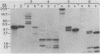
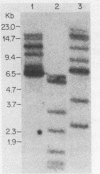
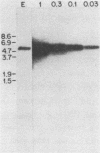
Abstract
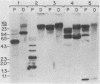
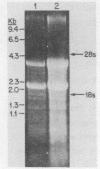
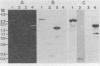
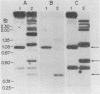
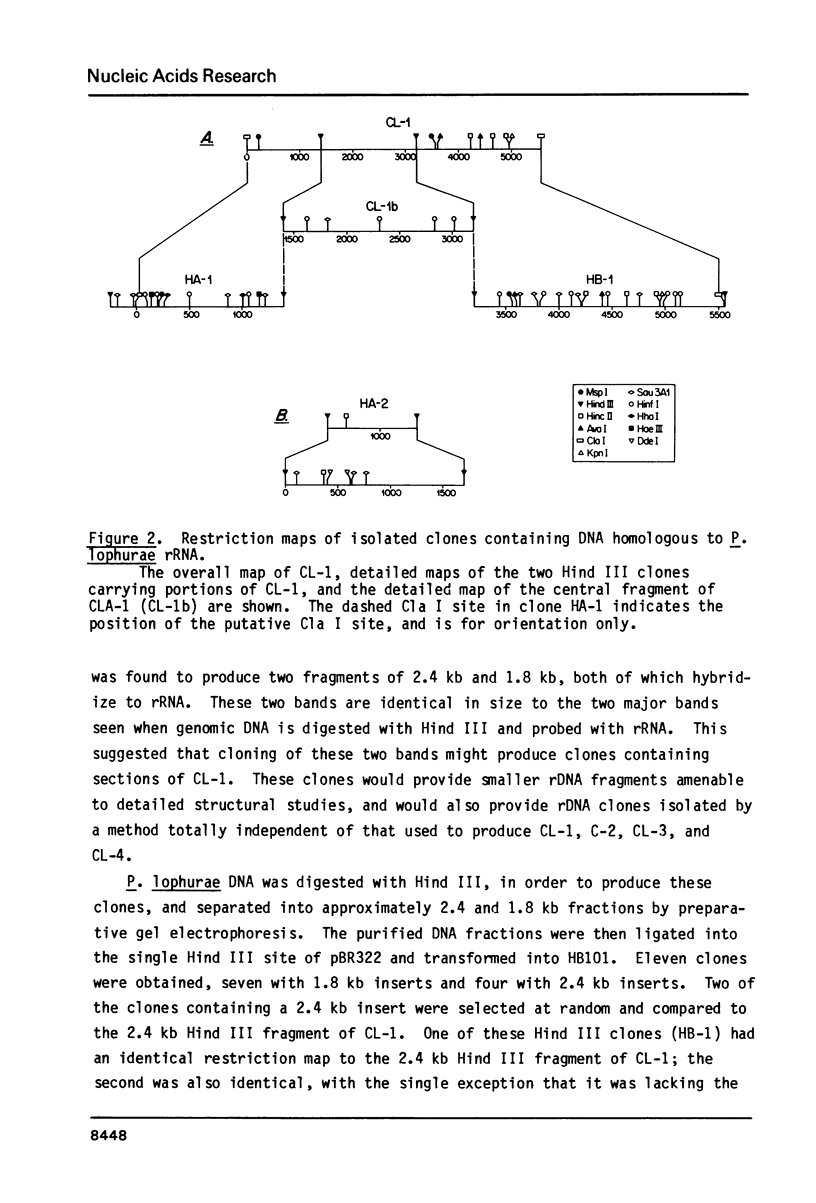
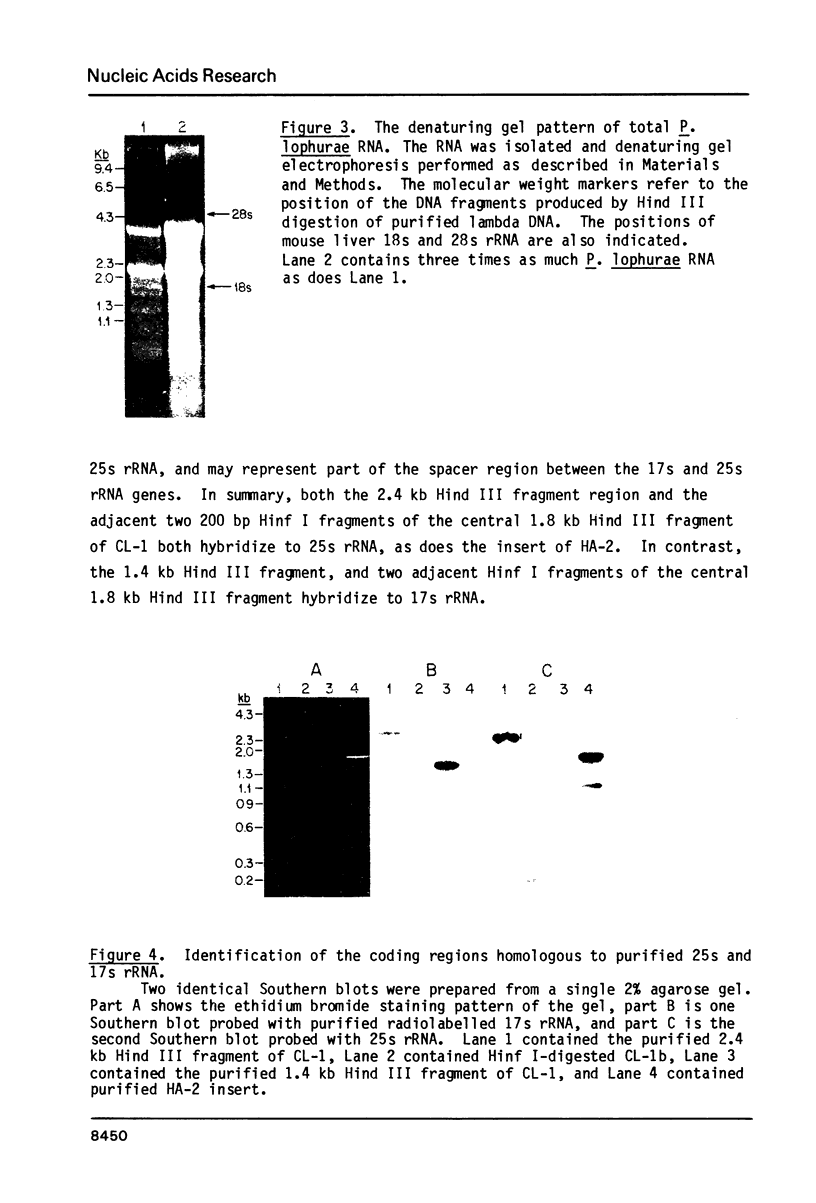
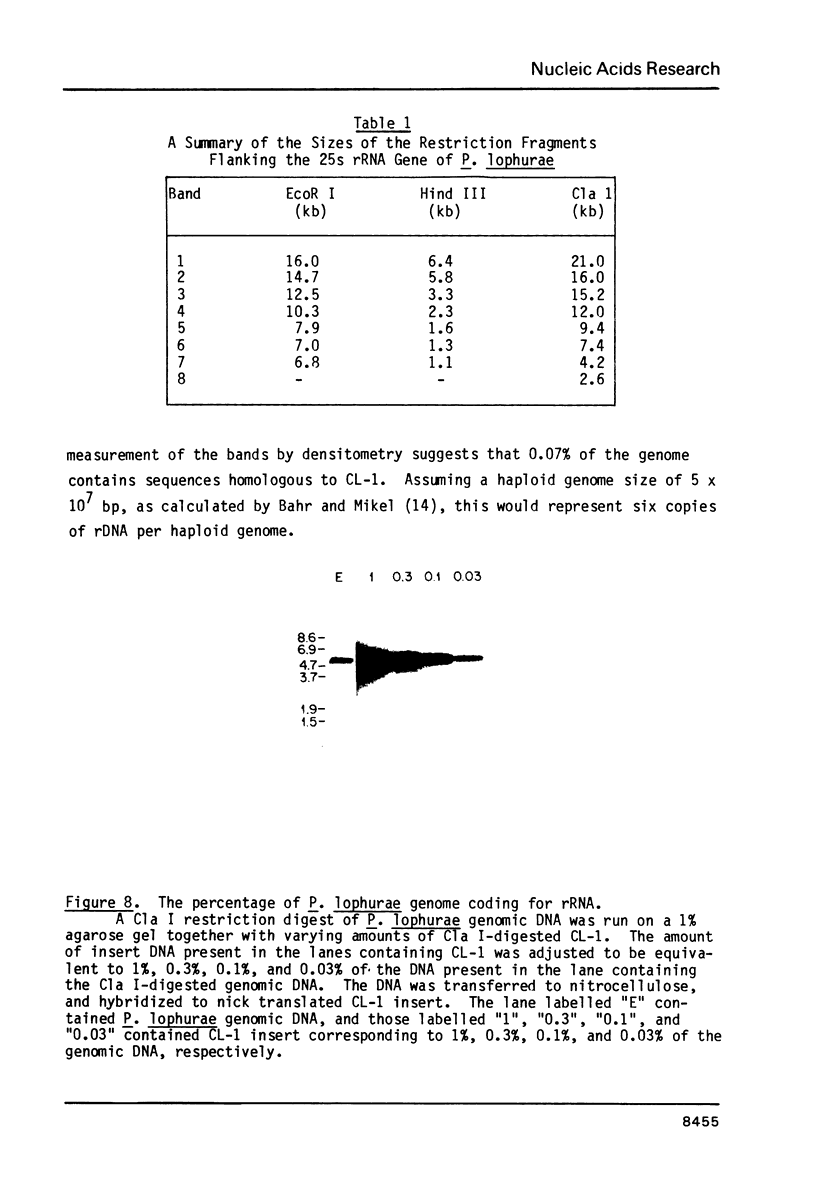
The structure and number of the ribosomal RNA (rRNA) genes of the avian malaria parasite Plasmodium lophurae has been examined using Southern blot analysis and recombinant DNA techniques. The ribosomal DNA (rDNA) of P. lophurae has been cloned into the plasmid pBR322, beginning with size-selected populations of Cla I- and Hind III-restricted parasite DNA. The structure of two clones (CL-1 and HA-2) is presented in detail. These two clones together probably represent the entire 17s and 25s coding regions of P. lophurae. Analysis of quantitative genomic Southern blots reveals that there are approximately six rRNA genes per haploid genome, and that the rRNA genes may be divided into four distinct classes by restriction analysis. Examination of the flanking regions of these genes indicates that they are not organized into easily recognizable tandem repeats.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheim N., Treco D., Taylor B., Eicher E. M. Distribution of ribosomal gene length variants among mouse chromosomes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4677–4680. doi: 10.1073/pnas.79.15.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boseley P. G., Tuyns A., Birnstiel M. L. Mapping of the Xenopus laevis 5.8S rDNA by restriction and DNA sequencing. Nucleic Acids Res. 1978 Apr;5(4):1121–1137. doi: 10.1093/nar/5.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boseley P., Moss T., Mächler M., Portmann R., Birnstiel M. Sequence organization of the spacer DNA in a ribosomal gene unit of Xenopus laevis. Cell. 1979 May;17(1):19–31. doi: 10.1016/0092-8674(79)90291-5. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Rio D. C. Localization of transcribed regions on extrachromosomal ribosomal RNA genes of Tetrahymena thermophila by R-loop mapping. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5051–5055. doi: 10.1073/pnas.76.10.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Gaubatz J., Prashad N., Cutler R. G. Ribosomal RNA gene dosage as a function of tissue and age for mouse and human. Biochim Biophys Acta. 1976 Feb 5;418(3):358–375. doi: 10.1016/0005-2787(76)90297-5. [DOI] [PubMed] [Google Scholar]
- Kopchick J. J., Cullen B. R., Stacey D. W. Rapid analysis of small nucleic acid samples by gel electrophoresis. Anal Biochem. 1981 Aug;115(2):419–423. doi: 10.1016/0003-2697(81)90027-0. [DOI] [PubMed] [Google Scholar]
- Leon W., Fouts D. L., Manning J. Sequence arrangement of the 16S and 26S rRNA genes in the pathogenic haemoflagellate Leishmania donovani. Nucleic Acids Res. 1978 Feb;5(2):491–504. doi: 10.1093/nar/5.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Collins M., Kiefer B. I., Dawid I. B. Expression of the ribosomal DNA insertions in bobbed mutants of Drosophila melanogaster. Mol Gen Genet. 1981;182(3):377–384. doi: 10.1007/BF00293925. [DOI] [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Expression of ribosomal DNA insertions in Drosophila melanogaster. Cell. 1979 Dec;18(4):1185–1196. doi: 10.1016/0092-8674(79)90231-9. [DOI] [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Repeated genes in eukaryotes. Annu Rev Biochem. 1980;49:727–764. doi: 10.1146/annurev.bi.49.070180.003455. [DOI] [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Restriction analysis of spacers in ribosomal DNA of Drosophila melanogaster. Nucleic Acids Res. 1979 Sep 11;7(1):205–215. doi: 10.1093/nar/7.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- RITOSSA F. M., SPIEGELMAN S. LOCALIZATION OF DNA COMPLEMENTARY TO RIBOSOMAL RNA IN THE NUCLEOLUS ORGANIZER REGION OF DROSOPHILA MELANOGASTER. Proc Natl Acad Sci U S A. 1965 Apr;53:737–745. doi: 10.1073/pnas.53.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schäfer M., Wyman A. R., White R. Length variation in the non-transcribed spacer of Calliphora erythrocephala ribosomal DNA is due to a 350 base-pair repeat. J Mol Biol. 1981 Feb 25;146(2):179–199. doi: 10.1016/0022-2836(81)90431-9. [DOI] [PubMed] [Google Scholar]
- Sherman I. W. Biochemistry of Plasmodium (malarial parasites). Microbiol Rev. 1979 Dec;43(4):453–495. doi: 10.1128/mr.43.4.453-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman I. W., Jones L. A. The Plasmodium lophurae (avian malaria) ribosome. J Protozool. 1977 May;24(2):331–334. doi: 10.1111/j.1550-7408.1977.tb00989.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]