Abstract
Rabs GTPases are key regulatory factors that specifically associate to organelles that integrate membrane transport pathways. Rabs, through their interactions with diverse effector proteins, regulate the formation, movement, tethering and fusion of transport carriers (vesicles and/or tubules). The mammalian Rab1b GTPase is required for ER to Golgi transport and interacts with multiple effectors localized at the ER-Golgi interface. Here, we focus on interactions between Rab1b and effectors that play essential roles in COPII and COPI vesicle formation/function. Based on evidence to date, we propose a model of Rab1b action at the ER exit sites.
Key words: Rab1, COPII, COPI, ERES, ER to Golgi transport
Introduction
Protein transport among membrane bound compartments that integrate the secretory pathway is mediated by transport intermediates (vesicles and/or tubules) generated in a donor compartment, which then fuse with a specific acceptor compartment. Protein sorting and the budding of membranes are the initial mechanisms that permit cargo concentration and the segregation of proteins.
Transport of membranes between the Endoplasmic Reticulum (ER) and the Golgi complex is the first step in the secretory pathway. Sorting and concentration of cargo takes place in specialized ER domains (in the rough ER) called ER exit sites (ERES) or transitional ER (tER), where COPII (coat protein complex II) coated vesicles bud from ERES. The COPII complex is composed of the small GTPase Sar1 and two heterodimeric protein complexes, Sec23/Sec24 and Sec13/Sec31.1,2 The presence of these proteins are indicators of ERES.3
COPII vesicle formation involves several steps. First, Sec12, an ER-localized transmembrane protein, catalyzes the GDP/GTP exchange on Sar1.4 Then, Sar1-GTP recruits the cargo adaptor complex Sec23/Sec24 to ER exit domains, where Sec23 is the GTPase activating protein (GAP) that stimulates the enzymatic activity of Sar1.5 Sec24 is the adaptor protein that captures specific transmembrane cargos and SNAREs in the nascent vesicle.6–8 Finally, the “pre-budding” complex of Sar1-GTP/Sec23/Sec24 recruits the Sec13/Sec31 complex, which provides the outer layer of the coat9,10 and supports further stimulation of GTP hydrolysis and the consequent release of Sar1.11 An additional upstream element of the COPII machinery is Sec16, which seems to operate as an ERES scaffold and allows Sar1 recruitment.12 Then, sequential interactions between the different COPII subunits give rise to coat polymerization, selective cargo recognition and sorting, membrane curvature formation and the release of small transport carriers from the ER membrane.
The newly budded COPII vesicles fuse with one another and also with the already fused vesicles to form the polymorphic tubular structures that have been referred to as ERGIC (ER-Golgi intermediate compartment) or vesicular tubular clusters (VTCs),13,14 which comprise a morphologically and functionally complex compartment that integrates the anterograde ER to the Golgi and the retrograde recycling transport pathways. During their limited lifespan, VTCs undergo maturation by the selective recycling and acquisition of specific proteins and the sequential exchange of COPII for COPI coat complex.15,16
The COPI coat is largely associated with the Golgi complex, VTCs and with the peripheral punctate structures adjacent to the COPII sites at the ERES (post-ERES, see below).17 The function of COPI on VTCs and the cis-Golgi is thought to be the sorting of cargo proteins for transport back to the ER. However, live-imaging has revealed that VSV-G-containing VTCs moving to the Golgi are coated with COPI, opening the possibility that COPI also participate in anterograde transport of VTCS.17–19 The COPI complex is composed of the small GTPase Arf1 and a protein complex of seven subunits (α-, β-, β'-, γ-, δ-, ε- and ζ-COP). Coatomer complex and Arf-GTP are the minimal machinery required to form buds and vesicles.20,21
The recruitment of COPI is mediated by the GTPase Arf1,22 with GBF1 [Golgi-specific brefeldin A (BFA) resistance factor 1] acting as the guanine nucleotide exchange factor (GEF) of Arf1, at the ER-Golgi interface.23 Association of COPI takes place on post-ERES, transient tubular compartments, adjacent to ERES, which are devoid of COPII subunits, but enriched in ERGIC53.24 Post-ERES are easily detected after Brefeldin A (BFA) treatment or by the expression of the inactive E794K GBF1 mutant.23
A considerable number of proteins are localized at the ER-Golgi interface and participate in ER to Golgi transport, among which are the Rab1a and Rab1b isoforms.25–27 Rab1 belong to the Rab GTPase family, the largest family of small GTPases. The Rabs function as molecular switches that change between two conformational states, the GTP-bound “active” form and the GDP-bound “inactive” form, with GEFs regulating the exchange of GDP to GTP and the GAPs catalyzing the GTP hydrolysis.28
In humans, there are more that 60 different Rab members localized in distinct intracellular membranes. The activation of a Rab is coupled with its association to a specific membrane and allows the recruitment of downstream effector proteins. In the yeast, Saccharomyces cerevisiae, the multi-subunit TRAPP complexes I and II act as a GEF of the Rab1 homolog, Ypt1. TRAPPI complex functions in ER to Golgi transport and is required for COPII vesicle tethering, while TRAPPII (which contains all TRAPPI subunits and three subunits more) regulates intra-Golgi and endosome to late Golgi traffic.29 In mammals, a single TRAPP complex, which correlates with yeast TRAPPII, has been identified and the mTrs130/TRAPPC10 subunit has been shown to act as a Rab1 GEF in the early Golgi compartment.30,31
Rabs regulate the formation, tethering, docking and fusion of vesicles through interaction with their effectors.28 Although it is well known that Rab1 regulates tethering events by interacting with p115,32 GM13033,34 and Golgin 84,35 the participation of Rab1 in coat recruitment/function is less recognized. In the following sections, we will focus on describing the role of Rab1b in COPII and COPI vesicle formation/function.
Rab1 and COPII
In yeast, the connection between the COPII complex and Rab1 was shown by the interaction between Bet3 (a component of the TRAPPI complex) and the Sec23p COPII subunit.36,37 By recruiting TRAPPI complex, and thus activating Ypt1p, the COPII vesicle coat was able to integrate the vesicle formation process with its tethering and fusion with Golgi membranes.36 In mammalian cells, the link between the COPII complex and Rab1 is provided by a subunit of the TRAPII complex, TRAPPC3 (the mammalian homolog of Bet3) and the tethering factor p115. TRAPPC3 localized to COPII structures, interacts with Sec23 and plays a role in VTC formation.38,39 In addition, in COPII vesicles generated in vitro, Rab1a interacts with p115 which forms a complex with SNAREs.32 Furthermore, we showed that Rab1b colocalizes with COPII markers and interacts with the Sec23, Sec24 and Sec31 COPII subunits, but we could not test whether these interactions are direct. Nevertheless, we did show that they took place in vivo.40
The functional significance of the Rab1b-COPII interaction was indicated by three different findings. First, Rab1b inhibition (by siRNA treatment or transfection with the Rab1bN121I mutant) induced size reduction of the punctate COPII structures. Second, FRAP assays performed on cells co-transfected with Rab1Q67L and Sec13-YFP indicated that only ∼50% of the initial Sec13-YFP fluorescence was recovered, and finally, in Rab1b depleted cells, the incorporation of ManII-CFP into COPII structures and ManII exit from the ER after BFA washout was delayed compared with control siRNA treated cells.40 Taken together these results imply that Rab1b modulates both COPII membrane association-dissociation dynamics and COPII sorting activity.
Rab1 and COPI
In yeast, the existence of a Rab1b-COPI interaction was proposed after a genetic interaction between Ypt1 and the yeast homologs of GBF1 (Gea1/2p) was reported.41 In mammalian cells, the connection between Rab1 function and COPI recruitment was shown in vivo by expression of the inactive form of Rab1b (the N121I mutant with impaired guanine nucleotide binding). Rab1bN121I blocked the forward transport of cargo and caused Golgi disruption,42 inducing relocation of resident Golgi proteins to the ER and redistribution of the ER-Golgi intermediate compartment proteins to punctate structures. Interestingly, Rab1bN121I induced a phenotype analogous to that induced by BFA, involving a complete release of COPI into the cytosol and relocation of GBF1 to the ER. Moreover, COPI dissociation induced by Rab1bN121I could be reversed by the overexpression of either Arf1 or GBF1 and the overexpression of the active form of Rab1b (Q67L mutant) reversed COPI dissociation by BFA treatment. Taken together, these data suggested the first link between Rab1b and COPI recruitment.42
Later, we showed that GST-Rab1b-GTP, pulled down GBF1 from rat liver cytosol, and that a GST-GBF1 construct, including its N-terminal domain [GST-GBF1 (1–380)] interacted with recombinant (bacterial purified) Rab1b loaded with GTPγ-S. These results indicated that Rab1b directly interact with GBF1 and provided the molecular basis that explained how Rab1b and Arf1 were able to interrelate in a regulatory cascade that mediated COPI recruitment. Furthermore, immunofluorescence analysis showed that overexpression of Rab1bQ67L increased the association of GBF1 and COPI to peripheral punctate structures localized at the ERES, indicating that Rab1b allowed recruitment of GBF1 to VTCs membranes (Fig. 1C). In agreement, cell fractionation assays performed with cells transfected with Rab1b siRNA indicated that the amount of GBF1 associated with membranes was decreased in Rab1b-depleted cells compared with control cells. Moreover, FRAP assays indicated that Rab1bQ67L stabilized activated Arf1 on Golgi membranes, suggesting that Rab1b also recruited GBF1 at the Golgi complex.43
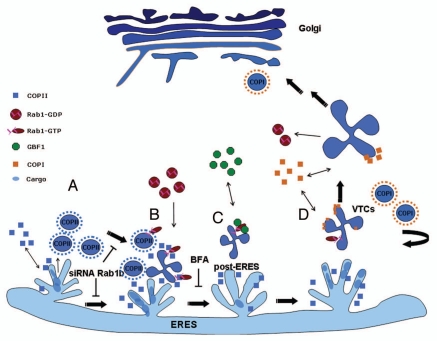
Figure 1.
Rab1b participation in sequential transport stages at the ERES: (A) Formation and budding of COPII vesicles; Rab1b depletion induces COPII vesicle accumulation. (B) Homotypic fusion of COPII vesicles and formation of VTCs: Rab1b co-localizes with these structures. (C and D) Maturation of VTCs: Rab1b recruits GBF1, which induces COPI recruitment required for the forward movement of VTCs to the cis-Golgi. BFA treatment inhibits GBF1 membrane association without disturbing Rab1b recruitment. More details are provided in the text.
Role of Rab1b in COPII and COPI Stages of Transport at the ERES
Analysis of GFP-Rab1b dynamics indicated that Rab1b-labeled punctate structures were relatively short-lived with limited-range movements. Furthermore, FRAP of GFP-Rab1b, localized at the Golgi, showed rapid recovery (t1/2 120 sec) with minimal dependence on microtubules.43 These results suggest that Rab1b is loaded independently at the Golgi complex and ERES via a direct exchange with the cytosolic pool. Moreover, correlative light electron microscopy assays indicated that Rab1b depletion, by siRNA treatment (Fig. 1A), induced Golgi disruption and accumulation of COPII vesicles located at the ERES (our unpublished results). Considering the published dynamic data and the Rab1b-COPII interaction we propose that Rab1b is associated to ERES (Fig. 1A and B), probably because Sec23 interacts with mBet3, which may then act as part of a GEF complex for Rab1b. Next, Rab1b-GTP interacts with COPII, regulates its membrane association/dissociation dynamics and contributes to sorting of cargo into COPII vesicles. Furthermore, in post-ERES structures, Rab1b recruits GBF1 which activates Arf1 that promotes COPI membrane association and VTCs maturation (Fig. 1C and D).40,43
It is clear that Rab1 is acting in two sequential stages at the ERES, but it is unknown how Rab1b coordinates the COPII-COPI transition. We speculate that Rab1b remains associated with the budded COPII vesicles and recruits its next effector, GFB1, which activates Arf1 and allows COPI recruitment. In the face of our theory, a number of mechanistic issues are in question. For example, besides interacting with Sec23, does Rab1b modulate Sec23 GAP activity to allow COPII dissociation? Is the pool of Rab1b molecules recruited to COPII vesicles the same pool that recruits GBF1 into VTCs? Or are different cycles of Rab1b membrane association/dissociations required during the process of VTCs maturation? How is the Rab1b-GDP/GTP cycling regulated at ERES? Future answers to these questions will improve our understanding of the mechanism by which Rab1b coordinates the COPII-COPI transition.
Acknowledgments
We thank Dr. P. Hobson (Asociación Argentina de Cultura Británica, Córdoba, Argentina) for revision of the manuscript, and former members of the lab (Pablo Monetta, Ileana Slavin and Nahuel Romero) for their rich discussion over the years. Work discussed here was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), FONCYT (BID 1728. PICT-10867 and PICT-00925), and Secyt (UNC). Secyt and FONCYT granted fellowships to I.A.G. and H.E.M. respectively.
Abbreviation
- Arf
ADP-ribosylation factor
- BFA
brefeldin A
- COP
coat protein complex
- ERES
endoplasmic reticulum exit sites
- GBF1
Golgi-specific BFA resistance factor 1
- GEF
guanine nucleotide exchange factors
- VTC
vesicular tubular cluster
Addendum to: Monetta P, Slavin I, Romero N, Alvarez C. Rab1b interacts with GBF1 and modulates both ARF1 dynamics and COPI association. Mol Biol Cell. 2007;18:2400–2410. doi: 10.1091/mbc.E06-11-1005. and Slavin I, Garcia IA, Monetta P, Martinez H, Romero N, Alvarez C. Role of Rab1b in COPII dynamics and function. Eur J Cell Biol. 2011;90:301–311. doi: 10.1016/j.ejcb.2010.10.001.
References
- 1.Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 2.Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, et al. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–275. doi: 10.1016/S0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- 3.Hammond AT, Glick BS. Dynamics of transitional endoplasmic reticulum sites in vertebrate cells. Mol Biol Cell. 2000;11:3013–3030. doi: 10.1091/mbc.11.9.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlowe C, d'Enfert C, Schekman R. Purification and characterization of SAR1p, a small GTP-binding protein required for transport vesicle formation from the endoplasmic reticulum. J Biol Chem. 1993;268:873–879. [PubMed] [Google Scholar]
- 5.Yoshihisa T, Barlowe C, Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science. 1993;259:1466–1468. doi: 10.1126/science.8451644. [DOI] [PubMed] [Google Scholar]
- 6.Kuehn MJ, Herrmann JM, Schekman R. COPII-cargo interactions direct protein sorting into ER-derived transport vesicles. Nature. 1998;391:187–190. doi: 10.1038/34438. [DOI] [PubMed] [Google Scholar]
- 7.Miller E, Antonny B, Hamamoto S, Schekman R. Cargo selection into COPII vesicles is driven by the Sec24p subunit. EMBO J. 2002;21:6105–6113. doi: 10.1093/emboj/cdf605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mossessova E, Bickford LC, Goldberg J. SNARE selectivity of the COPII coat. Cell. 2003;114:483–495. doi: 10.1016/S0092-8674(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 9.Lederkremer GZ, Cheng Y, Petre BM, Vogan E, Springer S, Schekman R, et al. Structure of the Sec23p/24p and Sec13p/31p complexes of COPII. Proc Natl Acad Sci USA. 2001;98:10704–10709. doi: 10.1073/pnas.191359398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stagg SM, Gurkan C, Fowler DM, LaPointe P, Foss TR, Potter CS, et al. Structure of the Sec13/31 COPII coat cage. Nature. 2006;439:234–238. doi: 10.1038/nature04339. [DOI] [PubMed] [Google Scholar]
- 11.Antonny B, Madden D, Hamamoto S, Orci L, Schekman R. Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol. 2001;3:531–537. doi: 10.1038/35078500. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharyya D, Glick BS. Two mammalian Sec16 homologues have nonredundant functions in endoplasmic reticulum (ER) export and transitional ER organization. Mol Biol Cell. 2007;18:839–849. doi: 10.1091/mbc. E06-08-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saraste J, Svensson K. Distribution of the intermediate elements operating in ER to Golgi transport. J Cell Sci. 1991;100:415–430. doi: 10.1242/jcs.100.3.415. [DOI] [PubMed] [Google Scholar]
- 14.Bannykh SI, Nishimura N, Balch WE. Getting into the Golgi. Trends Cell Biol. 1998;8:21–25. doi: 10.1016/S0962-8924(97)01184-7. [DOI] [PubMed] [Google Scholar]
- 15.Shima DT, Scales SJ, Kreis TE, Pepperkok R. Segregation of COPI-rich and anterograde-cargorich domains in endoplasmic-reticulum-to-Golgi transport complexes. Curr Biol. 1999;9:821–824. doi: 10.1016/S0960-9822(99)80365-0. [DOI] [PubMed] [Google Scholar]
- 16.Aridor M, Bannykh SI, Rowe T, Balch WE. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J Cell Biol. 1995;131:875–893. doi: 10.1083/jcb.131.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephens DJ, Lin-Marq N, Pagano A, Pepperkok R, Paccaud JP. COPI-coated ER-to-Golgi transport complexes segregate from COPII in close proximity to ER exit sites. J Cell Sci. 2000;113:2177–2185. doi: 10.1242/jcs.113.12.2177. [DOI] [PubMed] [Google Scholar]
- 18.Scales SJ, Pepperkok R, Kreis TE. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/S0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- 19.Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- 20.Orci L, Palmer DJ, Ravazzola M, Perrelet A, Amherdt M, Rothman JE. Budding from Golgi membranes requires the coatomer complex of non-clathrin coat proteins. Nature. 1993;362:648–652. doi: 10.1038/362648a0. [DOI] [PubMed] [Google Scholar]
- 21.Spang A, Matsuoka K, Hamamoto S, Schekman R, Orci L. Coatomer, Arf1p and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. Proc Natl Acad Sci USA. 1998;95:11199–11204. doi: 10.1073/pnas.95.19.11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/S0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 23.García-Mata R, Szul T, Alvarez C, Sztul E. ADP-ribosylation factor/COPI-dependent events at the endoplasmic reticulum-Golgi interface are regulated by the guanine nucleotide exchange factor GBF1. Mol Biol Cell. 2003;14:2250–2261. doi: 10.1091/mbc.E02-11-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward TH, Polishchuk RS, Caplan S, Hirschberg K, Lippincott-Schwartz J. Maintenance of Golgi structure and function depends on the integrity of ER export. J Cell Biol. 2001;155:557–570. doi: 10.1083/jcb.200107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plutner H, Cox AD, Pind S, Khosravi-Far R, Bourne JR, Schwaninger R, et al. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J Cell Biol. 1991;115:31–43. doi: 10.1083/jcb.115.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saraste J, Lahtinen U, Goud B. Localization of the small GTP-binding protein rab1p to early compartments of the secretory pathway. J Cell Sci. 1995;108:1541–1552. doi: 10.1242/jcs.108.4.1541. [DOI] [PubMed] [Google Scholar]
- 27.Tisdale EJ, Bourne JR, Khosravi-Far R, Der CJ, Balch WE. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol. 1992;119:749–761. doi: 10.1083/jcb.119.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morozova N, Liang Y, Tokarev AA, Chen SH, Cox R, Andrejic J, et al. TRAPPII subunits are required for the specificity switch of a Ypt-Rab GEF. Nat Cell Biol. 2006;8:1263–1269. doi: 10.1038/ncb1489. [DOI] [PubMed] [Google Scholar]
- 30.Choi C, Davey M, Schluter C, Pandher P, Fang Y, Foster LJ, et al. Organization and assembly of the TRAPPII complex. Traffic. 2011;12:715–725. doi: 10.1111/j.1600-0854.2011.01181.x. [DOI] [PubMed] [Google Scholar]
- 31.Yamasaki A, Menon S, Yu S, Barrowman J, Meerloo T, Oorschot V, et al. mTrs130 is a component of a mammalian TRAPPII complex, a Rab1 GEF that binds to COPI-coated vesicles. Mol Biol Cell. 2009;20:4205–4215. doi: 10.1091/mbc.E09-05-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allan BB, Moyer BD, Balch WE. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 2000;289:444–448. doi: 10.1126/science.289.5478.444. [DOI] [PubMed] [Google Scholar]
- 33.Weide T, Bayer M, Koster M, Siebrasse JP, Peters R, Barnekow A. The Golgi matrix protein GM130: a specific interacting partner of the small GTPase rab1b. EMBO Rep. 2001;2:336–341. doi: 10.1093/embo-reports/kve065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moyer BD, Allan BB, Balch WE. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis-Golgi tethering. Traffic. 2001;2:268–276. doi: 10.1034/j.1600-0854.2001.1o007.x. [DOI] [PubMed] [Google Scholar]
- 35.Satoh A, Wang Y, Malsam J, Beard MB, Warren G. Golgin-84 is a rab1 binding partner involved in Golgi structure. Traffic. 2003;4:153–161. doi: 10.1034/j.1600-0854.2003.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, et al. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–944. doi: 10.1038/nature05527. [DOI] [PubMed] [Google Scholar]
- 37.Sacher M, Barrowman J, Wang W, Horecka J, Zhang Y, Pypaert M, et al. TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport. Mol Cell. 2001;7:433–442. doi: 10.1016/S1097-2765(01)00190-3. [DOI] [PubMed] [Google Scholar]
- 38.Yu S, Satoh A, Pypaert M, Mullen K, Hay JC, Ferro-Novick S. mBet3p is required for homotypic COPII vesicle tethering in mammalian cells. J Cell Biol. 2006;174:359–368. doi: 10.1083/jcb.200603044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrowman J, Bhandari D, Reinisch K, Ferro-Novick S. TRAPP complexes in membrane traffic: convergence through a common Rab. Nat Rev Mol Cell Biol. 2010;11:759–763. doi: 10.1038/nrm2999. [DOI] [PubMed] [Google Scholar]
- 40.Slavin I, Garcia IA, Monetta P, Martinez H, Romero N, Alvarez C. Role of Rab1b in COPII dynamics and function. Eur J Cell Biol. 2011;90:301–311. doi: 10.1016/j.ejcb.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Jones S, Jedd G, Kahn RA, Franzusoff A, Bartolini F, Segev N. Genetic interactions in yeast between Ypt GTPases and Arf guanine nucleotide exchangers. Genetics. 1999;152:1543–1556. doi: 10.1093/genetics/152.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alvarez C, Garcia-Mata R, Brandon E, Sztul E. COPI recruitment is modulated by a Rab1b-dependent mechanism. Mol Biol Cell. 2003;14:2116–2127. doi: 10.1091/mbc.E02-09-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monetta P, Slavin I, Romero N, Alvarez C. Rab1b interacts with GBF1 and modulates both ARF1 dynamics and COPI association. Mol Biol Cell. 2007;18:2400–2410. doi: 10.1091/mbc.E06-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]