Abstract
Background
Cryopreserved ovarian tissue can be retransplanted to restore fertility after radiation or chemotherapy. To date, 15 live births after retransplantation have been reported worldwide. We report the first pregnancy and the first live birth after retransplantation in Germany.
Case report
A 25-year-old female patient received initial chemotherapy and radiation of the mediastinum for Hodgkin’s lymphoma in 2003 and suffered a relapse two years later. Ovarian tissue was laparoscopically removed and cryopreserved, and she was then treated with high-dose chemotherapy and stem cell transplantation. She remained in remission for 5 years and she could not conceive during this time. The cryopreserved ovarian tissue was thawed and laparoscopically retransplanted into a peritoneal pouch in the ovarian fossa of the right pelvic wall. Three months later, her menopausal symptoms resolved, and she had her first spontaneous menstruation. Six months after retransplantation, after two normal menstrual cycles, low-dose follicle stimulating hormone (FSH) treatment induced the appearance of a dominant follicle in the tissue graft. Ovulation was then induced with human chorionic gonadotropin (HCG), whereupon the patient conceived naturally. After an uncomplicated pregnancy, she bore a healthy child by Caesarean section on 10 October 2011. Histological examination of biopsy specimens revealed that the ovarian tissue of the graft contained follicles in various stages of development, while the original ovaries contained only structures without any reproductive potential.
Conclusion
This was the first live birth after retransplantation of cryopreserved ovarian tissue in Germany and also the first case with histological confirmation that the oocyte from which the patient conceived could only have come from the retransplanted tissue. In general, young women who will be undergoing chemotherapy and/or radiotherapy for cancer must be informed and counseled about the available options for fertility preservation.
The majority of young female patients who need radiotherapy or chemotherapy for treatment of a tumor are concerned about subsequent impairment of fertility (1). The ability to have children of their own is an important part of the quality of life they hope for after overcoming their disease (2). According to a survey in the USA, only half of American doctors give their female patients adequate information on preservation of fertility after oncological treatment (3). There are no published data on fertility counseling for young female cancer patients in Germany.
Alongside the well-established cryopreservation of oocytes and embryos, another option for preserving fertility is retransplantation of cryopreserved ovarian tissue (4). While cryopreservation of oocytes and embryos requires some 2 weeks of stimulation, removal of ovarian tissue can be carried out at any time and does not delay tumor treatment. The surgical technique for harvesting the ovarian tissue, the subsequent cryopreservation, and the first German case of successful retransplantation after completion of treatment for cancer have been described (4, 5). Around 15 live births following retransplantation of ovarian tissue have been reported by various teams across the world (6, 7), but before the case reported here there had been no pregnancy and birth after cryopreservation and retransplantation of ovarian tissue in Germany.
The patient
Hodgkin’s lymphoma (stage IIA according to the classification formulated at the Ann Arbor Conference in 1971) was diagnosed in a then 25-year-old woman in January 2003. The patient was initially treated with six cycles of ABVD (AdriamycinTM [doxorubicin]/bleomycin/vinblastine/DTIC) chemotherapy on days 1 and 15, in the framework of the HL-2 study, followed by involved-field irradiation of the mediastinum to a total dose of 30 Gy.
Follow-up imaging in August 2005 revealed a round pulmonary mass. Because the patient wanted to preserve her fertility, later that month tissue was laparoscopically removed from both ovaries for cryopreservation; the operation was carried out at the Department of Gynecology and Obstetrics, University Hospital Dresden. Using the facilities of the FertiPROTEKT network, the ovarian tissue was placed in precooled transport medium immediately after removal and transported in a special container to the Center for Gynecology and Obstetrics, Department of Endocrinology and Reproductive Medicine, University Hospital Bonn. There, the tissue was slow frozen, then cryopreserved with dimethyl sulfoxide as a cryoprotective agent and stored for later use (8, 9).
The patient then underwent chemotherapy according to the Dexa-BEAM protocol (BCNU/etoposide/cytarabine/melphalan) at the Department of Internal Medicine I, University Hospital Dresden. The pulmonary mass remained unaffected by two cycles of this regimen, so treatment continued with high-dose BEAM chemotherapy and autologous stem cell transplantation on 25 November 2005. However, this chemotherapy was followed by pronounced menopausal symptoms, so cyclic hormone substitution with a combination of estrogen and gestagen was commenced. This medication led to regular hormone withdrawal bleeding. On temporary discontinuation of hormone treatment in 2007 the patient experienced secondary amenorrhea with marked menopausal symptoms, so hormone substitution was recommenced. Clinical chemistry repeatedly showed primary ovarian insufficiency.
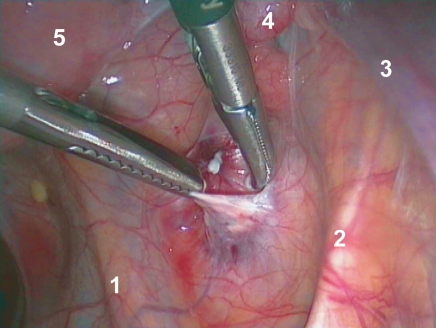
After over 5 years of freedom from recurrence, the patient requested retransplantation of the cryopreserved ovarian tissue to enable her to fulfill her wish to have a child. The cryopreserved material was therefore transported in a special container at a temperature of –196°C to the Department of Gynecology and Obstetrics, University Hospital Erlangen. There, on 24 June 2010, the ovarian tissue was thawed out and retransplanted into a peritoneal pouch in the area of the ovarian fossa on the right abdominal wall (Figure 1). Simultaneously, the patency of the fallopian tubes was tested by means of a dye solution (chromopertubation); both were immediately patent. Andrological investigation of the patient’s partner showed no limitations of fertility.
Figure 1.
Laparoscopic retransplantation of ovarian tissue in a peritoneal pouch of the ovarian fossa on the right abdominal wall near the hilum of the ovary (1, ureter; 2, lateral umbilical ligament; 3, infundibulopelvic ligament; 4, fallopian tube; 5, uterus)
Course after retransplantation
Following retransplantation the patient attended the Department of Gynecology and Obstetrics, University Hospital Dresden at intervals of around 2 weeks. Initially her secondary amenorrhea continued, albeit with marked amelioration of the menopausal symptoms. On 20 September 2010 her gonadotropins were in the normal range: serum estradiol 91.0 pg/mL, luteinizing hormone (LH) 7.7 IU/L, follicle stimulating hormone (FSH) 11.2 IU/L. The first spontaneous menstrual bleeding occurred exactly 3 months after retransplantation, lasting for 3 to 4 days. The next measurement of hormone levels on 5 October 2010 revealed serum estradiol 114 pg/mL, LH 3.9 IU/L, FSH 11.1 IU/L; thus, all endocrine parameters were in the normal range. Spontaneous menstruation without medication occurred again on 13 October 2010 and on 23 November 2010.
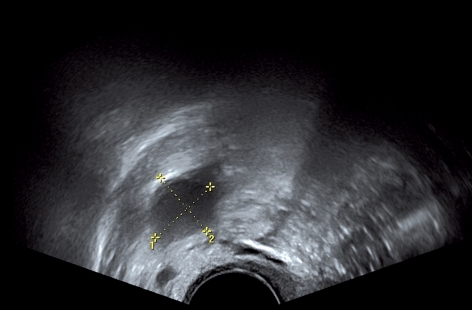
Because the patient wanted a child, and in the knowledge that retransplanted ovarian tissue remains viable for a limited time, her menstrual cycle was monitored and ovulation stimulated at the optimum time for conception (6, 7, 10). On 4 December 2010 and again on 31 December 2010, each time when there was a right-sided follicle with a sonographic diameter of 17 to 18 mm, ovulation was triggered with 5000 IU chorionic gonadotropin. However, pregnancy did not ensue on either occasion. In the third cycle, 6 months after retransplantation, the patient received daily subcutaneous injections of a low dose (25 IU) of FSH. On 22 January 2011, with a follicle 18 mm in diameter in the region of the transplant, ovulation was triggered by administering an intramuscular dose of 5000 IU chorionic gonadotropin (Figure 2).
Figure 2.
Dominant follicle in the area of the transplant at the time of triggered ovulation
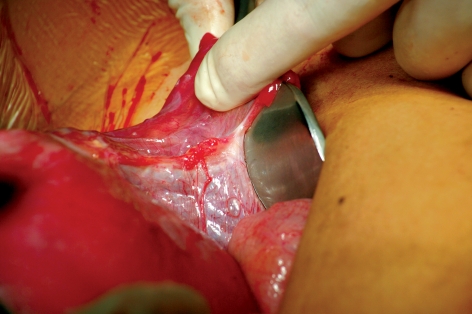
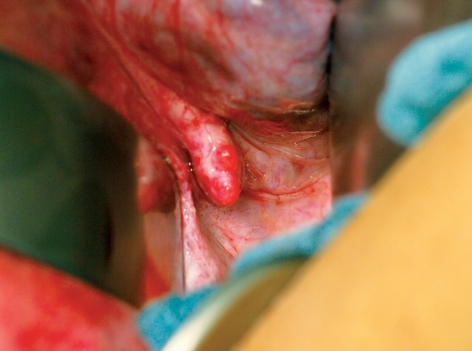
After the luteal phase had been supported with a daily dose of 600 mg progesterone, elevated levels of human chorionic gonadotropin (hCG) were measured on 8 February 2011 (155 IU/L) and 10 February 2011 (377 IU/L). On 28 February 2011 the fetus had a crown–rump length of 7.4 mm and its heartbeat was detected. First-trimester screening in the 12th week of gestation on 11 April 2011, including measurement of nuchal translucency, revealed no abnormalities. Detailed diagnostic sonography at the 21st week of gestation on 7 June 2011 showed a normal for gestational age fetus with normal sonographic anatomy. Pregnancy continued without incident. The patient had misgivings about spontaneous delivery because of her medical history, so we carried out a primary cesarean section on 10 October 2011. The patient was delivered of a healthy, mature male baby (Apgar 9/10/10, weight 3360 g, length 53 cm). In the course of the cesarean section the uterus was displaced towards the anterior abdominal wall to enable inspection of the ovaries and the retransplanted tissue in the right ovarian fossa. The ovaries were hardly recognizable as such; they were completely atrophied and could merely be identified as fibrotic, crescent-shaped strands of tissue in the adnexal area (Figure 3). In contrast, the retransplanted tissue on the right abdominal wall was clearly visible and follicles growing on the upper surface could be seen macroscopically (Figure 4). Tissue samples were taken from the retransplanted material and from the fibrotic ovarian structures and sent for histological analysis at the Institute of Pathology, University Hospital Erlangen. After the usual formalin fixation and embedding in paraffin, serial sections (3 µm) were prepared for complete tissue processing and then all sections were stained with hematoxylin and eosin. The biopsy samples from the retransplanted tissue displayed numerous follicles in various stages of development, while reproductively inactive tissue structures were found in both ovarian regions (Figure 5).
Figure 3.
Intraoperative view during cesarean section, showing residual right ovary: the right fallopian tube is being held up, revealing a crescent-shaped strand of tissue in place of the ovary
Figure 4.
Intraoperative view during cesarean section, showing retransplanted material on the right abdominal wall; small follicles are clearly seen in the area of the transplant, and ventrally a distinct venous complex in the right broad ligament
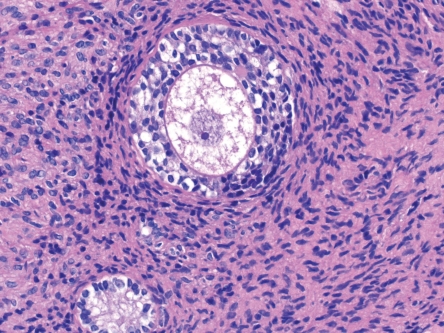
Figure 5.
Biopsy sample obtained from transplant during cesarean section, showing an intact secondary follicle with nucleus (hematoxylin-eosin staining, magnification 40×)
Discussion
We describe here the first birth after retransplantation of cryopreserved ovarian tissue in Germany, in a patient treated for Hodgkin’s lymphoma. This successful outcome was achieved by cooperation between physicians from three university hospitals in different parts of the country in the framework of a national network called FertiPROTEKT (www.fertiprotekt.com). For the first time anywhere in the world, histological examination revealed numerous follicles in all stages of development, and thus viability of the retransplanted ovarian tissue, after 5 years of cryopreservation. Given the pronounced atrophy and fibrosis of the original ovaries, the retransplanted material must be viewed as the source of the viable oocyte that resulted in the successfully completed pregnancy.
Sterility after successful treatment for cancer is a serious problem for young female patients who have not yet had any children at the time of diagnosis and treatment. Their quality of life is further impaired by the estrogen deficiency caused by the loss of ovarian function. It was thus worthwhile to explore the potential for protection of ovarian function by means of medication (contraceptives and GnRH analogs) during tumor treatment (11, 12), although the success of such measures has not been demonstrated conclusively; some authors are skeptical (13, 14).
Another option for preservation of fertility is cryopreservation of unfertilized or fertilized oocytes; both procedures are well established. However, German law permits fertilization of oocytes only when the woman concerned has a male partner. Moreover, both alternatives require 2 weeks of stimulation by gonadotropins, even when treatment can be started in the luteal phase (15). Therefore, there has to be a 2-week window before commencement of chemotherapy. If the cytostatic treatment cannot be delayed, however, enough ovarian tissue to enable cryopreservation of an adequate number of oocytes for a long period can be obtained by laparoscopy. Recommendations on how to proceed in the case of various malignant diseases have been drawn up as part of the FertiPROTEKT project (16) and can be found at www.fertiprotekt.de.
Little is known about the cellular mechanisms by which cytostatics lead to loss of the follicular apparatus or oocytes (17, 18). Only for alkylating agents has a direct, dose-dependent cytotoxic effect been described (19). High rates of amenorrhea have been reported, particularly in chemotherapy for Hodgkin’s lymphoma, where they reach 51% to 77% for the COPP (cyclophosphamide/Oncovin [vincristine]/procarbazine/prednisone) or ABVD (AdriamycinTM[doxorubicin]/bleomycin/vinblastine/DTIC) protocols or the escalated BEACOPP protocol (bleomycin/etoposide/AdriamycinTM [doxorubicin]/cyclophosphamide/OncovinTM [vincristine]/procarbazine/prednisone) (20, 21). Even one single application of cyclophosphamide is associated with dose-dependent impairment of ovarian function, and 8 Gy irradiation of the ovaries usually means complete radiomenolysis (18, 22). The extent to which fertility is affected and the potential for restoration of ovarian function depend mainly on the patient’s age, the chemotherapy regimen, and the radiation dose in the pelvic region (18, 22). However, the precise mechanisms that in some cases lead to restoration of ovarian function after chemotherapy are not yet known.
The ideal goal of measures to preserve fertility is to create conditions that primarily permit natural conception (23). This was the case in our patient; follicle maturation and ovulation took place in the transplant area, so pregnancy could occur by fully natural means. This requires orthotopic retransplantation of the tissue adjacent to the fallopian tube, patency of the tube, and normal andrological parameters. However, groups across the world have described pregnancy and birth following hormonal stimulation of the transplanted ovarian tissue and oocyte harvesting from the transplant by follicular aspiration in terms of in-vitro fertilization (24, 25, e1). Furthermore, several authors have reported pregnancy and birth in cases where one of a pair of monozygotic twins suffered premature ovarian failure syndrome and received repeated transplantations of ovarian tissue, or transplantation of a whole ovary, from the other twin (e2, e4).
It is unknown how many retransplantations of cryopreserved ovarian tissue have been carried out worldwide. Only case reports of pregnancy and birth have been published, so the actual pregnancy rate or “baby take home” rate cannot be established. However, the fact that all retransplantations in recent years have resulted in restoration of ovarian function can be interpreted as successful treatment (6, 7). Our own experience confirms this; all seven patients (n = 10 retransplantations) recommenced menstruation and had hormone levels in the normal range.
The majority of pregnancies reported after retransplantation of ovarian tissue have occurred spontaneously. The evolution of the hormone levels in each individual case confirms that the pregnancies can only have arisen from the area of the retransplanted tissue, as primary ovarian insufficiency was demonstrated anamnestically and by clinical chemistry (23, e5, e13).
It is theoretically feasible that the original ovarian tissue could be reactivated by the biological activity of the transplanted material. There is certainly some evidence suggesting that a critical mass of ovarian tissue is necessary for normal ovarian function. Pregnancies have been observed after heterotopic transplantation of ovarian tissue subcutaneously in the anterior abdominal wall; follicle development and ovulation was observed in the patients’ primarily insufficient ovaries (e12). Given the location of the transplanted tissues, these pregnancies must have arisen from the previously non-functioning original ovaries (e12). These cases support the hypothesis that transplantation of ovarian tissue can improve or even restore the function of the original ovaries.
The macroscopic and histological findings at the time of cesarean section clearly indicate that our patient’s fertilizable oocytes, and thus her pregnancy, arose from the retransplanted tissue. After 38 weeks of gestation follicles at various stages of maturity were histologically demonstrated in the transplant. This could not be shown in the previously published cases of retransplantation of cryopreserved ovarian tissue, because either the material was transplanted into an ovary, so it was no longer possible to differentiate between original ovary and transplant, or no histological investigations were carried out in the case of retransplantation into the abdominal wall. Thus, we are the first to prove, with this method of retransplantation, that the pregnancy can only have resulted from the retransplanted tissue.
Another important aspect of retransplantation is safety. Potentially, tumor cells could be transferred back to the patient or the tumor disease could be induced anew. However, preliminary findings in patients treated for Hodgkin’s lymphoma seem to confirm that retransplantation is safe in this scenario (e13, e14). The majority of successful retransplantations have been performed in women with Hodgkin’s lymphoma, and there have been no reports of recurrence to date.
The primary goal in any woman with a tumor is cure followed by long-term recurrence-free survival. Nevertheless, the patient’s subsequent quality of life must also be considered when planning treatment. In young female patients, this includes preservation of fertility so they can have children later on.
In summary, this is the first report of the birth of a child following retransplantation of cryopreserved ovarian tissue in Germany. Painstaking histological examination demonstrated that the pregnancy arose from the transplanted tissue. Conception was by natural means after low-dose follicular stimulation and triggering of ovulation.
In publishing this report, we hope we will motivate our colleagues to pay greater consideration to the potential for protection of fertility in the course of tumor treatment. All oncologists should be able to offer their female patients information on this subject or arrange counseling by members of the FertiPROTEKT network (www.fertiprotekt.de).
Key Messages.
This was the first German case of birth following retransplantation of ovarian tissue.
Histological examination demonstrated that the pregnancy arose from the transplanted tissue.
Conception was by natural means.
Three university departments of gynecology and obstetrics cooperated in this patient’s successful treatment.
All oncologists should pay attention to the fertility of young female patients.
Acknowledgments
Translated from the original German by David Roseveare.
The authors are grateful to the responsible physicians at the Department of Internal Medicine I (Chair: Prof. G. Ehninger), University Hospital Dresden for their kind cooperation.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–4183. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 2.West ER, Zelinski MB, Kondapalli LA, et al. Preserving female fertility following cancer treatment: current options and future possibilities. Pediatr Blood Cancer. 2009;53:289–295. doi: 10.1002/pbc.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn GP, Vadaparampil ST, Lee JH, et al. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. J Clin Oncol. 2009;27:5952–5957. doi: 10.1200/JCO.2009.23.0250. [DOI] [PubMed] [Google Scholar]
- 4.Dittrich R, Müller A, Binder H, et al. First retransplantation of cryopreserved ovarian tissue following cancer therapy in Germany. Dtsch Arztebl Int. 2008;105(15):274–278. doi: 10.3238/arztebl.2008.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller A, Oppelt PG, Renner SP, Hoffmann I, Beckmann MW, Dittrich R. Erste Retransplantation von kryokonserviertem Ovargewebe nach Krebserkrankung in Deutschland mit anschließender Eizellgewinnung und ICSI-Behandlung. Geburtsh Frauenheilk. 2009;69:124–130. [Google Scholar]
- 6.Donnez J, Silber S, Andersen CY, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43:437–450. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 7.Donnez J, Dolmans MM. Preservation of fertility in females with haematological malignancy. Br J Haematol. 2011;154:175–184. doi: 10.1111/j.1365-2141.2011.08723.x. [DOI] [PubMed] [Google Scholar]
- 8.Maltaris T, Dimmler A, Muller A, et al. The use of an open-freezing system with self-seeding for cryopreservation of mouse ovarian tissue. Reprod Dom Anim. 2005;40:250–254. doi: 10.1111/j.1439-0531.2005.00595.x. [DOI] [PubMed] [Google Scholar]
- 9.Maltaris T, Dimmler A, Muller A, Hoffmann I, Beckmann MW, Dittrich R. Comparison of two freezing protocols in an open freezing system for cryopreservation of rat ovarian tissue. J Obstet Gynaecol Res. 2006;32:273–279. doi: 10.1111/j.1447-0756.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 10.Dittrich R, Mueller A, Maltaris T, et al. Hormonal and histological findings in human cryopreserved ovarian autografts. Fertil Steril. 2009;91(4):1503–1506. doi: 10.1016/j.fertnstert.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Chapman RM, Sutcliffe SB. Protection of ovarian function by oral contraceptives in women receiving chemotherapy for Hodgkin’s disease. Blood. 1981;58:849–851. [PubMed] [Google Scholar]
- 12.Blumenfeld Z, Dann E, Avivi I, Epelbaum R, Rowe JM. Fertility after treatment for Hodgkin’s disease. Ann Oncol. 2002;1(13):138–147. doi: 10.1093/annonc/13.s1.138. [DOI] [PubMed] [Google Scholar]
- 13.Oktay K, Sönmezer M, Oktem O, Fox K, Emons G, Bang H. Absence of conclusive evidence for the safety and efficacy of gonadotropin-releasing hormone analogue treatment in protecting against chemotherapy-induced gonadal injury. Oncologist. 2007;12:1055–1066. doi: 10.1634/theoncologist.12-9-1055. [DOI] [PubMed] [Google Scholar]
- 14.Blumenfeld Z, von Wolff M. GnRH-analogues and oral contraceptives for fertility preservation in women during chemotherapy. Hum Reprod Update. 2008;14:543–552. doi: 10.1093/humupd/dmn022. [DOI] [PubMed] [Google Scholar]
- 15.von Wolff M, Thaler Cj, Frambach T, et al. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril. 2009;92:1360–1365. doi: 10.1016/j.fertnstert.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 16.von Wolff M, Montag M, Dittrich R, Denschlag D, Nawroth F, Lawrenz B. Fertility preservation in women-a practical guide to preservation techniques and therapeutic strategies in breast cancer, Hodgkin’s lymphoma and borderline ovarian tumours by the fertility preservation network FertiPROTEKT. Arch Gynecol Obstet. 2011;284:427–435. doi: 10.1007/s00404-011-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oktay K, Sönmezer M. Chemotherapy and amenorrhea: risks and treatment options. Curr Opin Obstet Gynecol. 2008;20:408–415. doi: 10.1097/GCO.0b013e328307ebad. [DOI] [PubMed] [Google Scholar]
- 18.Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53:727–739. doi: 10.1097/GRF.0b013e3181f96b54. [DOI] [PubMed] [Google Scholar]
- 19.Familiari G, Caggiati A, Nottola SA, Ermini M, Di Benedetto MR, Motta PM. Ultrastructure of human ovarian primordial follicles after combination chemotherapy for Hodgkin’s disease. Hum Reprod. 1993;8:2080–2087. doi: 10.1093/oxfordjournals.humrep.a137985. [DOI] [PubMed] [Google Scholar]
- 20.Kreuser ED, Klingmüller D, Thiel E. Diagnosis and prognosis of gonadal damage after chemotherapy and irradiation. Dtsch Med Wochenschr. 1992;117:1810–1817. doi: 10.1055/s-2008-1062514. [DOI] [PubMed] [Google Scholar]
- 21.Behringer K, Breuer K, Reineke T, et al. Secondary amenorrhea after Hodgkin’s lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2005;23:7555–7564. doi: 10.1200/JCO.2005.08.138. [DOI] [PubMed] [Google Scholar]
- 22.Dittrich R, Maltaris T, Hoffmann I, Oppelt PG, Beckmann MW, Mueller A. Fertility preservation in cancer patients. Minerva Ginecol. 2010;62:63–80. [PubMed] [Google Scholar]
- 23.Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 24.Meirow D, Levron J, Eldar-Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 25.Rosendahl M, Loft A, Byskov AG, et al. Biochemical pregnancy after fertilization of an oocyte aspirated from a heterotopic autotransplant of cryopreserved ovarian tissue: case report. Hum Reprod. 2006;21:2006–2009. doi: 10.1093/humrep/del140. [DOI] [PubMed] [Google Scholar]
- e1.Sánchez-Serrano M, Crespo J, Mirabet V, et al. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril. 2010;93 doi: 10.1016/j.fertnstert.2009.09.046. [DOI] [PubMed] [Google Scholar]
- e2.Silber SJ, Gosden RG. Ovarian transplantation in a series of monozygotic twins discordant for ovarian failure. N Engl J Med. 2007;356:1382–1384. doi: 10.1056/NEJMc066574. [DOI] [PubMed] [Google Scholar]
- e3.Silber SJ, DeRosa M, Pineda J, et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod. 2008;23:1531–1537. doi: 10.1093/humrep/den032. [DOI] [PubMed] [Google Scholar]
- e4.Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med. 2008;359:2617–2618. doi: 10.1056/NEJMc0804321. [DOI] [PubMed] [Google Scholar]
- e5.Silber SJ, Lenahan KM, Levine DJ, et al. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med. 2005;353:58–63. doi: 10.1056/NEJMoa043157. [DOI] [PubMed] [Google Scholar]
- e6.Demeestere I, Simon P, Buxant F, et al. Ovarian function and spontaneous pregnancy after combined heterotopic and orthotopic cryopreserved ovarian tissue transplantation in a patient previously treated with bone marrow transplantation: case report. Hum Reprod. 2006;21:2010–2014. doi: 10.1093/humrep/del092. [DOI] [PubMed] [Google Scholar]
- e7.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist. 2007;12:1437–1442. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- e8.Demeestere I, Simon P, Moffa F, Delbaere A, Englert Y. Birth of a second healthy girl more than 3 years after cryopreserved ovarian graft. Hum Reprod. 2010;25:1590–1591. doi: 10.1093/humrep/deq096. [DOI] [PubMed] [Google Scholar]
- e9.Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril. 2010;93 doi: 10.1016/j.fertnstert.2009.12.022. [DOI] [PubMed] [Google Scholar]
- e10.Andersen CY, Rosendahl M, Byskov AG, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- e11.Ernst E, Bergholdt S, Jørgensen JS, Andersen CY. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25:1280–1281. doi: 10.1093/humrep/deq033. [DOI] [PubMed] [Google Scholar]
- e12.Oktay K, Türkçüoðlu I, Rodriguez-Wallberg KA. Four spontaneous pregnancies and three live births following subcutaneous transplantation of frozen banked ovarian tissue: what is the explanation? Fertil Steril. 2011;95 doi: 10.1016/j.fertnstert.2010.07.1072. [DOI] [PubMed] [Google Scholar]
- e13.Kim SS, Radford J, Harris M, et al. Ovarian tissue harvested from lymphoma patients to preserve fertility may be safe for autotransplantation. Hum Reprod. 2001;16:2056–2060. doi: 10.1093/humrep/16.10.2056. [DOI] [PubMed] [Google Scholar]
- e14.Seshadri T, Gook D, Lade S, et al. Lack of evidence of disease contamination in ovarian tissue harvested for cryopreservation from patients with Hodgkin lymphoma and analysis of factors predictive of oocyte yield. Br J Cancer. 2006;94:1007–1010. doi: 10.1038/sj.bjc.6603050. [DOI] [PMC free article] [PubMed] [Google Scholar]