclear fractions, respectively.
As assembly of the TORC2 complex also requires SIN1 (25, 33), we next examined SIN1 expression and found that it was increased by DHT within 2–3 h (Fig. 4B). To determine whether this was due to decreased SIN1 degradation, steroid-depleted CWR22R3 cells were pre-treated for 2 h with DHT or vehicle. CHX was then added to block new protein synthesis, and SIN1 protein was followed for an additional 4 h. While SIN1 steadily declined in the vehicle-treated cells, there was no detectable decrease in SIN1 during 6 h in the DHT-treated cells, indicating that DHT was suppressing SIN1 degradation (Fig. 4C).
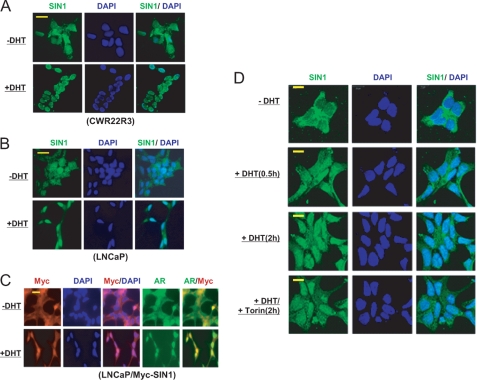
Mechanisms regulating SIN1 protein stability have not yet been identified. Therefore, as DHT stimulates the rapid nuclear translocation of AR, we next examined whether DHT altered the sub-cellular distribution of SIN1. Cellular fractionation revealed an increase in nuclear SIN1 and Rictor, but not Raptor, in response to DHT treatment (Fig. 4D). To confirm this result, we examined SIN1 localization by immunofluorescence. As expected, DHT caused a redistribution of AR from the cytoplasm to the nucleus in CWR22R3 (not shown) and LNCaP cells (see Fig. 5C). Significantly, DHT also stimulated a marked nuclear redistribution of endogenous SIN1 in both CWR22R3 and LNCaP cells (Fig. 5, A and B).
FIGURE 5.
DHT increases nuclear SIN1 accumulation independent of TORC2 activity. A, CWR22R3 or B, LNCaP cells cultured in medium with CDS serum were treated with DHT (10 nm) for 4 h. The cells were examined by immunofluoresence with an anti-SIN1 antibody. The nuclei are stained with DAPI. C, LNCaP cells were infected with Myc-tagged SIN1 expression virus, selected with puramycin, and similarly treated as above. The cells were immunostained with anti-AR and anti-Myc. D, CWR22R3 cells cultured in medium with CDS serum were treated without/with DHT (10 nm) alone or with added 250 nm Torin1 for 6 h. The cells were immunostained for SIN1 and examined with a confocal microscope. Bars: 25 μm.
To confirm that the antibody was specifically detecting SIN1, we carried out a similar study using LNCaP cells expressing Myc-tagged SIN1. This also showed markedly increased nuclear localization of the Myc-tagged SIN1 in response to DHT (Fig. 5C). It should be noted that this SIN1 redistribution into the nucleus by immunofluorescence appeared more dramatic than indicated by the nuclear fractionation. This indicates that the majority of nuclear SIN1 detected by immunofluorescence in DHT-treated cells is loosely associated with the nucleus, and therefore extracted into the cytoplasmic fraction during fractionation.
Although nuclear SIN1 was increased by DHT, it was not clear whether this was upstream of TORC2 or a downstream consequence of TORC2 activation. In support of this being upstream, the nuclear accumulation of SIN1 was observed as early as 0.5 h after DHT treatment (Fig. 5D). More importantly, treatment with Torin 1 did not prevent this SIN1 nuclear accumulation, confirming that it is independent of TORC2 activation (Fig. 5D).
DHT Stimulates AKT Activity toward Selective Substrates
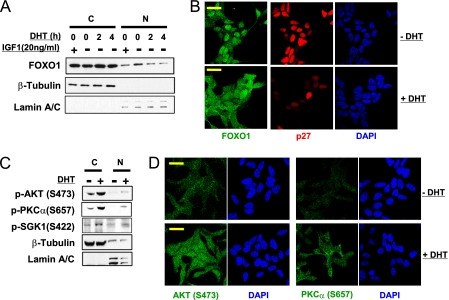
While the phosphorylation of p27 by AKT occurs in the cytoplasm and blocks p27 nuclear translocation (14), AKT-mediated phosphorylation of nuclear p27 cannot be excluded. The nuclear redistribution of Rictor and SIN1 in response to DHT, in conjunction with the failure to detect increased cytoplasmic AKT activity (based on the lack of PRAS40 phosphorylation or rapid TORC1 activation), suggested that activation of TORC2 and AKT may be occurring in the nucleus. Consistent with activation of nuclear AKT, DHT caused a decrease in nuclear FOXO1, which undergoes nuclear export in response to phosphorylation by AKT (Fig. 6A). The DHT-stimulated decrease in nuclear FOXO1 was comparable to the decrease mediated by IGF-1 (34). Immunofluorescence confirmed that DHT could stimulate a marked redistribution of FOXO1 from the nucleus to the cytoplasm (Fig. 6B).
FIGURE 6.
DHT stimulates nuclear and cytoplamic TORC2/AKT activity. A, CWR22R3 cells cultured in medium with CDS serum were treated without/with DHT (10 nm) for 2 or 4 h or IGF-1 (20 ng/ml) for 15 min, and were then fractioned into cytoplasmic (C) or nuclear (N) fractions, and were immunoblotted for FOXO1. B, CWR22R3 cells cultured in medium with CDS serum were treated without/with DHT (10 nm) for 4 h. The cells were immunostained for FOXO1 and p27 and examined with a confocal microscope. C, CWR22R3 cells cultured in medium with CDS serum were treated without/with DHT (10 nm) for 4 h and were then fractioned into cytoplasmic (C) or nuclear (N) fractions. D, CWR22R3 cells cultured in medium with CDS serum were treated without/with 10 nm DHT (−/+ DHT) for 4 h. The cells were immunostained for AKT or PKCα phosphorylation and examined with a confocal microscope. Bars: 20 μm.
These findings were consistent with previous data showing that Ser-473 phosphorylation is required for AKT targeting of a subset of substrates including FOXO1/3a (22, 23, 25, 35), and further suggested that DHT may be activating TORC2 in the nucleus. However, cellular fractionation and immunofluorescence results indicated that DHT was also stimulating cytoplasmic TORC2 activity as indicated by the increases in phosphorylation of both nuclear and cytoplasmic AKT, SGK1 and PKCα (Fig. 6, C and D). Further studies are clearly needed to determine the molecular mechanisms and contribution of AR-mediated TORC2 activation in each specific cellular compartment.
DISCUSSION
While TORC1 functions to regulate protein synthesis in response to cellular nutrient and energy levels, it is not yet clear what signals regulate TORC2. This study identifies such a pathway linking AR to the activation of TORC2 and AKT, p27 degradation, and cell-cycle progression in PCa cells. The rapid androgen-stimulated activation of TORC2, as assessed by increased phosphorylation of AKT, SGK1, and PKCα, was independent of TORC1. Significantly, the TORC2-mediated increase in AKT Ser-473 phosphorylation was not associated with an increase in PDK1-mediated Thr-308 phosphorylation, consistent with it being independent of the PI3K/AKT/TORC1 pathway.
The uncoupling of AKT Thr-308 and Ser-473 phosphorylation has been reported in cells that are selectively TORC2 deficient, where the absence of Ser-473 phosphorylation results in impaired AKT targeting of particular substrates including FOXO1. Our data similarly indicate that AKT Ser-473 phosphorylation regulates substrate specificity, as androgen-stimulated AKT selectively targeted FOXO1 and p27, but not PRAS40. The basis for this substrate specificity is not yet clear, but could reflect effects on AKT cellular localization or selective activation of an AKT isoform. Importantly, recent reports show that TORC2, but not TORC1, is required for tumor development in PTEN-deficient mouse prostate (36, 37). While the spectrum of AKT substrates in response to androgen-mediated TORC2 activation remains to be established, the targeting of p27 and FOXO1 further support a central role for TORC2 as a mediator of PCa proliferative and survival signals.
The DHT-stimulated nuclear translocation of AR was associated with a marked increase in nuclear SIN1 that was not blocked by a direct mTOR inhibitor (Torin 1), indicating that the androgen stimulated SIN1 nuclear translocation was upstream of TORC2 activation. It is not yet clear whether AR directly or indirectly facilitates this nuclear accumulation of SIN1. Androgens can stimulate the rapid nuclear accumulation of several AR interacting proteins including β-catenin and PP1 (38, 39), but we have not yet detected a direct AR interaction with SIN1. Alternatively, AR may indirectly enhance SIN1 nuclear localization through effects on nuclear import or export proteins or other mechanisms. In either case, the marked androgen-stimulated nuclear accumulation of SIN1, in conjunction with increased nuclear RICTOR, indicates that androgen-stimulated TORC2 activity may be primarily nuclear. Selective activation of TORC2 in the nucleus could also contribute to restricting AKT substrates, although our current data indicate that AKT activated in response to DHT is not solely confined to the nucleus. Further studies clearly are needed to determine whether TORC2 is being activated in the nucleus and to determine the basis for AKT substrate selectivity.
TORC2 also regulates SGK1, and androgen stimulation rapidly increased SGK1 phosphorylation and activity. Interestingly, SGK1 is a direct AR transcriptional target and can stimulate PCa growth (4, 40), although SGK1 protein levels did not increase during the short time courses of DHT treatment in this study (supplemental Fig. S8). These observations suggest that TORC2 activation and subsequent SGK1 phosphorylation also may contribute to PCa growth. Although our data indicate that SGK1 is not mediating the phosphorylation and degradation of p27, the androgen stimulation of TORC2 and subsequent increase in SGK1 activity may provide a mechanism to rapidly modulate other SGK1 substrates that contribute to tumor growth. Subsequent increases in AR-stimulated SGK1 protein expression may then maintain or further enhance these effects on SGK1 substrates.
Supplementary Material
Acknowledgments
We thank Dr. Masaki Mastsumoto for providing reagents, Drs. Wenyi Wei and Daming Guo for advice, and Dr. Alex Toker for critically reading the manuscript.
This work was supported, in whole or in part, by Grants R01DK079962 (to X. Y.) and R56AI085131 (to K. D. S.) from the National Institutes of Health. This work was also supported by Grant P50 CA 090381 from the Dana-Farber/Harvard SPORE in prostate cancer (to S. P. B.) and Grant PC020320 from the Department of Defense (to X. Y.).

This article contains supplemental Figs. S1–S8.
- PCa
- prostate cancer
- AR
- androgen receptor
- CRPC
- castration-recurrent/resistant prostate cancer
- CDS
- charcoal-dextran stripped
- CHX
- cyclohexamide
- DAPI
- 4,6-diamidino-2-phenylindole
- DHT
- dihydrotestosterone
- FOXO1
- Forkhead box protein O1
- mTOR
- mammalian target of rapamycin
- PDK1
- phosphoinositide-dependent protein kinase 1
- PI3K
- phosphoinositide 3-kinase
- TORC
- mTOR complex.
REFERENCES
- 1. Yuan X., Balk S. P. (2009) Mechanisms mediating androgen receptor reactivation after castration. Urol. Oncol. 27, 36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Knudsen K. E., Scher H. I. (2009) Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin. Cancer Res. 15, 4792–4798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knudsen K. E., Arden K. C., Cavenee W. K. (1998) Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J. Biol. Chem. 273, 20213–20222 [DOI] [PubMed] [Google Scholar]
- 4. Xu Y., Chen S. Y., Ross K. N., Balk S. P. (2006) Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 66, 7783–7792 [DOI] [PubMed] [Google Scholar]
- 5. Balk S. P., Knudsen K. E. (2008) AR, the cell cycle, and prostate cancer. Nucl. Recept. Signal. 6, e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuan X., Li T., Wang H., Zhang T., Barua M., Borgesi R. A., Bubley G. J., Lu M. L., Balk S. P. (2006) Androgen receptor remains critical for cell-cycle progression in androgen-independent CWR22 prostate cancer cells. Am. J. Pathol. 169, 682–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Z. X., Lane M. V., Kemppainen J. A., French F. S., Wilson E. M. (1995) Specificity of ligand-dependent androgen receptor stabilization: receptor domain interactions influence ligand dissociation and receptor stability. Mol. Endocrinol. 9, 208–218 [DOI] [PubMed] [Google Scholar]
- 8. Servant M. J., Coulombe P., Turgeon B., Meloche S. (2000) Differential regulation of p27(Kip1) expression by mitogenic and hypertrophic factors: Involvement of transcriptional and post-transcriptional mechanisms. J. Cell Biol. 148, 543–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Susaki E., Nakayama K. I. (2007) Multiple mechanisms for p27(Kip1) translocation and degradation. Cell Cycle 6, 3015–3020 [DOI] [PubMed] [Google Scholar]
- 10. Nakayama K., Nagahama H., Minamishima Y. A., Miyake S., Ishida N., Hatakeyama S., Kitagawa M., Iemura S., Natsume T., Nakayama K. I. (2004) Skp2-mediated degradation of p27 regulates progression into mitosis. Dev. Cell 6, 661–672 [DOI] [PubMed] [Google Scholar]
- 11. Wang H., Bauzon F., Ji P., Xu X., Sun D., Locker J., Sellers R. S., Nakayama K., Nakayama K. I., Cobrinik D., Zhu L. (2010) Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/− mice. Nat. Genet. 42, 83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamura T., Hara T., Matsumoto M., Ishida N., Okumura F., Hatakeyama S., Yoshida M., Nakayama K., Nakayama K. I. (2004) Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat. Cell Biol. 6, 1229–1235 [DOI] [PubMed] [Google Scholar]
- 13. Kotoshiba S., Kamura T., Hara T., Ishida N., Nakayama K. I. (2005) Molecular dissection of the interaction between p27 and Kip1 ubiquitylation-promoting complex, the ubiquitin ligase that regulates proteolysis of p27 in G1 phase. J. Biol. Chem. 280, 17694–17700 [DOI] [PubMed] [Google Scholar]
- 14. Liang J., Zubovitz J., Petrocelli T., Kotchetkov R., Connor M. K., Han K., Lee J. H., Ciarallo S., Catzavelos C., Beniston R., Franssen E., Slingerland J. M. (2002) PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat. Med. 8, 1153–1160 [DOI] [PubMed] [Google Scholar]
- 15. Shin I., Yakes F. M., Rojo F., Shin N. Y., Bakin A. V., Baselga J., Arteaga C. L. (2002) PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat. Med. 8, 1145–1152 [DOI] [PubMed] [Google Scholar]
- 16. Viglietto G., Motti M. L., Bruni P., Melillo R. M., D'Alessio A., Califano D., Vinci F., Chiappetta G., Tsichlis P., Bellacosa A., Fusco A., Santoro M. (2002) Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat. Med. 8, 1136–1144 [DOI] [PubMed] [Google Scholar]
- 17. Chu I., Sun J., Arnaout A., Kahn H., Hanna W., Narod S., Sun P., Tan C. K., Hengst L., Slingerland J. (2007) p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell 128, 281–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grimmler M., Wang Y., Mund T., Cilensek Z., Keidel E. M., Waddell M. B., Jäkel H., Kullmann M., Kriwacki R. W., Hengst L. (2007) Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 128, 269–280 [DOI] [PubMed] [Google Scholar]
- 19. Migliaccio A., Di Domenico M., Castoria G., Nanayakkara M., Lombardi M., de Falco A., Bilancio A., Varricchio L., Ciociola A., Auricchio F. (2005) Steroid receptor regulation of epidermal growth factor signaling through Src in breast and prostate cancer cells: steroid antagonist action. Cancer Res. 65, 10585–10593 [DOI] [PubMed] [Google Scholar]
- 20. Ishida N., Hara T., Kamura T., Yoshida M., Nakayama K., Nakayama K. I. (2002) Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. J. Biol. Chem. 277, 14355–14358 [DOI] [PubMed] [Google Scholar]
- 21. Connor M. K., Kotchetkov R., Cariou S., Resch A., Lupetti R., Beniston R. G., Melchior F., Hengst L., Slingerland J. M. (2003) CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis. Mol. Biol. Cell 14, 201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guertin D. A., Stevens D. M., Thoreen C. C., Burds A. A., Kalaany N. Y., Moffat J., Brown M., Fitzgerald K. J., Sabatini D. M. (2006) Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev. Cell 11, 859–871 [DOI] [PubMed] [Google Scholar]
- 23. Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S. Y., Huang Q., Qin J., Su B. (2006) SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127, 125–137 [DOI] [PubMed] [Google Scholar]
- 24. Sarbassov D. D., Ali S. M., Sengupta S., Sheen J. H., Hsu P. P., Bagley A. F., Markhard A. L., Sabatini D. M. (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168 [DOI] [PubMed] [Google Scholar]
- 25. Yang Q., Inoki K., Ikenoue T., Guan K. L. (2006) Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 20, 2820–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. García-Martínez J. M., Alessi D. R. (2008) mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem. J. 416, 375–385 [DOI] [PubMed] [Google Scholar]
- 27. Lu M., Wang J., Jones K. T., Ives H. E., Feldman M. E., Yao L. J., Shokat K. M., Ashrafi K., Pearce D. (2010) mTOR complex-2 activates ENaC by phosphorylating SGK1. J. Am. Soc. Nephrol. 21, 811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murray J. T., Campbell D. G., Morrice N., Auld G. C., Shpiro N., Marquez R., Peggie M., Bain J., Bloomberg G. B., Grahammer F., Lang F., Wulff P., Kuhl D., Cohen P. (2004) Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem. J. 384, 477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thoreen C. C., Kang S. A., Chang J. W., Liu Q., Zhang J., Gao Y., Reichling L. J., Sim T., Sabatini D. M., Gray N. S. (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 284, 8023–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hong F., Larrea M. D., Doughty C., Kwiatkowski D. J., Squillace R., Slingerland J. M. (2008) mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol. Cell 30, 701–711 [DOI] [PubMed] [Google Scholar]
- 31. Di Pietro N., Panel V., Hayes S., Bagattin A., Meruvu S., Pandolfi A., Hugendubler L., Fejes-Tóth G., Naray-Fejes-Tóth A., Mueller E. (2010) Serum- and glucocorticoid-inducible kinase 1 (SGK1) regulates adipocyte differentiation via forkhead box O1. Mol. Endocrinol. 24, 370–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calleja V., Laguerre M., Parker P. J., Larijani B. (2009) Role of a novel PH-kinase domain interface in PKB/Akt regulation: structural mechanism for allosteric inhibition. PLoS. Biol. 7, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ikenoue T., Inoki K., Yang Q., Zhou X., Guan K. L. (2008) Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signaling. EMBO J. 27, 1919–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan L., Lavin V. A., Moser L. R., Cui Q., Kanies C., Yang E. (2008) PP2A regulates the pro-apoptotic activity of FOXO1. J. Biol. Chem. 283, 7411–7420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bhaskar P. T., Hay N. (2007) The two TORCs and Akt. Dev. Cell 12, 487–502 [DOI] [PubMed] [Google Scholar]
- 36. Nardella C., Carracedo A., Alimonti A., Hobbs R. M., Clohessy J. G., Chen Z., Egia A., Fornari A., Fiorentino M., Loda M., Kozma S. C., Thomas G., Cordon-Cardo C., Pandolfi P. P. (2009) Differential requirement of mTOR in postmitotic tissues and tumorigenesis. Sci. Signal. 2, ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guertin D. A., Stevens D. M., Saitoh M., Kinkel S., Crosby K., Sheen J. H., Mullholland D. J., Magnuson M. A., Wu H., Sabatini D. M. (2009) mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell 15, 148–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Truica C. I., Byers S., Gelmann E. P. (2000) β-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 60, 4709–4713 [PubMed] [Google Scholar]
- 39. Chen S., Kesler C. T., Paschal B. M., Balk S. P. (2009) Androgen receptor phosphorylation and activity are regulated by an association with protein phosphatase 1. J. Biol. Chem. 284, 25576–25584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sherk A. B., Frigo D. E., Schnackenberg C. G., Bray J. D., Laping N. J., Trizna W., Hammond M., Patterson J. R., Thompson S. K., Kazmin D., Norris J. D., McDonnell D. P. (2008) Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res. 68, 7475–7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.