Abstract
BACKGROUND
Surgical ventricular reconstruction is a specific procedure designed to reduce left ventricular volume in patients with heart failure caused by coronary artery disease. We conducted a trial to address the question of whether surgical ventricular reconstruction added to coronary-artery bypass grafting (CABG) would decrease the rate of death or hospitalization for cardiac causes, as compared with CABG alone.
METHODS
Between September 2002 and January 2006, a total of 1000 patients with an ejection fraction of 35% or less, coronary artery disease that was amenable to CABG, and dominant anterior left ventricular dysfunction that was amenable to surgical ventricular reconstruction were randomly assigned to undergo either CABG alone (499 patients) or CABG with surgical ventricular reconstruction (501 patients). The primary outcome was a composite of death from any cause and hospitalization for cardiac causes. The median follow-up was 48 months.
RESULTS
Surgical ventricular reconstruction reduced the end-systolic volume index by 19%, as compared with a reduction of 6% with CABG alone. Cardiac symptoms and exercise tolerance improved from baseline to a similar degree in the two study groups. However, no significant difference was observed in the primary outcome, which occurred in 292 patients (59%) who were assigned to undergo CABG alone and in 289 patients (58%) who were assigned to undergo CABG with surgical ventricular reconstruction (hazard ratio for the combined approach, 0.99; 95% confidence interval, 0.84 to 1.17; P = 0.90).
CONCLUSIONS
Adding surgical ventricular reconstruction to CABG reduced the left ventricular volume, as compared with CABG alone. However, this anatomical change was not associated with a greater improvement in symptoms or exercise tolerance or with a reduction in the rate of death or hospitalization for cardiac causes. (ClinicalTrials.gov number, NCT00023595.)
Coronary artery disease is the predominant cause of heart failure, which is a major cause of death and disability throughout the world. Evidence-based medical therapy has been shown to reduce symptoms and increase survival in patients with heart failure and coronary artery disease.1 In addition, selected patients may benefit from surgical revascularization by means of coronary-artery bypass grafting (CABG), especially if the coronary anatomy is suitable for such surgery and if there is evidence of myocardial viability.2,3
The reduction in left ventricular function that can occur after myocardial infarction is typically accompanied by left ventricular remodeling, a process that includes left ventricular enlargement and changes in chamber geometry. Left ventricular remodeling is correlated with progression of heart failure and a poor prognosis,4,5 and the beneficial effects of therapeutic agents such as angiotensin-converting–enzyme (ACE) inhibitors and beta-blockers are associated with their effect on remodeling.4,6–9 These findings have generated considerable interest in the possibility that a surgical approach to remodeling through left ventricular volume reduction could improve outcomes for patients with coronary artery disease and heart failure.10
Surgical ventricular reconstruction is a specific surgical procedure developed for the management of heart failure with left ventricular remodeling caused by coronary artery disease.11 This operation has been shown to reduce the left ventricular volume, increase the ejection fraction, and improve ventricular function.12,13 On the basis of a small, nonrandomized, case–control study,14 it has been suggested that surgical ventricular reconstruction that is performed together with CABG may reduce the rate of hospitalization and improve ventricular function to a greater degree than CABG alone.
The Surgical Treatment for Ischemic Heart Failure (STICH) trial was designed to define the role of cardiac surgery in the treatment of patients with heart failure and coronary artery disease.15,16 One of the two major hypotheses of this trial (Hypothesis 2) was that surgical ventricular reconstruction, when added to CABG, would decrease the rate of death or hospitalization for a cardiac event, as compared with CABG alone.
METHODS
STUDY DESIGN
We conducted a multicenter, nonblinded, randomized trial at 127 clinical sites in 26 countries.15 The trial was sponsored by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health. Additional support was provided by Abbott Laboratories, Chase Medical, and CV Therapeutics, which had no role in the design, conduct, or reporting of the trial. The trial protocol was designed by the authors in collaboration with the NHLBI and was approved by the appropriate institutional review board or ethics committee at each study center. Trial operations, site management and monitoring, and data collection and analysis were coordinated by the Duke Clinical Research Institute. Oversight was provided by an independent data and safety monitoring board. A clinical events committee whose members were unaware of study-group assignments adjudicated primary outcome events. The authors wrote the manuscript and vouch for the completeness and accuracy of the data and the analyses.
SELECTION OF PATIENTS AND RANDOMIZATION
Patients were eligible for enrollment if they had coronary artery disease that was amenable to CABG and if they had a left ventricular ejection fraction of 35% or less (Fig. 1). Exclusion criteria were a recent myocardial infarction, a need for aortic-valve replacement, a planned percutaneous coronary intervention (PCI), and coexisting noncardiac disease resulting in a life expectancy of less than 3 years. All patients provided written informed consent.
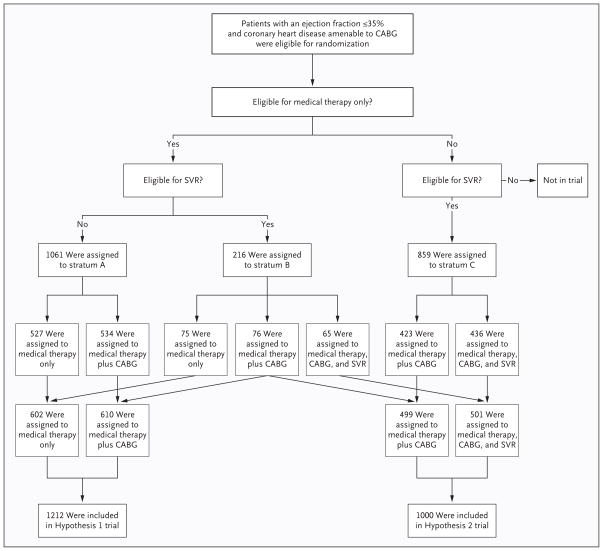
Figure 1. Enrollment and Outcomes in the STICH Hypothesis 1 and Hypothesis 2 Trials.
Eligible patients who had an ejection fraction of 35% or less and coronary artery disease that was amenable to coronary-artery bypass grafting (CABG) were stratified for randomization on the basis of protocol criteria regarding eligibility for medical therapy and for surgical ventricular reconstruction (SVR). Patients who were eligible for medical therapy with or without CABG but not for CABG with SVR were randomly assigned within stratum A. Data from these patients are being collected in the ongoing Hypothesis 1 component of the Surgical Treatment of Ischemic Heart Failure (STICH) trial. Patients who were eligible for medical therapy with or without CABG or for CABG with SVR were randomly assigned within stratum B. Patients who were not eligible for medical therapy alone but who were eligible for CABG or CABG with SVR were randomly assigned within stratum C. Patients in stratum B and stratum C who were then randomly assigned to undergo CABG with or without SVR are included in the 1000-patient cohort that was evaluated in the Hypothesis 2 trial.
After initial determination of overall eligibility for the trial, patients were evaluated to determine which component of the STICH program was appropriate for them on the basis of suitable therapeutic options for that patient (medical therapy alone, CABG alone, or CABG plus surgical ventricular reconstruction) (Fig. 1). Patients who had stenosis of the left main coronary artery of 50% or more or who had angina of Canadian Cardiovascular Society (CCS) class III or IV while receiving medical therapy were not eligible for medical therapy alone. All patients underwent cardiac imaging for assessment of left ventricular function and wall motion. Patients who were found to have dominant anterior akinesia or dyskinesia of the left ventricle were considered to have disease that was amenable to surgical ventricular reconstruction.
Using these guidelines, physicians who were responsible for the conduct of the trial selected the randomization stratum for each patient that appeared to offer treatment possibilities with equivalent potential risks and benefits. Permuted-block randomization was used, with stratification according to clinical site and according to whether the patient was a candidate for SVR, for medical therapy alone, or for both (Fig. 1).
The resulting trial included two major components. Patients in the Hypothesis 1 component were randomly assigned to receive either medical therapy alone or medical therapy plus CABG. The Hypothesis 1 component of the trial is ongoing. Patients in the Hypothesis 2 component were randomly assigned to receive either medical therapy plus CABG or medical therapy plus CABG and surgical ventricular reconstruction. The results of the Hypothesis 2 component are the subject of this report.
TREATMENT
Guideline-based recommendations for drug and device use were emphasized for all patients. The lead cardiologist at each site was responsible for monitoring to ensure that ACE inhibitors, angiotensin-receptor blockers, beta-blockers, aldosterone antagonists, antiplatelet agents, statins, diuretics, digitalis, pacemakers (for bradyarrhythmias or for cardiac resynchronization), and implantable cardioverter–defibrillators were used properly throughout the study.
Cardiac surgeons were individually certified to participate in the trial if they met prespecified performance criteria. For CABG certification, surgeons were required to provide data on at least 25 patients with a left ventricular ejection fraction of 40% or less who underwent CABG, with a death rate of 5% or less. Education in the operative technique for surgical ventricular reconstruction and perioperative management was made available before patient enrollment and during investigator meetings. Certification of individual surgeons for performing surgical ventricular reconstruction required evidence of a consistent postoperative decrease in left ventricular volume in five consecutive patients who survived the operation.
During CABG, arterial grafting for stenosis of the left anterior descending coronary artery was required for all patients without specific contraindications. The use of additional arterial conduits supplemented by vein grafts was recommended for revascularization of all major vessels with clinically significant stenoses. Concurrent mitral-valve surgery for regurgitation was performed at the discretion of the surgeon.
The technique of surgical ventricular reconstruction has been described previously.11,12,17 For patients who were assigned to undergo surgical ventricular reconstruction, this component of the operation was most commonly performed during a single period of cardioplegic arrest after construction of bypass grafts. However, the procedure could also be performed with the heart beating in order to facilitate identification of the noncontractile zone of scarring. In this procedure, after an anterior left ventriculotomy is centered in the zone of anterior asynergy, a suture is placed in the interior of the ventricle to encircle the scar at the boundary between the akinetic and viable tissue. Tightening of this suture brings the healthy portions of the ventricular walls together. Visual inspection and palpation facilitate the judgment of whether a patch is needed to optimize the chamber size without deforming the left ventricle during closure of the ventriculotomy.
PRIMARY AND SECONDARY OUTCOMES
Major perioperative events and specified end points were recorded at discharge or at 30 days for patients remaining in the hospital. Patients were evaluated at 4-month intervals after randomization during the first year and thereafter at 6-month intervals.
Symptoms of angina and heart failure were assessed at each follow-up visit. All patients who were able to do so performed a 6-minute walk test at baseline, at 4 months, and annually thereafter. Left ventricular volumes and function were assessed with the use of echocardiography, cardiac magnetic resonance imaging, or single photon emission computed tomography at baseline, at 4 months, and at 2 years.
The primary outcome was the time to death from any cause or hospitalization for cardiac causes. Secondary outcomes included death from any cause at 30 days, hospitalization for any cause and for cardiovascular causes, myocardial infarction, and stroke.
STATISTICAL ANALYSIS
We calculated that we would need to enroll 1000 patients in the trial for a power of 90% to detect a 20% reduction in the relative risk of death or hospitalization for cardiac causes, assuming a 3-year event rate in the CABG-only group of 45% or more and allowing for a crossover rate of up to 20%.
All major study-group comparisons were performed according to the intention-to-treat principle. Supplementary analyses tabulated postoperative complications and clinical events occurring within 30 days, according to the type of operation that was performed. All statistical tests were two-tailed. Cumulative event rates from the time of randomization were calculated with the use of the Kaplan–Meier method.18 The log-rank test for time-to-event data was used for the statistical comparison of study groups with respect to the primary outcome and overall mortality.19 Hazard ratios with associated 95% confidence intervals were derived with the use of the Cox proportional-hazards model.20 The Cox model was also used to assess the consistency of treatment effects by testing for interactions between the type of surgery and prespecified baseline characteristics.
Eight interim analyses of the data were performed and reviewed by the data and safety monitoring board. Interim comparisons between study groups were monitored with the use of two-sided, symmetric O’Brien–Fleming boundaries generated with the alpha-spending-function approach to group-sequential testing.21,22 A P value of 0.05 or less was considered to indicate statistical significance. Because of the sequential monitoring, the level of significance that was required for the primary analysis at the completion of the study was 0.04.
RESULTS
STUDY POPULATION
Between July 24, 2002, and May 5, 2007, 2136 patients were enrolled in the overall STICH trial (Fig. 1). Of these patients, 1000 were enrolled in the Hypothesis 2 component at 96 clinical sites between September 12, 2002, and January 24, 2006, and were randomly assigned to undergo either CABG alone (499 patients) or CABG with surgical ventricular reconstruction (501 patients), with follow-up continued through December 31, 2008. No significant differences between the two study groups were observed in baseline demographic or clinical characteristics (Table 1). The median age was 62 years, and 147 of the 1000 patients were women. The median left ventricular ejection fraction was 28%. The median end-systolic volume index was 82 ml per square meter of body-surface area. Multivessel coronary artery disease was present in 913 patients; 197 patients had stenosis of the left main coronary artery.
Table 1.
Baseline Characteristics of the Patients.*
| Variable | CABG Alone (N = 499) | CABG with Surgical Ventricular Reconstruction (N = 501) |
|---|---|---|
| Demographic characteristics | ||
| Age — yr
| ||
| Median | 62 | 62 |
|
| ||
| Interquartile range | 54–69 | 55–69 |
|
| ||
| Female sex — no. (%) | 78 (16) | 69 (14) |
|
| ||
| Race — no. (%)†
| ||
| White | 451 (90) | 460 (92) |
|
| ||
| Black or other | 48 (10) | 41 (8) |
|
| ||
| Body-mass index
| ||
| Median | 27 | 27 |
|
| ||
| Interquartile range | 25–30 | 24–30 |
|
| ||
|
Medical history
| ||
| Myocardial infarction — no. (%) | 435 (87) | 437 (87) |
|
| ||
| Hyperlipidemia — no. (%) | 367 (74) | 351 (70) |
|
| ||
| Hypertension — no. (%) | 289 (58) | 296 (59) |
|
| ||
| Diabetes — no. (%) | 173 (35) | 171 (34) |
|
| ||
| Current smoker — no. (%) | 117 (23) | 100 (20) |
|
| ||
| Previous percutaneous coronary intervention — no. (%) | 100 (20) | 95 (19) |
|
| ||
| Chronic renal insufficiency — no. (%) | 42 (8) | 43 (9) |
|
| ||
| Stroke — no. (%) | 28 (6) | 28 (6) |
|
| ||
| Previous CABG — no. (%) | 15 (3) | 9 (2) |
|
| ||
| Current Canadian Cardiovascular Society angina class — no. (%)
| ||
| No angina | 121 (24) | 128 (26) |
|
| ||
| I | 36 (7) | 35 (7) |
|
| ||
| II | 94 (19) | 94 (19) |
|
| ||
| III | 203 (41) | 205 (41) |
|
| ||
| IV | 45 (9) | 39 (8) |
|
| ||
| Current New York Heart Association heart failure class — no. (%)
| ||
| I | 36 (7) | 50 (10) |
|
| ||
| II | 222 (44) | 207 (41) |
|
| ||
| III | 210 (42) | 218 (44) |
|
| ||
| IV | 31 (6) | 26 (5) |
|
| ||
| Blood pressure — mm Hg
| ||
| Systolic
| ||
| Median | 120 | 120 |
|
| ||
| Interquartile range | 110–130 | 110–130 |
|
| ||
| Diastolic
| ||
| Median | 71 | 74 |
|
| ||
| Interquartile range | 65–80 | 66–80 |
|
| ||
| Pulse — beats/min
| ||
| Median | 70 | 72 |
|
| ||
| Interquartile range | 64–80 | 64–80 |
|
| ||
|
Baseline laboratory measure
| ||
| Creatinine — mg/dl | ||
|
| ||
| Median | 1.1 | 1.1 |
|
| ||
| Interquartile range | 0.9–1.3 | 0.9–1.3 |
|
| ||
| Left ventricular function | ||
|
| ||
| Left ventricular ejection fraction — %
| ||
| Median | 28 | 28 |
|
| ||
| Interquartile range | 23–31 | 24–31 |
|
| ||
| End-systolic volume index — ml/m2
| ||
| Median | 82 | 82 |
|
| ||
| Interquartile range | 65–102 | 66–105 |
|
| ||
| Akinesia or dyskinesia of anterior wall — %
| ||
| Median | 56 | 50 |
|
| ||
| Interquartile range | 40–60 | 40–60 |
|
| ||
| Mitral regurgitation — no. (%)‡
| ||
| None or trace | 173 (35) | 190 (38) |
|
| ||
| Mild (≤2+) | 233 (47) | 216 (43) |
|
| ||
| Moderate (3+) | 72 (14) | 70 (14) |
|
| ||
| Severe (4+) | 16 (3) | 20 (4) |
|
| ||
| Not assessed | 5 (1) | 5 (1) |
|
| ||
|
Coronary anatomy
| ||
| No. of vessels with stenosis of ≥50% — no. (%) | ||
|
| ||
| One | 36 (7) | 51 (10) |
|
| ||
| Two | 144 (29) | 131 (26) |
|
| ||
| Three | 319 (64) | 319 (64) |
|
| ||
| Stenosis of left main coronary artery — no. (%)
| ||
| 50–74% | 72 (14) | 61 (12) |
|
| ||
| ≥75% | 31 (6) | 33 (7) |
|
| ||
| ≥75% Stenosis of proximal left anterior descending coronary artery — no. (%) | 388 (78) | 369 (74) |
|
| ||
| Duke Coronary Artery Disease Severity Index§
| ||
| Median — % | 65 | 65 |
|
| ||
| Interquartile range — % | 43–91 | 39–91 |
|
| ||
|
Medication at baseline — %
| ||
| Beta-blocker | 85 | 87 |
|
| ||
| ACE inhibitor or angiotensin-receptor blocker | 87 | 89 |
|
| ||
| ACE inhibitor | 80 | 82 |
|
| ||
| Digoxin | 17 | 14 |
|
| ||
| Diuretic | 69 | 66 |
|
| ||
| Aspirin | 77 | 77 |
|
| ||
| Aspirin or warfarin | 81 | 83 |
|
| ||
| Statin | 79 | 75 |
P>0.05 for all between-group comparisons. The body-mass index is the weight in kilograms divided by the square of the height in meters. ACE denotes angiotensin-converting enzyme, and CABG coronary-artery bypass grafting.
Race was self-reported.
Mitral regurgitation was rated with the use of color Doppler and semiquantitative methods.
The Duke Coronary Artery Disease Severity Index ranges from 0 to 100, with higher values indicating greater severity.
SURGICAL PROCEDURES
Of the 499 patients who were assigned to undergo CABG alone, 463 (93%) underwent the assigned procedure; 9 did not undergo any surgery, and 27 underwent CABG with surgical ventricular reconstruction (Table 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Of the 501 patients who were assigned to undergo CABG with surgical ventricular reconstruction, 454 (91%) underwent the assigned procedure; 12 patients did not undergo any surgery, and 35 patients underwent CABG without surgical ventricular reconstruction.
Of the 979 patients who underwent surgery, the procedure was elective in 819 patients (84%), urgent in 127 (13%), performed for ongoing ischemia in 21 (2%), and performed under emergency conditions in 11 (1%) (Table 2 in the Supplementary Appendix). Mitral-valve surgery was performed in 178 patients (18%) undergoing surgery. More arterial conduits were used in patients undergoing CABG alone than in patients undergoing CABG with surgical ventricular reconstruction (P=0.008). Surgical ventricular reconstruction added a median of 27 minutes of cardiopulmonary bypass time to the CABG procedure (P<0.001). The duration of aortic cross-clamping, the time to endotrachael extubation, and the duration of postoperative hospitalization were longer for patients undergoing CABG with surgical ventricular reconstruction (P<0.001 for all comparisons).
FOLLOW-UP
The median follow-up for all surviving patients was 48 months (minimum, 30). Only four patients withdrew consent, and six patients were lost to follow-up before the last visit. Of the 1000 patients, 990 (99%) underwent complete follow-up that began at randomization and concluded between August 1 and December 31, 2008.
LEFT VENTRICULAR VOLUME
A core-laboratory quantitative assessment of the end-systolic volume index on echocardiography was performed at baseline and at 4 months in a total of 373 patients (212 patients who were assigned to undergo CABG alone and 161 who were assigned to undergo CABG with surgical ventricular reconstruction). The mean end-systolic volume index in patients assigned to undergo CABG alone decreased by an average of 5 ml per square meter, from 82 to 77 ml per square meter (a reduction of 6%). For patients who were assigned to undergo CABG with surgical ventricular reconstruction, the average decrease was 16 ml per square meter, from 83 to 67 ml per square meter (a reduction of 19%) (Fig. 1 in the Supplementary Appendix). The difference between the two groups in the change from baseline was significant (P<0.001).
SYMPTOMS
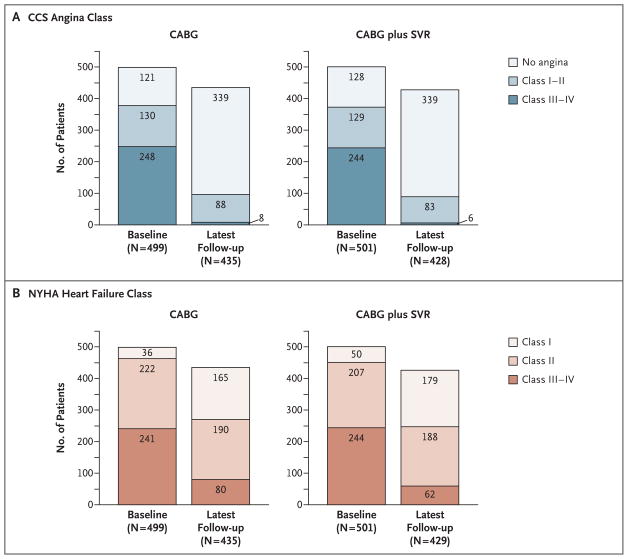
Among patients in both study groups, the proportion with no angina increased and the proportion with CCS class III or IV angina decreased during the interval from baseline to the last follow-up visit (Fig. 2A). The symptoms of patients in both study groups improved by an average of 1.7 classes (P = 0.84 for the difference between the two groups in the change from baseline). Likewise, in the two groups, the proportion with New York Heart Association (NYHA) class I heart failure (no symptoms) increased and the proportion with class III or IV heart failure decreased during the interval from baseline to the last follow-up visit (Fig. 2B). The symptoms in the two study groups improved by an average of one NYHA class (P = 0.70 for the difference between the two groups in the change from baseline).
Figure 2. Angina and Heart-Failure Symptoms at Baseline and at the Last Follow-up Visit.
Symptoms of angina, classified according to criteria of the Canadian Cardiovascular Society (CCS), and symptoms of heart failure, classified according to criteria of the New York Heart Association (NYHA), improved significantly for the 499 patients who underwent coronary-artery bypass grafting (CABG) alone and for the 501 patients who under-went CABG with surgical ventricular reconstruction (SVR) between baseline and the latest follow-up visit, at a median of 48 months. Angina symptoms improved by an average of 1.7 classes in both cohorts (P = 0.84) (Panel A). Heart-failure symptoms improved by an average of one class in both cohorts (P = 0.70) (Panel B).
6-MINUTE WALK TEST
In the two study groups, approximately three quarters of patients performed the 6-minute walk test at baseline, and a similar proportion did so at 4 months (Fig. 2 in the Supplementary Appendix). The median distance walked was 350 m at baseline and 350 m at 4 months for patients assigned to undergo CABG and 358 m at baseline and 410 m at 4 months for those assigned to undergo CABG with surgical ventricular reconstruction. The increase in the median distance walked was similar in the two groups (48 m among patients who were assigned to undergo CABG and 52 m among patients assigned to undergo CABG with surgical ventricular reconstruction, P = 0.80). Among patients assigned to undergo CABG who performed the 6-minute walk test and were assessed for symptoms, 34% were symptomatic during the baseline test and 9% were symptomatic at 4 months. The corresponding rates among patients assigned to undergo CABG with surgical ventricular reconstruction were 33% and 11%.
PRIMARY OUTCOME
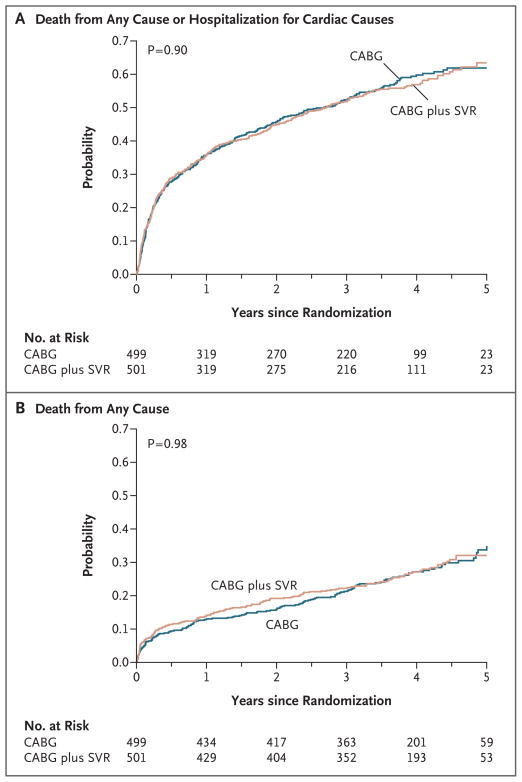
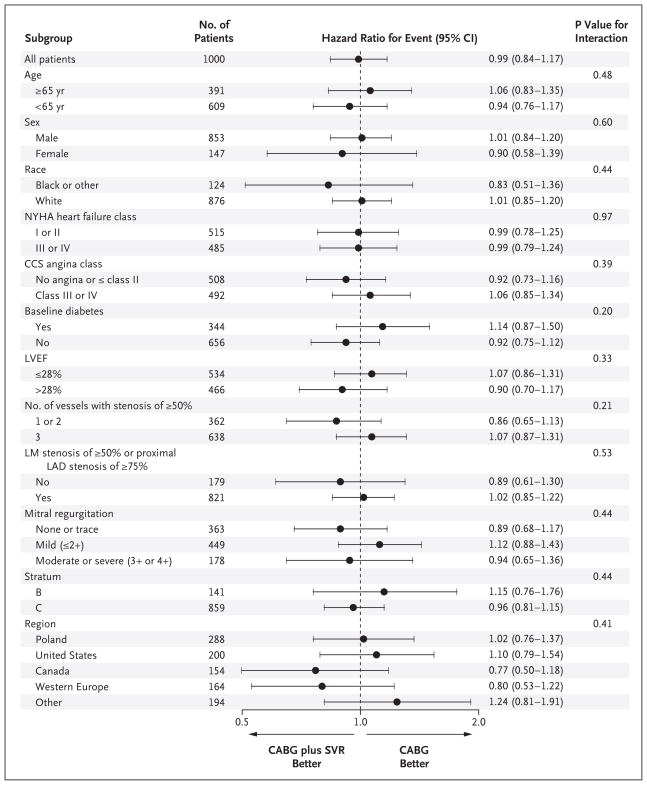
The primary outcome of death from any cause or hospitalization for cardiac causes occurred in 292 of 499 patients (59%) assigned to undergo CABG and in 289 of 501 patients (58%) assigned to undergo CABG with surgical ventricular reconstruction (hazard ratio for the combined approach, 0.99; 95% confidence interval [CI], 0.84 to 1.17; P = 0.90) (Table 2 and Fig. 3A). Fatal events of any cause occurred in 141 patients (28%) assigned to undergo CABG and in 138 patients (28%) assigned to undergo CABG with surgical ventricular reconstruction (hazard ratio, 1.00; 95% CI, 0.79 to 1.26; P = 0.98) (Table 2 and Fig. 3B). Hospitalization for cardiac causes occurred in 211 patients (42%) and in 204 patients (41%), respectively (P = 0.73). Hazard-ratio plots showed no interaction for the primary outcome between study-group assignment and baseline characteristics of interest (Fig. 4).
Table 2.
Outcomes and Subsequent Procedures.*
| Variable | CABG Alone (N = 499) | CABG with Surgical Ventricular Reconstruction (N = 501) | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|
| number (percent) | ||||
|
Outcome
| ||||
| Death from any cause or hospitalization for cardiac causes | 292 (59) | 289 (58) | 0.99 (0.84–1.17) | 0.90 |
|
| ||||
| Death from any cause | 141 (28) | 138 (28) | 1.00 (0.79–1.26) | 0.98 |
|
| ||||
| Death from any cause within 30 days after randomization (intention-to-treat analysis) | 22 (4) | 30 (6) | 0.26 | |
|
| ||||
| Death from any cause within 30 days after surgery†
| ||||
| Intention-to-treat analysis | 25 (5) | 26 (5) | 0.88 | |
|
| ||||
| As-treated analysis | 23 (5) | 28 (6) | 0.40 | |
|
| ||||
| Hospitalization
| ||||
| For any cause | 272 (55) | 268 (53) | 0.98 (0.83–1.16) | 0.82 |
|
| ||||
| For cardiac causes | 211 (42) | 204 (41) | 0.97 (0.80–1.18) | 0.73 |
|
| ||||
| Acute myocardial infarction | 22 (4) | 20 (4) | 1.01 (0.54–1.87) | 0.96 |
|
| ||||
| Stroke | 31 (6) | 23 (5) | 0.77 (0.45–1.32) | 0.35 |
|
| ||||
|
Subsequent procedure‡
| ||||
| CABG | 0 | 1 (<1) | ||
|
| ||||
| Placement of left ventricular assist device | 2 (<1) | 2 (<1) | ||
|
| ||||
| Heart transplantation | 2 (<1) | 7 (1) | ||
|
| ||||
| Percutaneous coronary intervention | 32 (6) | 17 (3) | ||
|
| ||||
| Placement of pacemaker
| ||||
| For resynchronization | 31 (6) | 30 (6) | ||
|
| ||||
| For heart rate | 44 (9) | 47 (9) | ||
|
| ||||
| Placement of implantable cardioverter–defibrillator | 100 (20) | 86 (17) | ||
Hazard ratios are for coronary-artery bypass grafting (CABG) with surgical ventricular reconstruction as compared with CABG alone.
For the analysis of death within 30 days after surgery, in the group that was assigned to undergo CABG alone, 490 patients were evaluated in the intention-to-treat analysis and 498 patients in the as-treated analysis; in the group that was assigned to undergo CABG with surgical ventricular reconstruction, 489 patients were evaluated in the intention-to-treat analysis and 481 patients in the as-treated analysis.
Hazard ratios and P values are not provided for subsequent procedures because of the low incidence of these events.
Figure 3. Kaplan–Meier Estimates of Outcomes.
Panel A shows the probability of the primary outcome (death from any cause or hospitalization for cardiac causes), which did not differ significantly between the two groups. The primary outcome occurred in 292 patients (59%) assigned to undergo coronary-artery bypass grafting (CABG) alone and in 289 patients (58%) assigned to undergo CABG with surgical ventricular reconstruction (SVR) (hazard ratio, 0.99; 95% CI, 0.84 to 1.17). Panel B shows the probability of death from any cause, which occurred in 141 patients (28%) assigned to undergo CABG and in 138 patients (28%) assigned to undergo CABG with SVR (hazard ratio, 1.00; 95% CI, 0.79 to 1.26).
Figure 4. Primary Outcome in Subgroups of Interest.
Hazard-ratio plots for selected characteristics of patients at baseline show no significant interaction with the study-group assignment. CABG denotes coronary-artery bypass grafting, CCS Canadian Cardiovascular Society, LAD left anterior descending coronary artery, LM left main coronary artery, LVEF left ventricular ejection fraction, NYHA New York Heart Association, and SVR surgical ventricular reconstruction.
SECONDARY OUTCOMES AND EVENTS
Operative rates of death (i.e., deaths occurring within 30 days after the procedure) did not differ significantly between the two study groups, according to either the intention-to-treat analysis or the as-treated analysis (Table 2). The rates of seven secondary procedures, of acute myocardial infarction (P=0.96), and of stroke (P=0.35) were low and similar in the two groups.
DISCUSSION
We compared the efficacy of CABG alone with that of CABG combined with surgical ventricular reconstruction in patients with coronary artery disease and left ventricular systolic dysfunction. As anticipated, the addition of surgical ventricular reconstruction resulted in a significantly greater reduction in left ventricular volume than was achieved with CABG alone. However, this improvement in ventricular volume did not translate into a measurable benefit for the patients. Symptomatic improvement after surgery was similar in the two study groups. There was no significant between-group difference in the primary outcome of death or hospitalization for cardiac causes or in any other clinical outcome. Operative, intubation, and initial hospitalization times were longer in patients treated with the combined procedure. The findings of this study do not support the use of surgical ventricular reconstruction in the population studied.
Two reasons might be offered for the negative outcome of this trial. Perhaps experienced surgeons decided to enroll patients for whom they recognized that surgical ventricular reconstruction would prove unnecessary but offered this procedure directly, instead of enrollment in the trial, to all concurrently evaluated patients for whom they were confident the procedure would be beneficial. Making such precise decisions about patient selection is not consistent with the diverse opinions about the eligibility of specific patients for randomized assignment to surgical ventricular reconstruction, as discussed at STICH investigator meetings. Participating investigators appeared to make randomization decisions from a broad spectrum of positions of equipoise on the basis of differences in judgment in weighing baseline clinical and imaging characteristics of potential participants in the trial.
The more plausible reason for the lack of benefit seen with surgical ventricular reconstruction is that benefits anticipated from surgical reduction of left ventricular volume (reduced wall stress improvement in systolic function) are counterbalanced by a reduction in diastolic distensibility. Previous work has shown that an important predictor of survival in patients with systolic dysfunction is the left ventricular ejection fraction during exercise.23 The most favorable dynamic response of the ventricle to the demand for increased cardiac output is associated with both an increase in end-diastolic volume and a decrease in end-systolic volume. Surgical ventricular reconstruction may impede this enhanced filling response.
One limitation of the design of the STICH trial was that physicians and surgeons caring for patients were aware of the treatment received. We sought to mitigate this limitation as much as possible by seeking complete follow-up for all patients and by selecting trial end points that were not primarily subjective ones. It is conceivable that the decision to hospitalize a patient during the follow-up period could have been influenced by the physician’s knowledge of the specific operation performed. However, rates of death, myocardial infarction, and stroke were similar in the two study groups.
A total of 83 of 1000 patients (8%) did not receive the assigned treatment. Patients who were identified before randomization as eligible for either treatment could be expected to have a higher preoperative crossover rate than patients for whom one operative strategy was preferred. Moreover, physician-directed crossovers were similar in the two study groups. Analyses that were performed on the intention-to-treat principle and according to the surgery received had similar results.
In summary, our trial compared the efficacy of CABG alone with that of CABG with surgical ventricular reconstruction in patients with coronary artery disease and left ventricular systolic dysfunction. There was no significant difference between the two study groups in the degree of symptom improvement or in the rate of death or hospitalization for cardiac causes.
Supplementary Material
Acknowledgments
Supported by grants (5U01-HL-69015, 5U01-HL-69013, and 5U01-HL-69010) from the National Heart, Lung, and Blood Institute.
We thank Vanessa Moore for her assistance in the preparation of the manuscript and Kerry Bassett and Anthony Doll for their work in the initial preparation of the figures.
Footnotes
Dr. Velazquez reports receiving grant support from Cardiokinetix; Dr. O’Connor, receiving grant support from Scios and NovaCardia; and Dr. Rouleau, receiving consulting fees from Novartis, Pfizer, and Scios. No other potential conflict of interest relevant to this article was reported.
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Bax JJ, van der Wall EE, Harbinson M. Radionuclide techniques for the assessment of myocardial viability and hibernation. Heart. 2004;90(Suppl 5):v26–v33. doi: 10.1136/hrt.2002.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery) Circulation. 2004;110(14):e340–e437. [Erratum, Circulation 2005:111:2014.] [PubMed] [Google Scholar]
- 4.Sutton MGS, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–8. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 5.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling — concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–82. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Lamas GA, Vaughan DE, Parisi AF, Braunwald E. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med. 1988;319:80–6. doi: 10.1056/NEJM198807143190204. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg B, Quinones MA, Koilpillai C, et al. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction: results of the SOLVD echocardiography substudy. Circulation. 1995;91:2573–81. doi: 10.1161/01.cir.91.10.2573. [DOI] [PubMed] [Google Scholar]
- 8.Doughty RN, Whalley GA, Gamble G, MacMahon S, Sharpe N. Left ventricular remodeling with carvedilol in patients with congestive heart failure due to ischemic heart disease. J Am Coll Cardiol. 1997;29:1060–6. doi: 10.1016/s0735-1097(97)00012-0. [DOI] [PubMed] [Google Scholar]
- 9.Doughty RN, Whalley GA, Walsh HA, Gamble GD, López-Sendón J, Sharpe N. Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: the CAPRICORN Echo Substudy. Circulation. 2004;109:201–6. doi: 10.1161/01.CIR.0000108928.25690.94. [DOI] [PubMed] [Google Scholar]
- 10.Alfieri O, Maisano F, Schreuder JJ. Surgical methods to reverse left ventricular remodeling in congestive heart failure. Am J Cardiol. 2003;91(Suppl 9A):81F–87F. doi: 10.1016/s0002-9149(02)03342-8. [DOI] [PubMed] [Google Scholar]
- 11.Athanasuleas CL, Stanley AWH, Jr, Buckberg GD. Restoration of contractile function in the enlarged left ventricle by exclusion of remodeled akinetic anterior segment: surgical strategy, myocardial protection, and angiographic results. J Card Surg. 1998;13:418–28. doi: 10.1111/j.1540-8191.1998.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 12.Athanasuleas CL, Buckberg GD, Stanley AWH, et al. Surgical ventricular restoration in the treatment of congestive heart failure due to post-infarction ventricular dilation. J Am Coll Cardiol. 2004;44:1439–45. doi: 10.1016/j.jacc.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Menicanti L, Castelvecchio S, Ranucci M, et al. Surgical therapy for ischemic heart failure: single-center experience with surgical anterior ventricular restoration. J Thorac Cardiovasc Surg. 2007;134:433–41. doi: 10.1016/j.jtcvs.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 14.Prucz RB, Weiss ES, Patel ND, Nwakanna LU, Baumgartner WA, Conte JV. Coronary artery bypass grafting with or without surgical ventricular restoration: a comparison. Ann Thorac Surg. 2008;86:806–14. doi: 10.1016/j.athoracsur.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Velazquez EJ, Lee KL, O’Connor CM, et al. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007;134:1540–7. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones RH. Is it time for a randomized trial of surgical treatment of ischemic heart failure? J Am Coll Cardiol. 2001;37:1210–3. doi: 10.1016/s0735-1097(01)01123-8. [DOI] [PubMed] [Google Scholar]
- 17.Athanasuleas CL, Stanley AWH, Jr, Buckberg GD, Dor V, Di Donato M, Blackstone EH. Surgical anterior ventricular endocardial restoration (SAVER) in the dilated remodeled ventricle after anterior myocardial infarction. J Am Coll Cardiol. 2001;37:1199–209. doi: 10.1016/s0735-1097(01)01119-6. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 19.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2. New York: John Wiley; 2002. [Google Scholar]
- 20.Cox DR. Regression models and life-tables. J R Stat Soc [B] 1972;34:187–220. [Google Scholar]
- 21.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 22.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;79:659–63. [Google Scholar]
- 23.Lee KL, Pryor DB, Pieper KS, et al. Prognostic value of radionuclide angiography in medically treated patients with coronary artery disease: a comparison with clinical and catheterization variables. Circulation. 1990;82:1705–17. doi: 10.1161/01.cir.82.5.1705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.