Abstract
We examined the impact of surgical treatments (breast-conserving surgery [BCS], mastectomy alone, mastectomy with reconstruction) and surgical side-effects severity on early stage (0–IIA) breast cancer patients' body image over time. We interviewed patients at 4–6 weeks (T1), six (T2), 12 (T3), and 24 months (T4) following definitive surgical treatment. We examined longitudinal relationships among body image problems, surgery type, and surgical side-effects severity using the Generalized Estimating Equation approach, controlling for demographic, clinical, and psychosocial factors. We compared regression coefficients of surgery type from two models, one with and one without surgical side-effects severity. Of 549 patients enrolled (mean age 58; 75% White; 65% BCS, 12% mastectomy, 23% mastectomy with reconstruction), 514 (94%) completed all four interviews. In the model without surgical side-effects severity, patients who underwent mastectomy with reconstruction reported poorer body image than patients who underwent BCS at T1–T3 (each P < 0.02), but not at T4. At T2, patients who underwent mastectomy with reconstruction also reported poorer body image than patients who underwent mastectomy alone (P = 0.0106). Adjusting for surgical side-effects severity, body image scores did not differ significantly between patients with BCS and mastectomy with reconstruction at any interview; however, patients who underwent mastectomy alone had better body image at T2 than patients who underwent mastectomy with reconstruction (P = 0.011). The impact of surgery type on body image within the first year of definitive surgical treatment was explained by surgical side-effects severity. After 2 years, body image problems did not differ significantly by surgery type.
Keywords: Body image, Breast cancer, Psychosocial factors, Quality of life, Surgical side effects, Surgery type
Introduction
Breast cancer is the most frequently diagnosed cancer in women and ranks second in cancer deaths. Treatment for early stage breast cancer involves either breast-conserving surgery (BCS) or mastectomy, often followed by some combination of radiation, chemotherapy, and/or endocrine therapy [1]. Each treatment has the potential to impact a patient's quality of life (QOL) across several domains. Body image is one domain related to QOL that can be affected by the type of surgical treatment a breast cancer patient receives.
Many studies have examined the effects of surgical treatment on breast cancer patients' body image [2–24]. Since breast-conserving surgery (BCS) with radiation therapy is as effective as mastectomy in terms of long-term survival in the treatment of ductal carcinoma in situ (DCIS) and early stage invasive breast cancer [25–31], current research has turned to examining body image, a psychosocial outcome. Several studies have reported better body image in patients undergoing BCS when compared with mastectomy [2, 4, 8–10, 12, 13, 16–22, 24]. However, other studies have found no significant differences in body image by surgery type [3, 6, 7, 15, 23, 32]. Many of these studies did not distinguish between mastectomy alone and with reconstruction; none examined the effect of surgical side effects on body image. Patients who received mastectomy with reconstruction have reported more favorable body image when compared with patients who received mastectomy alone, but not compared with patients who received BCS [19]. Other studies have found that patients who received BCS reported better body image than those who received mastectomy with reconstruction [11, 17, 22]. The inconsistent findings of these cross-sectional studies suggested that longitudinal research investigating the impact of surgical treatments, surgical side effects, and other cancer treatments on early stage breast cancer patients' body image might contribute new knowledge to our understanding of this issue. We hypothesized that lingering surgical side effects might be particularly important to understanding the relationships between surgery type and body image, especially since recovery from reconstructive surgery after mastectomy can be prolonged.
As part of a longitudinal QOL study, we developed new measures of body image and side effects of breast cancer surgery. We report on the psychometric properties of these measures, differences in body image and surgical side-effects severity by type of surgical treatment (BCS, mastectomy alone, mastectomy with reconstruction), and changes in body image after surgical treatment over a 2-year follow-up period.
Methods
Participants
Between October 2003 and July 2007, we prospectively identified and enrolled incident cases of pathologically confirmed DCIS and early stage (I and IIA) invasive breast cancer patients who were diagnosed and treated at the Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis and at Saint Louis University School of Medicine. We included women age 40 and older because screening mammography is recommended for women in this age group [33–35]. Women were eligible for participation if they had completed their definitive surgical treatment, had not received neoadjuvant chemotherapy, had no prior history of any breast cancer, spoke English, and did not demonstrate cognitive impairment on the Orientation-Memory-Concentration Test [36].
This study was approved by the Institutional Review Boards at Washington University and Saint Louis University Schools of Medicine. Participants were paid $20 per interview.
Procedures
After obtaining informed consent, specially trained interviewers administered computer-assisted telephone interviews at 4–6 weeks (T1), 6 months (T2), 12 months (T3), and 24 months (T4) after definitive surgical treatment. We included variables based on the vast literature of factors associated with QOL in breast cancer survivors. At each interview, we collected demographic information, calculated body-mass index (BMI) using patients' self-reported height and weight, and administered measures of body image, surgical side-effects severity, comorbidity, anxiety, and depressed mood. From the medical record, we obtained clinical data regarding stage at diagnosis (DCIS, I, or IIA), surgery type (BCS, mastectomy alone, or mastectomy with reconstruction), and adjuvant therapies (radiation, chemo-therapy, and endocrine therapy).
We developed an 8-item body image questionnaire using modified items from the Cancer Rehabilitation Evaluation System (CARES) [37] and other studies [2, 38]. We also developed a list of commonly reported surgical side effects based on collaborating physicians' anecdotal reports and the literature [37]. Participants were asked to rate the extent to which each statement (Table 1) applied to them in the last month using a 5-point scale ranging from “not at all” (1) to “all of the time” (5). Higher scores indicated more problems with body image and self-consciousness about the way one looks and more severe surgical side effects.
Table 1.
Number (%) of patients choosing each response to items measuring body image problems and surgical side-effects severity at T1
| Body image | Not at all | A little of the time | Some of the time | Most of the time | All of the time |
|---|---|---|---|---|---|
| You are dissatisfied with the way your clothes fit | 300 (54.7) | 90 (16.4) | 77 (14.1) | 51 (9.3) | 30 (5.5) |
| You think your clothes do not look good on you | 348 (63.5) | 59 (10.8) | 74 (13.5) | 43 (7.8) | 24 (4.4) |
| You have difficulty finding clothes that fit | 411 (74.9) | 43 (4.7) | 42 (11.5) | 27 (5.8) | 35 (3.1) |
| You feel self-conscious about being seen nude by your husband/partner | 314 (68.1) | 26 (9.3) | 63 (9.1) | 32 (5.9) | 17 (7.6) |
| You are embarrassed to look at your nude body | 454 (82.7) | 28 (5.1) | 28 (5.1) | 18 (3.3) | 20 (2.6) |
| You are embarrassed to wear a swimming suit | 289 (20.6) | 31 (5.6) | 44 (8.0) | 58 (10.6) | 113 (20.6) |
| You feel self-conscious about your appearance | 344 (62.7) | 56 (10.2) | 78 (14.2) | 41 (7.5) | 29 (5.3) |
| You feel ashamed of your body | 450 (82.0) | 29 (5.3) | 25 (4.6) | 23 (4.2) | 21 (3.8) |
| Surgical side-effects severity | Not at all | A little bit | Somewhat | Quite a bit | Very much |
|---|---|---|---|---|---|
| You have limited arm mobility/frozen shoulder | 386 (70.3) | 58 (10.6) | 60 (10.9) | 34 (6.2) | 11 (2.0) |
| You have tightness/tenderness/discomfort in your chest wall | 330 (60.1) | 90 (16.4) | 70 (12.8) | 45 (8.2) | 14 (2.6) |
| You have tightness/tenderness/discomfort in your breast | 206 (37.5) | 148 (27.0) | 110 (20.0) | 68 (12.4) | 17 (3.1) |
| You have arm weakness | 389 (70.9) | 65 (11.8) | 55 (10.0) | 29 (5.3) | 11 (2.0) |
| You have lymphedema (swelling) of the arm | 476 (86.7) | 33 (6.0) | 23 (4.2) | 12 (2.2) | 5 (0.9) |
| You have swelling of the chest/breast/axilla (underarm) wall | 361 (65.8) | 88 (16.0) | 50 (9.1) | 39 (7.1) | 9 (1.6) |
| You have numbness/tingling, pins and needles | 311 (56.6) | 88 (16.0) | 71 (12.9) | 59 (10.7) | 20 (3.6) |
| You have tightness/pulling/stretching in your arm/axilla (underarm) | 314 (57.2) | 104 (18.9) | 69 (12.6) | 49 (8.9) | 13 (2.4) |
We included a validated interview measure of comorbidity [39], which is based on the Charlson Index [40]. This measure inquires about the presence of several medical conditions having prognostic significance. We computed a weighted index that takes into account the number and severity of comorbid diseases reported.
The 21-item Beck Anxiety Inventory® (BAI®) measured the severity of anxiety symptoms in the past week [41]. Items are rated on a 4-point scale from 0 = “Not at all” to 3 = “Severely, I could barely stand it,” with total scores ranging from 0 to 63.
The 20-item Center for Epidemiologic Studies Depression (CES-D) questionnaire [42, 43] measured the extent to which participants have experienced depressive symptoms during the past week. Each item is scored from 0 (rarely or none of the time) to 3 (most or all of the time). After reverse scoring four items, a total score is computed (range 0–60).
Statistical analyses
The factor structure of the newly developed measures of body image and surgical side-effects severity was analyzed using exploratory principal components analysis with varimax rotation and Lautenschlager's parallel analysis criteria [44] to determine the number of factors. Lautenschlager's criteria are based on Velicer's Minimum Average Partial (MAP) Method [45], which uses partial correlation matrices to determine the number of components to be retained. We used Cronbach's alpha to measure the internal consistency of items on the new body image and surgical side effects measures at each interview.
To decide which variables to include as covariates in our multivariable analysis, we identified factors that were associated with body image in analyses of variance (ANOVA) or Pearson product-moment correlations at P ≤ 0.05 at T1. We used χ2 tests to determine associations between surgery type and the categorical demographic and clinical variables. These tests were performed using SPSS version 16.0 (SPSS, Inc., Chicago, IL). We decided a priori to include receipt of adjuvant treatments as covariates in our multivariable models, whether or not they were significantly associated with body image in bivariate tests, since treatment variables were of intrinsic interest to our study. Two separate linear regression analyses were performed with Generalized Estimating Equation (GEE) models to examine the associations between body image and surgery type. The two models—one including and one excluding the surgical side effects measure—were run to determine whether the association between surgery type and body image was explained by surgical side-effects severity. Since body image was measured repeatedly over the study period, we used the GEE approach with an unstructured working matrix to adjust for correlations among the observations within a subject, setting BCS as the reference group. We also compared body image between patients who underwent mastectomy alone and patients who had mastectomy with reconstruction. The GEE approach handles missing values of the dependent variable, so a balanced data set was not required. All the available data could be used to assess the effect of surgery type on body image. The change over time in body image by surgery type was estimated by the interaction between time and surgery type in the model adjusted for covariates. The procedure GENMOD in SAS v. 9.1 was used to fit the GEE models (SAS Institute, Cary, NC). Two-sided P values < 0.05 were considered statistically significant.
Results
We enrolled 549 of 772 (71.1%) eligible patients over 45 months. Participants were interviewed four times a mean 6.3 weeks (T1), 6.2 months (T2), and 12.3 months (T3), and 24.3 months (T4) after surgery. The mean age of the sample was 58 years old (range 40–91). Retention rates were very high, with 537 (97.8%) participants completing T2, 528 (96.2%) completing T3, and 514 (94%) completing T4 interviews. Non-participants were less likely than participants to be married and more likely to be non-white (each P ≤ 0.001), but the groups did not differ significantly by age or stage at diagnosis.
Table 2 provides the clinical and the demographic characteristics for patients in each surgical-treatment group. There was a significant association between stage at diagnosis and surgery type (P ≤ 0.001); DCIS patients were more likely to have received mastectomy with reconstruction (42%) when compared with stage I (34%) and stage IIA (24%) patients. Women who underwent BCS were more likely than women who received mastectomy (with or without reconstruction) to have received radiation and endocrine therapy (each P ≤ 0.001); BCS with radiation is standard of care. Women who received mastectomy with reconstruction were more likely to be younger, married, and have completed at least some college education. As would be expected, women who were diagnosed with stage IIA disease were more likely to have received chemotherapy than women who were diagnosed with DCIS or stage I breast cancer (data not shown; P ≤ 0.001).
Table 2.
Demographic and clinical characteristics of patients, by type of surgery
| Characteristics | N = 549 (%) | BCS n = 356 (65%) | Mastectomy alone n = 66 (12%) | Mastectomy with reconstructionan = 127 (23%) | P |
|---|---|---|---|---|---|
| Mean age (SD) at diagnosis | 58.3 (10.6) | 59.7 (10.8) | 61.5 (10.3) | 52.9 (8.2) | <0.001 |
| Race | 0.071 | ||||
| White | 439 (80.0) | 292 (82.0) | 46 (69.7) | 101 (79.5) | |
| Non-white | 110 (20.0) | 64 (18.0) | 20 (30.3) | 26 (20.5) | |
| Marital status | 0.008 | ||||
| Married/domestic partner | 333 (60.7) | 203 (57.0) | 38 (57.6) | 92 (72.4) | |
| Not married | 216 (39.3) | 153 (43.0) | 28 (42.4) | 35 (27.6) | |
| Education | 0.008 | ||||
| <12 years | 43 (7.8) | 25 (7.1) | 11 (16.7) | 7 (5.5) | |
| High school graduate/GED | 128 (23.3) | 90 (25.3) | 17 (25.8) | 21 (16.5) | |
| >12 years | 378 (68.9) | 241 (67.7) | 38 (57.6) | 99 (78.0) | |
| Stage at diagnosis | <0.001 | ||||
| DCIS | 184 (33.5) | 111 (31.2) | 20 (30.3) | 53 (41.7) | |
| Stage I | 282 (51.4) | 203 (57.0) | 36 (54.5) | 43 (33.9) | |
| Stage IIA | 83 (15.1) | 42 (11.8) | 10 (15.2) | 31 (24.4) | |
| Radiation therapy | |||||
| No | 199 (36.2) | 24 (6.7) | 62 (93.9) | 113 (89.0) | <0.001 |
| Yes | 350 (63.8) | 332 (93.3) | 4 (6.1) | 14 (11.0) | |
| Chemotherapy | |||||
| No | 413 (75.2) | 274(77.0) | 48 (72.7) | 91 (71.7) | 0.434 |
| Yes | 136 (24.7) | 82 (23.0) | 18 (27.3) | 36 (28.3) | |
| Endocrine therapyb | |||||
| No | 200 (36.7) | 104 (29.4) | 36 (55.4) | 60 (48.0) | <0.001 |
| Yes | 344 (63.2) | 250 (70.6) | 29 (44.6) | 65 (52.0) |
BCS breast-conserving surgery
Includes 93 patients with implant, 33 with autogenous tissue (10 transverse rectus abdominis muscle [TRAM] flap; 23 latissimus dorsi flap), and 1 with unknown type of reconstruction
N = 544 (5 patients' records did not include this information)
Factor analyses
Separate principal components analysis and Lautensch-lager's parallel analysis criteria [44] of the body image and surgical side effects items yielded a single-factor solution for each measure. Cronbach's alpha ranged from 0.87 to 0.89 for the eight-item body image scale and ranged between 0.77 and 0.83 for the surgical side-effects severity scale at the four interviews.
Table 3 shows differences in body image by demographic and clinical characteristics at T1. Body image problems differed significantly by surgery type, but not by breast cancer stage at diagnosis, even though a greater proportion of DCIS patients than stage I and IIA patients received mastectomy with reconstruction (Table 2). Among patients who received mastectomy with reconstruction, body image problems did not differ significantly at any interview by type of reconstructive surgery (implant or autogenous tissue reconstruction), even after adjustment for surgical side-effects severity. Table 4 shows the Pearson correlations among the measure of body image problems and other continuous variables at T1. The correlation coefficients at the three subsequent interviews were similar in magnitude and direction to those at T1 (data not shown).
Table 3.
Means (standard deviations) of body image problems reported at T1, by demographic and clinical characteristics
| Characteristics | T1 N = 549 | P |
|---|---|---|
| Race | 0.178 | |
| White | 1.79 (0.91) | |
| Non-White | 1.66 (0.85) | |
| Marital status | 0.906 | |
| Married/domestic partner | 1.75 (0.90) | |
| Not married | 1.76 (0.90) | |
| Education | 0.059 | |
| <12 years | 2.00 (0.95) | |
| High school graduate/GED | 1.87 (1.04) | |
| >12 years | 1.70 (0.84) | |
| Surgery type | 0.000 | |
| BCS | 1.66 (0.83) | |
| Mastectomy alone | 1.75 (0.92) | |
| Mastectomy with reconstruction | 2.04 (1.01) | |
| Stage at diagnosis | 0.380 | |
| DCIS | 1.79 (0.95) | |
| Stage I | 1.71 (0.87) | |
| Stage IIA | 1.86 (0.90) | |
| Radiation therapy | 0.156 | |
| No | 1.83 (0.95) | |
| Yes | 1.72 (0.87) | |
| Chemotherapy | 0.133 | |
| No | 1.73 (0.90) | |
| Yes | 1.86 (0.89) | |
| Endocrine therapya | 0.100 | |
| No | 1.85 (0.98) | |
| Yes | 1.71 (0.85) |
Note: tests of significance were analyses of variance
N = 544 (5 patients' records did not include this information)
Table 4.
Pearson correlations among body image problems and covariates at T1 (N = 549)
| Age | Depressed mood | Anxiety | Body-mass index | Surgical side-effects severity | Comorbidity | |
|---|---|---|---|---|---|---|
| Body image | −0.202† | 0.503† | 0.370† | 0.228† | 0.370† | 0.110* |
| Age | −0.318† | −0.289† | −0.004 | −0.347† | 0.167† | |
| Depressed mood | 0.736† | 0.085* | 0.353† | 0.067 | ||
| Anxiety | 0.056 | 0.373† | 0.046 | |||
| Body-mass index | 0.002 | 0.191† | ||||
| Surgical side-effects severity | −0.051 | |||||
| Comorbidity | 1.00 |
Body-mass index at T1, N = 546
P < 0.05.
P < 0.01
GEE models
The mean differences in body image and 95% confidence intervals (CI) are reported in Table 5. In Model 1, we included age, depressed mood, anxiety, BMI, comorbidity, chemotherapy, endocrine therapy, and radiation in addition to surgery type. We found that reporting more problems with body image was associated with younger age and higher levels of depressed mood, anxiety, and BMI. Patients who underwent mastectomy with reconstruction reported worse body image than patients who underwent BCS at T1–T3. By T4, patients who received mastectomy with reconstruction reported similar body image as BCS patients. Patients who underwent mastectomy alone reported similar body image as BCS patients across all four interviews. At T2, patients who underwent mastectomy with reconstruction reported worse body image when compared with patients who underwent mastectomy alone (data not shown in Table 5; mean difference = 0.2836; 95% CI 0.0660–0.5012; P = 0.0106).
Table 5.
Mean differences in body image and 95% confidence intervals from Generalized Estimating Equations Models that were unadjusted and adjusted for surgical side-effects severity
| Model 1 Unadjusted for surgical side-effects severity |
Model 2 Adjusted for surgical side-effects severity |
|||||
|---|---|---|---|---|---|---|
| Mean difference | 95% confidence interval | P | Mean difference | 95% confidence interval | P | |
| Age at diagnosis | −0.0066 | −0.0118, −0.0013 | 0.0148 | −0.0050 | −0.0102, 0.0002 | 0.0592 |
| Depressed mood | 0.0254 | 0.0192, 0.0317 | <0.0001 | 0.0251 | 0.0189, 0.0313 | <0.0001 |
| Anxiety | 0.0107 | 0.0035, 0.0179 | 0.0035 | 0.0069 | −0.0005, 0.0143 | 0.0663 |
| Body-mass index | 0.0346 | 0.0257, 0.0435 | <0.0001 | 0.0338 | 0.0249, 0.0426 | <0.0001 |
| Comorbidity index | −0.0137 | −0.0499, 0.0225 | 0.4582 | −0.0166 | −0.0524, 0.0190 | 0.3611 |
| Surgical side-effects severity | − | − | − | 0.1727 | 0.1027, 0.2427 | <0.0001 |
| Adjuvant endocrine therapy | ||||||
| No | Reference | Reference | ||||
| Yes | 0.0069 | −0.0718, 0.0857 | 0.8630 | 0.0154 | −0.0627, 0.0935 | 0.6999 |
| Radiation therapy | ||||||
| No | Reference | Reference | ||||
| Yes | −0.0482 | −0.0449, 0.1413 | 0.3102 | 0.0194 | −0.0742, 0.1130 | 0.6849 |
| Chemotherapy | ||||||
| No | Reference | Reference | ||||
| Yes | −0.0422 | −0.1616, 0.0771 | 0.4877 | −0.0272 | −0.1450, 0.0907 | 0.6515 |
| Time after surgery | ||||||
| T1 | ||||||
| BCS | Reference | Reference | ||||
| Mastectomy alone | 0.0790 | −0.1117, 0.2697 | 0.4169 | −0.0183 | −0.2063, 0.1698 | 0.8490 |
| Mastectomy with reconstruction | 0.2808 | 0.0971, 0.4644 | 0.0027 | 0.1359 | −0.0600, 0.3318 | 0.1739 |
| T2 | ||||||
| BCS | Reference | Reference | ||||
| Mastectomy alone | −0.0365 | −0.2202, 0.1471 | 0.6966 | −0.1038 | −0.2884, 0.0808 | 0.2703 |
| Mastectomy with reconstruction | 0.2470 | 0.0586, 0.4355 | 0.0102 | 0.1727 | −0.0145, 0.3600 | 0.0705 |
| T3 | ||||||
| BCS | Reference | Reference | ||||
| Mastectomy alone | 0.0091 | −0.1991, 0.2173 | 0.9319 | −0.0440 | −0.2603, 0.1723 | 0.6901 |
| Mastectomy with reconstruction | 0.2286 | 0.0384, 0.4187 | 0.0185 | 0.1706 | −0.0197, 0.3610 | 0.0790 |
| T4 | ||||||
| BCS | Reference | Reference | ||||
| Mastectomy alone | 0.0063 | −0.1981, 0.2107 | 0.9517 | −0.0513 | −0.2581, 0.1555 | 0.6268 |
| Mastectomy with reconstruction | 0.1406 | −0.0317, 0.3130 | 0.1098 | 0.0951 | −0.0750, 0.2652 | 0.2733 |
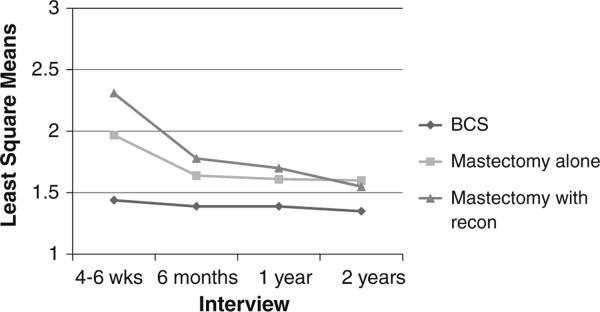
Figure 1 shows change in surgical side-effects severity by type of surgical treatment over the 2-year period. Although surgical side-effects severity declined over time for patients who had mastectomies alone or with reconstruction, these two groups reported more severe surgical side effects than patients who had BCS at all four interviews (P ≤ 0.01).
Fig. 1.
Least square means of surgical side-effects severity over time, by type of surgical treatment. Patients who received mastectomy alone and mastectomy with reconstruction reported more severe surgical side effects than patients who received BCS at all four time points, P < 0.01
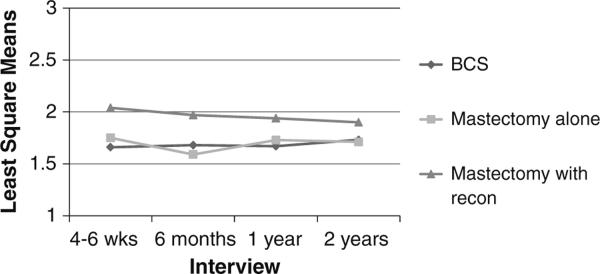
We next sought to determine whether surgical side-effects severity explained the association between surgery type and body image. Therefore, in Model 2 (Table 5), we adjusted for all the variables in Model 1 as well as for surgical side-effects severity. As shown in Model 2, worse body image was associated with more severe depressed mood, higher BMI, and more severe surgical side effects, but the significant differences at the first three interviews between patients who received mastectomy with reconstruction and patients who received BCS (shown in Model 1) were attenuated. By T4, patients' body image did not differ significantly by surgery type. However, at T2, patients who underwent mastectomy with reconstruction reported worse body image than patients who underwent mastectomy alone (data not shown in Table 5; mean difference = 0.2766, 95% CI 0.0635–0.4896; P = 0.0110), after controlling for surgical side-effects severity (Fig. 2).
Fig. 2.
Least square means of body image problems over time, by type of surgical treatment. Patients who received mastectomy with reconstruction reported more problems with body image than those patients who received mastectomy alone at T2, even after controlling for surgical side-effects severity, P = 0.011
Discussion
We examined the differences in two new measures of body image and surgical side-effects severity by type of surgical treatment and changes in body image after surgical treatment over a 2-year follow-up period. Items on each measure showed a high degree of internal consistency at each interview and good construct and discriminant validity as measured by their associations with other psychosocial and clinical measures. The measure of body image problems was significantly correlated with each of the psychosocial measures and BMI at each interview, but these first-order correlation coefficients were low to moderate in magnitude indicating that they are not measuring the same construct. The very low correlations with our measure of comorbidity show that conditions included in this index would not be expected to be highly correlated with body image. We did not include another measure of body image in our study, because the outcome of interest in the parent study was QOL, not body image. Our measure of body image problems included modifications of previously developed items [2, 37, 38], thus further validation of this new measure is warranted. As expected, surgical side effects were reported to be more severe in patients who received mastectomies alone or with reconstruction at each time point when compared with patients who received BCS (P ≤ 0.01), although these differences were most pronounced at the first interview (Fig. 1). This relationship between surgical side-effects severity and surgery type provides evidence of the construct validity of the new surgical side-effects severity measure.
We found that early stage breast cancer patients' body image differed by type of surgical treatment received. After adjustment for several potential confounders (Model 1), we found that patients who received mastectomy with reconstruction had worse body image than patients who received BCS, at each interview during the first year after surgical treatment. After adding surgical side effects in Model 2, the difference in body image between patients who received mastectomy with reconstruction and patients who received BCS was no longer significant. Thus, patients' experience of surgical side effects explained the relationship between surgery type and body image. However, after adding the surgical side-effects severity measure in Model 2, patients who received mastectomy with reconstruction still reported significantly more body image problems at T2 when compared with patients who received mastectomy alone. At 6 months after patients' surgical treatment, patients who received mastectomy with reconstruction might continue to experience the side effects of their reconstructive surgery, as well as an increased likelihood of surgical-wound infections [46–48], which delays healing. Mastectomy is associated with greater morbidity, in general, and greater likelihood of surgical-site infection, in particular, compared with BCS [46, 48]. Surgical morbidity following reconstructive surgery would be expected to add to the risk of surgical-wound infections associated with mastectomy alone. Future research might examine whether surgical-site infections have an impact on body image over and above the surgical side effects that we measured, especially in women who have a mastectomy either with or without reconstruction.
Surgical side-effects severity explained the differences in body image between patients who received BCS and mastectomy with reconstruction within the first year (T1–T3) after surgical treatment. But by the 2-year follow-up, when the lengthy process of breast reconstruction is likely completed, body image did not differ significantly by surgery type in either of the two models, even though surgical side-effects severity was still significantly greater for patients who received mastectomies alone or with reconstruction when compared with patients who received BCS (Fig. 1). Although there was a sharp decline in surgical side-effects severity from T1 to T2 both for patients who received mastectomy alone and mastectomy with reconstruction (Fig. 1), patients who received a mastectomy alone or with reconstruction continued to endure more severe surgical side effects after T2 when compared with patients who received BCS.
As shown in Table 5, Model 2, surgical side-effects severity did not substantially weaken the effects of elevated depressed mood and BMI on patients' body image problems. Thus, the effects of elevated depressed mood and BMI on body image remain, regardless of the pain or discomfort patients may feel after surgical treatment.
Our study contributes to the body image literature in early stage breast cancer patients not only in the development of two new measures of body image problems and of surgical side-effects severity, but also in its longitudinal design and in distinguishing between patients who received mastectomy alone and mastectomy with reconstruction. In two cross-sectional studies, patients who received either mastectomy alone or mastectomy with reconstruction had significantly more body image problems than patients who received BCS [11, 22]; and the body image benefits of mastectomy with reconstruction were less than expected [22]. However, patients in these studies were interviewed once, at various times within 1-year of surgical treatment [11] or up to 5 years after diagnosis [22]. A third study found that patients who received BCS reported fewer body image concerns when compared with patients who received mastectomy alone [9]; but this study, too, surveyed patients at varying times after surgical treatment, some <20 weeks post surgery. In another cross-sectional study [19], body image did not differ significantly between patients who received BCS and mastectomy with reconstruction, similar to our findings in the model that adjusted for surgical side-effects severity. However, both the groups reported significantly fewer body image problems than patients who received mastectomy alone [19], whereas we found that body image did not differ significantly between patients who received BCS and mastectomy alone across all four interviews. Importantly, none of these cross-sectional studies included a measure of surgical side-effects severity in their analysis, which ultimately explained the differences that we detected in body image problems by surgery type. As surgical side-effects severity diminished over time, so, too, did the extent of body image problems.
Kraus [14] observed early stage breast cancer patients experienced a significant decline in body image satisfaction 8 weeks post-surgical treatment. A descriptive trend in the data suggested that mastectomy patients were more satisfied with their body image than BCS patients, both before and after surgery. Patients reported basing their treatment decisions on what they believed would offer them the best opportunities for long-term survival and not on how they would look after surgery. Clinicians should be aware of the possible impact that breast cancer treatment may have on a woman's body image and other psychological outcomes (e.g., elevated depressed mood or anxiety), which were positively correlated with body image problems in our study. Although Kraus's study was limited by a small sample size of 31 patients, measuring body image scores both before and after surgical treatment was a strength of her study and a limitation of ours, since we did not measure body image before surgical treatment.
An important strength of our study was the longitudinal cohort design allowing for repeated measures of individual patients over time. In addition, since the measures of body image and surgical side-effects severity correlated with other validated psychosocial measures and surgery type in the expected direction and magnitude, we believe that measurement (response) bias was minimal. Although not population based, our sample was representative of the racial/ethnic population in the St. Louis metropolitan area. However, we excluded women younger than 40 and with more advanced breast cancers. Therefore, our results may not be generalizable to younger breast cancer patients and patients with more advanced disease, who may receive different treatment. Also, it is possible that patients who received mastectomy with reconstruction had more problems with their body image prior to surgical treatment [14] and chose to undergo reconstruction with the hopes of improving their body image. However, since reconstruction can take as long as 1 year or more to complete, these patients still may not have been satisfied with the outcomes of their reconstructive surgery at 6 months, and body image problems prior to surgical treatment (or other unmeasured factors) may account for our observed difference in body image problems between patients who received mastectomy alone and mastectomy with reconstruction at T2.
In summary, we developed new measures of body image problems and surgical side-effects severity that demonstrated adequate reliability and validity in this sample of early stage breast cancer survivors. Although body image problems were quite low, problems were significantly higher among patients who received mastectomy with reconstruction than patients with BCS. However, the effects of surgery type on body image problems were attenuated once we adjusted for surgical side-effects severity in the model. Patients who had mastectomy with reconstruction still reported significantly more problems with body image than patients with mastectomy alone 6 months after surgical treatment, after controlling for surgical side-effects severity. Since early stage breast cancer patients' long-term survival is similar between women treated with BCS and mastectomy (with or without reconstruction) [26, 27], breast cancer patients can make surgical decisions based not only on their surgeons' clinical judgment and recommendations but also with consideration of the pros and cons of different types of surgery and the potential short- and long-term effects of each option, including effects on body image, an important QOL outcome of breast cancer treatment.
Acknowledgments
This study was supported by a Grant from the National Cancer Institute and Breast Cancer Stamp Fund (R01 CA102777) to Dr. Jeffe and by the National Cancer Institute Cancer Center Support Grant (P30 CA91842) to the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri. We thank our patient participants, the interviewers, and the Siteman Cancer Center's Health Behavior, Communication and Outreach Core and Biostatistics Core for data management and statistical services. We also thank the physicians who helped us recruit their patients for this study, including Drs. Barbara Monsees, Jill Dietz, Julie Margenthaler, Virginia Herrmann, Timothy Eberlein, Matthew Ellis, Imran Zoberi, Marie Taylor, Michael Naughton, Antonella Rastelli, Donald Lombardi, Cynthia Ma, Loren Michel, and Rama Suresh at Washington University School of Medicine and Dr. Eddie Hsueh and Pam Hun-borg, RN, at Saint Louis University School of Medicine. The Beck Anxiety Inventory® and BAI® (copyright 1990, 1993 by Aaron T. Beck) are trademarks of The Psychological Corporation, a Harcourt Assessment Company. The BAI® was adapted and used by permission of the publisher, The Psychological Corporation. All rights reserved.
References
- 1.American Cancer Society . Cancer facts & figures. American Cancer Society; Atlanta: 2007. [Google Scholar]
- 2.Curran D, van Dongen JP, Aaronson NK, Kiebert G, Fentiman IS, Mignolet F, Bartelink H, the European Organization for Research and Treatment of Cancer (EORTC) Breast Cancer Co-operative Group (BCCG) Quality of life of early-stage breast cancer patients treated with radical mastectomy or breast-conserving procedures: results of EORTC Trial 10801. Eur J Cancer. 1998;34:307–314. doi: 10.1016/s0959-8049(97)00312-2. [DOI] [PubMed] [Google Scholar]
- 3.de Haes J, Curran D, Aaronson N, Fentiman I. Quality of life in breast cancer patients aged over 70 years, participating in the EORTC 10850 randomized clinical trial. Eur J Cancer. 2003;39(7):945–951. doi: 10.1016/s0959-8049(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 4.Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Holzel D. Quality of life following breast-conserving therapy or mastectomy: results of a 5-year prospective study. Breast J. 2004;10(3):223–231. doi: 10.1111/j.1075-122X.2004.21323.x. [DOI] [PubMed] [Google Scholar]
- 5.Fobair P, Stewart SL, Chang S, D'Onofrio C, Banks P, Bloom JR. Body image and sexual problems in young women with breast cancer. Psychooncology. 2006;15(7):579–594. doi: 10.1002/pon.991. [DOI] [PubMed] [Google Scholar]
- 6.Ganz PA, Schag CA, Lee JJ, Polinsky ML, Tan SJ. Breast conservation versus mastectomy: is there a difference in psychological adjustment or quality of life in the year after surgery? Cancer. 1992;69:1729–1738. doi: 10.1002/1097-0142(19920401)69:7<1729::aid-cncr2820690714>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg J, Scott R, Davidson P, Murray G, Stallard S, George W, Maguire G. Psychological morbidity in the first year after breast surgery. Eur J Surg Oncol. 1992;18:327–331. [PubMed] [Google Scholar]
- 8.Hartl K, Janni W, Kastner R, Sommer H, Strobl B, Rack B, Stauber M. Impact of medical and demographic factors on long-term quality of life and body image of breast cancer patients. Ann Oncol. 2003;14(7):1064–1071. doi: 10.1093/annonc/mdg289. [DOI] [PubMed] [Google Scholar]
- 9.Hopwood C, Haviland J, Mills J, Sumo G, Bliss J. The impact of age and clinical factors on quality of life in early breast cancer: an analysis of 2208 woman recruited to the UK START Trial (Standardisation of Breast Radiotherapy Trial) Breast. 2007;16:241–251. doi: 10.1016/j.breast.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Janni W, Rjosk D, Dimpfl T, Haertl K, Strobl B, Hepp F, Henke A, Bergauer F, Sommer H. Quality of life influenced by primary surgical treatment for stage I-III breast cancer: Long-term follow-up of a matched-pair analysis. Ann Surg Oncol. 2001;8(6):542–548. doi: 10.1007/s10434-001-0542-2. [DOI] [PubMed] [Google Scholar]
- 11.Janz NK, Mujahid MS, Lantz PM, Fagerlin A, Salem B, Morrow M, Deapen D, Katz SJ. Population-based study of the relationships of treatment and sociodemographics on quality of life for early stage breast cancer. Qual Life Res. 2005;14:1467–1479. doi: 10.1007/s11136-005-0288-6. [DOI] [PubMed] [Google Scholar]
- 12.Kiebert GM, de Haes JCM, van de Velde CJH. The impact of breast-conserving treatment and mastectomy on the quality of life of early-stage breast cancer patients. J Clin Oncol. 1991;9:1059–1070. doi: 10.1200/JCO.1991.9.6.1059. [DOI] [PubMed] [Google Scholar]
- 13.Kissane DW, Clarke DM, Ikin J, Bloch S, Smith GC, Vitetta L, McKenzie DP. Psychological morbidity and quality of life in Australian women with early-staged breast cancer: a cross sectional survey. Med J Aust. 1998;169(4):192–196. doi: 10.5694/j.1326-5377.1998.tb140220.x. [DOI] [PubMed] [Google Scholar]
- 14.Kraus P. Body image, decision making and breast cancer treatment. Cancer Nurs. 1999;22(6):421–427. doi: 10.1097/00002820-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Langer M, Prohaska R, Schreiner-Frech I, Ringler M, Kubista E. Coping and body image after different operative techniques in breast cancer patients. Psychother Psychosom Med Psychol. 1991;41:379–384. [PubMed] [Google Scholar]
- 16.Lasry JCM, Margolese RG, Poisson R, Shibata H, Fleischer D, Lafleur D, Legault S, Taillefer S. Depression and body image following mastectomy and lumpectomy. J Chron Dis. 1987;40:529–534. doi: 10.1016/0021-9681(87)90010-5. [DOI] [PubMed] [Google Scholar]
- 17.Mock V. Body image in women treated for breast cancer. Nurs Res. 1993;42:153–157. [PubMed] [Google Scholar]
- 18.Moyer A. Psychosocial outcomes of breast-conserving surgery versus mastectomy: a meta-analytic review. Health Psychol. 1997;16:284–293. doi: 10.1037//0278-6133.16.3.284. [DOI] [PubMed] [Google Scholar]
- 19.Nano MT, Grantly G, Kollias J, Bochner MA, Malycha P, Winefield HR. Psychological impact and cosmetic outcome of surgical breast cancer strategies. ANZ J Surg. 2005;75:940–947. doi: 10.1111/j.1445-2197.2005.03517.x. [DOI] [PubMed] [Google Scholar]
- 20.Noguchi M, Kitagawa H, Kinoshita K, Earashi M, Miyazaki I, Tatsukuchi S, Saito Y, Mizukami Y, Nonmura A, Nakamura S, et al. Psychologic and cosmetic self-assessments of breast conserving therapy compared with mastectomy and immediate breast reconstruction. J Surg Oncol. 1993;54:260–266. doi: 10.1002/jso.2930540416. [DOI] [PubMed] [Google Scholar]
- 21.Poulson B, Graversen HP, Beckmann J, Blichert-Toft M. A comparative study of post-operative psychosocial function in women with primary operable breast cancer randomized to breast conservation therapy or mastectomy. Eur J Surg Oncol. 1997;23:327–334. doi: 10.1016/s0748-7983(97)90804-0. [DOI] [PubMed] [Google Scholar]
- 22.Rowland JH, Desmond KA, Meyerowitz BE, Belin TR, Wyatt GE, Ganz PA. Role of breast reconstructive surgery in physical and emotional outcomes among breast cancer survivors. J Natl Cancer Inst. 2000;92(17):1422–1429. doi: 10.1093/jnci/92.17.1422. [DOI] [PubMed] [Google Scholar]
- 23.Schover LR, Yetman RJ, Tuason LJ, Meisler E, Esselstyn CB, Hermann RE, Grundfest-Broniatowski S, Dowden RV. Partial mastectomy and breast reconstruction. A comparison of their effects on psychosocial adjustment, body image, and sexuality. Cancer. 1995;75(1):54–64. doi: 10.1002/1097-0142(19950101)75:1<54::aid-cncr2820750111>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 24.Wellisch DK, DiMatteo R, Silverstein M, Landsverk J, Hoffman R, Waisman J, Handel N, Waisman-Smith E, Schain W. Psychosocial outcomes of breast cancer therapies: lumpectomy versus mastectomy. Pasychosomatics. 1989;30:365–373. doi: 10.1016/S0033-3182(89)72241-6. [DOI] [PubMed] [Google Scholar]
- 25.Carrera C, Payne S. Ductal carcinoma in situ (DCIS) of the breast: the need for psychosocial research. Psychooncology. 1999;8:538–545. doi: 10.1002/(sici)1099-1611(199911/12)8:6<538::aid-pon426>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized study comparing total mastectomy, lumpectomy and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 27.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 28.Baxter NN, Virnig BA, Durham SB, Tuttle TM. Trends in treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96:443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz GF, Solin LJ, Olivotto IA, Ernster VL, Pressman PI, Consensus Conference Committee Consensus conference on the treatment of in situ ductal carcinoma of the breast, April 22–25, 1999. Cancer. 2000;88:946–954. [PubMed] [Google Scholar]
- 30.Morrow M, Strom E, Bassett L, Dershaw DD, Fowble B, Harris JR, O'Malley F, Schnitt SJ, Singletary SE, Winchester DP. Standard for the management of ductal carcinoma in situ of the breast (DCIS) CA Cancer J Clin. 2002;52(5):256–276. doi: 10.3322/canjclin.52.5.256. [DOI] [PubMed] [Google Scholar]
- 31.Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C. Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA. 1996;275:913–918. [PubMed] [Google Scholar]
- 32.Parker PA, Youssef A, Walker S, Basen-Engquist K, Cohen L, Gritz E, Wei QX, Robb GL. Short-term and long-term psychosocial adjustment and quality of life in women undergoing different surgical procedures for breast cancer. Ann Surg Oncol. 2007;14(11):3078–3089. doi: 10.1245/s10434-007-9413-9. [DOI] [PubMed] [Google Scholar]
- 33.Feig SA. Ductal carcinoma in situ: implications for screening mammography. Radiol Clin North Am. 2000;38:653–668. doi: 10.1016/s0033-8389(05)70192-5. [DOI] [PubMed] [Google Scholar]
- 34.Leitch AM, Dodd GD, Costanza M, Linver M, Pressman P, McGinnis L, Smith RA. American Cancer Society Guidelines for the early detection of breast cancer: update 1997. CA Cancer J Clin. 1997;47:150–153. doi: 10.3322/canjclin.47.3.150. [DOI] [PubMed] [Google Scholar]
- 35.Smith RA, Mettlin CJ, Davis KJ, Eyre H. American Cancer Society guidelines for the early detection of cancer. CA Cancer J Clin. 2000;50(1):34–49. doi: 10.3322/canjclin.50.1.34. [DOI] [PubMed] [Google Scholar]
- 36.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 37.Schag CA, Ganz PA, Polinsky ML, Fred C, Hirji K, Petersen L. Characteristics of women at risk for psychosocial distress in the year after breast cancer. J Clin Oncol. 1993;11:783–793. doi: 10.1200/JCO.1993.11.4.783. [DOI] [PubMed] [Google Scholar]
- 38.Wapnir IL, Cody RP, Greco RS. Subtle differences in quality of life after breast cancer surgery. Ann Surg Oncol. 1999;6:359–366. doi: 10.1007/s10434-999-0359-y. [DOI] [PubMed] [Google Scholar]
- 39.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Charlson ME, Pompei P, Ales K, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 41.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 42.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 43.Radloff LS, Locke BZ. The community mental health assessment survey and CES-D Scale. In: Weissman MM, Myers JK, Ross CE, editors. Community surveys of psychiatric disorders. Rutgers University Press; New Brunswick: 1986. pp. 177–187. [Google Scholar]
- 44.Lautenschlager GJ. A comparison of alternatives to conducting Monte Carlo analyses for determining parallel analysis criteria. Multivariate Behav Res. 1989;24(3):365–395. doi: 10.1207/s15327906mbr2403_6. [DOI] [PubMed] [Google Scholar]
- 45.Velicer WF. Determining the number of components form the matrix of partial correlations. Psychometrika. 1976;41(3):321. [Google Scholar]
- 46.Olsen M, Lefta M, Dietz J, Brandt KE, Aft R, Matthews P, Mayfield J, Fraser VJ. Risk factors for surgical site infection after major breast operation. J Am Coll Surg. 2008;207(3):326–335. doi: 10.1016/j.jamcollsurg.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olsen MA, Chu-Ongsakul S, Brandt KE, Dietz JR, Mayfield J, Fraser VJ. Hospital-associated costs due to surgical site infection after breast surgery. Arch Surg. 2008;143(1):53–60. doi: 10.1001/archsurg.2007.11. [DOI] [PubMed] [Google Scholar]
- 48.El-Tamer MB, Ward BM, Schifftner T, Neumayer L, Khuri S, Henderson W. Morbidity and mortality following breast cancer surgery in women. Ann Surg. 2007;245(5):665–671. doi: 10.1097/01.sla.0000245833.48399.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]