Abstract
Plasma membrane receptor proteins play a key role in signal transduction and nutrient uptake, thereby controlling quality of receptor proteins is one of the most important issues in cellular logistics. After endocytosis, receptor proteins are generally delivered to lysosomes for degradation or recycled back to the plasma membrane for recycling. Transferrin receptor (TfR) is a well-known representative of recycling receptor proteins, which are traveled between plasma membrane and recycling endosomes. Although the molecular mechanism of the TfR recycling pathway has been extensively investigated in the literature, almost nothing is known about its degradation mechanism. We have recently shown that small GTPase Rab12 and its upstream activator Dennd3 regulate the constitutive degradation of TfR without modulating a conventional endocytic degradation pathway or TfR recycling pathway. Our findings suggest that Rab12 regulates membrane trafficking of TfR from recycling endosomes to lysosomes. In this addendum, we discuss the physiological significance of TfR degradation and the fate of determination of TfR (recycling or degradation).
Key words: degradation, lysosome, membrane traffic, recycling endosome, small GTPase Rab, transferrin receptor
Discovery of a Novel Degradation Pathway of Transferrin Receptor (TfR) from Recycling Endosomes to Lysosomes
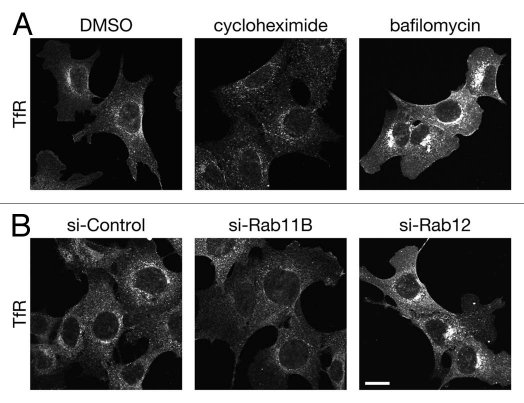
TfR and transferrin (Tf) involve maintenance of intracellular iron homeostasis.1,2 After binding of diferric Tf (Fe2Tf), internalized TfR is first transported to early endosomes, where iron is released from Tf-TfR complex. TfR is then transported to recycling endosomes and finally to the plasma membrane (so-called recycling pathway).3 However, since TfR protein could be damaged during recycling process, e.g., by acidic pH in early/recycling endosomes, it seems unreasonable to use the same TfR protein forever, and we anticipated that TfR is constitutively degraded and synthesized, the same as other intracellular proteins. In contrast to the TfR recycling pathway,4–7 however, almost nothing is known about the mechanism of TfR degradation. Recently, we found that treatment of mouse embryonic fibroblast (MEF) cells with cycloheximide, an inhibitor of protein synthesis, caused a dramatic reduction in TfR signals (Fig. 1A, compare left and middle parts), indicating that TfR is constitutively degraded in MEF cells.8 On the contrary, treatment of MEF cells with bafilomycin A1, an inhibitor of V-ATPase, that blocks lysosomal degradation, caused a dramatic increase in TfR signals (Fig. 1A, right part), indicating that some portions of TfR protein are actually degraded by lysosomes.8
Figure 1.
(A) TfR is constitutively degraded by lysosomes in MEF cells. MEF cells were treated with DMSO (left part), 50 µg/ml cycloheximide (middle part) for 4 h or 100 nM bafilomycin A1 (bafilomycin; right part) for 9 h. The cells were fixed and immunostained with anti-TfR antibody. Note that TfR signals in cycloheximide-treated and bafilomycin-treated cells were weaker and stronger, respectively, than those in control cells. (B) Rab11 and Rab12 differently regulate the trafficking of TfR protein from recycling endosomes. MEF cells transfected with control siRNA (si-Control; left part), Rab11B siRNA (si-Rab11; middle part) or Rab12 siRNA (si-Rab12; right part) were immunostained with anti-TfR antibody. Note that TfR signals in Rab11B-knockdown and Rab12-knockdown cells were weaker and stronger, respectively, than those in control cells. Scale bar, 20 µm.
To unravel the molecular mechanism of TfR degradation, we recently employed a comprehensive analysis of the mammalian Rab family small GTPases9,10 and successfully identified Rab12 as a novel regulator of TfR degradation.8 We found that over-expression of a constitutive active mutant of Rab12 caused a reduction in the amount of TfR protein, whereas functional ablation of Rab12 by knockdown of either Rab12 itself or its upstream activator Dennd3 (i.e., guanine nucleotide exchange factor (GEF) for Rab1211) caused an increase in the amount of TfR protein, indicating that Rab12 functions as a positive regulator of TfR degradation. Most importantly, Rab12 knockdown has no effect on degradation of epidermal growth factor receptor (EGFR), which is known to be degraded by the conventional degradation pathway,12–14 or TfR recycling pathway. Furthermore, Rab12 co-localizes with TfR-positive recycling endosomes and partially with lysosomes, but not with early endosomes or late endosomes/multi vesicular bodies (MVBs). These findings strongly indicated the presence of a novel membrane trafficking pathway, in which Rab12 regulates TfR protein trafficking from recycling endosomes to lysosomes.
Physiological Significance of TfR Degradation Pathway
What is the physiological significance of TfR degradation pathway? One might expect that cells actively degrade TfR protein when they are placed under iron-rich conditions. Actually, Tachiyama and coworkers very recently reported that excess iron treatment induced TfR degradation at lysosomes.15 In contrast to our finding,8 they also showed that ubiquitylated TfR protein is accumulated at late endosomes/MVBs in cells expressing a dominant negative mutant of SKD1/Vps4, which is required for the conventional degradation pathway,16–19 and that ubiquitylation of TfR is increased after excess iron treatment. So far, however, there was no report on ubiquitylation of TfR under “basal” conditions, suggesting that ubiquitylation-dependent degradation of TfR may occur under “selective” conditions (e.g., excess irons). Since iron treatment also affects transcription of TfR gene,20,21 we speculate that cells rapidly decrease TfR protein level both by reducing transcription of TfR gene and by ubiquitylation-dependent degradation of TfR protein in response to excess iron concentrations.
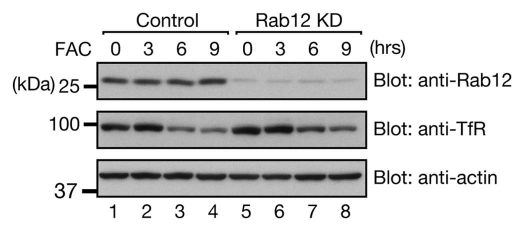
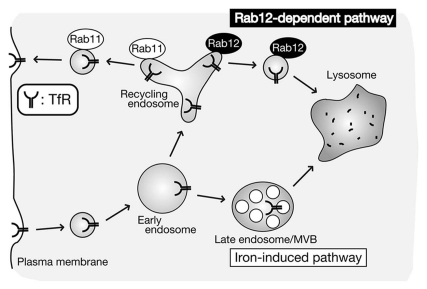
An interesting question is as to whether Rab12 is involved in iron-induced TfR degradation described above. The answer is probably no, because our data showed that iron-induced TfR degradation normally occurs even in Rab12 knockdown cells (Fig. 2). We therefore propose that two distinct TfR degradation pathways exist in cells: (1) iron-induced degradation pathway for reducing iron uptake under “selective” conditions15 and (2) Rab12-dependent constitutive degradation pathway8 for quality control of TfR protein under “basal” conditions (Fig. 3).
Figure 2.
Rab12 is not involved in iron-induced TfR degradation. MEF cells transfected with control siRNA or Rab12 siRNA were treated with 50 µg/ml FAC (ferric ammonium citrate, an iron supplier) for the indicated times. Cell lysates were analyzed by 10% SDS-PAGE followed by immunoblotting with anti-Rab12 antibody (top part), anti-TfR antibody (middle part), and anti-β-actin antibody (bottom part). The positions of the molecular mass markers (in kDa) are shown on the left.
Figure 3.
A model for two distinct TfR degradation pathways. Internalized TfR is recycled back to the plasma membrane through Rab11-depdenent recycling pathway or delivered to lysosomes through either iron-induced “selective” pathway15 or Rab12-dependent “constitutive” pathway.8 Adapted with permission from reference 8.
The Fate Determination of TfR Protein
What is the molecular determinant of the fate of TfR, i.e., recycling back to the plasma membrane or to lysosomes? One possible mechanism for the determination of the final destination of TfR protein is the mechanism regulated by two small GTPase Rabs, Rab11 and Rab12. Rab11 functions as a crucial regulator for TfR trafficking from recycling endosomes to the plasma membrane,4–6 and knockdown of Rab11B, a major Rab11 isoform in MEF cells, caused a reduction in TfR signals (Fig. 1B, compare left and middle parts). In contrast, knockdown of Rab12 in MEF cells caused an increase in TfR signals, the same as the bafilomycin A1 treatment did (Fig. 1A and B, right parts). Recruitment of Rab11 and Rab12 to TfR-positive recycling endosomes may be regulated by a random process. Alternatively, some sort of competition between Rab11 and Rab12 on TfR-positive recycling endosomes may occur. Further elucidation of the regulatory mechanism of their upstream regulators (e.g., Dennd3 for Rab12 and unidentified Rab11-GEF) will be necessary to determine when and where Rab11 and Rab12 are activated.
Perspectives
Two recent reports, including our own work, indicated that TfR, which is previously thought to be recycled back to the plasma membrane, undergo degradation at lysosomes under “selective” conditions (e.g., excess iron concentrations)15 and “basal” conditions8 (Fig. 3). The latter pathway is extremely interesting, because TfR protein is degraded by a novel Rab12-dependent pathway, which is likely to travel directly from recycling endosomes to lysosomes, but not by the conventional endocytic pathway (from late endosomes/MVBs to lysosomes). One important remaining question is as to whether recycling receptor proteins other than TfR (e.g., LDL receptor, TGFβ receptor and β2-adrenergic receptor) also undergo Rab12-dependent constitutive degradation. Future investigation of the degradation of other recycling receptor proteins in Rab12-knockdown cells or of the cargos of Rab12-bearing vesicles/membranes will be necessary to determine whether Rab12-dependent membrane trafficking pathway has a more general role in lysosomal degradation of recycling receptor proteins.
Acknowledgments
We are grateful to the Ministry of Education, Culture, Sports and Technology (MEXT) of Japan for support of this study (to M.F.) and members of the Fukuda Laboratory for valuable discussions.
References
- 1.Dautry-Varsat A, Ciechanover A, Lodish HF. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci USA. 1983;80:2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klausner RD, van Renswoude J, Ashwell G, Kempf C, Schechter AN, Dean A, et al. Receptor-mediated endocytosis of transferrin in K562 cells. J Biol Chem. 1983;258:4715–4724. [PubMed] [Google Scholar]
- 3.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 4.Ullrich O, Reinsch S, Urbé S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren M, Xu G, Zeng J, De Lemos-Chiarandini C, Adesnik M, Sabatini DD. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc Natl Acad Sci USA. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlierf B, Fey GH, Hauber J, Hocke GM, Rosorius O. Rab11b is essential for recycling of transferrin to the plasma membrane. Exp Cell Res. 2000;259:257–265. doi: 10.1006/excr.2000.4947. [DOI] [PubMed] [Google Scholar]
- 7.Weigert R, Yeung AC, Li J, Donaldson JG. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell. 2004;15:3758–3770. doi: 10.1091/mbc.E04-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsui T, Itoh T, Fukuda M. Small GTPase Rab12 regulates constitutive degradation of transferrin receptor. Traffic. 2011;12:1432–1443. doi: 10.1111/j.1600-0854.2011.01240.x. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuda M. How can mammalian Rab small GTPases be comprehensively analyzed?: development of new tools to comprehensively analyze mammalian Rabs in membrane traffic. Histol Histopathol. 2010;25:1473–1480. doi: 10.14670/HH-25.1473. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura S, Gerondopoulos A, Linford A, Rigden DJ, Barr FA. Family-wide characterization of the DENN domain Rab GDP-GTP exchange factors. J Cell Biol. 2010;191:367–381. doi: 10.1083/jcb.201008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbieri MA, Roberts RL, Gumusboga A, Highfield H, Alvarez-Dominguez C, Wells A, et al. Epidermal growth factor and membrane trafficking Egf receptor activation of endocytosis requires Rab5a. J Cell Biol. 2000;151:539–550. doi: 10.1083/jcb.151.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanlandingham PA, Ceresa BP. Rab7 regulates late endocytic trafficking downstream of multivesicular body biogenesis and cargo sequestration. J Biol Chem. 2009;284:12110–12124. doi: 10.1074/jbc.M809277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tachiyama R, Ishikawa D, Matsumoto M, Nakayama KI, Yoshimori T, Yokota S, et al. Proteome of ubiquitin/MVB pathway: possible involvement of iron-induced ubiquitylation of transferrin receptor in lysosomal degradation. Genes Cells. 2011;16:448–466. doi: 10.1111/j.1365-2443.2011.01499.x. [DOI] [PubMed] [Google Scholar]
- 16.Babst M, Sato TK, Banta LM, Emr SD. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 1997;16:1820–1831. doi: 10.1093/emboj/16.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bishop N, Woodman P. ATPase-defective mammalian VPS4 localizes to aberrant endosomes and impairs cholesterol trafficking. Mol Biol Cell. 2000;11:227–239. doi: 10.1091/mbc.11.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshimori T, Yamagata F, Yamamoto A, Mizushima N, Kabeya Y, Nara A, et al. The mouse SKD1, a homologue of yeast Vps4p, is required for normal endosomal trafficking and morphology in mammalian cells. Mol Biol Cell. 2000;11:747–763. doi: 10.1091/mbc.11.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita H, Yamanaka M, Imamura K, Tanaka Y, Nara A, Yoshimori T, et al. A dominant negative form of the AAA ATPase SKD1/VPS4 impairs membrane trafficking out of endosomal/lysosomal compartments: class E vps phenotype in mammalian cells. J Cell Sci. 2003;116:401–414. doi: 10.1242/jcs.00213. [DOI] [PubMed] [Google Scholar]
- 20.Owen D, Kuhn LC. Noncoding 3′ sequences of the transferrin receptor gene are required for mRNA regulation by iron. EMBO J. 1987;6:1287–1293. doi: 10.1002/j.1460-2075.1987.tb02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]