Background: Scaffold proteins, such as the pro-apoptotic scaffold POSH (Plenty of SH3s), organize MAP kinase pathways into functional modules.
Results: Sh3rf2 promotes the degradation of POSH and prevents apoptosis in multiple cell types.
Conclusion: Sh3rf2 antagonizes POSH-JNK signaling under basal conditions and provides a “brake” on apoptosis.
Significance: Sh3rf2 may provide a target in neoplasia and apoptosis involving POSH such as trophic factor deprivation.
Keywords: Apoptosis, Cell Signaling, MAP Kinases (MAPKs), Proteasome, Scaffold Proteins
Abstract
We report that Sh3rf2, a homologue of the pro-apoptotic scaffold POSH (Plenty of SH3s), acts as an anti-apoptotic regulator for the c-Jun N-terminal kinase (JNK) pathway. siRNA-mediated knockdown of Sh3rf2 promotes apoptosis of neuronal PC12 cells, cultured cortical neurons, and C6 glioma cells. This death appears to result from activation of JNK signaling. Loss of Sh3rf2 triggers activation of JNK and its target c-Jun. Also, apoptosis promoted by Sh3rf2 knockdown is inhibited by dominant-negative c-Jun as well as by a JNK inhibitor. Investigation of the mechanism by which Sh3rf2 regulates cell survival implicates POSH, a scaffold required for activation of pro-apoptotic JNK/c-Jun signaling. In cells lacking POSH, Sh3rf2 knockdown is unable to activate JNK. We further find that Sh3rf2 binds POSH to reduce its levels by a mechanism that requires the RING domains of both proteins and that appears to involve proteasomal POSH degradation. Conversely, knockdown of Sh3rf2 promotes the stabilization of POSH protein and activation of JNK signaling. Finally, we show that endogenous Sh3rf2 protein rapidly decreases following several different apoptotic stimuli and that knockdown of Sh3rf2 activates the pro-apoptotic JNK pathway in neuronal cells. These findings support a model in which Sh3rf2 promotes proteasomal degradation of pro-apoptotic POSH in healthy cells and in which apoptotic stimuli lead to rapid loss of Sh3rf2 expression, and consequently to stabilization of POSH and JNK activation and cell death. On the basis of these observations, we propose the alternative name POSHER (POSH-eliminating RING protein) for the Sh3rf2 protein.
Introduction
Eukaryotic cells depend on signal transduction pathways to allow them to respond to various extracellular stimuli. The evolutionarily conserved mitogen-activated protein kinase (MAPK) pathways are sequential kinase cascades involved in a variety of complex physiologic processes such as cell survival, apoptosis, differentiation, proliferation, and migration (1). In these pathways, a stimulus causes the activation of a serine/threonine protein kinase (MAPK kinase kinase), which in turn activates a MAPK kinase. The MAPK kinases activate the MAPKs through dual phosphorylation on threonine and tyrosine residues within a Thr-Xaa-Tyr motif. The MAPKs then phosphorylate various downstream effectors including members of the AP1 family of transcription factors, which alter the transcription of target genes.
In mammals, three families of MAPKs, the p38 MAPKs, the extracellular-signal related kinases (ERKs), and the c-Jun N-terminal kinase (JNKs) have been identified (1). Stresses including DNA damage, trophic factor deprivation, hypoxia, and oxidative stress activate the JNKs, which play a key role in apoptosis following these stimuli (2–4). Three distinct genes encode at least 10 distinct isoforms of JNKs.
JNK isoforms appear to have especially important physiologic roles in cell death within the nervous system. Gene deletion studies identified a key role for jnk1 and jnk2 in apoptosis in the developing brain (5). In addition, hippocampal neurons from jnk3−/− mice are resistant to excitotoxicity-induced death by kainic acid (6) and sympathetic neurons cultured from these animals exhibit decreases in c-Jun phosphorylation and in apoptosis in response to nerve growth factor (NGF) deprivation (2). The JNK pathway also participates in neuronal injury in vivo following a variety of insults including acoustic trauma (7) and cerebral hypoxia-ischemia in adult and juvenile animals (3, 8) and has been implicated in several neurodegenerative disorders (9–11).
Increasing evidence suggests that kinase cascades organize into functional modules, in some cases with the help of scaffold proteins. In yeast, members of the MAPK pheromone response pathway interact with the scaffold protein Ste5, and this interaction is required for effective signaling through this pathway (12). In mammals, the JNK-interacting proteins, JIP1, JIP2, and JIP3, are homologous scaffold proteins involved in the JNK pathway (13). The JIPs interact with MKK4/7 and the JNKs and potentiate activation of JNK by upstream signals. Importantly, activation of the JNK pathway in response to excitotoxicity or oxygen-glucose deprivation in hippocampal cultures requires JIP1 (14). Interestingly, JIP1 appeared to be anti-apoptotic when overexpressed following specific stimuli (15). One possibility is that the molar ratio of individual pathway components is important for determining the fate of the cell. Thus, over- or underexpression of a scaffold protein might promote or prevent apoptosis, and this might be context specific. For example, an increase in JIP1 might segregate components of the pathway from one another, preventing their efficient activation, whereas underexpression may also prevent apoptosis by impairing assembly of the signaling cascade.
Another JNK scaffold protein POSH (Plenty of SH3s)4 was initially identified as a Rac1-binding protein with four SH3 domains (16). POSH binds to the active (GTP-bound) form of Rac and promotes apoptosis when overexpressed (16–18). As part of its scaffold function, POSH interacts directly with members of the MLK (mixed lineage kinase) MKKK family and promotes their activation (16, 17). It also interacts indirectly with MKK4/7 and the JNKs through its interaction with the JIPs (19). In this capacity, the POSH-JIP interaction leads to formation of a multiprotein complex (POSH-JIP apoptotic complex or PJAC) that brings together key components of the JNK activation cascade. As with the JIPs, POSH is required for c-Jun activation and apoptosis in response to multiple stimuli including NGF deprivation in neuronal PC12 cells and sympathetic neurons (17) and in vivo cerebral ischemia (20).
Because of its pro-apoptotic activity, levels of POSH appear to be tightly controlled. In healthy cells, POSH levels are low due to rapid turnover of the protein. In response to apoptotic stimuli, POSH appears to be stabilized, thereby permitting formation of a sufficient number of PJAC complexes to drive apoptosis (18). The mechanisms by which cellular levels of POSH protein are regulated are presently only partially understood. POSH has a RING domain that has a putative E3 ligase function (17, 21), and the presence of the RING domain appears to be required for the rapid turnover of POSH. Beyond this, however, the means of POSH turnover in healthy cells are unknown. Likewise, the events that lead to POSH stabilization under apoptotic conditions are not fully clear. One mechanism appears to be a “feed-forward” loop in which activation of JNKs in turn promotes POSH stability (18). However, this does not fully account for the increase in POSH levels with apoptotic stimuli and additional mechanisms remain to be described.
We previously identified a homologue of POSH, “POSH2,” which contains three SH3 domains as well as a RING domain (22) (NCBI Ref Seq NM_001034187, Sh3rf2). However, a different POSH homologue in humans has also been identified that contains four SH3 domains and is therefore more appropriately called POSH2 (23). We therefore use the names Sh3rf2 or POSHER (POSH-eliminating RING protein) throughout this manuscript. As in the case of POSH, overexpression of Sh3rf2 promotes JNK activation and apoptosis of neuronal PC12 cells (22). Here we report that in contrast to siRNA-mediated knockdown of POSH, which is protective, siRNA-mediated knockdown of Sh3rf2 promotes apoptotic cell death. This indicates that unlike POSH, endogenous Sh3rf2 has anti-apoptotic activity. We further show that siRNA-mediated “knockdown” of endogenous Sh3rf2 promotes POSH stabilization and, consequentially, activation of the JNK cascade and apoptosis of multiple cell types. In healthy cells, Sh3rf2 binds to POSH and promotes its degradation via a mechanism that requires the RING domains of both proteins, although this domain is not required for their interaction. Finally, we show that degradation of Sh3rf2 rapidly occurs following trophic factor deprivation and DNA damage and knockdown of Sh3rf2 promotes apoptosis of healthy neurons. These findings support a model in which Sh3rf2/POSHER protects healthy cells from apoptotic death by promoting turnover of POSH, and in which apoptotic stimuli lead to degradation of Sh3rf2 and consequent stabilization of POSH and activation of apoptotic JNK signaling.
EXPERIMENTAL PROCEDURES
Materials
Adult rat brain RNA was obtained from Clontech (Mountain View, CA). Cell culture media RPMI 1640, Neurobasal, and B27 and Dulbecco's modified Eagle's medium (DMEM) were obtained from Mediatech (Herndon, VA). SP600125 and MG132 were from Calbiochem. The cell-permeable JNK inhibitor DJNKi1 (24) was obtained from BioMol (Plymouth Meeting, PA). Lipofectamine 2000 was obtained from Invitrogen. Recombinant human NGF (hrNGF) was kindly supplied by Genentech (South San Francisco, CA). Hoechst dye 33342 and anti-hrNGF antiserum were obtained from Sigma. JNK/phospho-JNK, c-Jun/phospho-c-Jun, and β-actin antibodies were from Cell Signaling (Denvers, MA). Monoclonal anti-POSH and anti-Sh3rf2 were from Abnova (Beijing, China). Anti-FLAG, anti-c-Myc, and anti-ERK2 were obtained from Santa Cruz (Paso Robles, CA). Anti-FLAG beads and anti-Myc beads were from Sigma. siRNAs were obtained from Dharmacon (Lafayette, CO). Constitutively active (V) and inactive (N) Rac1 in PRK5 were graciously provided by Dr. Alan Hall. GST fusion proteins for Rac1 (N- and V-) were generated by PCR using these constructs as templates. PCR products, not including the start codon, were ligated in-frame into the pcDNA3-GST vector. POSH, MLK3, MLK2, DLK, and JIP constructs have been previously described (17, 19) as have Sh3rf2 and the RING mutant of Sh3rf2 (22).
Generation of Plasmids and Primers
The sequence of all newly generated constructs was confirmed by sequencing at the DNA facility of the Columbia University Health Sciences or University of Wisconsin-Madison campus using appropriate primers. A 5′ Myc tag was added to Sh3rf2 using the primer, 5′-AACCATGGAGCAGAAGCTGATCTCCGGGAGGACCTGGTGATTTGACGTTACTTGATCTC. The synthetic siRNA for rat Sh3rf2 targets the sequence AAGCAAGTCAAAACCGTGAGA, corresponding to base pairs 1930–1950. An siRNA designed against both rat and the putative human Sh3rf2 orthologue (sihuSh3rf2) targets the sequence GTGGAAGTCATCAAGCAGCTGCC, corresponding to base pairs 535–557 of the rat sequence. We generated a vector expressing shSh3rf2 under control of the U6 promoter, which targets this same sequence, using the instructions for the pU6-siRNA vector. For PCR of Sh3rf2 from various tissues, we used primers 5′-GAGTGAGAACCAGGATTGCCTGACCTTCCTC-3′, and GGCTGTAATTCTGAAATCTCACGGTTTTGAC. For qRT-PCR we generated intron-spanning primers for the housekeeping gene cyclophilin A (PPIA) 5′-GGTCCTGGCATCTTGTCCAT and 3′-GCCTTCTTTCACCTTCCCAAA and for Sh3rf2, 5′-TTATCCACCTCCTCTGTGTCCTC and 3′-TCCCTGATTGAACTGGTCTCTG using Primer3 software. Adenovirus expressing shRNAs were produced using the BLOCK-IT Adenoviral RNAi Expression System (Invitrogen) following the manufacturer's instructions. The inserted sequences were: Sh3rf2 (top), CACCGAAGCAAGTCAAAACCGTGAGACGAATCTCACGGTTTTGACTTG, Sh3rf2 (bottom), AAAAGCAAGTCAAAACCGTGAGATTCGTCTCACGGTTTTGACTTGCTTC, and control (top), CACCGAATTCTCCGAACGTGTCACGTCGAAACGTGACACGTTCGGAGAA, control (bottom), AAAATTCTCCGAACGTGTCACGTTTCGACGTGACACGTTCGGAGAATTC. These sequences generate an shRNA targeting the same sequence as siSh3rf2 (bp 1930–1950). Lentiviral expression constructs were generated using the Sigma Mission lentiviral pLKO.1-puro vector as per the manufacturer's instructions. We used one of the predetermined Sh3rf2 targeting vectors with the cDNA insert CCGGGACATCAGTTATCACGCACAACTCGAGTTGTGCGTGATAACTGATGTCTTTTTG, which targets bp 794–814 of the mouse Sh3rf2 sequence. The Sigma nonsilencing vector was used as the control.
Cell Culture and Transfections
HEK293 cells were maintained in 1× DMEM supplemented with 10% fetal bovine serum (FBS). Culture of PC12 cells, both naive and neuronally differentiated was performed as previously described as was NGF deprivation of PC12 cells (17). HEK293 cells were transfected using the calcium phosphate method as previously described (17). PC12 cells were transfected 2 days after NGF treatment using Lipofectamine 2000 per the manufacturer's instructions. siPOSH 293 cells have been previously described (22, 25). Primary cortical cultures were prepared from E18 mouse or rat pups as previously described (26). Cortical neurons were maintained in Neurobasal medium supplemented with B27. The final concentration of SP600125 was 20 μm. The final concentration of MG132 was 20 μm. The final concentration of DJNKi1 was 5 μm.
Immunoprecipitation, Western Immunoblotting, and in Vitro Binding Assays
Co-immunoprecipitation and Western immunoblotting and in vitro binding assays were performed as previously described (17). Chemiluminescent signals were digitally acquired using a Kodak Image Station 4000MM and Kodak Molecular Imaging Software version 4.0.0 or with the LiCor Odyssey system. Bands were identified visually and relative intensity was determined using either Kodak or Odyssey software or NIH ImageJ.
For the in vitro binding assays, constitutively active (V-) and inactive (N-) Rac1 were subcloned into the PGEX vector. GST-Rac1 (V- or N-) were purified as previously described (17). POSH and Sh3rf2 were used for in vitro transcription and translation using the TnT-coupled reticulocyte lysate system (Promega, Madison, WI). Incubation, washing, and resolution of radiolabeled proteins was performed as previously described (27).
Quantitative PCR
Neuronally differentiated PC12 cells were subjected to NGF deprivation as previously described (17) and collected at 0, 2, and 4 h after treatment. Cellular material was mixed extensively in 1 ml of TRIzol Reagent (Invitrogen). Initial extraction of total RNA was performed in chloroform followed by precipitation in 75% ethanol. Clean up of RNA was performed using the RNeasy Mini Protocol for RNA cleanup (Qiagen, Valencia, CA) with final ethanol precipitation in 3 m NaOAc. Purity and concentration of each sample was verified by determination of the A260/A280 nm ratio, with an output of 1.8–2. cDNA for qRT-PCR was synthesized using 1 μg of purified RNA and random hexamers (Invitrogen). Reactions were run on a Lightcycler 2.0 (Roche Diagnostics) for 45 cycles. Data were normalized to expression in the housekeeping gene, cyclophilin A, and relative values were determined.
Determination of Cell Death
Strip counting to determine cell survival was performed as previously described (17). Nuclear morphology was examined using Hoechst 33342 staining. The individual who carried out the counting was blinded to the experimental groups in all cases. TUNEL labeling was performed using the Click-iT® TUNEL Alexa Fluor® 488 Imaging Assay. A minimum of 100 cells were counted in each condition. Each experiment (in triplicate) was performed a minimum of three separate times.
Statistics
Comparisons between groups were performed by one-way analysis of variance followed by pairwise t-tests or Student-Newman-Keuls for comparisons between more than one pair.
RESULTS
Sh3rf2 Binds MLKs and JIPs
Given the structural homology to POSH, and the JNK activation that occurs when Sh3rf2 is overexpressed (22), we postulated that Sh3rf2 might bind to components of the JNK pathway. To determine whether Sh3rf2 is capable of binding to upstream members of the JNK pathway, we performed a series of co-immunoprecipitations. As shown in supplemental Fig. S1A, MLK2 and DLK were immunoprecipitated by anti-FLAG antiserum when co-expressed with FLAG-Sh3rf2, but not when co-expressed with empty GFP vector. Furthermore, JIP1, -2, and -3 also co-immunoprecipitated with Sh3rf2 (supplemental Fig. S1B). The reciprocal co-immunoprecipitations revealed similar results (data not shown). As seen in supplemental Fig. S1B, the interaction between Sh3rf2 and JIP1 appears stronger than that with JIP2 and JIP3. These observations indicate that overexpressed Sh3rf2, like POSH with which it bears considerable structural similarity, binds multiple members of the JNK pathway. However, because such studies were performed under conditions of overexpression, they left open the role of Sh3rf2 under physiologic conditions.
Sh3rf2 Binds Both Active and Inactive Rac1
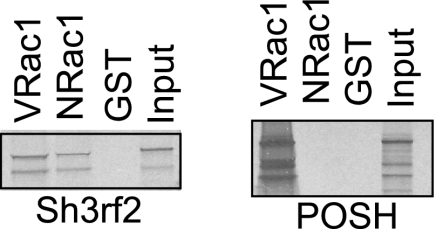
Because POSH was originally detected as a binding partner for activated Rac1, we assessed whether this was also the case for Sh3rf2. When the sequences of POSH and Sh3rf2 are compared, the region of Sh3rf2 corresponding to the putative Rac1-binding site of POSH identified by Tapon et al. (16) has lower homology than other defined domains (22). To determine whether Sh3rf2 also binds selectively to the activated (GTP-bound) form of Rac1, we performed in vitro binding assays using in vitro translated, radiolabeled POSH and Sh3rf2. GST fusion proteins of constitutively active (V-) and inactive (N-) Rac1 were used to bind these radiolabeled proteins in parallel assays. As shown in Fig. 1, radiolabeled Sh3rf2 binds directly to both the active and inactive forms of Rac1, but not to GST alone. Reciprocal in vitro binding assays using a Sh3rf2-GST fusion protein confirmed these results (data not shown). This provides an interesting contrast to POSH which, in agreement with previously reported data (16), binds selectively only to active Rac1. This suggests a functional difference from POSH, and we therefore sought to determine whether other functional differences exist.
FIGURE 1.
Sh3rf2, unlike POSH, binds to both active and inactive Rac1. POSH and Sh3rf2 were transcribed and translated in vitro in the presence of radiolabeled methionine. An aliquot was reserved as input. Products were incubated with beads bound to either NRac-GST or VRac-GST fusion proteins or uncoupled GST, washed, and resolved by SDS-PAGE and visualized by autoradiography. Smaller bands represent incompletely translated protein, which is still capable of interacting.
Sh3rf2 Is an Anti-apoptotic Protein in Several Cell Types
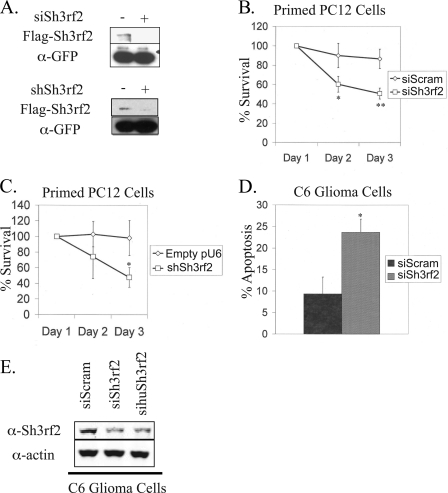
As we previously reported, overexpressed Sh3rf2 activates JNK in HEK293 cells and promotes apoptosis in neuronally differentiated (“neuronal”) PC12 cells, which is blocked by a dominant-negative form of c-Jun (22). This is consistent with a JNK scaffold activity when overexpressed as suggested by our above data. We therefore initially postulated that Sh3rf2 performs a complimentary or additive function with POSH following apoptotic stimuli. To test this idea, we designed a small inhibitory RNA (siRNA) to suppress expression of endogenous Sh3rf2 (siSh3rf2, see “Experimental Procedures”). Fig. 2A (upper panel) shows that siSh3rf2 effectively reduces expression of overexpressed, FLAG-tagged Sh3rf2.
FIGURE 2.
Sh3rf2 knockdown promotes JNK-dependent apoptosis in both proliferating and post-mitotic cells. A, upper panel, FLAG-tagged Sh3rf2 in pCMS-EGFP was expressed in HEK293 cells in the presence of either scrambled (nonsilencing) siRNA or siSh3rf2. Lysates were collected 24 h later and subjected to immunoblot for FLAG. Membranes were reprobed for GFP to confirm equal loading and transfection efficiency. In the lower panel, FLAG-tagged Sh3rf2 was co-expressed with 4-fold excess of shRNA targeting Sh3rf2 in the pU6 vector (shSh3rf2), or empty vector. Cells were collected 24 h later and immunoblotting was performed for FLAG-Sh3rf2. The membrane was reprobed with GFP to ensure equal transfection efficiency for Sh3rf2. B, neuronal PC12 cells were co-transfected with pCMS-EGFP and either scrambled siRNA or siSh3rf2. Transfected cells were counted starting 24 h after transfection (Day 1). Shown is one experiment performed in triplicate (*, p < 0.03; **, p < 0.01). The experiment was performed three times with similar results. C, an shRNA plasmid (shSh3rf2) targeting Sh3rf2 was transfected into primed PC12 cells in 4-fold excess to pCMS-EGFP ensuring all fluorescent cells express shRNA. Again EGFP+ cells were counted starting 24 h after transfection (Day 1). Shown is one experiment performed in triplicate (*, p < 0.03). The experiment was performed three times with similar results. D, C6 glioma cells were transfected with pCMS-EGFP and the indicated pre-synthesized siRNAs. Cells were fixed 24 h later, stained for eGFP, and stained with Hoechst 33342 to visualize nuclear morphology. The percentage of cells with condensed and fragmented nuclei was determined for each condition. Shown is one experiment performed in triplicate (*, p < 0.01). The experiment was performed three times with similar results. E, to confirm knockdown of endogenous Sh3rf2, C6 glioma cells were transfected with the indicated presynthesized siRNAs. Twenty hours later, cells were collected and subjected to immunoblot as indicated. The experiment was performed three times with similar results.
Surprisingly, unlike the case with POSH siRNA, which protects against death (17), expression of a Sh3rf2 siRNA (siSh3rf2) in neuronal PC12 cells promoted enhanced apoptosis upon NGF deprivation relative to a scrambled siRNA (supplemental Fig. S2). Moreover, siSh3rf2 reduced the survival of neuronal PC12 cells, even in the absence of a death stimulus as shown in Fig. 2B. To confirm that the reduced survival was a consequence of Sh3rf2 knockdown, we generated an shRNA plasmid (shSh3rf2) expressing an shRNA directed against a different region of Sh3rf2 (see “Experimental Procedures”) under control of the U6 promoter. shSh3rf2 also effectively knocks down Sh3rf2, as shown in Fig. 2A (lower panel). This construct also reduced survival of neuronal PC12 cells when compared with empty pU6 vector (Fig. 2C).
We next determined whether Sh3rf2 knockdown promotes apoptosis in another cell type. We examined nuclear morphology of proliferating rat C6 glioma cells expressing siSh3rf2. Co-transfection of pCMS-EGFP was used to identify transfected cells. siSh3rf2 significantly increased the percentage of EGFP+ cells with apoptotic nuclei 48 h after transfection (Fig. 2D). Transfection efficiency was ∼25% in these experiments. Similar results were also obtained with a second siRNA sequence, sihuSh3rf2 (see “Experimental Procedures,” data not shown). Supplemental Fig. S3 shows the efficiency of knockdown by sihuSh3rf2. Both siRNAs induced substantial knockdown of endogenous Sh3rf2 protein (Fig. 2E), despite the low transfection efficiency. Thus, loss of Sh3rf2 promotes death in at least two very different cell types and it therefore appears that endogenous Sh3rf2 has an anti-apoptotic role.
Knockdown of Sh3rf2 Promotes JNK Activation, Which Depends on POSH
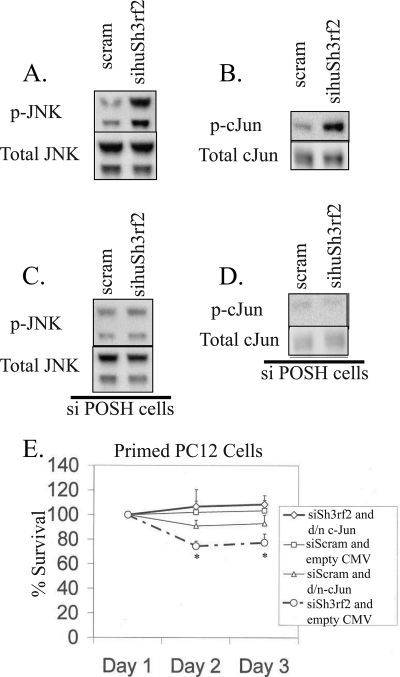
We next explored the mechanisms by which Sh3rf2 knockdown could promote cell death. We tested the hypothesis that endogenous Sh3rf2 might act to suppress JNK activation and that loss of Sh3rf2 would therefore promote activation of pro-apoptotic JNK signaling. To carry out this evaluation we turned to HEK293 cells, which permit high transfection efficiency and thus examination of endogenous JNKs after Sh3rf2 knockdown. After transfection of sihuSh3rf2 (or scrambled siRNA) into HEK293 cells, we performed immunoblots for activated (phospho-) JNK and total JNK. Co-transfection with pCMS-EGFP confirmed transfection efficiencies greater than 80% for each experiment (data not shown). As shown in Fig. 3A, sihuSh3rf2, but not the scrambled siRNA, induces JNK activation without affecting total levels of JNK protein. Similarly, sihuSh3rf2 activates the downstream transcription factor c-Jun as shown in Fig. 3B.
FIGURE 3.
Knockdown of Sh3rf2 promotes JNK and c-Jun activation in a POSH-dependent fashion. A, HEK293 cells were transfected with a siRNA targeting human Sh3rf2 (sihuSh3rf2) or scrambled siRNA. Lysates were collected 20 h later and subjected to immunoblotting using an antibody specific for phospho-JNK. Membranes were then reprobed for total JNK. B, HEK293 cells were treated as above and lysates were subjected to immunoblot using an antibody specific for phospho-c-Jun. Membranes were reprobed with an antibody detecting total c-Jun. C and D, HEK293 cells constitutively expressing siRNA targeting POSH (siPOSH cells) were treated as in A and B, respectively. Note the lack of JNK and c-Jun activation in these cells. E, primed PC12 cells were co-transfected with pCMS-EGFP and the indicated constructs in pCMV. Cells were transfected with the indicated synthetic siRNAs and transfected (EGFP+) cells were counted beginning 24 h later (Day 1). Shown is one experiment performed in triplicate. The experiment was performed three times with similar results (*, p < 0.01 relative to same time point for siSh3rf2 with dominant-negative c-Jun and siSCRAM with empty vector).
We next sought to determine whether such JNK activation requires POSH, which would implicate Sh3rf2 in antagonizing POSH under basal conditions. For these experiments we utilized HEK293 cells that constitutively express siRNA targeting POSH (siPOSH cells) and that consequently have significantly reduced expression of endogenous POSH (27). We examined wild-type (WT) and siPOSH cells transfected with sihuSh3rf2 or a scrambled siRNA for JNK and c-Jun activation. Transfection efficiency was again >80% as determined visually by EGFP positive cells. As shown in Fig. 3, C and D, activation of JNK and c-Jun by knockdown of Sh3rf2 was greatly reduced in the siPOSH cells. These findings thus support a model in which POSH is required for siSh3rf2-induced JNK activation.
Death Promoted by Knockdown of Sh3rf2 Is Mediated by c-Jun
If loss of Sh3rf2 activates the JNK pathway and promotes activation of c-Jun, then this suggests that death evoked by Sh3rf2 knockdown may be mediated by JNK/c-Jun signaling. To assess this possibility, we co-expressed dominant-negative c-Jun (or empty CMV vector) with pCMS·EGFP and siSh3rf2 in neuronal PC12 cells. Under these conditions, dominant-negative c-Jun completely restored cell survival to levels seen in cells expressing scrambled siRNA (Fig. 3E). Furthermore, a cell-permeable specific inhibitor of the JNK pathway (DJNKi1)(24) protects neuronal PC12 cells from apoptosis induced by siSh3rf2 (supplemental Fig. S4). Thus, knockdown of Sh3rf2 promotes POSH-dependent activation of JNK and c-Jun, which is required for induction of apoptosis. This raises the possibility that Sh3rf2 interacts with POSH to regulate pro-apoptotic JNK signaling.
POSH and Sh3rf2 Interact in Mammalian Cells
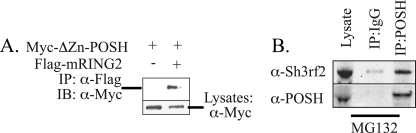
To determine whether Sh3rf2 physically interacts with POSH, we carried out co-immunoprecipitation experiments. A FLAG-tagged RING mutant of Sh3rf2 (RING2) (22), or empty vector was co-expressed in 293 cells with a Myc-tagged RING deletion mutant of POSH (ΔZn-POSH) (17). These RING mutant forms were used because they are much more stable than the wild-type forms, yet function similarly in other ways when overexpressed (17, 22). As shown in Fig. 4A, after immunoprecipitation with anti-Myc, RING2 was detected only in immunoprecipitates from cells co-expressing Myc-ΔZn-POSH. Reciprocal immunoprecipitation experiments confirmed this interaction (data not shown).
FIGURE 4.
Sh3rf2 and POSH physically interact in mammalian cells. A, Sh3rf2 co-immunoprecipitates (IP) with POSH. Myc-tagged ΔZn-POSH was co-expressed with a FLAG-tagged RING mutant of Sh3rf2 (mRING2) or empty pCMS-EGFP vector. Lysates were collected 20 h later, before significant death occurred. An aliquot of total lysate was reserved and the remaining sample was immunoprecipitated with anti-FLAG beads and the immunoprecpitates were subjected to Western immunoblotting and probed with anti-Myc. Lysates were also subjected to immunoblot for Myc to confirm equal expression of POSH under both conditions. B, mouse cortical neurons were treated with the proteasome inhibitor MG132 (20 μm) for 20 h. Cells were collected, lysed in immunoprecipitation (IP) buffer, and split into equal quantities for immunoprecipitation with anti-POSH or mouse IgG. Immunoprecipitates were resolved by SDS-PAGE and immunoblots were performed for Sh3rf2. Total cell lysate was examined in parallel. The experiment was performed three times with similar results.
We next sought to determine whether endogenous (nontagged) Sh3rf2 and POSH interact by co-immunoprecipitation. Because both proteins are degraded by the proteasome, and therefore expressed at low levels under basal conditions (18) (and see Fig. 6 and supplemental Fig. S5), we treated cortical neurons with the proteasome inhibitor MG-132. 20 h later, cell lysates were divided into equal aliquots and subjected to immunoprecipitation with either monoclonal POSH antibody or an equal quantity of mouse IgG. As shown in Fig. 4B, a significant amount of endogenous Sh3rf2 was detected only in POSH immunocomplexes. Identical results were obtained from PC12 cells treated with MG-132 (data not shown).
FIGURE 6.
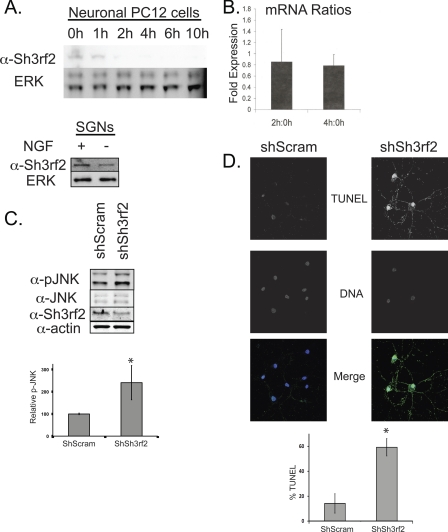
Decrease in Sh3rf2 protein is an early event in POSH-JNK-dependent apoptosis induced by trophic factor deprivation. A, top panel, PC12 cells were treated with NGF for 5–7 days and then subjected to NGF deprivation as described under “Experimental Procedures.” Cells were collected at the indicated times following treatment, and equal quantities were subjected to immunoblot as shown. Membranes were reprobed with anti-ERK2 to confirm equal loading. Bottom panel, sympathetic cervical ganglion neurons (SGNs) were treated with NGF deprivation as described under “Experimental Procedures.” Cells were collected 8 h after treatment and subjected to immunoblot as shown. B, after priming for 5 days, PC12 cells were subjected to NGF deprivation for 0, 2, or 4 h. mRNA was collected and quantitative PCR was performed as described under “Experimental Procedures.” The ratios of Sh3rf2 mRNA relative to a housekeeping gene, cyclophilin (PPIA), for each time pair were determined as indicated. C, cortical neurons were cultured for 14–21 days prior to treatment with lentivirus expressing shRNAs as indicated. 96 h later, cells were collected and subjected to immunoblot with the indicated antibodies. The same blot was reprobed serially. The Sh3rf2 band shown here runs at 55 kDa. shSh3rf2 induced a 2-fold increase of p-JNK when normalized to total JNK. The graph shows combined results from 3 experiments (*, p < 0.002 relative to control shRNA). Nearly identical results were obtained when normalizing to actin (not shown). D, murine cortical neurons were treated identically to those in C. 5 days later, cells were fixed and TUNEL-labeled as described under “Experimental Procedures.” DNA (Hoechst 33342) and TUNEL (Alexa 488) images are shown in grey scale and the merged image shown in color. Note the marked increase in TUNEL positivity and contracted, fragmented nuclei in the Sh3rf2-treated neurons. Greater than 100 cells were counted per condition. This experiment was performed twice and the graph represents the results from one experiment performed in triplicate (*, p < 0.05 versus control shRNA).
Sh3rf2 Promotes Degradation of POSH under Basal (Survival) Conditions
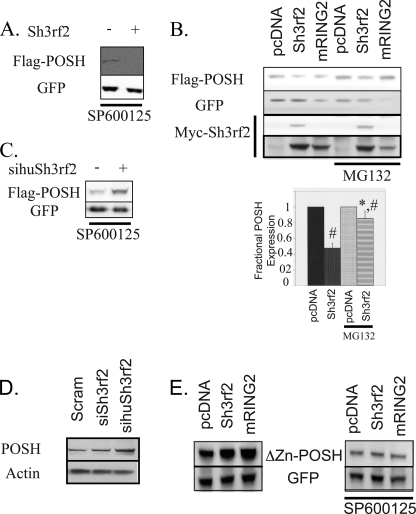
The present and past data indicate that 1) knockdown of Sh3rf2 promotes POSH-dependent JNK activation; 2) POSH and Sh3rf2 interact physically; 3) Sh3rf2 has a putative E3 ligase activity (22); and 4) POSH overexpression drives JNK and c-Jun activation and cell death (17). We therefore hypothesized that Sh3rf2 might promote the degradation of POSH under basal conditions, thereby preventing POSH accumulation and consequent JNK activation and apoptosis. To assess this possibility, we co-expressed pCMS·EGFP·POSH with pcDNA·Sh3rf2 in HEK293 cells. This was carried out in the presence of the JNK inhibitor SP600125 to prevent stabilization of POSH via the previously identified JNK-dependent feed-forward loop (18). Under these conditions, levels of POSH expression were dramatically reduced compared with cells co-expressing empty pcDNA (Fig. 5A). Blot membranes were reprobed for eGFP expression, which indicated similar transfection efficiencies and cell survival under all conditions (Fig. 5A).
FIGURE 5.
Sh3rf2 reduces POSH levels under basal conditions by a proteasome-dependent mechanism, which requires the RING domain of both proteins. A, FLAG-tagged, wild-type POSH in pCMS·EGFP was co-expressed in HEK293 cells with either Sh3rf2 in the pcDNA3.1 vector or pcDNA3.1 alone. Cells were collected 20 h later and subjected to immunoblotting for FLAG. Membranes were reprobed for GFP to confirm equal transfection. SP600125 (20 μm) was added to prevent JNK activation by overexpressed POSH and self-stabilization of POSH. B, FLAG-tagged POSH in pCMS·EGFP was expressed in HEK293 cells with the indicated cDNAs in the pcDNA3.1 vector. The experiment was again performed in the presence of SP600125 (20 μm), and MG132 (20 μm) was added as indicated 20 h after transfection. Cells were collected 6 h after the addition of MG132. Equal quantities of cell lysate were subjected to immunoblotting for FLAG. Membranes were reprobed for GFP to confirm equal transfection efficiency and loading. Membranes were then reprobed for Myc to determine expression of tagged Sh3rf2. The lower panel shows a darker exposure of the Myc blot to show expression levels of the RING mutant. The graph at the bottom shows the results from three consecutive experiments. Band intensity in each case was determined, with POSH expression in the presence of pcDNA being set to 1. EGFP intensity was used to control for transfection efficiency and loading. The marked reduction in GFP in lanes containing RING2 is due to enhanced cell death in these cells. C, FLAG-tagged, wild-type POSH in pCMS-EGFP was expressed in HEK293 cells. Cells were treated with siRNA targeting human Sh3rf2 (sihuSh3rf2) or control siRNA in the presence of SP600125 (20 μm). Cells were collected 20 h later and subjected to immunoblot for FLAG. Membranes were reprobed for GFP to confirm equal transfection. D, HEK293 cells were treated with the indicated synthetic siRNAs in the presence of SP600125 (20 μm) for 20 h. Equal quantities of lysates were subjected to immunoblot for endogenous POSH. Membranes were reprobed for β-actin to ensure equal loading. E, Myc-tagged POSH lacking the RING domain (ΔZn-POSH) in pCMS-EGFP was co-expressed with the indicated cDNAs in the pcDNA3.1 vector in the presence or absence of SP600125 (20 μm). Lysates were collected 20 h later and subjected to Western immunoblot as indicated. Membranes were reprobed with anti-GFP to confirm equal loading and transfection.
Because POSH is regulated by the proteasome, and the tagged protein was expressed under control of the CMV promoter of the vector, this suggests the Sh3rf2 promotes the degradation (rather than blocking the transcription) of POSH under basal conditions. To confirm a role of the proteasome in degradation of POSH by Sh3rf2, we repeated this experiment in the presence and absence of the proteasome inhibitor MG132. MG132 was added for 6 h beginning 20 h after transfection to allow near equilibrium as the half-life is less than 1 h of POSH.5 As shown in Fig. 5B, MG132 treatment markedly reduced the effects of Sh3rf2 on POSH protein levels. We performed three consecutive experiments and in each the expression of POSH (normalized to GFP to control for transfection efficiency and surviving cell number) co-expressed with empty vector was used as control (100% expression). In the presence of Sh3rf2, POSH levels are reduced to 48% of levels in the presence of vector alone (95% CI, 41–55%) (Fig. 5B, lower panel). However, when MG132 was added for 6 h, POSH levels in the presence of Sh3rf2 were 85% of control levels (95% CI, 74–96%). This was not due to decreased expression of Sh3rf2 as levels of Sh3rf2 were slightly increased in the presence of MG132 (1.7 ± 0.6-fold), consistent with its own proteasomal turnover (22).
If Sh3rf2 acts to promote POSH turnover, then loss of Sh3rf2 should in turn result in POSH accumulation. To determine whether endogenous Sh3rf2 performs such a function, we examined the effects of siSh3rf2 on levels of tagged POSH expressed in HEK293 cells. Once again, these experiments were performed in the presence of the JNK inhibitor SP600125 (to prevent POSH stabilization via the feed-forward loop) and the EGFP signal was used to control for transfection efficiency and cell survival. As shown in Fig. 5C, siSh3rf2 promotes increased POSH expression compared with scrambled siRNA.
We also examined levels of endogenous POSH protein in HEK293 cells treated with scrambled siRNA, an siRNA (siSh3rf2) that targets only rat (and not human) Sh3rf2 or an siRNA targeting human Sh3rf2 (sihuSh3rf2), which is expected to decrease endogenous Sh3rf2 in the human-derived HEK293 cells. Co-transfection of pCMS·EGFP confirmed greater than 80% efficiency in all cases. Fig. 5D shows that knockdown of human Sh3rf2 promotes increased levels of endogenous POSH protein. Blot membranes were reprobed for actin to confirm equal levels of total protein under each condition.
Destabilization of POSH in the Presence of Sh3rf2 Requires RING Domain of Both Proteins
The RING domains of POSH and Sh3rf2 both possess putative E3 ligase activity. To assess whether reduction of POSH protein levels by Sh3rf2 might require the latter's RING domain, we co-expressed wild-type POSH with a mutant of Sh3rf2 (mRING2) lacking a functional RING domain or with empty vector. As shown in Fig. 5B, under these conditions and in contrast to findings with wild-type Sh3rf2, POSH expression levels did not decrease (and somewhat increased). The lower GFP protein levels observed in cells co-expressing POSH and mRING2 reflect a marked reduction in cell survival, which was confirmed by visual inspection. Thus, the increase in POSH protein is even more dramatic when this is taken into account. This suggests that the RING domain of Sh3rf2 plays an important role in reducing POSH protein levels. Not surprisingly, there is no effect of the proteasome inhibitor MG132 under these conditions (Fig. 5B, far right lane). In addition, neither wild-type Sh3rf2, nor the RING mutant decreased expression of mutant POSH protein with a deleted RING domain (ΔZnPOSH) (Fig. 5E). Thus it appears that the RING domains of both proteins participate in promotion of POSH degradation by Sh3rf2.
Sh3rf2 Is Rapidly Degraded following Trophic Factor Deprivation
As Sh3rf2 appears to regulate cell survival through its effects on POSH levels, we hypothesized that loss of Sh3rf2 might occur in death paradigms involving POSH. We therefore subjected neuronal PC12 cells to trophic factor deprivation as previously described (17) and monitored Sh3rf2 expression. Endogenous Sh3rf2 levels decreased within 1–2 h following NGF deprivation and were nearly undetectable beyond this time (Fig. 6A, top panel). Consistent with the idea that Sh3rf2 degrades POSH under basal conditions, the time of Sh3rf2 loss is earlier than that previously reported for POSH stabilization following NGF deprivation (19).
To confirm similar events in primary neurons, we next examined Sh3rf2 levels in sympathetic ganglion neurons at several time points following NGF deprivation. Although Sh3rf2 remained detectable at all time points examined, levels are reduced as early as 6 h after NGF deprivation (Fig. 6A, bottom, shows data at 8 h of NGF deprivation). Reduction in Sh3rf2 levels was observed at 8, 10, and 16 h of trophic factor deprivation; levels were reduced on average to 57 ± 14% of basal (n = 3, 95% CI: 41–73%). A similar loss of Sh3rf2 occurred in cortical neurons treated with the DNA damaging agent camptothecin, a neuron death model previously shown to involve POSH-dependent JNK activation (18) (supplemental Fig. S5).
The rapid decrease in Sh3rf2 following NGF withdrawal suggests a post-translational mechanism. However, because Sh3rf2 is itself degraded by the proteasome under basal conditions (22), a decrease in the corresponding mRNA levels might result in a rapid fall of cellular Sh3rf2 protein. We therefore used quantitative PCR to determine whether Sh3rf2 mRNA levels decrease following trophic factor deprivation of neuronal PC12 cells. Cells were examined 2 and 4 h after NGF deprivation, times corresponding to partial and complete reduction in the Sh3rf2 protein (Fig. 6A). As shown in Fig. 6B, Sh3rf2 mRNA levels were not significantly changed from baseline at both time points, supporting a post-translational mechanism of POSH degradation.
These observations are consistent with a model in which Sh3rf2 is degraded in response to apoptotic stimuli, leading to POSH stabilization and subsequent JNK activation and apoptosis. To determine whether knockdown of Sh3rf2 promotes JNK activation and death in primary neurons as predicted by this model, we examined cultured cortical neurons infected with lentivirus expressing shRNAs. As shown in Fig. 6C, neuron cultures infected with shSh3rf2-expressing lentivirus had a significant increase in phosphorylated JNK as determined by Western blot when compared with neurons transduced with lentivirus expressing a nonsilencing shRNA. Furthermore, treatment with shSh3rf2-epxressing lentivurus also increased the number of TUNEL-positive (apoptotic) neurons (Fig. 6D). This occurs despite a relatively modest overall reduction in Sh3rf2 protein levels (Fig. 6C). Similar results on neuronal survival were obtained using adenovirus expressing a different Sh3rf2-targeting shRNA sequence (supplemental Fig. S6). These data confirm that reduction of cellular Sh3rf2, as occurs following a death stimulus, is sufficient to promote activation of the pro-apoptotic JNK-POSH pathway and cell death in primary cortical neurons.
DISCUSSION
Several scaffold proteins interact with the JNK pathway to regulate JNK activation and cell survival in different paradigms. The JIPs and POSH have been shown to enhance JNK signaling and promote apoptosis in neuronal and nonneuronal cells, at least following certain apoptotic stimuli. Interestingly, some research has suggested that JIP1 is anti-apoptotic in certain settings (28). Thus, not only are levels of individual proteins in the JNK pathway tightly regulated, their expression relative to one another may also determine cell fate. Here we have identified another protein that regulates apoptosis through the JNK pathway, albeit with a different mechanism than for those previously described.
Although initial work suggested that overexpression of Sh3rf2 promotes JNK activation and apoptosis (22), siRNA-mediated knockdown revealed an anti-apoptotic function of endogenous Sh3rf2. Furthermore, knockdown of Sh3rf2 promotes stabilization of POSH as well as JNK activation. This appears to be the mechanism by which Sh3rf2 knockdown promotes apoptosis as dominant-negative c-Jun protects cells following Sh3rf2 knockdown. The lack of JNK and c-Jun phosphorylation by siSh3rf2 in HEK293 cells lacking POSH also supports this model. Although this suggests that the basal function of Sh3rf2 in cells is to inhibit apoptosis, this does not exclude the possibility that induction of extremely high levels of Sh3rf2 protein could also promote apoptosis. Other proteins have been identified that promote apoptosis with both high and absent levels (e.g. Omi/HtrA2)(29). Whether high levels of Sh3rf2 are induced with specific pro-apoptotic stimuli remains an active area of investigation in our laboratory.
Levels of pro-apoptotic proteins must be tightly controlled to prevent unregulated apoptosis. This is particularly important for proteins such as POSH, which are able to amplify the apoptotic signal once activated (18). POSH is proteolytically cleaved by the proteasome; a function that is dependent on its own RING domain. The RING domain of Sh3rf2, which regulates its own proteasomal degradation, also promotes the degradation of POSH. The RING domain of Sh3rf2 therefore has two potential anti-apoptotic functions. First, it keeps basal levels of POSH below the apoptotic threshold. Second, it may prevent excessive levels of Sh3rf2 expression, which also promote JNK activation and apoptosis in unstressed cells. Interestingly, Sh3rf2 is unable to degrade POSH lacking a functional RING domain. The mechanism by which these two RING domains interact to promote degradation of POSH requires further investigation. However, our results indicate that the two proteins are capable of physical interaction in the absence of both RING domains (Fig. 4A).
The degradation of Sh3rf2 at early time points following trophic factor deprivation of PC12 cells strongly suggests that it is responsible for POSH stabilization in this model. We previously showed that siRNA-mediated knockdown of POSH protects these cells. Although it would be ideal to show that preventing Sh3rf2 degradation is also protective, the death invoked by overexpression of Sh3rf2 prohibits these experiments. Future work will determine the mechanism(s) by which Sh3rf2 is degraded in this model. This should allow blockade of degradation to determine its effect on cell survival. Another approach would be to determine whether specific Sh3rf2 mutants do not promote apoptosis when overexpressed, and to examine whether they can protect in trophic factor deprivation. Ongoing work in our lab is aimed at identifying such Sh3rf2 mutants.
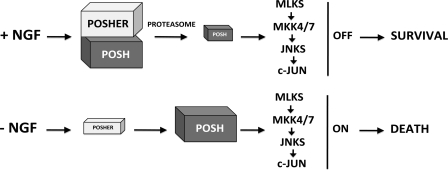
Our work identifies, to our knowledge, the first anti-apoptotic JNK scaffold. Sh3rf2 promotes the proteolytic degradation of POSH under basal conditions, and therefore prevents unrestrained JNK activation and apoptosis. We propose the model shown in Fig. 7 for the regulation of POSH by Sh3rf2. In the presence of NGF (top), Sh3rf2 promotes the proteasomal degradation of POSH and thereby prevents activation of pro-apoptotic JNK signaling. Once trophic factor is removed (Fig. 7, bottom), levels of Sh3rf2 decrease, promoting increased POSH protein, formation of the POSH·JIP apoptotic complex (PJAC), and JNK-dependent apoptosis. Because Sh3rf2 appears to limit POSH levels under basal conditions, we now propose that this protein be referred to as POSHER (POSH-eliminating RING protein). Importantly, degradation of Sh3rf2/POSHER appears to play a role in trophic factor deprivation-induced death of neuronal PC12 cells and sympathetic ganglion cells and in camptothecin-induced death of cortical neurons, presumably through stabilization and elevation of cellular POSH levels.
FIGURE 7.
Model for regulation of POSH-dependent JNK signaling by Sh3rf2.
Our findings indicate that basal levels of Sh3rf2/POSHER are required to maintain survival of neuronal PC12 cells as well as of cultured cortical neurons. In addition to neuronal cells, we found that Sh3rf2/POSHER regulates cell survival of a second cell type derived from a glioma. Moreover, loss of Sh3rf2/POSHER also regulates POSH levels and JNK activity in embryonic kidney cells. Prior observations (22) indicated that POSH and Sh3rf2/POSHER are widely expressed in various tissues, thus suggesting that these may regulate JNK pathway signaling and cell survival/death in a variety of cell types and conditions. Future work will be needed to determine whether Sh3rf2/POSHER has physiologic functions other than antagonizing pro-apoptotic JNK signaling by POSH.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants 5RO1NS033689 and 5PO1 NS38370 (to L. A. G.) and National Basic Research Program of China 973 Program number 2006CB504100/2006CB500701 (to Z. X.).

This article contains supplemental Figs. S1–S6.
M. Wilhelm, unpublished data.
- SH3
- Src homology domain 3
- MLK
- mixed lineage kinase
- JIP
- JNK-interacting protein.
REFERENCES
- 1. Chang L., Karin M. (2001) Mammalian MAP kinase signalling cascades. Nature 410, 37–40 [DOI] [PubMed] [Google Scholar]
- 2. Bruckner S. R., Tammariello S. P., Kuan C. Y., Flavell R. A., Rakic P., Estus S. (2001) JNK3 contributes to c-Jun activation and apoptosis but not oxidative stress in nerve growth factor-deprived sympathetic neurons. J. Neurochem. 78, 298–303 [DOI] [PubMed] [Google Scholar]
- 3. Kuan C. Y., Whitmarsh A. J., Yang D. D., Liao G., Schloemer A. J., Dong C., Bao J., Banasiak K. J., Haddad G. G., Flavell R. A., Davis R. J., Rakic P. (2003) A critical role of neural-specific JNK3 for ischemic apoptosis. Proc. Natl. Acad. Sci. U.S.A. 100, 15184–15189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maroney A. C., Finn J. P., Bozyczko-Coyne D., O'Kane T. M., Neff N. T., Tolkovsky A. M., Park D. S., Yan C. Y., Troy C. M., Greene L. A. (1999) CEP-1347 (KT7515), an inhibitor of JNK activation, rescues sympathetic neurons and neuronally differentiated PC12 cells from death evoked by three distinct insults. J. Neurochem. 73, 1901–1912 [PubMed] [Google Scholar]
- 5. Kuan C. Y., Yang D. D., Samanta Roy D. R., Davis R. J., Rakic P., Flavell R. A. (1999) The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22, 667–676 [DOI] [PubMed] [Google Scholar]
- 6. Yang D. D., Kuan C. Y., Whitmarsh A. J., Rincón M., Zheng T. S., Davis R. J., Rakic P., Flavell R. A. (1997) Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 389, 865–870 [DOI] [PubMed] [Google Scholar]
- 7. Wang J., Van De Water T. R., Bonny C., de Ribaupierre F., Puel J. L., Zine A. (2003) A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J. Neurosci. 23, 8596–8607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pirianov G., Brywe K. G., Mallard C., Edwards A. D., Flavell R. A., Hagberg H., Mehmet H. (2007) Deletion of the c-Jun N-terminal kinase 3 gene protects neonatal mice against cerebral hypoxic-ischaemic injury. J. Cereb. Blood Flow Metab. 27, 1022–1032 [DOI] [PubMed] [Google Scholar]
- 9. Choi W. S., Yoon S. Y., Oh T. H., Choi E. J., O'Malley K. L., Oh Y. J. (1999) Two distinct mechanisms are involved in 6-hydroxydopamine- and MPP+-induced dopaminergic neuronal cell death. Role of caspases, ROS, and JNK. J. Neurosci. Res. 57, 86–94 [DOI] [PubMed] [Google Scholar]
- 10. Kuan C. Y., Burke R. E. (2005) Targeting the JNK signaling pathway for stroke and Parkinsons diseases therapy. Curr. Drug Targets CNS Neurol. Disord. 4, 63–67 [DOI] [PubMed] [Google Scholar]
- 11. Troy C. M., Rabacchi S. A., Xu Z., Maroney A. C., Connors T. J., Shelanski M. L., Greene L. A. (2001) β-Amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J. Neurochem. 77, 157–164 [DOI] [PubMed] [Google Scholar]
- 12. Choi K. Y., Satterberg B., Lyons D. M., Elion E. A. (1994) Ste5 tethers multiple protein kinases in the MAP kinase cascade required for mating in S. cerevisiae. Cell 78, 499–512 [DOI] [PubMed] [Google Scholar]
- 13. Yasuda J., Whitmarsh A. J., Cavanagh J., Sharma M., Davis R. J. (1999) The JIP group of mitogen-activated protein kinase scaffold proteins. Mol. Cell. Biol. 19, 7245–7254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whitmarsh A. J., Kuan C. Y., Kennedy N. J., Kelkar N., Haydar T. F., Mordes J. P., Appel M., Rossini A. A., Jones S. N., Flavell R. A., Rakic P., Davis R. J. (2001) Requirement of the JIP1 scaffold protein for stress-induced JNK activation. Genes Dev. 15, 2421–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dickens M., Rogers J. S., Cavanagh J., Raitano A., Xia Z., Halpern J. R., Greenberg M. E., Sawyers C. L., Davis R. J. (1997) A cytoplasmic inhibitor of the JNK signal transduction pathway. Science 277, 693–696 [DOI] [PubMed] [Google Scholar]
- 16. Tapon N., Nagata K., Lamarche N., Hall A. (1998) A new rac target POSH is an SH3-containing scaffold protein involved in the JNK and NF-κB signalling pathways. EMBO J. 17, 1395–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu Z., Kukekov N. V., Greene L. A. (2003) POSH acts as a scaffold for a multiprotein complex that mediates JNK activation in apoptosis. EMBO J. 22, 252–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu Z., Kukekov N. V., Greene L. A. (2005) Regulation of apoptotic c-Jun N-terminal kinase signaling by a stabilization-based feed-forward loop. Mol. Cell. Biol. 25, 9949–9959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kukekov N. V., Xu Z., Greene L. A. (2006) Direct interaction of the molecular scaffolds POSH and JIP is required for apoptotic activation of JNKs. J. Biol. Chem. 281, 15517–15524 [DOI] [PubMed] [Google Scholar]
- 20. Zhang Q. G., Wang R. M., Yin X. H., Pan J., Xu T. L., Zhang G. Y. (2005) Knock-down of POSH expression is neuroprotective through down-regulating activation of the MLK3-MKK4-JNK pathway following cerebral ischaemia in the rat hippocampal CA1 subfield. J. Neurochem. 95, 784–795 [DOI] [PubMed] [Google Scholar]
- 21. Kim G. H., Park E., Kong Y. Y., Han J. K. (2006) Novel function of POSH, a JNK scaffold, as an E3 ubiquitin ligase for the Hrs stability on early endosomes. Cell. Signal. 18, 553–563 [DOI] [PubMed] [Google Scholar]
- 22. Wilhelm M., Kukekov N. V., Xu Z., Greene L. A. (2007) Identification of POSH2, a novel homologue of the c-Jun N-terminal kinase scaffold protein POSH. Dev. Neurosci. 29, 355–362 [DOI] [PubMed] [Google Scholar]
- 23. Kärkkäinen S., Hiipakka M., Wang J. H., Kleino I., Vähä-Jaakkola M., Renkema G. H., Liss M., Wagner R., Saksela K. (2006) Identification of preferred protein interactions by phage-display of the human Src homology-3 proteome. EMBO Rep. 7, 186–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borsello T., Clarke P. G., Hirt L., Vercelli A., Repici M., Schorderet D. F., Bogousslavsky J., Bonny C. (2003) A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 9, 1180–1186 [DOI] [PubMed] [Google Scholar]
- 25. Xu Z., Sproul A., Wang W., Kukekov N., Greene L. A. (2006) Siah1 interacts with the scaffold protein POSH to promote JNK activation and apoptosis. J. Biol. Chem. 281, 303–312 [DOI] [PubMed] [Google Scholar]
- 26. Westmark C. J., Malter J. S. (2007) FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 5, e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilhelm M., Xu Z., Kukekov N. V., Gire S., Greene L. A. (2007) Proapoptotic Nix activates the JNK pathway by interacting with POSH and mediates death in a Parkinson disease model. J. Biol. Chem. 282, 1288–1295 [DOI] [PubMed] [Google Scholar]
- 28. Dong Z., Zhou L., Del Villar K., Ghanevati M., Tashjian V., Miller C. A. (2005) JIP1 regulates neuronal apoptosis in response to stress. Mol. Brain Res. 134, 282–293 [DOI] [PubMed] [Google Scholar]
- 29. Vande Walle L., Lamkanfi M., Vandenabeele P. (2008) The mitochondrial serine protease HtrA2/Omi. An overview. Cell Death Differ. 15, 453–460 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.