Abstract
Bacterial pathogens are renowned cell biologists that subvert detrimental host responses by manipulating eukaryotic protein function. A select group of pathogens use a specialized type IV secretion system (T4SS) as a conduit to deliver an arsenal of proteins into the host cytosol where they interact with host proteins. The translocated “effectors” have garnered increased attention because they uncover novel aspects of host-pathogen interactions at the subcellular level. This review presents a group of effectors termed Anks that possess eukaryotic-like ankyrin repeat domains that mediate proteinprotein interactions and are critical for effector function. Interestingly, most known prokaryotic Anks are produced by bacteria that devote much of their time to replicating inside eukaryotic cells. Ank proteins represent a fascinating and versatile family of effectors exploited by bacterial pathogens and are proving useful as tools to study eukaryotic cell biology.
Key words: ankyrin repeat, Ank, intracellular pathogen, type IV secretion, effector
Ankyrin Repeat Domain-Containing Proteins
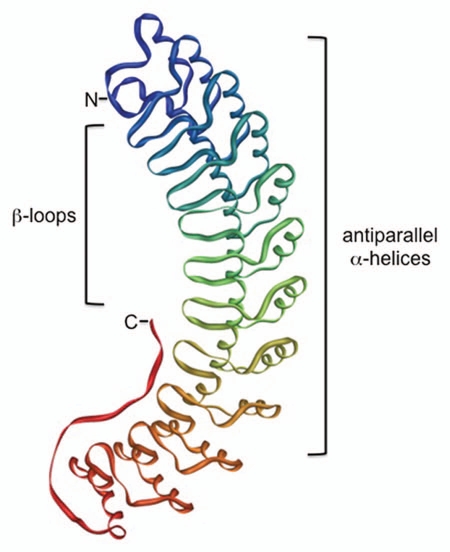
Ankyrin repeat domains are one of the most common protein domains historically associated with eukaryotic organisms. Proteins containing these repeats are referred to as Anks and mediate many cellular processes including cell cycle progression, transcription and cytoskeletal organization.1 Anks have also been implicated in significant human diseases including tumor formation and progression.2 The first Anks described were yeast cell cycle proteins and the Drosophila NOTCH protein.2 The repeat's name is derived from the cytoskeletal protein ankyrin, which contains 22 repeats.3 A typical ankyrin repeat consists of 33-residue repeating segments that adapt helix-turn-helix confirmations and comprise antiparallel α-helices. A group of these regions are collectively arranged in a curved concave structure with exposed flexible loop regions (Fig. 1).4 The overall Ank structure allows for incredible versatility in the types of protein-protein interactions the molecule can direct. Loop regions in each repeat vary greatly in composition to provide binding specificity while the helices provide support for the overall curved structure of the protein. Thus, Anks can direct many diverse, yet specific, protein-protein interactions. Stacking of individual repeats gives the overall Ank structure a flexible and elastic nature that functions as a spring, providing the versatility required to mediate these interactions.5 Two repeats are necessary for a functional protein-protein interaction platform as one repeat cannot fold properly.6
Figure 1.
Ank protein structure. Anks consist of repeating regions that adapt a helix-turn-helix configuration containing antiparallel α-helices. A series of repeats stack into a spring-like curved structure, with β-loops in each turn playing a crucial role in mediating protein-protein interactions. The example shown is a 12-repeat region of mammalian Ankyrin-R, which was solved by Michaely et al.49 The structure was rendered with iMol50 using Protein Data Bank coordinates 1N11.
Bacterial Anks
Although originally described as eukaryote-specific, many recent reports have demonstrated the utility of Anks in bacterial infections, particularly those caused by intracellular pathogens. Species that encode Anks include Legionella pneumophila, Anaplasma phagocytophilum, Coxiella burnetii, Rickettsia spp and Orientia tsutsugamuchi. The unifying feature of these pathogens is their ability to infect and replicate within eukaryotic cells, in some cases, in an obligatory fashion. Anaplasma, Rickettsia and Orientia each require a host cell for their infectious cycle and cannot currently be cultured in axenic medium. Due to their close association with host cells, intracellular bacteria adeptly alter a wide range of host cellular processes to establish a niche that supports replication. One mechanism by which these pathogens manipulate their host cell is through the use of a type IV secretion system (T4SS). T4SSs are versatile multi-protein complexes that translocate a diverse panel of bacterial proteins, termed “effectors,” directly into the host cytosol where they interact with host components and are often required for efficient infection.7
Interestingly, the pathogens mentioned above share very few, if any, common effectors. However, co-evolution of intracellular bacteria with their eukaryotic hosts has resulted in incorporation of several common eukaryotic-like motifs/domains into bacterial effectors.8 This is predicted to arise from interdomain horizontal gene transfer between bacteria and their hosts. Commonly incorporated eukaryotic domains include ankyrin repeats, coiled coils and tetratricopeptide repeats, which regulate protein-protein interactions in eukaryotic systems. Following translocation into the eukaryotic cytoplasm, effectors are predicted to bind specifically to and alter the activity of a host protein(s). Therefore, bacteria have shrewdly exploited eukaryotic domains, in particular ankyrin repeats, to control host responses to infection. Use of the ankyrin repeat allows the bacterial effector to promote specific interactions with a host protein, followed by alteration of host activity either by the simple recruitment or mislocalization of the protein or via activity of a separate domain present in the effector. Many examples now exist demonstrating bacterial control of infection events using Ank effector proteins (Table 1).9 Three intracellular pathogen examples are provided below.
Table 1.
Ank T4SS effectors with known functions
| Ank | Number of repeats | Other domains/motifs | Interacting host protein | Function | Pathogen host cell | References |
| Anaplasma | ||||||
| AnkA | 11 | EPIYA motif | SHP-1, Abi-1 | Binds eukaryotic DNA, influences CYBB expression | Neutrophil | 13–16 |
| Legionella | ||||||
| AnkB | 2 | F-box domain | Parvin B | Recruitment of ubiquitinated proteins to the Legionella-containing vacuole | 26, 28–30, 33, 34 | |
| AnkH AnkJ |
2 3 |
Unknown | Required for intracellular replication | Macrophage | 22 | |
| AnkX | 4 | FIC domain | Rab1 and Rab35 | Phosphocholine transferase that regulates Rab1 and Rab35 activity | 25 | |
| Coxiella | ||||||
| AnkG | 2 | p32 (gC1qR) | Inhibits apoptosis | Macrophage | 41 |
Anaplasma
AnkA from Anaplasma phagocytophilum was the first Ank described and characterized for an intracellular pathogen. Anaplasma spp are obligate intracellular pathogens that replicate in neutrophils and cause human anaplasmosis, a disease that is not typically life threatening, but causes a debilitating condition characterized by thrombocytopenia, malaise, fever and headache.10 Inside neutrophils, Anaplasma generates a host-derived membrane bound vacuole in which to replicate. However, the pathogen avoids the phagolysosomal maturation pathway prior to fusion with lysosomes, a condition that would degrade the bacterium.11 Phagosome maturation and other infection events are likely controlled by secreted T4SS effectors such as AnkA.
The combined efforts of three laboratories have shown that AnkA is a versatile effector with activity in the host cytoplasm and nucleus. Caturegli et al. reported the initial identification of AnkA as a protein with 11 ankyrin repeats that is recognized by sera collected from animals infected with A. phagocytophilum.12 Using immunoelectron microscopy, AnkA was observed in both the host cytoplasm and nucleus, suggesting the protein was secreted by Anaplasma during infection. This work was followed by experiments showing that AnkA binds specifically to eukaryotic DNA and three DNA-associated proteins, suggesting the effector influences host gene expression.13 Indeed, AnkA interacts with regulatory regions in host chromatin and specifically downregulates expression of CYBB, or gp91phox, which encodes a component of the phagocyte oxidase complex known to target Anaplasma during intracellular growth.14 AnkA activity also causes downregulation of rac2, mpo, bpi and myc expression, suggesting multiple regulatory features of AnkA in the host nucleus. Negative regulation of these host response genes is likely critical for Anaplasma infection and the pathogen efficiently uses one effector to regulate numerous transcriptional events.
In addition to a regulatory role in transcription, AnkA is found in the host cytoplasm during infection. Lin et al. showed that AnkA is delivered to the cytosol via the T4SS and binds to at least two phosphorylation-related proteins. AnkA binds to Abl-interactor 1, which recruits the tyrosine kinase Abl-1.15 This interaction allows phosphorylation of AnkA, which occurs early during the infectious process; however, it is currently unknown how phosphorylation affects AnkA function. AnkA is also phosphorylated by Src kinase and binds to host SHP-1 (Src homology phosphatase-1) via Src homology domains.16 SHP-1 is a phosphatase that controls cellular activation events, including production of bactericidal reactive oxygen species, which Anaplasma must combat to survive in its host cell. Phosphorylation by Src kinase occurs at regions containing the amino acid residues EPIYA and is required for optimal Anaplasma infection. Presence of AnkA or phosphotyrosine antibodies inhibits infection, suggesting AnkA is a major Anaplasma virulence factor.15 Additionally, silencing of Abl-1 expression antagonizes infection, demonstrating the importance of effector interactions with host proteins. Furthermore, AnkA is recognized by sera from infected humans, indicating the protein is detected by the host immune response during Anaplasma infection. Collectively, current AnkA reports demonstrate the incredible versatility of a single bacterial effector in mediating transcriptional and post-translational events.
Intracellular pathogens in the same family (Anaplasmataceae) as A. phagocytophilum also encode Anks. The livestock pathogen A. marginale encodes three Anks, including an AnkA homo-log, that are expressed during infection of mammalian cells.17,18 Additionally, a recent report showed that A. marginale AnkA is translocated into the host cell cytosol using Legionella as a surrogate model of secretion.19 Ehrlichia chaffeensis is the macrophage-tropic agent of ehrlichiosis, a tick-borne disease similar to anaplasmosis. Ehrlichia encodes four Anks,20 one of which, termed p200, is an immunoreactive protein found in the host nucleus during infection that interacts with promoters of genes involved in apoptosis and cytokine production.21 p200 is likely translocated into the host cytoplasm by the Ehrlichia T4SS; however, due to difficulty in genetically manipulating the organism, this has not been shown experimentally.
Legionella
Legionella pneumophila is a facultative intracellular pathogen that parasitizes macrophages and causes Legionnaires' disease, a pneumonia that causes complications in immunocompromised individuals. Inside susceptible cells, Legionella generates a phagosome that eludes lysosome fusion and recruits components of the endoplasmic reticulum. Amazingly, Legionella translocates over 300 different effectors via its T4SS, making it currently the most prolific T4SS-producing pathogen. Among these many effectors are 11 Anks that are conserved among L. pneumophila strains.22 AnkX contains four ankyrin repeats and is involved in microtubule-associated transport during Legionella infection.23 When ectopically expressed in mammalian cells, AnkX impairs microtubule transport of vesicles but does not cause complete breakdown of the cytoskeletal network. Mutational analysis revealed that the ankyrin repeats in AnkX are required for disruption of vesicular transport. In addition to this activity, AnkX possesses an AMPylation-related FIC domain. FIC domains direct AMPylation to post-translationally regulate eukaryotic proteins such as small GTPases.24 Through the use of its FIC domain, AnkX phosphocholinates Rab1 and Rab35 to regulate their activity.25 Thus, the ankyrin repeats in AnkX likely mediate binding to Rab1 and Rab35, allowing close contact for phosphocholination to occur. Alteration of these two small GTPases allows Legionella to regulate host secretory transport that relies on a properly functioning microtubule network.
A collection of recent reports defined the function of Legionella AnkB during infection of macrophages and protozoan hosts. AnkB contains two ankyrin repeats and a ubiquitination-related F-box domain.26 F-box-containing proteins form ubiquitination complexes termed SCFs (Skp1-Cullin-F-box) with eukaryotic Skp1 and Cullin.27 The F-box protein typically recruits the protein to be ubiquitinated into the SCF complex for modification by attachment of ubiquitin moieties. AnkB-deficient Legionella are unable to replicate efficiently in macrophages and amoeba26 and do not cause disease in a mouse model of Legionnaires' disease.28 AnkB localizes to the host cell periphery and co-localizes with Skp1, which is a prerequisite for assembling a SCF complex. A portion of AnkB is also found on the cytosolic face of the Legionella-containing vacuole where the effector regulates recruitment of polyubiquitinated proteins. AnkB's ankyrin repeats are predicted to direct binding to a host protein that then is targeted for ubiquitination and potential degradation via activity of the F-box domain in the SCF complex. This prediction is supported by experimental evidence showing the Ank domains29 and F-box region28 of AnkB are needed for optimal Legionella intracellular replication and decoration of the Legionella-containing vacuole with ubiquitinated proteins. AnkB recruits at least one protein, parvin B, but does not increase ubiquitination or degradation of the protein.30 Parvin B is a member of a family of proteins involved in cell spreading and motility,31 suggesting Legionella modulates these events during intracellular growth. Optimal AnkB activity and recruitment of ubiquitinated proteins also requires farnesylation, likely via activity of the farnesyltransferase RCE-1.32 Farnesylation of AnkB occurs at a CaaX motif present in the effector C-terminus33 and is independent of the F-box and ankyrin repeat regions.34 Taken together, dissection of AnkB activity has revealed the potential complexity and versatility of a single Ank in the context of intracellular pathogenesis.
Finally, AnkH and AnkJ are critical for Legionella infection, as mutations in either encoding gene render the pathogen incapable of replication in macrophages and amoebal hosts.22 However, the function of these Anks is currently unknown.
Coxiella
Coxiella burnetii is the highly infectious agent of human Q fever and targets host phagocytic cells following aerosol-mediated delivery to alveolar spaces in the lung. Coxiella is unique among intracellular bacterial pathogens in that the organism promotes formation of a replication vacuole that fuses with host lysosomes.35 However, harbored organisms are not degraded and bacterial metabolism is activated by the acidic pH of the vacuole lumen. Coxiella uses a T4SS to promote vacuole formation, allow intracellular replication and inhibit host cell apoptosis.36,37 However, the T4SS effectors used by Coxiella to direct these events are largely unknown. Recent reports have collectively demonstrated translocation of over 60 Coxiella effectors,23,36,38–40 suggesting the pathogen employs a battery of translocated proteins similar to Legionella. One large family of Coxiella effectors contain ankyrin repeats and are highly diverse among isolates that cause differing forms of Q fever. Eleven proteins of this 15-member Ank family are translocated by the T4SS23,40 and several traffic to unique subcellular regions when ectopically expressed, suggesting they perform specific roles associated with host components at these sites.40 Although many Coxiella Anks have been identified, only AnkG has a defined activity to date. Using a clever experimental approach, Luhrmann et al. showed that expression of AnkG by Legionella inhibited death of infected dendritic cells.41 AnkG binds to and inhibits activity of host p32 (gC1qR), a protein that normally triggers mitochondrial-dependent apoptosis. Anti-apoptotic effectors like AnkG are critical for Coxiella infection, as the pathogen potently inhibits apoptosis of its host cell to provide a viable niche for intracellular growth.42,43
Untested Bacterial Anks
Finally, a number of additional intracellular pathogens encode Anks that have not been confirmed as T4SS effectors. Rickettsia spp cause tick borne infections including Rocky Mountain spotted fever and epidemic typhus.44 Collectively, Rickettsia spp encode numerous Anks, with R. felis alone containing 22 Ank genes.45 Unfortunately, due to a lack of tractable genetic systems, we do not currently know if rickettsial Anks are T4SS effectors. Orientia tsutsugamuchi causes scrub typhus and also replicates in a membrane bound vacuole in host cells. Sequencing of the Orientia genome demonstrated the presence of an astonishing 50 Ank genes,46 suggesting the organism may exploit this domain to a much further extent than other intracellular pathogens. Furthermore, a non-pathogenic symbiont of insects, Wolbachia, encodes 60 Anks,47 indicating Anks are not always determinants of virulence, but can also benefit an organism living in harmony with its host. Testing of these Anks in a translocation model should shed light on their viability as bona fide T4SS effectors.
Conclusion
Intracellular bacterial pathogens clearly exploit eukaryotic protein features to manipulate host cells. Ankyrin repeats are apparently a favored domain, likely due to Ank versatility in directing protein-protein interactions. We are just beginning to appreciate the quantity and versatility of bacterial Anks, and further functional characterization will undoubtedly uncover novel cellular functions critical for intracellular pathogen subversion of the host response. Currently, several questions remain regarding Ank function in bacterial pathogenesis. First, which Anks are essential virulence determinants required for disease presentation in animals? Data from Legionella AnkB studies in a mouse model of Legionnaires' disease suggest Anks are critical for development of disease.28 Second, why do pathogens such as Rickettsia and Orientia encode such a high number of Anks and are these secreted into the host cytoplasm? It is possible that a high degree of redundancy exists among these larger Ank families, as has been suggested for Legionella effectors. Third, what are the interacting host proteins targeted by individual Anks? Finally, can Anks be used as therapeutic targets to combat infectious diseases? Originally thought to be masked from the host immune response, intracellular pathogen effectors may be detected by intracellular eukaryotic surveillance systems. Additionally, Anaplasma AnkA and Coxiella AnkG are immunoreactive, suggesting they could be targeted therapeutically. As mechanistic aspects of bacterial Ank function are further defined, these questions can be resolved and Anks should prove quite useful as tools to study eukaryotic biology. Indeed, molecules designed based on Ank structure hold promise as inhibitors to treat non-infectious diseases. A new class of compounds termed DARPins (designed ankyrin repeat proteins) is being tested as alternatives to monoclonal antibodies to stimulate adaptive immune responses and selectively target tumor cells.48
Acknowledgments
I thank members of the Voth laboratory for critical reading of the manuscript. T4SS-related research in the Voth laboratory is supported by funding from NIH/NIAID (R01AI087669) to D.E.V.
Abbreviation
- Ank
ankyrin repeat-containing protein
- T4SS
type IV secretion system
Note
An editorial about this paper can be found online at: www.landesbioscience.com/journals/cellularlogistics/article/18984
References
- 1.Mosavi LK, Cammett TJ, Desrosiers DC, Peng ZY. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 2004;13:1435–1448. doi: 10.1110/ps.03554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Mahajan A, Tsai MD. Ankyrin repeat: a unique motif mediating protein-protein interactions. Biochemistry. 2006;45:15168–15178. doi: 10.1021/bi062188q. [DOI] [PubMed] [Google Scholar]
- 3.Lux SE, John KM, Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell cycle control proteins. Nature. 1990;344:36–42. doi: 10.1038/344036a0. [DOI] [PubMed] [Google Scholar]
- 4.Kohl A, Binz HK, Forrer P, Stumpp MT, Pluckthun A, Grutter MG. Designed to be stable: crystal structure of a consensus ankyrin repeat protein. Proc Natl Acad Sci USA. 2003;100:1700–1705. doi: 10.1073/pnas.0337680100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee G, Abdi K, Jiang Y, Michaely P, Bennett V, Marszalek PE. Nanospring behaviour of ankyrin repeats. Nature. 2006;440:246–249. doi: 10.1038/nature04437. [DOI] [PubMed] [Google Scholar]
- 6.Sklenovsý P, Banas P, Otyepka M. Two C-terminal ankyrin repeats form the minimal stable unit of the ankyrin repeat protein p18INK4c. J Mol Model. 2008;14:747–759. doi: 10.1007/s00894-008-0300-5. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Martinez CE, Christie PJ. Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev. 2009;73:775–808. doi: 10.1128/MMBR.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Felipe KS, Pampou S, Jovanovic OS, Pericone CD, Ye SF, Kalachikov S, et al. Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J Bacteriol. 2005;187:7716–7726. doi: 10.1128/JB.187.22.7716-26.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Khodor S, Price CT, Kalia A, Abu Kwaik Y. Functional diversity of ankyrin repeats in microbial proteins. Trends Microbiol. 2010;18:132–139. doi: 10.1016/j.tim.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11:1828–1834. doi: 10.3201/eid1112.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mott J, Barnewall RE, Rikihisa Y. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect Immun. 1999;67:1368–1378. doi: 10.1128/iai.67.3.1368-1378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caturegli P, Asanovich KM, Walls JJ, Bakken JS, Madigan JE, Popov VL, et al. ankA: an Ehrlichia phagocytophila group gene encoding a cytoplasmic protein antigen with ankyrin repeats. Infect Immun. 2000;68:5277–5283. doi: 10.1128/IAI.68.9.5277-83.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J, Kim KJ, Choi KS, Grab DJ, Dumler JS. Anaplasma phagocytophilum AnkA binds to granulocyte DNA and nuclear proteins. Cell Microbiol. 2004;6:743–751. doi: 10.1111/j.1462-5822.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Garcia JC, Rennoll-Bankert KE, Pelly S, Milstone AM, Dumler JS. Silencing of host cell CYBB gene expression by the nuclear effector AnkA of the intracellular pathogen Anaplasma phagocytophilum. Infect Immun. 2009;77:2385–2391. doi: 10.1128/IAI.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin M, den Dulk-Ras A, Hooykaas PJ, Rikihisa Y. Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection. Cell Microbiol. 2007;9:2644–2657. doi: 10.1111/j.1462-5822.2007.00985.x. [DOI] [PubMed] [Google Scholar]
- 16.IJdo JW, Carlson AC, Kennedy EL. Anaplasma phagocytophilum AnkA is tyrosine-phosphorylated at EPIYA motifs and recruits SHP-1 during early infection. Cell Microbiol. 2007;9:1284–1296. doi: 10.1111/j.1462-5822.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 17.Ramabu SS, Schneider DA, Brayton KA, Ueti MW, Graca T, Futse JE, et al. Expression of Anaplasma marginale ankyrin repeat-containing proteins during infection of the mammalian host and tick vector. Infect Immun. 2011;79:2847–2855. doi: 10.1128/IAI.05097-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brayton KA, Kappmeyer LS, Herndon DR, Dark MJ, Tibbals DL, Palmer GH, et al. Complete genome sequencing of Anaplasma marginale reveals that the surface is skewed to two superfamilies of outer membrane proteins. Proc Natl Acad Sci USA. 2005;102:844–849. doi: 10.1073/pnas.0406656102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lockwood S, Voth DE, Brayton KA, Beare PA, Brown WC, Heinzen RA, et al. Identification of Anaplasma marginale type IV secretion system effector proteins. PLoS ONE. 2011;6:e27724. doi: 10.1371/journal.pone.0027724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rikihisa Y, Lin M. Anaplasma phagocytophilum and Ehrlichia chaffeensis type IV secretion and Ank proteins. Curr Opin Microbiol. 2010;13:59–66. doi: 10.1016/j.mib.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu B, Nethery KA, Kuriakose JA, Wakeel A, Zhang X, McBride JW. Nuclear translocated Ehrlichia chaffeensis ankyrin protein interacts with a specific adenine-rich motif of host promoter and intronic Alu elements. Infect Immun. 2009;77:4243–4255. doi: 10.1128/IAI.00376-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habyarimana F, Al-Khodor S, Kalia A, Graham JE, Price CT, Garcia MT, et al. Role for the Ankyrin eukaryotic-like genes of Legionella pneumophila in parasitism of protozoan hosts and human macro-phages. Environ Microbiol. 2008;10:1460–1474. doi: 10.1111/j.1462-2920.2007.01560.x. [DOI] [PubMed] [Google Scholar]
- 23.Pan X, Luhrmann A, Satoh A, Laskowski-Arce MA, Roy CR. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science. 2008;320:1651–1654. doi: 10.1126/science.1158160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy CR, Mukherjee S. Bacterial FIC Proteins AMP Up Infection. Sci Signal. 2009;2:14. doi: 10.1126/scisignal.262pe14. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee S, Liu X, Arasaki K, McDonough J, Galan JE, Roy CR. Modulation of Rab GTPase function by a protein phosphocholine transferase. Nature. 2011;477:103–106. doi: 10.1038/nature10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Khodor S, Price CT, Habyarimana F, Kalia A, Abu Kwaik YA. Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol Microbiol. 2008;70:908–923. doi: 10.1111/j.1365-2958.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho MS, Tsai PI, Chien CT. F-box proteins: the key to protein degradation. J Biomed Sci. 2006;13:181–191. doi: 10.1007/s11373-005-9058-2. [DOI] [PubMed] [Google Scholar]
- 28.Price CT, Al-Khodor S, Al-Quadan T, Santic M, Habyarimana F, Kalia A, et al. Molecular mimicry by an F-box effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa. PLoS Pathog. 2009;5:1000704. doi: 10.1371/journal.ppat.1000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price CT, Al-Khodor S, Al-Quadan T, Abu Kwaik Y. Indispensable role for the eukaryotic-like ankyrin domains of the ankyrin B effector of Legionella pneumophila within macrophages and amoebae. Infect Immun. 2010;78:2079–2088. doi: 10.1128/IAI.01450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lomma M, Dervins-Ravault D, Rolando M, Nora T, Newton HJ, Sansom FM, et al. The Legionella pneumophila F-box protein Lpp2082 (AnkB) modulates ubiquitination of the host protein parvin B and promotes intracellular replication. Cell Microbiol. 2010;12:1272–1291. doi: 10.1111/j.1462-5822.2010.01467.x. [DOI] [PubMed] [Google Scholar]
- 31.Sepulveda JL, Wu C. The parvins. Cell Mol Life Sci. 2006;63:25–35. doi: 10.1007/s00018-005-5355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price CT, Al-Quadan T, Santic M, Jones SC, Abu Kwaik Y. Exploitation of conserved eukaryotic host cell farnesylation machinery by an F-box effector of Legionella pneumophila. J Exp Med. 2010;207:1713–1726. doi: 10.1084/jem.20100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price CT, Jones SC, Amundson KE, Kwaik YA. Host-mediated post-translational prenylation of novel Dot/Icm-translocated effectors of Legionella pneumophila. Front Microbiol. 2010;1:131. doi: 10.3389/fmicb.2010.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Quadan T, Kwaik YA. Molecular characterization of exploitation of the polyubiquitination and farnesylation machineries of Dictyostelium discoideum by the AnkB F-box effector of Legionella pneumophila. Front Microbiol. 2011;2:23. doi: 10.3389/fmicb.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voth DE, Heinzen RA. Lounging in a lysosome: the intracellular lifestyle of Coxiella burnetii. Cell Microbiol. 2007;9:829–840. doi: 10.1111/j.1462-5822.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 36.Carey KL, Newton HJ, Luhrmann A, Roy CR. The Coxiella burnetii Dot/Icm system delivers a unique repertoire of type IV effectors into host cells and is required for intracellular replication. PLoS Pathog. 2011;7:1002056. doi: 10.1371/journal.ppat.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beare PA, Gilk SD, Larson CL, Hill J, Stead CM, Omsland A, et al. Dot/Icm type IVB secretion system requirements for Coxiella burnetii growth in human macrophages. MBio. 2011;2:175–211. doi: 10.1128/mBio.00175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C, Banga S, Mertens K, Weber MM, Gorbaslieva I, Tan Y, et al. Large-scale identification and translocation of type IV secretion substrates by Coxiella burnetii. Proc Natl Acad Sci USA. 2010;107:21755–21760. doi: 10.1073/pnas.1010485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voth DE, Beare PA, Howe D, Sharma UM, Samoilis G, Cockrell DC, et al. The Coxiella burnetii cryptic plasmid is enriched in genes encoding type IV secretion system substrates. J Bacteriol. 2011;193:1493–1503. doi: 10.1128/JB.01359-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voth DE, Howe D, Beare PA, Vogel JP, Unsworth N, Samuel JE, et al. The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J Bacteriol. 2009;191:4232–4242. doi: 10.1128/JB.01656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luhrmann A, Nogueira CV, Carey KL, Roy CR. Inhibition of pathogen-induced apoptosis by a Coxiella burnetii type IV effector protein. Proc Natl Acad Sci USA. 2010;107:18997–19001. doi: 10.1073/pnas.1004380107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luhrmann A, Roy CR. Coxiella burnetii inhibits activation of host cell apoptosis through a mechanism that involves preventing cytochrome c release from mitochondria. Infect Immun. 2007;75:5282–5289. doi: 10.1128/IAI.00863-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voth DE, Howe D, Heinzen RA. Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect Immun. 2007;75:4263–4271. doi: 10.1128/IAI.00594-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker DH, Ismail N. Emerging and re-emerging rickettsioses: endothelial cell infection and early disease events. Nat Rev Microbiol. 2008;6:375–386. doi: 10.1038/nrmicro1866. [DOI] [PubMed] [Google Scholar]
- 45.Ogata H, Renesto P, Audic S, Robert C, Blanc G, Fournier PE, et al. The genome sequence of Rickettsia felis identifies the first putative conjugative plasmid in an obligate intracellular parasite. PLoS Biol. 2005;3:248. doi: 10.1371/journal.pbio.0030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho NH, Kim HR, Lee JH, Kim SY, Kim J, Cha S, et al. The Orientia tsutsugamushi genome reveals massive proliferation of conjugative type IV secretion system and host-cell interaction genes. Proc Natl Acad Sci USA. 2007;104:7981–7986. doi: 10.1073/pnas.0611553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker T, Klasson L, Sebaihia M, Sanders MJ, Thomson NR, Parkhill J, et al. Ankyrin repeat domain-encoding genes in the wPip strain of Wolbachia from the Culex pipiens group. BMC Biol. 2007;5:39. doi: 10.1186/1741-7007-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stumpp MT, Binz HK, Amstutz P. DARPins: a new generation of protein therapeutics. Drug Discov Today. 2008;13:695–701. doi: 10.1016/j.drudis.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Michaely P, Tomchick DR, Machius M, Anderson RG. Crystal structure of a 12 ANK repeat stack from human ankyrinR. EMBO J. 2002;21:6387–6396. doi: 10.1093/emboj/cdf651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rotkiewicz P. iMol molecular visualization program 2007 [Google Scholar]