Abstract
DNA ligases are crucial for most DNA transactions, including DNA replication, repair and recombination. Recently, DNA ligase III (Lig3) has been demonstrated to be crucial for cell survival due to its catalytic function in mitochondria. This review summarizes these recent results and reports on a hitherto unappreciated widespread phylogenetic presence of Lig3 in eukaryotes, including in some organisms before the divergence of metazoa. Analysis of these putative Lig3 homologs suggests that many of them are likely to be found in mitochondria and to be critical for mitochondrial function.
Key words: genetics, molecular biology, DNA ligases, genome integrity, DNA repair
Introduction
DNA ligation is critical for joining DNA ends that arise during replication or recombination and from DNA damage.1,2 DNA ligases catalyze an energetically favorable multistep reaction in which adenosine 5′-monophosphate (AMP) is sequentially transferred from ATP or NAD+ to an active-site lysine on the enzyme and then to the 5′ phosphate of a DNA end; adenylation of DNA activates the 5′ phosphate for nucleophilic attack by a 3′ hydroxyl from another DNA end, displacing AMP and covalently joining the ends of two DNA strands.
Mammals have three ATP-dependent DNA ligases, Lig1, Lig3 and Lig4.1 Although they have varied accessory domains that specify particular functions, their catalytic core region (CC) and associated DNA binding domain (DBD) share significant sequence identity. Both Lig1 and Lig4 are found throughout eukaryotes. Lig1 is considered to be the critical ligase during replication of the nuclear genome, while Lig4 is critical for the repair of double-strand breaks (DSBs) by nonhomologous end joining (NHEJ). Lig3 has been implicated both in nuclear DNA repair and in mitochondrial genome maintenance.3,4 Although Lig3 has a more restricted phylogenetic distribution in eukaryotes than the other two ligases, it is found more broadly in eukaryotic groups than originally thought, as will be discussed below.
Lig3 is Essential for Cellular Viability due to its Role in Mitochondria
Recent work using both cell complementation and tissue-specific deletion in the animal has shed light on the role of Lig3.5–7 Lig3-null mouse embryos die at ∼8.5 dpc and previous attempts to generate Lig3-null cells were unsuccessful,8 suggesting that Lig3 is required for cell survival. To directly determine whether Lig3 is an essential gene in cells, we developed a pre-emptive complementation strategy in mouse embryonic stem (ES) cells (Fig. 1). For this approach, a Lig3KO/cKOneo+ cell line was constructed to contain one Lig3-null allele and one conditional allele in which loxP sites flank several Lig3 exons and a drug selection marker.7 A wild-type Lig3 transgene was stably integrated into the Lig3KO/cKOneo+ cells; Cre recombinase was then expressed to transform the endogenous Lig3 conditional allele into a second null allele. With this approach, drug sensitive clones (Lig3KO/KO) were readily obtained if the cells contained the integrated Lig3 transgene (Fig. 1A). However, drug sensitive clones were not obtained without prior transgene integration, proving that Lig3 is essential for cell survival.
Figure 1.
Strategy for pre-emptive complementation of Lig3-null mouse embryonic stem (ES) cells. DNA ligase transgenes (Tg) are stably introduced into Lig3 conditional cells to determine which allow the survival of Lig3KO/KO cells. In the parental Lig3KO/cKOneo+ cells, the Lig3KO allele is null due to deletion of exons 6–14 which encode the Lig3 catalytic core and the Lig3cKOneo+ allele is conditional, with LoxP sites flanking exons 6–14 and an intronic neomycin resistance gene (neo). Cre recombinase is transiently expressed and colonies are grown in normal (drug-free) media. Colonies are replica plated into media containing G418 to determine which had successfully undergone Cre-mediated recombination to delete the neo gene and hence had become Lig3KO/KO at the endogenous Lig3 locus; colonies that had retained the neo gene continue to grow in G418 and hence maintain the parental genotype (Lig3KO/cKOneo+). (A) A Lig3 transgene which expresses both nuclear and mitochondrial Lig3 allows the survival of Lig3KO/KO colonies. After Cre expression, both G418S colonies (with Cre deletion; Lig3KO/KO) and G418R colonies (parental, without Cre deletion; Lig3KO/cKOneo+) are recovered. (B) Expression of nuclear Lig3 from a transgene (NucLig3) does not allow the survival of Lig3KO/KO colonies, indicating that mitochondrial localization of Lig3 is critical for cell survival. Only G418R colonies (Lig3KO/cKOneo+) are recovered after Cre expression.
Lig3 encodes both nuclear and mitochondrial proteins. Evidence suggests that an alternate translation start site leads to a mitochondrial leader sequence (MLS) which targets the protein into mitochondria.9 To determine the basis for the cellular viability requirement for Lig3, preemptive complementation was taken with transgenes expressing either nuclear or mitochondrial Lig3 proteins.7 Expression of Lig3 deleted for the MLS, such that it only localizes to the nucleus (NucLig3), did not allow the formation of drug sensitive colonies, indicating that MLS deletion does not support the viability of Lig3KO/KO cells (Fig. 1B). As mutation of the activesite lysine in mitochondria-targeted Lig3 also did not permit cell survival, catalytic function of the protein is apparently essential. These results demonstrate a requirement for Lig3 in mitochondria. Consistent with our findings, deletion of Lig3 in the developing nervous system using Nestin-cre leads to ataxic mice with smaller brains; importantly, mitochondria have an abnormal morphology and a reduced DNA content.6 Not surprisingly, loss of Lig3 in the heart resulting from Ckmm-cre expression is also problematic.
We also explored the requirement for Lig3 in the nucleus of mouse ES cells. One approach to address this question was the use of heterologous ligases such as Lig1 for pre-emptive complementation. Lig1 was introduced into cells fused to an MLS to ensure its entry into mitochondria (Fig. 2A). This form of Lig1 (MtLig1), but not unmodified Lig1, was able to support cell survival (Fig. 2B), indicating that Lig3 itself is not essential in either the nucleus or mitochondria, but that it is essential because it provides DNA ligase activity to mitochondria. As Lig1 is already expressed in the nucleus from the endogenous Lig1 gene, the added MtLig1 activity is unlikely to be providing essential nuclear activity to compensate for loss of Lig3. However, to rule this out, MtLig1 was modified to be excluded from the nucleus by deletion of the nuclear localization signal (NLS) and addition of a nuclear export signal (NES). Exclusion from the nucleus was verified by examining the GFP fusion of the protein (MtLig1-ΔNLS-GFP-NES) (Figs. 2C and S1). Expression of this protein was able to support survival of Lig3-null cells, as indicated by the ability to efficiently obtain G418S colonies (Fig. 2B) that were devoid of Lig3 (Fig. 2D). These results indicate that nuclear Lig3 is not essential for cellular survival.
Figure 2.
Mitochondria-targeted Lig1 is sufficient for survival of Lig3-null cells. (A) Structure of Lig1 proteins expressed in Lig3 conditional cells. Mitochondria-targeted (Mt) proteins contain a Lig3 MLS (mitochondrial leader sequence); ΔNLS proteins are deleted for the Lig1 nuclear localization signal, and the NES protein contains a nuclear export signal derived from MAPKK. Each protein was also fused to GFP. (B) Mitochondria-targeted Lig1 is sufficient for survival of Lig3-null cells. Lig3 conditional cells expressing the various Lig1 proteins from transgenes were transfected with a Cre vector and colonies were obtained in normal media, which were then screened for survival in media containing G418. Total number of colonies screened and number of G418S clones obtained are indicated, along with the percent G418S. n, number of independent transgene-expressing clones tested. Data for MtLig1 ΔNLS GFP NES are new to this paper; the remaining data are from reference 7. (C) Live cell imaging to examine subcellular localization of the GFP-tagged Lig1 forms. Deletion of the Lig1 NLS results in reduced levels of the protein in the nucleus, but it is not absent. Addition of the potent NES signal, however, results in efficient export of the protein. Plasmids expressing the various GFP-tagged DNA ligases were transiently transfected into mouse ES cells and imaged to visualize protein localization. Overexpression in this way is sensitive for visualizing the protein but the levels of protein are much higher than in cells with integrated ligase transgenes. (D) Protein gel blot analysis showing loss of Lig3 in Lig3KO/KO clones stably expressing MtLig1 ΔNLS GFP NES.
Heterologous DNA Ligases can Substitute for Lig3 in Mitochondria
The ability of MtLig1 to rescue the survival of cells demonstrates that Lig3 itself is not essential for ligation activity in mitochondria. We further tested Lig4, the remaining DNA ligase known to exist in mammalian cells. As with Lig1, Lig4 is able to substitute for Lig3 in mitochondria when fused to an MLS (MtLig4; Simsek D and Jasin M, unpublished data). Interestingly, coexpression of a mitochondria-targeted XRCC4, the partner protein of Lig4, is not necessary for complementation despite the requirement for XRCC4 for stabilization of Lig4 in the nucleus, indicating that MtLig4 is sufficiently stable in mitochondria.
The smallest eukaryal ligase known is Chlorella virus DNA ligase (ChVLig; 298 amino acids), consisting solely of a CC with conserved motifs found in the ATP-dependent ligases.10 ChVLig is able to rescue the survival of Lig3-null cells, further implying that mitochondrial catalytic activity is sufficient for cell viability.7 Lig3-null cells rescued by MtChVLig, as with MtLig1, show no apparent defect in mitochondrial DNA maintenance or repair after treatment of cells with hydrogen peroxide (H2O2).
The other co-factor for DNA ligases is NAD+, use of which is usually restricted to prokaryotes.1 We observed that the Escherichia coli NAD-dependent ligase, LigA, was also able to rescue the survival of Lig3-null cells, indicating that DNA ligase cofactor preference is not strict in mammalian mitochondria.7 Previously, LigA and ChVLig were each introduced into Saccharomyces cerevisiae deleted for the endogenous DNA ligases, rescuing viability of the resultant strains with no obvious mitochondrial defects.11 The reverse experiment, replacement of a NAD-dependent DNA ligase with an ATP-dependent ligase, has also been performed in Salmonella typhimurium.12 Together, these results indicate that cofactor requirement does not restrict DNA ligase use.
Mitochondria are vital organelles of nucleated cells, as they generate the majority of the cellular energy through oxidative phosphorylation.13 During this process, reactive oxygen species are produced as unavoidable byproducts14 and have the potential to damage the mitochondrial genome. Several inherited diseases result from mutations in the mitochondrial genome itself or from mutations in nuclear genes that encode mitochondrial components, including DNA polymerase γ.15,16 Our studies raise the question whether mutations in Lig3 might be responsible for some of the human syndromes associated with defects in replication and/or repair of the mitochondrial genome.
In mammals, mitochondria are transmitted through the female germ line and mature oocytes contain at least 50,000 mitochondria.17 It seems possible that the store of maternal mitochondria could support cellular viability for a time after fertilization, which could account for the delay in embryonic lethality seen in Lig3-null mice.8 Our cellular results imply that directing Lig1 to the mitochondria would further prolong the survival of Lig3-null embryos. Whether it would completely rescue embryonic lethality and lead to healthy, fertile animals will be an important question to address.
Nuclear DNA Functions for Lig3 and the Role of Accessory Domains
Two accessory domains are found in Lig3, a CCHC zinc finger (ZnF) at its N terminus and a BRCT at the C terminus (Fig. 3).1 (Note: the BRCT is only found in the Lig3α isoform; the Lig3β isoform found in testis contains an alternate C terminus). While not required for Lig3 catalytic activity, the ZnF affects substrate specificity and catalytic rate.18,19 The Lig3 ZnF is related to ZnFs in poly(ADP-ribose) polymerase PARP1 and this domain interacts with PARP1.20,21 In response to DNA strand breaks, PARP1 uses NAD+ as a substrate to catalyze the ADP ribosylation of a number of proteins.22 The BRCT domain interacts with the BRCT domain of the XRCC1 protein, which is also involved in DNA single-strand break repair.3 Importantly, neither the ZnF nor the BRCT is essential for mitochondrial function.7
Figure 3.
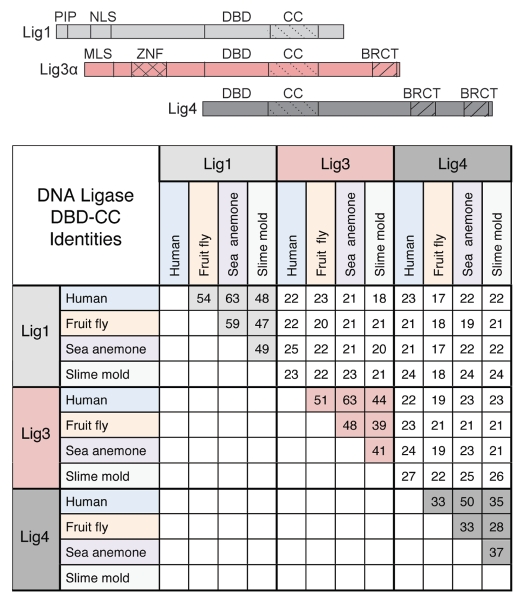
Sequence identity comparisons for the DBD-CC domain of the three DNA ligases from 4 diverse eukaryotes. Lig3 proteins are predicted based on conservation of the DBD-CC. The overall domain structure of the DNA ligases is shown on top. PIP, PCNA-interacting peptide; NLS, nuclear localization signal; DBD, DNA binding domain; CC, catalytic core; MLS, mitochondrial leader sequence; ZnF, zinc finger domain; BRCT, related to the C-terminal domain of BRCA1. Lig3a differs from Lig3β in containing a BRCT domain at the C terminus.
Strand break repair in the nucleus.
Interactions with XRCC1 and PARP1, as well as biochemical experiments, suggest a role for Lig3 in strand-break repair in the nucleus.1 To test this, Lig3KO/KO cells expressing MtLig1 were exposed to several DNA damaging agents, including H2O2 and ionizing radiation (IR).7 These cells were not any more sensitive than cells expressing Lig3, raising questions about the role of Lig3 in strand break repair. Parallel studies in mice using tissue-specific deletion of Lig3 in quiescent astrocytes gave similar results.6,23 In this case, alkaline comet assays were performed after treatment of cells with either H2O2 or IR. Whereas XRCC1-deficient cells showed increased comet tail moments with these agents, Lig3-deficient cells were no different from wild type.
To address which DNA ligase is crucial for DNA strand break repair, Gao et al. performed knockdowns in embryonic fibroblasts.6 Lig1 knockdown, but not knockdown of either Lig3 or Lig4, was found to reduce repair of H2O2 damage. However, the mean comet moment increased further with dual knockdown of Lig1 and Lig3, revealing a role for Lig3 in the absence of Lig1. How Lig1 works with XRCC1, and whether PCNA-XRCC1 interaction1 is important for this needs further investigation. Also, whether the nuclear repair role of Lig3 in the absence of Lig1 requires interaction with XRCC1 has yet to be tested. After IR, which creates mostly single-strand breaks but also DSBs, both Lig1 and Lig4 were found to be important for repair, and loss of both of these ligases resulted in a more severe phenotype. As with repair of H2O2-induced DNA damage, a role for Lig3 in repair of IR-induced damage was uncovered by concomitant knockdown of Lig1. Thus, Lig3 plays a role in nuclear DNA damage repair, but at least in these assays with mouse cells, its role is subsidiary to those of the other two ligases.
Alt-NHEJ and chromosomal translocations.
Lig3 has also been implicated in alternative NHEJ (alt-NHEJ), named as such to distinguish it from canonical NHEJ involving Lig4, which is critical for the repair of DSBs generated by IR.24,25 The cellular role of alt-NHEJ is not clear, although in mouse cells it is the primary route for the formation of chromosomal translocations after the induction of site-specific DSBs, in contrast to canonical NHEJ involving Lig4, which suppresses translocation formation.26 Using various Lig3-null cells, we determined that Lig3 is the primary DNA ligase that promotes translocation formation in mouse cells.5 This role for Lig3 in alt-NHEJ is true in otherwise wild-type or Lig4-deficient cells. In the absence of Lig3, Lig1 provides activity; thus, loss of both Lig3 and Lig1 leads to a strong reduction in translocation frequency. The roles of the two ligases in translocation formation are therefore reversed compared with strand break repair, where Lig1 is the primary ligase and Lig3 acts in the absence of Lig1.6
Analysis of translocation junctions revealed interesting findings regarding the use of microhomologies.5 Alt-NHEJ has a greater dependence on joining at microhomologies (sequence identities of 1 or a few bp) than expected by chance. Thus, translocation junctions in mouse cells show a fairly flat distribution of junctions having 0, 1, 2, 3 and 4 bp of microhomology. In the absence of Lig3, the junctions shift in distribution toward no microhomology, such that the 0 bp class predominates, as expected by the random chance of encountering microhomology during joining. These results are consistent with Lig3 joining ends that have base paired after DNA ends are resected to single strands. Like Lig3, loss of the resection factor CtIP reduces translocations and microhomology use, further implicating end resection in the joining events.27
Translocation experiments were also performed with Lig3 deleted for either the ZnF or BRCT.5 Loss of the BRCT had no effect on either the translocation frequency or the microhomology distribution for junctions, indicating that interaction with XRCC1 is not important for these alt-NHEJ events. However, loss of the ZnF reduced translocation frequency. Microhomology distribution was not altered, suggesting that the Lig3 ΔZnF was participating in translocations but that it was impaired kinetically. One class of junctions was notably altered, those with insertions, in that the insertions were significantly longer. Both of these results may be explained by the ability of the ZnF to promote intermolecular ligation in vitro,19 such that reduced kinetics of joining from the ZnF deletion could reduce the frequency of joining but also allow for longer polymerization giving rise to insertions in a subset of junctions.5
Evolutionary Conservation of Lig3
DBD-CC.
Lig3 was initially thought to have arisen in vertebrates.1 Database examination, however, indicates that Lig3 homologs exist in eukaryotes as diverged as slime mold (Figs. S2 and S3). This conclusion is based on comparisons of the DBD-CC for the three eukaryotic ligases (Fig. 3). For example, in human, fruit fly, sea anemone and slime mold, the sequence identities for the same DNA ligase ranges from ∼30–60% for the DBD-CC: Lig1 homologs have ∼50–60% sequence identity, Lig3 homologs have ∼40–60% identity and Lig4 homologs have ∼30–50% identity. By contrast, within any one organism, the sequence identity between pairs of different ligases is ∼20%.
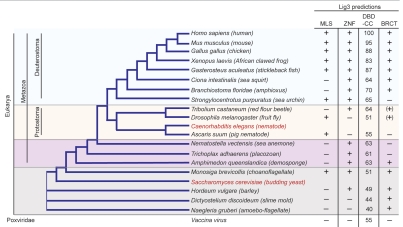
Lig3 homologs are identifiable in the genomes of most metazoans as well as in choanoflagellates, the closest relatives of metazoans,28 (Figs. 4, S2 and S3). Notably, an intact Lig3 is not evident in reported Caenorhabditis genomes, although it is observed in other nematodes, e.g., Ascaris and Brugia. What appears to be a remnant of Lig3 in the Caenorhabditis genomes contains a conserved DBD, but no CC, suggesting a deletion occurred within the gene prior to the divergence of the genus.
Figure 4.
Evolutionary conservation of Lig3. Predicted Lig3 homologs are found throughout many metazoan branches and also in organisms in other ancestral eukaryotic groups. It is absent from other eukaryotic groups, including most plants, as well as model organisms C. elegans and S. cerevisiae (in red). The Lig3 DBD-CC is related to the DNA ligase from poxviruses, such as vaccinia.
Lig3 accessory domains.
We compared the putative Lig3 homologs for conservation of ligase accessory domains (Figs. 4, S2 and S3). An MLS was predicted if there was an N-terminal extension upstream of the conserved domains and by use of a prediction program.29 In some cases, an N-terminal extension was not present in the annotated sequence but could be identified by examining genomic sequence upstream of the annotated ATG. A CCHC ZnF was scored based on the conservation and spacing of Zn binding residues (i.e., CXXCX30HXXC) and a BRCT, which usually contains a conserved WXXXC/S motif, was determined from the NCBI Conserved Domains and Protein Classification Database.30 Vertebrates have these three domains (MLS, ZnF and BRCT), but the only non-vertebrates in which all three domains were identified thus far are choanoflagellates. The most stripped down homolog is the vaccinia virus DNA ligase, which only contains the DBD-CC, as previously noted,31 although most non-metazoan proteins contained at least one accessory domains.
MLS.
The prediction of an MLS in Lig3 of some non-vertebrates (e.g., fruit flies) suggests that Lig3 provides DNA ligase activity in the mitochondria of these organisms as it does in mammals (Figs. 4, S2 and S3). In line with this, Lig1 in Drosophila does not have an N-terminal extension upstream of the PCNA-interacting peptide (PIP) box, as does Lig1 in Saccharomyces cerevisiae,32 to function in mitochondrial transport. However, a PIP box sequence is also at the immediate N terminus of Lig1 in other organisms where Lig3 does not have an obvious MLS (e.g., amphioxus),33 making it uncertain which ligase is to be found in mitochondria in these organisms. Given the lack of a clear understanding of the signals for mitochondrial transport,34 localization of Lig3 to mitochondria in such organisms cannot be ruled out. Further, mistakes in genome annotations could also confound MLS predictions for either Lig3 or Lig1.
PARP1-related ZnF.
A well-conserved CCHC ZnF is evident in Lig3 homologs of most metazoans and the near neighbor, the choanoflagellate Monosiga (Figs. 4 and S2–S4). Fruit flies are an exception, as the putative MLS abuts the DBD, although Tribolium and other insect Lig3 homologs (honeybee, wasp, louse, tick and pea aphid) have a ZnF. As mentioned previously, this domain in the human ZnF binds DNA to stimulate nick joining and intermolecular ligation,19 which may facilitate an alt-NHEJ pathway in these organisms.5 Binding to PARP1,21 which is found in the genome of metazoans and Monosiga, is also another potential function of the ZnF.
BRCT.
A BRCT is predicted in Lig3 homologs of several non-vertebrates as well as vertebrates (Figs. 4 and S2–S4), suggesting a possible conserved interaction with XRCC1.3 Lig3 and XRCC1 are annotated in a similarly limited set of eukaryotic genomes, consistent with an overlapping role for these proteins.
Additional Lig3 motifs not found in vertebrate Lig3.
Two other domains are identifiable in some non-vertebrate Lig3 proteins, both of which have been reported to bind poly(ADP-ribose). A CCHH ZnF is found C-terminal to the DBD-CC in several organisms. This ZnF differs from the PARP1-like CCHC ZnF in both the arrangement of cysteine and histidine residues and also in the spacing between them (Figs. S2 and S4). Instead, it is related to the tandem ZnFs in APLF, an XRCC1 binding protein, which promote APLF localization to DNA damage sites.35 Of note, the ZnFs of APLF bind poly(ADP-ribose), such that they have been termed PBZ, for poly(ADP-ribose)-binding ZnF.36 A macrodomain, which also binds poly(ADP-ribose),37 is predicted in the slime mold protein (Figs. S2 and S4). This domain is found in a variety of proteins, including some PARPs.
Lig3 in other ancestral eukaryotic groups.
Beyond the ancestral eukaryotic groups which include metazoans, choanoflagellates and slime mold, a putative homolog of Lig3 is found in the free-living amoeba-flagellate, Naegleria gruberi (Figs. 4 and S2).38 The reported sequence contains an insertion between the DBD and CC relative to that of other Lig3 proteins. The CC of the putative Naegleria protein shares 46% identity with the human protein, a little less than the CC from Dictyostelium (51%), which is otherwise the most distant Lig3 from the human protein. The Naegleria protein is most similar in domain organization to that of Dictyostelium in that it does not contain a ZnF but does contains both a potential ADP-ribose binding module and a BRCT domain C-terminal to the DBD-CC.
A homolog to Lig3 has also been recently reported in a cDNA collection from the barley Hordeum vulgare (Figs. 4 and S2).39 In addition to a conserved DBD-CC, the protein is predicted to have an N-terminal ZnF and a C-terminal BRCT, although not an N-terminal extension that could encode an MLS. Lig3 homologs, however, do not appear to be present in other plants, like Arabidopsis, whose genomes have been sequenced, and the barley genome sequence has yet to be reported to confirm the cDNA find. However, if confirmed, the presence of Lig3 in plants further indicates its wide phylogenetic distribution, although it remains possible that the barley Lig3 arose through horizontal gene transfer.
Conservation of Lig3.
With the recent reports from the Naegleria and barley sequencing efforts, potential Lig3 homologs have been identified thus far in four ancestral eukaryotic groups. Lig3 homologs have yet to be identified in two other ancestral groups that include organisms as diverse as Paramecium and Giardia, although the latter does not have mitochondria.40 Thus, genome sequencing provides evidence that Lig3 arose much earlier in eukaryotic evolution than previously suspected. Although deciphering the root of eukaryotes is still a challenge, its presence in several ancestral groups suggests that Lig3 arose very early in eukaryotes. However, the absence of Lig3 from many eukaryotes also indicates that it was lost at several times during evolution. In some cases, this may be part of a global loss of genes that occurs in parasitic organisms (e.g., trypansomes and Giardia). In other cases the reason is not evident, as related organisms can differ in the presence of Lig3 (Ascaris vs. Caenorhabditis; Drosophila vs. Anopheles).
Despite its wide presence in eukaryotic groups, Lig3 is nonetheless absent from large groups of organisms, including fungi and most protists. For example, whereas Lig1 is present in the annotated genomes of most organisms, Lig3 is present in only about one third of the genomes listed in the KEGG database (www.genome.jp/kegg). The prevalence of Lig1 is consistent with a requirement in nuclear DNA replication. Given the yeast paradigm41 and the ability of MtLig1 to provide mitochondrial ligase activity to Lig3-null mouse cells,7 it seems likely that Lig1 provides mitochondrial DNA ligase activity in those organisms without a Lig3 homolog. Notably, Lig1 homologs from several plants (Arabidopsis, grape, corn, rice) are predicted to have an MLS, suggesting that Lig1 provides mitochondrial DNA ligase activity in these organisms. (It also remains possible that Lig1 provides mitochondrial activity in some organisms that have Lig3). Lig4 is also a candidate mitochondrial DNA ligase, although MLS sequences are not obvious for the Lig4 homologs we have examined.
If either Lig1 or Lig3 can function in mitochondrial DNA metabolism the question arises as to whether either can function interchangeably in the nucleus. In addition to the widespread presence of Lig1 in eukaryotes, several lines of biochemical and cellular evidence suggest that Lig1 is specialized for DNA replication. For example, Lig1 binds PCNA.42 And although Lig1 is missing from databases of a few organisms (chicken, turkey, dog), genome annotation is likely to be the problem, rather than actual absence of Lig1. For example, in the cases of chicken and turkey, a likely explanation for the lack of an annotated Lig1 is the lack of sequence data for some of their microchromosomes, since the syntenic region that encompasses human Lig1 is mostly absent from the annotated genome sequences.43,44 (XRCC1 is likely absent from their genomes for the same reason).
Given the spotty presence of Lig3 in eukaryotes and the much more universal presence of Lig1, why is Lig3 retained in as many organisms as it is and why are the accessory domains as conserved as they are if they are not required for mitochondrial activity? Thus far, Lig3 appears to be mostly present in metazoan genomes, suggesting that the presence of a second DNA ligase may be advantageous to the metazoan state. Lig1 is expressed at a high level in proliferating cells, in line with its role in DNA replication, whereas Lig3 has been reported to be expressed at similar levels in proliferating and quiescent cells (reviewed in ref. 45). For cells that have exited the cell cycle, having a second DNA ligase which can be independently regulated may be advantageous for providing mitochondrial ligase activity in non-dividing cells. The nuclear activities of Lig3, i.e., backup to Lig1 in single-strand break repair and in alt-NHEJ, are likely to be physiologically important under some circumstances. As for the domains, the ZnF, while not required, enhances alt-NHEJ,5,19 and the BRCT interaction with XRCC1 is likely to be important for Lig3 activity in single-strand break repair.3 It is important to note that the ability of MtLig1 to substitute for Lig3 has only been tested in a cell line; as yet, it is not certain whether MtLig1 can rescue the viability of Lig3-null mice.
As with Lig1, Lig4 is also more widespread evolutionarily than Lig3 and seems to be present in all organisms that contain Lig3, consistent with Lig4 being the principle NHEJ ligase. (One exception is platypus, where a Lig3 homolog is evident but Lig4 is not). The organisms in which Lig4 is absent include parasites and some used in fermentation, which have adapted genomes. The retention of Lig4 in most eukaryotes is in line with the notion that canonical NHEJ is less error prone and more efficient than alt-NHEJ.
Conclusions
A wide phylogenetic distribution for Lig3 in eukaryotes was unexpected, given its absence from several model organisms. The recently reported knockout approaches for Lig3 in both mouse cell lines and tissues have now provided a framework in which one can understand its roles in both the mitochondrion and nucleus in a number of organisms.
Acknowledgments
We thank Mansi Srivastava for helpful discussions and Jerome Artus for help with live cell imaging. This work was supported by National Institutes of Health grant GM54668 (to M.J.).
Supplementary Material
References
- 1.Ellenberger T, Tomkinson AE. Eukaryotic DNA ligases: structural and functional insights. Annu Rev Biochem. 2008;77:313–338. doi: 10.1146/annurev.biochem.77.061306.123941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuman S. DNA ligases: progress and prospects. J Biol Chem. 2009;284:17365–17369. doi: 10.1074/jbc.R900017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldecott KW, McKeown CK, Tucker JD, Ljungquist S, Thompson LH. An interaction between the mammalian DNA repair protein XRCC1 and DNA ligase III. Mol Cell Biol. 1994;14:68–76. doi: 10.1128/mcb.14.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakshmipathy U, Campbell C. Antisense-mediated decrease in DNA ligase III expression results in reduced mitochondrial DNA integrity. Nucleic Acids Res. 2001;29:668–676. doi: 10.1093/nar.29.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simsek D, Brunet E, Wong SY, Katyal S, Gao Y, McKinnon PJ, et al. DNA ligase III promotes alternative nonhomologous end joining during chromosomal translocation formation. PLoS Genet. 2011;7:1002080. doi: 10.1371/journal.pgen.1002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao Y, Katyal S, Lee Y, Zhao J, Rehg JE, Russell HR, et al. DNA ligase III is critical for mtDNA integrity but not Xrcc1-mediated nuclear DNA repair. Nature. 2011;471:240–244. doi: 10.1038/nature09773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simsek D, Furda A, Gao Y, Artus J, Brunet E, Hadjantonakis AK, et al. Crucial role for DNA ligase III in mitochondria but not in Xrcc1-dependent repair. Nature. 2011;471:245–248. doi: 10.1038/nature09794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puebla-Osorio N, Lacey DB, Alt FW, Zhu C. Early embryonic lethality due to targeted inactivation of DNA ligase III. Mol Cell Biol. 2006;26:3935–3941. doi: 10.1128/MCB.26.10.3935-41.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakshmipathy U, Campbell C. The human DNA ligase III gene encodes nuclear and mitochondrial proteins. Mol Cell Biol. 1999;19:3869–3876. doi: 10.1128/mcb.19.5.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho CK, Van Etten JL, Shuman S. Characterization of an ATP-dependent DNA ligase encoded by Chlorella virus PBCV-1. J Virol. 1997;71:1931–1937. doi: 10.1128/jvi.71.3.1931-1937.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sriskanda V, Schwer B, Ho CK, Shuman S. Mutational analysis of Escherichia coli DNA ligase identifies amino acids required for nick-ligation in vitro and for in vivo complementation of the growth of yeast cells deleted for CDC9 and LIG4. Nucleic Acids Res. 1999;27:3953–3963. doi: 10.1093/nar.27.20.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park UE, Olivera BM, Hughes KT, Roth JR, Hillyard DR. DNA ligase and the pyridine nucleotide cycle in Salmonella typhimurium. J Bacteriol. 1989;171:2173–2180. doi: 10.1128/jb.171.4.2173-2180.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexeyev MF. Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS J. 2009;276:5768–5787. doi: 10.1111/j.1742-4658.2009.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan SS, Copeland WC. DNA polymerase gamma and mitochondrial disease: understanding the consequence of POLG mutations. Biochim Biophys Acta. 2009;1787:312–319. doi: 10.1016/j.bbabio.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuppen HA, Blakely EL, Turnbull DM, Taylor RW. Mitochondrial DNA mutations and human disease. Biochim Biophys Acta. 2010;1797:113–128. doi: 10.1016/j.bbabio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Shoubridge EA, Wai T. Mitochondrial DNA and the mammalian oocyte. Curr Top Dev Biol. 2007;77:87–111. doi: 10.1016/S0070-2153(06)77004-1. [DOI] [PubMed] [Google Scholar]
- 18.Mackey ZB, Niedergang C, Murcia JM, Leppard J, Au K, Chen J, et al. DNA ligase III is recruited to DNA strand breaks by a zinc finger motif homologous to that of poly(ADP-ribose) polymerase. Identification of two functionally distinct DNA binding regions within DNA ligase III. J Biol Chem. 1999;274:21679–21687. doi: 10.1074/jbc.274.31.21679. [DOI] [PubMed] [Google Scholar]
- 19.Cotner-Gohara E, Kim IK, Tomkinson AE, Ellenberger T. Two DNA-binding and nick recognition modules in human DNA ligase III. J Biol Chem. 2008;283:10764–10772. doi: 10.1074/jbc.M708175200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei YF, Robins P, Carter K, Caldecott K, Pappin DJ, Yu GL, et al. Molecular cloning and expression of human cDNAs encoding a novel DNA ligase IV and DNA ligase III, an enzyme active in DNA repair and recombination. Mol Cell Biol. 1995;15:3206–3216. doi: 10.1128/mcb.15.6.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leppard JB, Dong Z, Mackey ZB, Tomkinson AE. Physical and functional interaction between DNA ligase IIIalpha and poly(ADP-Ribose) polymerase 1 in DNA single-strand break repair. Mol Cell Biol. 2003;23:5919–5927. doi: 10.1128/MCB.23.16.5919-27.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 23.Katyal S, McKinnon PJ. Disconnecting XRCC1 and DNA ligase III. Cell Cycle. 2011;10:2269–2275. doi: 10.4161/cc.10.14.16495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieber MR. The Mechanism of Double-Strand DNA Break Repair by the Nonhomologous DNA End joining Pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Rosidi B, Perrault R, Wang M, Zhang L, Windhofer F, et al. DNA ligase III as a candidate component of backup pathways of nonhomologous end joining. Cancer Res. 2005;65:4020–4030. doi: 10.1158/0008-5472.CAN-04-3055. [DOI] [PubMed] [Google Scholar]
- 26.Simsek D, Jasin M. Alternative end joining is suppressed by the canonical NHEJ component Xrcc4-ligase IV during chromosomal tran slocation formation. Nat Struct Mol Biol. 2010;17:410–416. doi: 10.1038/nsmb.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Jasin M. An essential role for CtIP in chromosomal translocation formation through an alternative end joining pathway. Nat Struct Mol Biol. 2011;18:80–84. doi: 10.1038/nsmb.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, et al. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451:783–788. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claros MG, Vincens P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem. 1996;241:779–786. doi: 10.1111/j.1432-033.1996.00779.x. [DOI] [PubMed] [Google Scholar]
- 30.Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, et al. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011;39:225–229. doi: 10.1093/nar.gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Tomkinson AE, Ramos W, Mackey ZB, Danehower S, Walter CA, et al. Mammalian DNA ligase III: molecular cloning, chromosomal localization and expression in spermatocytes undergoing meiotic recombination. Mol Cell Biol. 1995;15:5412–5422. doi: 10.1128/mcb.15.10.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vijayakumar S, Chapados BR, Schmidt KH, Kolodner RD, Tainer JA, Tomkinson AE. The C-terminal domain of yeast PCNA is required for physical and functional interactions with Cdc9 DNA ligase. Nucleic Acids Res. 2007;35:1624–1637. doi: 10.1093/nar.gkm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putnam NH, Butts T, Ferrier DE, Furlong RF, Hellsten U, Kawashima T, et al. The amphioxus genome and the evolution of the chordate karyotype. Nature. 2008;453:1064–1071. doi: 10.1038/nature06967. [DOI] [PubMed] [Google Scholar]
- 34.Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- 35.Rulten SL, Cortes-Ledesma F, Guo L, Iles NJ, Caldecott KW. APLF (C2orf13) is a novel component of poly(ADP-ribose) signaling in mammalian cells. Mol Cell Biol. 2008;28:4620–4628. doi: 10.1128/MCB.02243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahel I, Ahel D, Matsusaka T, Clark AJ, Pines J, Boulton SJ, et al. Poly(ADP-ribose)-binding zinc finger motifs in DNA repair/checkpoint proteins. Nature. 2008;451:81–85. doi: 10.1038/nature06420. [DOI] [PubMed] [Google Scholar]
- 37.Karras GI, Kustatscher G, Buhecha HR, Allen MD, Pugieux C, Sait F, et al. The macro domain is an ADP-ribose binding module. EMBO J. 2005;24:1911–1920. doi: 10.1038/sj.emboj.7600664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fritz-Laylin LK, Prochnik SE, Ginger ML, Dacks JB, Carpenter ML, Field MC, et al. The genome of Naegleria gruberi illuminates early eukaryotic versatility. Cell. 2010;140:631–642. doi: 10.1016/j.cell.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto T, Tanaka T, Sakai H, Amano N, Kanamori H, Kurita K, et al. Comprehensive sequence analysis of 24,783 barley full-length cDNAs derived from 12 clone libraries. Plant Physiol. 2011;156:20–28. doi: 10.1104/pp.110.171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tovar J, Leon-Avila G, Sanchez LB, Sutak R, Tachezy J, van der Giezen M, et al. Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature. 2003;426:172–176. doi: 10.1038/nature01945. [DOI] [PubMed] [Google Scholar]
- 41.Willer M, Rainey M, Pullen T, Stirling CJ. The yeast CDC9 gene encodes both a nuclear and a mitochondrial form of DNA ligase I. Curr Biol. 1999;9:1085–1094. doi: 10.1016/S0960-9822(99)80477-1. [DOI] [PubMed] [Google Scholar]
- 42.Montecucco A, Rossi R, Levin DS, Gary R, Park MS, Motycka TA, et al. DNA ligase I is recruited to sites of DNA replication by an interaction with proliferating cell nuclear antigen: identification of a common targeting mechanism for the assembly of replication factories. EMBO J. 1998;17:3786–3795. doi: 10.1093/emboj.17.13.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalloul RA, Long JA, Zimin AV, Aslam L, Beal K, Blomberg Le A, et al. Multi-platform next-generation sequencing of the domestic turkey (Meleagris gallopavo): genome assembly and analysis. PLoS Biol. 2010;8:20838655. doi: 10.1371/journal.pbio.1000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.International Chicken Genome Sequencing Consortium, author. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- 45.Paran N, De Silva FS, Senkevich TG, Moss B. Cellular DNA ligase I is recruited to cytoplasmic vaccinia virus factories and masks the role of the vaccinia ligase in viral DNA replication. Cell Host Microbe. 2009;6:563–569. doi: 10.1016/j.chom.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.