Abstract
Successful pathogens are equipped to exploit the signaling pathways of their host cell to establish a niche conducive for their survival and proliferation. One emerging example is the modulation of the small GTPase Rab1 by virulence factors of the intracellular pathogen Legionella pneumophila. Besides proteins that mimic host regulatory factors involved in controlling Rab1 activity, this bacterium temporally locks this small GTPase in its active form by AMPylation. Efficient release of Rab1 from the bacterial phagosome requires deAMPylation prior to being inactivated by the bacterial GAP protein LepB. Whether Rab activity is similarly regulated under native condition is unknown, but it is clear that virulence factors from pathogens can be invaluable tools in dissecting the intricacy of host cellular processes.
Key words: Legionella pneumophila, effectors, AMPylation, post-translational modification, deAMPylation
Virulence factors produced by pathogenic bacteria contribute significantly to the symptoms of diseases caused by the bacteria. L. pneumophila is a parasite of amoebae, which are professional phagocytes that prey on bacteria.1 Amoeba is believed to be the training site for the pathogenicity of L. pneumophila, which conditions the bacterium with the necessary traits to cause Legionnaires' disease in susceptible individuals following colonization in the alveolar macrophages.2 Among the many host processes modulated by L. pneumophila, the manipulation of membrane trafficking pathways arguably is the best understood.3 Right after entry by phagocytosis, the Legionella containing vacuole (LCV) undergoes efficient membrane remodeling to convert the plasma membranes into membranes with properties resembling those of the endoplasmic reticulum (ER).4 Successful membrane remodeling by L. pneumophila is achieved by the functions of protein substrates of a specialized protein translocation system termed Dot/Icm (deficiency in organelle trafficking or intracellular multiplication).5 The Dot/Icm machinery is a member of type IV secretion systems, which are believed to be adapted from bacterial conjugation apparatuses.6 Whereas type IV secretion systems are the essential pathogenic determinant in many pathogens,6,7 the Dot/Icm system is distinctive by its extremely large substrate repertoire of more than 270 proteins.8
Rab1 is one of the several host proteins found to be enriched on the LCV in the early phase of L. pneumophila infection.9,10 Further, inhibition of Rab1 activity impaired intracellular growth of L. pneumophila, suggesting an important role of this protein in the intracellular life cycle of this pathogen.11 Investigations in the last several years have revealed that L. pneumophila employs specific proteins to control each step of the Rab1 activation cycle. Among these, the SidM/DrrA protein dominates the process by several distinctive activities. By binding to phosphatidylinositol-4-phosphate,12 this protein is associated with the LCV to which it recruits Rab1 by its activity as a GDI displacement factor (GDF) and probably by its high binding affinity to this small GTPase.13,14 Second, SidM/DrrA functions as GEF to activate Rab1.15,16 As infection proceeds to about 2 h after bacterial uptake, the bacterial GAP protein LepB begins to kick in to inactivate Rab1 and facilitate its removal from the LCV.13 Controlling Rab1 activity by these proteins is believed to function in parallel with yet unidentified pathways to facilitate membrane remodeling of the LCV by redirecting trafficking vesicles originating from the ER to the bacterial phagosome.3
The most potent pathogenic determinants enter host cells and covalently modify target substrates within the cytosol, leading to alternations in host cell physiology and the establishment of a successful infection. Thus, it is fulfilling to discover that L. pneumophila covalently modifies Rab1 by AMPylation.17 First found in the VopS protein of Vibrio parahaemolyticus,18 Fic domain-dependent post-translational modification (PTM) of proteins by AMPylation is a newly identified pathogenic strategy employed by bacterial pathogens.19 Further, the fact that the mammalian protein HYPE contains a functional Fic domain indicates that this PTM is a signaling mechanism in eukaryotic cells.20 Adding to this excitement is the discovery that SidM/DrrA contains the GX11DXD signature resembling the catalytic motif of the glutamine synthetase adenylyl transferase (GS-ATase) and its ability to AMPylate tyrosine 77 within the switch II region of Rab1.17 This finding reveals that non-Fic domain proteins can catalyze the AMPylation reaction, suggesting potentially wider utilization of this posttranslational modification in cell signaling. So far, all characterized AMPylated proteins from eukaryotic cells are small GTPases, and in each case, the modification interferes with the enzymatic activity of the modified proteins or their interactions with their cellular binding partners, or both.19,21 Probably because of the steric hindrance caused by the bulky AMP moiety, AMPylated Rab1 became inaccessible to GAP proteins such as the Legionella protein LepB and thus was locked into its active GTP binding form17 (Fig. 1A). Keeping Rab1 in its active form for several hours during L. pneumophila infection may allow the bacterial phagosome to maximally receive trafficking vesicles for membrane materials. Alternatively, AMPylation may prevent the activity of Rab1 from being interrupted by bacterial or host factors, such as the eukaryotic GAP TBC1D20.17
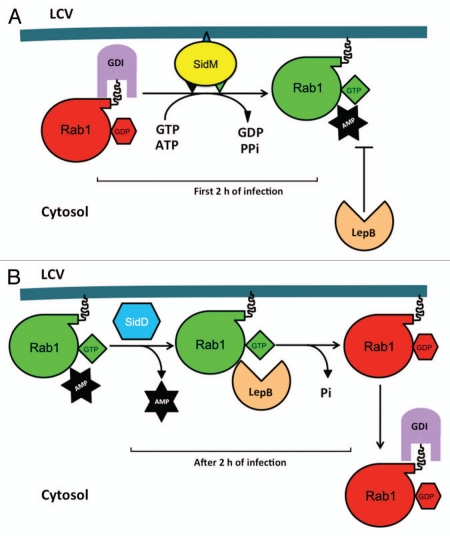
Figure 1.
A model for the regulation of Rab1 activity by Legionella proteins. (A) SidM associated with the LCV membrane extracts Rab1 from the Rab1-GDI complex and subsequently activates Rab1 by its GEF and AMPylation activity. AMPylated Rab1 becomes inaccessible to GAP proteins such as LepB and TBC1D20, thereby is locked into its active state. (B) The deAMPylase SidD removes the AMP moiety from Rab1, making it accessible to LepB, which stimulates its GTPase activity, leading to the extraction of the inactive Rab1 from the bacterial phagosome by an unknown GDI. The time frame for each event occurring during infection is indicated.
Rab1 begins to dissociate from the LCV within 4 h of bacterial uptake, presumably becomes sequestered by a host GDP association inhibitor (GDI) after being inactivated by LepB.13 The fact that LepB cannot inactivate AMPylated GTP-Rab1 suggested the need for an enzyme to remove the AMP moiety.17 Two research groups have independently identified the deAM-Pylase involved in this process by very different methods.22,23 By the observation that total cell lysate of L. pneumophila was able to remove the AMP moiety from Rab1 and gene synteny of the sidM/drrA/sidD locus on several sequenced Legionella genomes, Machner and colleagues identified sidD,24 which localizes next to sidM/drrA, as the gene coding for the deAMPylase.23 Our discovery of SidD was driven by the search of L. pneumophila proteins capable of regulating SidM activity by screening from a random bacterial genomic library for genes that suppress the SidM cytotoxicity to yeast.22 SidD efficiently removes the AMP moiety from modified Rab122,23 as well as several members of the Rab family.22 Consistent with the model in which deAMPylation of Rab1 is required for the inactivation of Rab1 and its subsequent removal from the bacterial phagosome, deletion of sidD caused a delay in the disassociation of Rab1 from the LCVs.22,23 The identification of SidD added another layer of regulation to the intricate modulation of Rab1 activity by L. pneumophila. Two hours after phagocytosis, as the amounts of translocated SidM start to decrease,23 the effects of SidD become apparent, leading to the inactivation of Rab1 and its subsequent removal from the LCV (Fig. 1B).
Despite the clear step-wise regulation, the mechanism of this temporal regulation is unknown. It is possible that expression of sidM/drrA and sidD by L. pneumophila is differently regulated when the bacterium begins to replicate or that the AMPylator is more susceptible to the host protein degradation machinery than SidD. Different sensitivity to host proteasome-mediated degradation has been attributed to the temporal regulation of Rac1/Cdc42 activity by the bacterial GEF SopE and the GAP SptP during Salmonella invasion.25 Interestingly, SidM contains an -L192PRY195-motif, which matches the recognition site (PPxY/LPxY) of NEDD4, a member of the HECT family of ubiquitin ligases.26 It is possible that ubiquitination plays a role in the regulation of SidM/DrrA activity during infection.
The association of Rab1 with the phagosome of the sidD mutant lasted for only two extra hours,22 suggesting the involvement of other bacterial or host factors in the inactivation of Rab1. Very often a post-translational modification introduced by a pathogen is to mimic a naturally occurring reaction, it will be interesting to investigate whether the activity of Rab1 is regulated by eukaryotic AMPylator or deAMPylase under normal physiological condition. Enzymes involved in such modifications may only be induced or activated when maximal Rab1 activity is required in certain tissues or cell types. Alternatively, the decreased abundance of active (and AMPylated) Rab1 on the LCV is a result of its intrinsic GTPase activity.
Because AMP was attached to the protein via a phosphodiester bond, the reaction is predicted to be reversible, probably by some phosphodiesterases.20 However, SidD does not share detectable homology with any phosphodiesterase. Consistently, SidD does not hydrolyze cyclic AMP, a molecule with an internal phosphodiester bond,23 suggesting that deAMPylation is catalyzed by a mechanism different from that of phosphodiesterases. In support of this notion, the predicted secondary structure of the N-terminal portion of SidD is similar to two protein phosphatases.22 Further, Asp residues at positions 92 and 110 of SidD conserved with residues critical for the biochemical activity of two phosphatases are essential for the deAMPylase activity.22 Thus, it is likely that deAMPylation is catalyzed by a mechanism similar to dephosphorylation. Further study, particularly the structural analysis of the protein complex formed between SidD and AMPylated Rab1 will surely be instrumental in determining the catalytic mechanism of this deAMPylase. Such information will also be useful in the identification of deAMPylases in other domains of organisms.
Acknowledgments
The work in our laboratory was supported by NIH-NIAID grants R01AI069344, K02AI085403 and R21AI092043.
Note
An editorial about this paper can be found online at: www.landesbioscience.com/journals/cellularlogistics/article/18984
References
- 1.Fields BS, Benson RF, Besser RE. Legionella and Legionnaires' disease: 25 years of investigation. Clin Microbiol Rev. 2002;15:506–526. doi: 10.1128/CMR.15.3.506-26.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swanson MS, Hammer BK. Legionella pneumophila pathogesesis: a fateful journey from amoebae to macrophages. Annu Rev Microbiol. 2000;54:567–613. doi: 10.1146/annurev.micro.54.1.567. [DOI] [PubMed] [Google Scholar]
- 3.Hubber A, Roy CR. Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol. 2010;26:261–283. doi: 10.1146/annurev-cellbio-100109-4034. [DOI] [PubMed] [Google Scholar]
- 4.Tilney LG, Harb OS, Connelly PS, Robinson CG, Roy CR. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J Cell Sci. 2001;114:4637–4650. doi: 10.1242/jcs.114.24.4637. [DOI] [PubMed] [Google Scholar]
- 5.Isberg RR, O'Connor TJ, Heidtman M. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol. 2009;7:13–24. doi: 10.1038/nrmicro1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christie PJ, Atmakuri K, Krishnamoorthy V, Jakubowski S, Cascales E. Biogenesis, architecture and function of bacterial type IV secretion systems. Annu Rev Microbiol. 2005;59:451–485. doi: 10.1146/annurev.micro.58.030603.123630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backert S, Meyer TF. Type IV secretion systems and their effectors in bacterial pathogenesis. Curr Opin Microbiol. 2006;9:207–217. doi: 10.1016/j.mib.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Zhu W, Banga S, Tan Y, Zheng C, Stephenson R, Gately J, et al. Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PLoS ONE. 2011;6:17638. doi: 10.1371/journal.pone.0017638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derré I, Isberg RR. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect Immun. 2004;72:3048–3053. doi: 10.1128/IAI.72.5.3048-53.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kagan JC, Stein MP, Pypaert M, Roy CR. Legionella subvert the functions of Rab1 and Sec22b to create a replicative organelle. J Exp Med. 2004;199:1201–1211. doi: 10.1084/jem.20031706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kagan JC, Roy CR. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol. 2002;4:945–954. doi: 10.1038/ncb883. [DOI] [PubMed] [Google Scholar]
- 12.Brombacher E, Urwyler S, Ragaz C, Weber SS, Kami K, Overduin M, et al. Rab1 guanine nucleotide exchange factor SidM is a major phosphatidylinositol-4-phosphate-binding effector protein of Legionella pneumophila. J Biol Chem. 2009;284:4846–4856. doi: 10.1074/jbc.M807505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingmundson A, Delprato A, Lambright DG, Roy CR. Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature. 2007;450:365–369. doi: 10.1038/nature06336. [DOI] [PubMed] [Google Scholar]
- 14.Machner MP, Isberg RR. A bifunctional bacterial protein links GDI displacement to Rab1 activation. Science. 2007;318:974–977. doi: 10.1126/science.1149121. [DOI] [PubMed] [Google Scholar]
- 15.Machner MP, Isberg RR. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell. 2006;11:47–56. doi: 10.1016/j.devcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol. 2006;8:971–977. doi: 10.1038/ncb1463. [DOI] [PubMed] [Google Scholar]
- 17.Müller MP, Peters H, Blumer J, Blankenfeldt W, Goody RS, Itzen A. The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science. 2010;329:946–949. doi: 10.1126/science.1192276. [DOI] [PubMed] [Google Scholar]
- 18.Yarbrough ML, Li Y, Kinch LN, Grishin NV, Ball HL, Orth K. AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science. 2009;323:269–272. doi: 10.1126/science.1166382. [DOI] [PubMed] [Google Scholar]
- 19.Woolery AR, Luong P, Broberg CA, Orth K. AMPylation: Something old is new again. Front Microbiol. 2010;1:113. doi: 10.3389/fmicb.2010.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Worby CA, Mattoo S, Kruger RP, Corbeil LB, Koller A, Mendez JC, et al. The fic domain: regulation of cell signaling by adenylylation. Mol Cell. 2009;34:93–103. doi: 10.1016/j.molcel.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yarbrough ML, Orth K. AMPylation is a new post-translational modiFICation. Nat Chem Biol. 2009;5:378–379. doi: 10.1038/nchembio0609-378. [DOI] [PubMed] [Google Scholar]
- 22.Tan Y, Luo ZQ. Legionella pneumophila SidD is a deAMPylase that modifies Rab1. Nature. 2011;475:506–509. doi: 10.1038/nature10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neunuebel MR, Chen Y, Gaspar AH, Backlund PS, Jr, Yergey A, Machner MP. De-AMPylation of the small GTPase Rab1 by the pathogen Legionella pneumophila. Science. 2011;333:453–456. doi: 10.1126/science.1207193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo ZQ, Isberg RR. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc Natl Acad Sci USA. 2004;101:841–846. doi: 10.1073/pnas.0304916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubori T, Galan JE. Temporal regulation of salmonella virulence effector function by proteasome-dependent protein degradation. Cell. 2003;115:333–342. doi: 10.1016/S0092-8674(03)00849-3. [DOI] [PubMed] [Google Scholar]
- 26.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009;10:398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]