Abstract
Background
5% to 8% of 70-year-olds and some 10% of persons over age 80 have atrial fibrillation (AF).
Methods
Selective literature review.
Results
New scoring schemes (CHA2DS2-VASc score, HAS-BLED score) have been introduced to enable more accurate estimation of the risk of stroke and hemorrhage in patients with AF. These scores are calculated on the basis of clinical data (left ventricular dysfunction, hypertension, age, diabetes, prior stroke, vascular diseases, sex, renal or hepatic dysfunction, bleeding, labile INR values, consumption of medications and alcohol) and are used to determine the potential indication for, and appropriate type of, anticoagulation in the individual AF patient. Hemodynamically unstable patients with rapid AF should undergo DC cardioversion at once. Patients with permanent AF should be given beta-blockers, calcium antagonists, or digitalis for rate control, with a target rate below 110/minute. A recently introduced drug, dronedarone, is used for rhythm control and has relatively few side effects. Patients with AF and impaired left ventricular function should be given amiodarone. Rhythm control has not been found to prolong life any more than rate control. Patients with a CHA2DS2-VASc score of 2 or above should be orally anticoagulated. Those with a score of 1 can be treated with aspirin (75 to 325 mg daily); those with a score of 0 do not need antithrombotic treatment. A HAS-BLED score of 3 or above is associated with a high risk of bleeding. Pulmonary vein isolation is an established method of treating symptomatic AF, with a success rate of 60% to 80%. Surgical procedures are possible in AF patients who need additional cardiac surgery.
Conclusion
The treatment strategy for AF must be individualized on the basis of the patient’s clinical manifestations. The mainstay of treatment is anticoagulation; the indication for anticoagulation depends on the patient’s age, underlying disease, and left ventricular function.
The prevalence of atrial fibrillation (AF) rises with advancing age, from 2% in persons over age 40 to 6% in persons over age 70 and about 15% in those over age 90 (1). The number of people with this cardiac arrhythmia has increased considerably during the past 30 years. In the United States, some 2.3 million people are affected. The aging of the population is certainly one reason, and improved detection of AF is another (2).
Methods
This review is based on the current guidelines of the German, European, and American cardiological societies (3, 4).
Acute treatment of atrial fibrillation
Electrical cardioversion to sinus rhythm
If the patient is hemodynamically unstable (i.e., has clinical manifestations of presyncope or shock), or if sinus rhythm has not returned after treatment with antiarrhythmic drugs, then electric direct-current (DC) cardioversion should be performed under sedation with a short-acting anesthetic (class I recommendation) (3). Biphasic cardioversion, with success rates over 90%, seems to be clearly superior to the application of monophasic current, although the available data from clinical trials on this question are still sparse (5). DC cardioversion, triggered by R spikes, should be carried out under short-acting general anesthesia and with continuous ECG monitoring, at an energy of 200 to 360 joules (monophasic) or 150 to 200 joules (biphasic). Electric cardioversion is now the preferred treatment for AF in Germany (Figures 1 and 2).
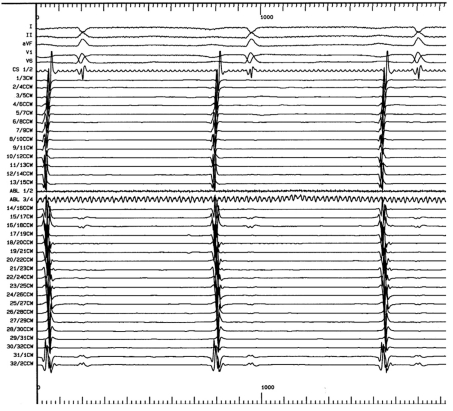
Figure 1.
Intracardiac recordings before pulmonary vein isolation. The observed potentials (electrograms) from the pulmonary veins serve as triggers for the initiation of atrial fibrillation. They are the target potentials in pulmonary vein isolation: The goal of ablation in the treatment of atrial fibrillation is to eliminate them entirely. The absence of these potentials is taken to indicate successful ablation
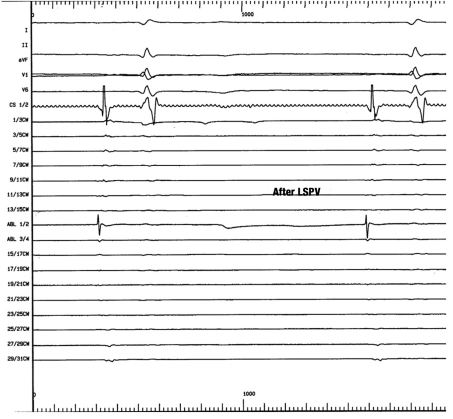
Figure 2.
Intracardiac recordings after pulmonary vein isolation. Total isolation of the pulmonary veins by interruption of the conducting pathways from the pulmonary veins to the left atrium (entrance block after ablation). The pulmonary vein electrograms that were seen before ablation (Figure 1) are no longer present; thus, pulmonary vein isolation is likely to be successful in this case. Pulmonary vein isolation is now an established technique, with success rates above 70%
LSPV, left superior pulmonary vein
Conversion to sinus rhythm by pharmacotherapy
Effective antiarrhythmic drugs for the pharmacological conversion of AF to sinus rhythm include substances of classes IA, IC, and III (Table I). Flecainide and propafenone take effect relatively rapidly; the use of either drug has been reported to yield cardioversion rates ranging from 40% to 60%. Amiodarone is considered less useful for cardioversion, partly but not only because of its slower effect. Ibutilide yields cardioversion rates of 50% to 70% but is not available in Germany.
Table 1. Medications for pharmacological cardioversion in atrial fibrillation*1.
| Drug | Dose | Follow-up dose | Risks |
| Amiodarone | 5 mg/kg IV in 1 hour | 50 mg/hourr | Hypotension |
| Flecainide | 2 mg/kg IV in 10 min,or 200–300 mg PO | – | Not for patients with SHD |
| Ibutilide | 1 mg IV in 10 min | 1 mg IV in 10 min after a 10-minute waiting period | QT prolongation;TdP |
| Propafenone | 2 mg/kg IV in 10 min,or 450–600 mg PO | – | Not for patients with SHD; QT prolongation; proarrhythmic effect (atrial flutter) |
| Vernakalant | 3 mg/kg IV in 10 min | 2 mg/kg IV in 10 min after a 15-minute waiting period | Not for patients with severe AS; SBP < 100 mmhg; nyha iii–iv ; qt interval >440 ms |
*1Modified from the guidelines of the European Society of Cardiology (3); AS, aortic stenosis; IV, intravenously; kg, kilograms; mg, milligrams; min, minutes; ms, milliseconds; PO, per os (by mouth); SBP, systolic blood pressure; SHD, structural heart disease; TdP, torsade-de-pointes tachycardia
Rate control in tachycardic permanent atrial fibrillation
When cardioversion with either drugs or electricity is not indicated, the goal of treatment is rate control. Effective drugs for rate control in chronic AF include digitalis, calcium-channel blockers of the verapamil class, diltiazem, and beta-blockers, either as monotherapy or in combination.
Anticoagulation
Oral anticoagulation with coumarins is an effective way to prevent stroke and thromboembolic events in persons with AF. A meta-analysis revealed that oral anticoagulation lowers the risk of stroke by 64% overall, or, stated another way, by 2.7% per year (6). In the ACTIVE-A trial, patients who did not take coumarin because of medical contraindications or personal objection, but instead took acetylsalicylic acid (ASA) and clopidogrel, had a 6.8% rate of “major vascular events” during a follow-up period of 3.6 years (mean), compared to 7.6% in patients who took ASA and placebo (p = 0.01). The annual stroke rates in these two groups were 2.4% and 3.3%, respectively (p<0.001). ASA is much less effective than clopidogrel for stroke prevention in AF patients; it plays only a secondary role, compared to coumarins, in the current guidelines of the European Society of Cardiology (3).
Anticoagulation before and after electrical or pharmacological cardioversion
Patients who have had AF for less than 48 hours have a low risk of thromboembolic events. Thus, patients who are currently in AF and who have had an ECG documenting sinus rhythm at some time in the past 48 hours, or whose AF-related symptoms clearly began less than 48 hours ago, would seem not to need either transesophageal cardiac ultrasonography to rule out a thrombus in the atrium or atrial appendage, or the periprocedural anticoagulation that accompanies it. There is nonetheless a class I recommendation to manage these patients in this way (3, 7). Low-molecular-weight heparin is at least as effective as either unfractionated heparin or oral anticoagulation and is considered an equally valid alternative in view of its greater practicality and controllability. Patients at increased risk of thromboembolic events (i.e., those who are over age 65 or have hypertension, diabetes mellitus, impaired left ventricular function, coronary heart disease, mitral valve dysfunction, or a history of thromboembolic events) should undergo three weeks of effective anticoagulation, and then either pharmacological cardioversion or electrical cardioversion with direct current (DC) shock (class I recommendation). In general, anticoagulation should be maintained for a further four weeks after cardioversion, to allow time for renormalization of atrial mechanical activity. The possible indication for permanent anticoagulation depends on the patient’s age, underlying cardiac disease, and left-ventricular pump function; these factors, taken together, are summarized in the CHADS2 score. The indication does not depend on whether AF is s paroxysmal, persistent, or permanent, as patients with all of these types of AF have a comparable risk of stroke.
Rhythm control or rate control?
In the PIAF trial, 252 patients with AF were treated with either diltiazem (125 patients) or amiodarone (127 patients) (8). There was no difference in mortality (two deaths, or 1.6%, in each group), but the mean heart rate was lower under treatment with diltiazem. On the other hand, patients receiving amiodarone had better exercise tolerance in a 6-minute walking test. The AFFIRM trial included 4060 elderly persons (age 69.7 ± 9.0 years, mean follow-up 3.5 years) at high risk of stroke or death who were randomly allotted to either “rate-limiting” or “rhythm-controlling” treatment (2027 and 2033 patients, respectively) (9). The primary endpoint, death of any cause, occurred at nearly the same frequency in both groups (310 patients with rate control, or 26%, vs. 356 patients with rhythm control, or 27%), but the patients receiving antiarrhythmic drugs suffered more frequent adverse events of various types: asystole (0.6% vs. <0.1%), torsade de pointes tachycardia (0.8% vs. 0.2%), central nervous system complications (8.9% vs. 7.4%), of which 7.1% vs. 5.5% were strokes. In the RACE trial, patients with persistent AF after electrical cardioversion were randomly allotted to receive either rate or rhythm control (256 and 266 patients, respectively) (10). After a mean follow-up interval of 3.5 years, the two groups did not differ significantly in the frequency of the composite primary endpoint, which was defined as cardiovascular death, thromboembolic complications, hemorrhage, and/or pacemaker treatment (18 patients in each group; 7.0% vs. 6.8%). Nonetheless, in this trial as in the AFFIRM trial, the rhythm-controlled patients had a markedly higher rate of arrhythmic complications than the rate-controlled-patients did (4.5% vs. 0.8%). The STAF and HOT CAFÉ trials yielded similar findings (11). In 2008, Roy et al. (12) reported on a trial of rhythm control versus rate control in a study population of 1376 patients with a left ventricular ejection fraction of 35% or less: cardiovascular death occurred in 27% of rhythm-controlled patients and in 25% of rate-controlled patients (hazard ratio 1.06) over a mean follow-up period of 37 months. The secondary endpoints were reached at comparable frequencies in the two groups: death of any cause 32% vs. 33%, stroke 3% vs. 4%, worsening of congestive heart failure 28% vs. 31%; combination of cardiovascular death, stroke, and worsening of congestive heart failure, 43% vs. 46%.
New developments
The classification of atrial fibrillation and its clinical manifestations
Beyond the classification of AF that has been commonly used to date, there is a newly defined entity called “persistent AF of long duration,” i.e., AF that has been present for more than a year; in such cases, treatment for the restoration of sinus rhythm should be attempted. There is also a new scoring system for the clinical manifestations of atrial fibrillation, which has been introduced by the European Heart Rhythm Association (EHRA).
EHRA I: asymptomatic state;
EHRA II: mild symptoms that do not affect everyday life;
EHRA III: marked symptoms that interfere with everyday activities;
EHRA IV: symptoms severe enough to make normal everyday life impossible (3).
The CHA2DS2-VASc score
The CHA2DS2-VASc score has been introduced as a means of estimating the risk of stroke in AF patients more precisely. This score is intended to be useful in identifying AF patients at low risk for stroke. It is based on the same predictive factors as the CHADS2 score, with three additional ones (Table 2):
Table 2. Definitions of the CHADS2 and CHA2DS2-VASc scores, with distribution of points*1.
| Score | Variable | Points |
| CHADS2 Score | ||
| C | Congestive heart failure | 1 point |
| H | Hypertension | 1 point |
| A | Age 75 or above | 1 point |
| D | Diabetes mellitus | 1 point |
| S2 | Prior stroke, TIA, or embolic event | 2 points |
| CHA2DS2-VASc Score | ||
| C | Congestive heart failure | 1 point |
| H | Hypertension | 1 point |
| A2 | Age 75 or above | 2 points |
| D | Diabetes mellitus | 1 point |
| S2 | Prior stroke, TIA, or embolic event | 2 points |
| V | Prior myocardial infarction and/or peripheral arterial occlusive disease | 1 point |
| A | Age 65–74 | 1 point |
| S | Female sex | 1 point |
TIA, transient ischemic attack;
*1Modified from the guidelines of the European Society of Cardiology (3)
Existing vascular disease
Age 65 to 74
Female sex.
It remains to be seen whether this more complex score will supplant the CHADS2 score for general use. It still needs to be validated.
The HAS-BLED score
This score is used to estimate the risk of hemorrhage. It is based on seven variables, each of which is scored in a point system, just as in the CHADS2 and CHA2DS2-VASc scores (Table 3). A total score of three or more points indicates a high hemorrhagic risk. When AF is to be treated with anticoagulant drugs, the HAS-BLED score indicates the risk of bleeding associated with the treatment.
Table 3. Definition of the HAS-BLED score, with point distribution*1.
| Score | Variable | Points |
| H | Hypertension | 1 point |
| A | Abnormal renal or hepatic function | 1–2 points |
| S | Prior stroke | 1 point |
| B | Bleeding | 1 point |
| L | Labile INR values | 1 point |
| E | Elderly, i.e., over age 65 | 1 point |
| D | Concomitant use of other drugs or alcohol | 1–2 points |
INR, International Normalized Ratio.
*1Modified from the guidelines of the European Society of Cardiology (3)
Pharmacological cardioversion
The newly approved drug vernakalant delays atrial conduction and prolongs the refractory period (Table 1). Its half-life is only two to three hours (13). In two trials, called ACT-I and ACT-III, its efficacy was tested against that of placebo in 390 hemodynamically stable patients with AF of short duration (3 hours to 7 days). In the ACT-I trial, vernakalant brought about conversion to sinus rhythm in 74 (51%) of the patients treated with this drug, compared to 3 (4%) of those who received a placebo (p<0.0001). Comparable results were obtained in the ACT-III trial, with cardioversion in 44 (51.2%) of the patients treated with vernakalant and 3 (3.6%) of those treated with placebo (p<0.0001).
The ACT-II trial, in contrast, dealt with the treatment of AF of 3 to 72 hours’ duration that had arisen at some time from 24 hours to 7 days after aortocoronary bypass grafting and/or an operation on the valves of the heart. The rates of cardioversion in such patients were 47% with vernakalant and 14% with placebo (p = 0.0001).
A further trial (the AVRO trial) compared vernakalant to amiodarone in the treatment of AF of 3 to 48 hours’ duration. There were 116 patients in each arm of the trial; the rates of conversion to sinus rhythm within 90 minutes were 51.7% with vernakalant and 5.2% with amiodarone, indicating significantly more rapid cardioversion with vernakalant. At present, however, the use of vernakalant is limited by its high cost of 453.47 euros per package (personal communication from the Drug Commission of the German Medical Association [Arzneimittelkommission der deutschen Ärzteschaft, AkdÄ], 12 January 2011).
Rhythm-maintaining treatment
Dronedarone, a new antiarrhythmic drug, was tested for the treatment of AF in the DAFNE trial: 800 mg of dronedarone per day significantly prolonged the interval to the first recurrence of AF compared to placebo (60 days vs. 5.3 days, p<0.001). Six months after the beginning of treatment, 35% of the patients taking dronedarone were in sinus rhythm, compared to 10% with placebo. Dronedarone in a dose of 800 mg per day was compared against placebo in two further trials, EURIDIS (612 patients) and ADONIS (625 patients). In the combined analysis of these two studies, dronedarone was found to double the mean time to the recurrence of AF compared to placebo (116 days vs. 53 days, p<0.01); at 12 months, 36% of the patients taking dronedarone and 25% of those taking placebo were in sinus rhythm (p<0.001). The rate-controlling effect of dronedarone (800 mg daily) was studied in the ERATO trial: in 174 patients, the mean heart rate dropped after 14 days of treatment by 11.7/min at rest, and by 24.5/min under stress (p<0.0001 for both findings).
In the ANDROMEDA trial, dronedarone (800 mg/day) was tested for the treatment of patients with congestive heart failure and ventricular dysfunction (ejection fraction ≤ 35%). The trial was stopped at seven months, because 25 deaths occurred in the dronedarone group (most of them due to worsened congestive heart failure), compared to 12 in the placebo group (p = 0.12). Because of the premature termination of the trial, these findings do not permit the drawing of any definitive conclusions. In the ATHENA trial, which included 4628 patients, the main endpoint was mortality on dronedarone (800 mg/day) compared to placebo (14). After 21 ± 5 months of observation, mortality was significantly lower in patients taking dronedarone (31.9% vs. 39.4%) (p<0.001).
In the DIONYSOS trial (504 patients), a comparison of dronedarone (800 mg/day) with amiodarone (200 mg/day) revealed that the combined endpoint “recurrent AF”/”premature discontinuation of drug because of side effects” was reached by 75% of patients taking dronedarone, and by 59% of those taking amiodarone (p<0.00001). AF recurred more often under dronedarone than under amiodarone (64% vs. 42%), but premature discontinuation of the drug was less common with dronedarone (39% vs. 45%, p = 0.13).
In a study published in the New England Journal of Medicine in December 2011, dromedarone was found to increase the rates of congestive heart failure, stroke, and cardiovascular death in comparison to placebo when it was given to treat atrial fibrillation of more than six months’ duration in patients over age 65 with cardiovascular risk factors. Such patients should not be treated with dromedarone.
In the author’s view, the current state of the evidence, after a relatively short period of observation of the effects of dronedarone, does not justify the prominent position accorded to this drug in the published guidelines.
Anticoagulation
Patients undergoing cardioversion need not be anticoagulated if the presence of an atrial thrombus has been excluded by transesophageal echocardiography, or if AF has been present for less than 48 hours (class IB recommendation) (3). In all other circumstances, the patient should be effectively anticoagulated for three weeks before and four weeks after cardioversion (International Normalized Ratio [INR] 2.0–3.0) (class IB recommendation).
According to the current ESC (European Society of Cardiology) guidelines, patients without risk factors (CHADS2 and CHA2DS2-VASc scores of 0) need not be anticoagulated for four weeks after cardioversion: “Anticoagulation should normally be continued for 4 weeks after a cardioversion attempt, except when atrial fibrillation is of recent onset and no risk factors are present“ (3).
The potential benefit of long-term anticoagulation must be weighed against the risk of hemorrhage: Patients with a CHA2DS2-VASc score of 2 or above should be treated with oral anticoagulation to an INR of 2.0 to 3.0 (class IA recommendation). For patients with a CHA2DS2-VASc score of 1, either oral anticoagulation or ASA (75 to 325 mg daily) can be given, although the former is favored. Patients with a CHA2DS2-VASc score of 0 can be given either ASA (75 to 325 mg daily) or no antithrombotic drug at all, according to the guidelines of the European Society of Cardiology (class IB recommendation). In patients with a HAS-BLED score of 3 or above, who are at high risk of hemorrhage, the risks of antithrombotic treatment with ASA or oral anticoagulation must be carefully weighed against its benefits (class IIa recommendation) (Table 4).
Table 4. The risk of stroke as a function of CHADS2 and CHA2DS2-VASc scores (in 1733 and 7329 patients, respectively)*1.
| Points | Number of patients | Annual risk of stroke |
| CHADS2-Score | ||
| 0 | 120 | 1,9 % |
| 1 | 463 | 2,8 % |
| 2 | 523 | 4,0 % |
| 3 | 337 | 5,9 % |
| 4 | 220 | 8,5 % |
| 5 | 65 | 12,5 % |
| 6 | 5 | 18,2 % |
| CHA2DS2-VASc-Score | ||
| 0 | 1 | 0 % |
| 1 | 422 | 1,3 % |
| 2 | 1 230 | 2,2 % |
| 3 | 1 730 | 3,2 % |
| 4 | 1 718 | 4,0 % |
| 5 | 1 159 | 6,7 % |
| 6 | 679 | 9,8 % |
| 7 | 294 | 9,6 % |
| 8 | 82 | 6,7 % |
| 9 | 14 | 15,2 % |
*1Modified from the guidelines of the European Society for Cardiology (3)
New anticoagulant drugs
The search for new anticoagulant drugs that can be given as an alternative to warfarin has been going on for years. In the SPORTIF-III trial, the rates of acute myocardial infarction with the oral thrombin inhibitor ximelagatran and with warfarin were not significantly different (1.1% vs. 0.6%); the same was found in the SPORTIF-V trial (1.0% vs. 1.4%) (15, 16). Ximelagatran was withdrawn from the market, however, because it induces abnormalities of liver function.
The RE-LY trial included 18 113 patients with atrial fibrillation of non-valvular origin. In these patients, the new direct thrombin inhibitor dabigatran (150 mg PO b.i.d.) led to a significant reduction of the rate of stroke and systemic embolic events (the combined primary endpoint), compared to warfarin (1.11%/year vs. 1.69%/year, p<0.001), while the rates of hemorrhage did not differ significantly in the two treatment groups (3.36%/year vs. 3.11%/year, p = 0.31) (17). The relative risk of achieving the primary endpoint for this dose of dabigatran, compared to warfarin, was 0.66. On the other hand, when dabigatran was given at a lower dose (110 mg PO b.i.d.), its therapeutic benefit over warfarin disappeared (rate of achieving the primary endpoint 1.53%, relative risk compared to warfarin 0.91), but severe hemorrhage became significantly less common than under warfarin (2.71%/year vs. 3.36%/year, p = 0.003). Dabigatran is now approved for the prevention of venous thromboembolism after operative joint replacement; on 1 September 2011, it was also approved for the prevention of stroke in patients with AF. In the first few weeks of clinical use of dabigatran after its approval, however, a number of fatal hemorrhages were observed that may have been causally related to this drug. Renal function should always be tested before dabigatran is given; if the creatinine clearance is lower than 30 mL/min, dabigatran is contraindicated.
The orally administered factor Xa inhibitor rivaroxaban was tested in the ROCKET-AF trial (“rivaroxaban once daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation“), a randomized, double-blind phase III trial that included 14 264 patients in 45 countries. The participating patients received either rivaroxaban once daily or warfarin in an adapted dose. With respect to the primary endpoint—stroke or systemic embolization outside the central nervous system—rivaroxaban was found to be at least as effective as warfarin in an intention-to-treat analysis (2.1%/year vs. 2.4%/year; p<0.001 for non-inferiority, p = 0.12 for superiority). Rivaroxaban was associated with significantly fewer intracranial hemorrhages, but also with significantly more gastrointestinal hemorrhages, than warfarin (0.5% vs. 0.7%, p = 0.02; 3.2% vs. 2.2%, p<0.001). Overall, the rates of major hemorrhage in the two groups were comparable (3.6% for rivaroxaban and 3.4% for warfarin, p = 0.58). Rivaroxaban was associated with statistically insignificant trends toward less frequent myocardial infarction (0.9% vs. 1.1%, p = 0.12) and lower overall mortality (1.9% vs. 2.2%, p = 0.07). This medication is given at a dose of 20 mg daily and has been approved for clinical use in Germany since December 2011.
Key Messages.
Atrial fibrillation (AF) is the most common arrhythmia in adults. Its prevalence is 5-8% among 70-year-olds and 10% or more among persons over age 80.
Although patients who have been in AF for less than 48 hours are at low risk for thromboembolic events, it is recommended that they should undergo transesophageal echocardiography to rule out thrombi in the atrium or atrial appendage, with periprocedural anticoagulation.
The extended CHA2DS2-VASc score was introduced alongside the CHADS2score to enable more accurate estimation of the risk of stroke in patients with AF.
The HAS-BLED score is a total of subscores relating to seven different variables. It yields an estimate of the risk of hemorrhage in patients with AF and can thus serve as an aid to decision-making about anticoagulation.
Patients with a CHA2DS2-VASc score of 2 or above should be orally anticoagulated to an INR of 2.0 to 3.0.
Acknowledgments
Translated from the original German by Ethan Taub, MD.
Footnotes
Conflict of interest statement
Prof. Trappe has received reimbursement of travel and lodging expenses from St. Jude Medical, Boston Scientific for ESC and DGK Mannheim. He has also received honoraria for scientific educational presentations from St. Jude Medical and Boston Scientific.
References
- 1.Sack S. Epidemiologie des Vorhofflimmerns. Herz. 2002;27:294–300. doi: 10.1007/s00059-002-2395-2. [DOI] [PubMed] [Google Scholar]
- 2.Khairallah F, Ezzedine R, Ganz LI, London B, Saba S. Epidemiology and determinants of outcome of admissions for atrial fibrillation in the United States from 1996 to 2001. Am J Cardiol. 2004;94:500–504. doi: 10.1016/j.amjcard.2004.04.068. [DOI] [PubMed] [Google Scholar]
- 3.Camm AJ, Kirchhof P, Lip GYH, et al. Guidelines for the management of atrial fibrillation. The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 4.2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline) A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:104–123. doi: 10.1161/CIR.0b013e3181fa3cf4. [DOI] [PubMed] [Google Scholar]
- 5.Trappe HJ, Meine M. Wie funktioniert und wie wirkt die biphasische Defibrillation? Intensivmed. 2003;40:147–157. [Google Scholar]
- 6.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 7.Klein AL, Grimm RA, Murray RD, et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001;344:1411–1420. doi: 10.1056/NEJM200105103441901. [DOI] [PubMed] [Google Scholar]
- 8.Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356:1789–1794. doi: 10.1016/s0140-6736(00)03230-x. [DOI] [PubMed] [Google Scholar]
- 9.AFFIRM Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 10.Van Gelder I, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 11.Opolski G, Torbicki A, Kosior DA, Szulc M, Wozakowska-Kaplon B, Kolodziej P, Achremczyk P. for the Investigators of the Polish HOT CAFE Trial. Rate control versus rhythm control in patients with nonvalvular persistent atrial fibrillation. Chest. 2004;126:476–486. doi: 10.1378/chest.126.2.476. [DOI] [PubMed] [Google Scholar]
- 12.Roy D, Talajic M, Nattel S, et al. for the Atrial Fibrillation and Congestive Heart Failure Investigators: Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 13.Heinzl S, Zylka-Menhorn V. Vernakalant: Vorhofselektive Kardioversion. Dtsch Arztebl. 2010;107(37) [Google Scholar]
- 14.Hohnloser SH, Crijns HJGM, Van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, Connolly SJ. for the ATHENA Investigators: Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668–678. doi: 10.1056/NEJMoa0803778. [DOI] [PubMed] [Google Scholar]
- 15.Olsson SB. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF-III): randomised controlled trial. Lancet. 2003;362:1691–1698. doi: 10.1016/s0140-6736(03)14841-6. [DOI] [PubMed] [Google Scholar]
- 16.Albers GW, Diener HC, Frison L, et al. Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomised trial. JAMA. 2005;293:690–698. doi: 10.1001/jama.293.6.690. [DOI] [PubMed] [Google Scholar]
- 17.Conolly SJ, Ezekowitz MD, Yusuf S, Eikeboom J, Oldgren P, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;3611:139–151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]