Abstract
Successful pregnancy requires a functionally normal blastocyst encountering a receptive maternal endometrium. Interestingly, the cell cycle regulator and tumor suppressor p53 has been reported to support reproduction in mice by regulating the expression of the leukemia inhibitory factor gene in the maternal endometrium. However, in humans the hormonal system orchestrating successful pregnancy is considerably different from rodents. Particularly, the primatespecific dimeric glycoprotein hormone human chorionic gonadotropin (hCG) is essential for blastocyst implantation and maintenance of early human pregnancy. Here we provide evidence that p53 selectively induces expression of the hCGβ7 (CGB7) gene. None of the other CGB genes was found to be regulated by p53. We show that expression of the CGB7 gene is upregulated upon p53 induction in human HFF, HCT116 and DLD1 cells as well as in cell preparations enriched in human primary first-trimester trophoblasts. The increase in CGB7 levels upon doxorubicin treatment is lost after siRNA-directed knockdown of p53. Furthermore, we describe CGB7 as a direct transcriptional target gene of p53 by identifying a p53-responsive element in the CGB7 promoter using reporter assays, electrophoretic mobility shift assays and chromatin immunoprecipitations. With these results we provide a new link between p53 transcriptional activity and human reproduction.
Key words: p53, hCG, CGB7, reproduction, tumor suppressor, transcriptional regulation, blastocyst implantation, promoter
Introduction
The tumor suppressor p53 is a major regulator of cell division with well-established functions in controlling cell cycle arrest, apoptosis and senescence.1,2 As a transcription factor, p53 binds to specific palindromic sequences3 and is able to activate a large number of target genes.4 Recently, p53 has been shown to also be involved in reproduction. The reduced reproductive success of p53−/− mice has been attributed to a reduction in expression of the leukemia inhibitory factor (LIF),5,6 an endometrial cytokine whose maternal expression positively influences blastocyst implantation in mice.7 In wild-type mice, LIF is transcriptionally activated by p53 through a canonical binding site in the LIF promoter.5 In humans the association of single-nucleotide polymorphisms (SNPs) in the p53 gene with reduced fertility was shown, which led to the notion that p53 also plays a role in human reproduction.6,8 For example the p53 allele coding for proline at the codon 72 polymorphism is significantly overrepresented in in vitro fertilization (IVF) patients and is associated with recurrent implantation failure following IVF.8–10 However, the system controlling reproductive success in humans is significantly different from that in mice. Particularly, the primate-specific glycoprotein hormone human chorionic gonadotropin (hCG) is one of the earliest blastocyst-derived signals that plays an essential role in the establishment and maintenance of early human pregnancy by supporting corpus luteum survival to maintain progesterone production and by inducing local immune tolerance of the maternal endometrium toward the fetal semi-allograft. hCG also plays a role in placentation by promoting angiogenesis at the implantation site.11–13 It was shown that implantation and pregnancy rates after IVF increase following treatment with hCG preparations underlining the important role of this hormonal stimulus.14
hCG is active as a highly glycosylated heterodimer with the α-subunit common to luteinizing hormone (LH), folliclestimulating hormone (FSH) and thyrotropin (TSH), the distinct β-subunits of which confer the respective biological specificities.15 The common α-subunit of this glycoprotein hormone family (GPHα, CGA) is expressed in both the placenta and the pituitary gland.16 It is well established that heterodimeric hCG can act through binding to a G-protein-coupled receptor shared with LH as alternative ligand in either an endocrine or a paracrine manner.12 More recently, hCG functions independent of the LH/CG receptor by interactions with the TGFβ receptor and the mannose receptor and independent of the α-subunit by formation of hCGββ homodimers have been described.17–20 Interestingly, six CGB (hCGβ) genes are found in the human genome together with their ancestor, the LHB (LHβ) gene, in a cluster on chromosome 19.21,22 Of these six genes containing CGB sequences, CGB1 and 2 had originally thought to be pseudogenes.23,24 However, recently CGB1 and 2 proteins were detected mainly in the testes, possibly playing a role in the male reproductive system.25 CGB3, 5 and 8 code for identical proteins secreted in large quantities by the placenta and by some tumor types. They differ in three amino acids from CGB7, which is produced to a lesser extent by several tissues and does not appear to be induced upon malignant transformation.26
CGB mRNA and protein can be detected in the preimplantation embryo in increasing amounts beginning already at the two-cell stage.27,28 In the maternal circulation CGB protein is observed around implantation time.27,29 It is interesting to note that it was the β-subunit of hCG detected in these early stages, however without distinguishing between the different β isoforms. Additionally, a hyperglycosylated form of hCG was described in very early pregnancy,20 but different glycosylation patterns as well as distinct functions of the various β-subunits have yet to be defined.
Here we provide evidence that p53 selectively induces expression of the CGB7 gene which we show to be a direct transcriptional target gene of p53. This implies a new role of p53 in human reproduction.
Results
p53 induces CGB7 expression in a human first trimester trophoblast-enriched cell population.
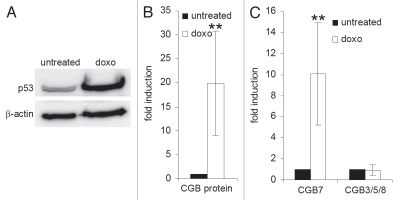
The expression of p53 has previously been described in first trimester trophoblasts,30 raising the possibility that p53 may influence hCG expression over the decisive period of blastocyst implantation. To test for a regulatory connection between p53 and hCG, we utilized cell preparations enriched in human primary first-trimester trophoblasts. We added the intercalating agent doxorubicin to the culture media in order to increase p53 protein levels and determined the expression of hCG at the protein and mRNA levels. We did indeed detect a rise in secreted CGB protein levels in the culture supernatant together with an increase in cellular p53 following stimulation with the chemotherapeutic drug (Fig. 1A and B). Isotype-specific RT-PCR analysis revealed the expression of CGA, LHB and CGB1/2 mRNAs to be below the detection limit (data not shown), and the levels of CGB3, 5 and 8 transcripts to be unaffected by doxorubicin. In contrast, CGB7 mRNA expression was significantly induced together with p53 protein by doxorubicin treatment (Fig. 1C).
Figure 1.
CGB7 expression increases after induction of p53 in a trophoblast-enriched cell population. Cells were treated with doxorubicin for 48 h (doxo). (A) p53 protein was detected by protein gel blot. β-actin served as loading control. (B) CGB protein levels in the cell culture supernatant were measured by an immunoassay detecting all isoforms (mean ± SD, n = 3). (C) mRNA levels of CGB isoforms were determined by real-time RT-PCR with isoform-specific primers (mean ± SD, n = 4). **p < 0.01.
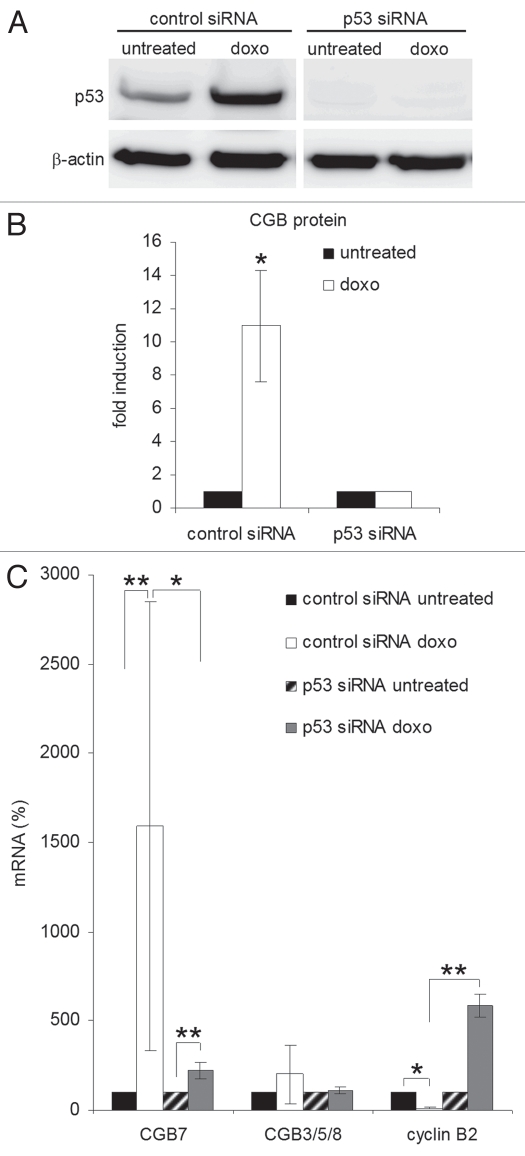
To determine whether or not this induction is dependent on p53, we performed siRNA-directed knockdown of p53 in the trophoblast-enriched cell population and found the rise in secreted CGB protein upon doxorubicin treatment to be lost upon siRNA-mediated abrogation of p53 protein (Fig. 2A and B). Furthermore, the strong induction of CGB7 mRNA expression on doxorubicin treatment was also significantly reduced in these cells. In contrast, the expression of CGB3/5/8 mRNAs was barely affected. The expression of cyclin B2, which is transcriptionally repressed by p53,31 served as a control with cyclin B2 mRNA levels slightly increasing upon p53-specific siRNA treatment (Fig. 2C).
Figure 2.
Induction of CGB7 expression in a primary trophoblast cell population depends on p53. A cell population enriched in primary trophoblasts was transfected with p53-directed or control siRNA. Starting 48 h after transfection cells were treated with doxorubicin for further 48 h (doxo). (A) p53 and β-actin proteins were detected by immunoblot. (B) CGB protein levels in culture media were measured by immunoassay (mean ± SD, n = 3). (C) Levels of CGB mRNAs were determined by real-time RT-PCR (mean ± SD, n = 5). Cyclin B2 expression was monitored as control (n = 3). *p < 0.05, **p < 0.01.
CGB7 expression is upregulated by p53 in various cellular systems.
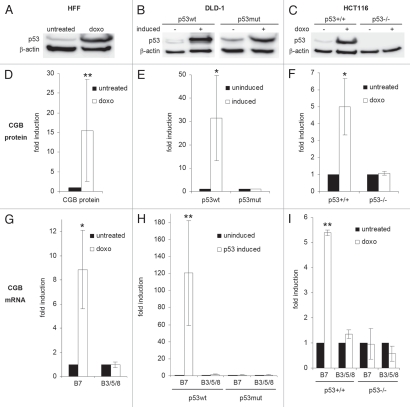
We then tested whether p53-dependent CGB7 induction is also observed in other cell systems. In human foreskin fibroblasts (HFF) the induction of wild-type (wt) p53 protein by doxorubicin treatment was associated with an increase in CGB protein and CGB7 mRNA levels without induction of the CGB3, 5 and 8 genes (Fig. 3A, D and G). In the p53-mutant colorectal carcinoma cell line DLD-1,32 the induction of transgenic wt p53 expression was also associated with the very strong and specific induction of CGB7, while no change in CGB expression was observed in DLD-1 cells carrying a mutant p53 transgene (Fig. 3B, E and H). The p53+/+ and p53−/− HCT116 human colon cancer cells provide a similar experimental system enabling inducible p53 expression at a more physiological level. Comparison of gene regulation in HCT116 p53+/+ cells with that in cells carrying deletions in both p53 alleles (HCT116 p53−/−) allows direct testing of p53 function in a constant human cellular background.33 CGB protein levels in the culture media from p53-positive HCT116 but not from HCT116 p53−/− cells were increased 5-fold after p53 induction by doxorubicin. On the mRNA level CGB7 but not CGB3/5/8 mRNA was induced upon doxorubicin treatment in HCT116 p53+/+ but not in HCT116 p53−/− cells (Fig. 3C, F and I). Thus, we show that p53 increases expression of CGB7 in several cellular systems.
Figure 3.
p53 induces CGB7 expression in different cell systems. (A–C) p53 protein was detected by immunoblot, β-actin serving as loading control. (D–F) CGB protein levels were measured by immunoassay. (G–I) mRNA levels of CGB isoforms were determined by real-time RT-PCR. (A, D and G) Human foreskin fibroblasts (HFF) were treated with doxorubicin (doxo) for 48 h. (B, E and H) In the human colorectal adenocarcinoma cell line DLD-1 carrying tet-off inducible p53 wild-type (p53wt) or R175H mutant (p53mut) transgenes in a functionally p53-negative background the expression of transgenes was induced for 9 h. (C, F and I) HCT116 cells with wild-type p53 (HCT116 p53+/+) or with targeted deletions in both p53 alleles (HCT116 p53−/−) were treated with doxorubicin for 48 h. (D–I) Mean ± SD, n = 3, *p < 0.05, **p < 0.01.
Identification of a functional p53 response element in the CGB7 promoter.
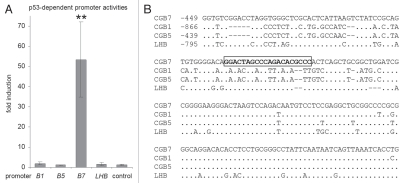
The remarkably selective p53-dependent induction of CGB7 prompted us to test for direct transcriptional regulation of the CGB promoters by p53. To this end, we performed dual luciferase-reporter assays using LHB, CGB1, CGB5 and CGB7 promoter constructs (GenBank accession numbers EU526020, EU526021, EU526022 and EU526023). Due to their nearly identical sequences, the CGB1 promoter is representative for CGB2 and the CGB5 upstream DNA is nearly identical to CGB3 and 8, respectively. The reporter constructs were cotransfected with plasmids expressing wild-type or a DNA-binding deficient mutant of p53 in the p53-negative osteosarcoma cell line SaOS-2. Consistent with our observations at the mRNA level, activity of the CGB7 promoter, but not that of either of the LHB, CGB1 or CGB5 promoters, was significantly upregulated upon p53 expression (Fig. 4A). Deletion mutants of the CGB7 promoter were tested in reporter assays narrowing down the region responsible for p53-dependent regulation to a segment which was found to contain an element closely related to established p53 binding sites defined as two copies of the ten base-pair motif 5′-PuPuPuC(A/T)(T/A)GPyPyPy-3′ separated by a spacer of 0 to 13 base pairs3 (Fig. 5). After mutation of one or both half-sites of this putative p53 response element, induction of the CGB7 promoter by p53 was strongly diminished suggesting that this site, whose second core motif does not perfectly match the consensus, is indeed required for the direct p53-dependent regulation (Fig. 5). Considering that the sequences of CGB7 and CGB3/5/8 proteins differ only in three amino acids, it is important to note that the CGB and LHB promoters also share large sequence homologies. The region directly downstream of the p53 consensus is highly conserved between the CGB promoters whereas the p53 binding site itself as well as the upstream sequences lack this strong conservation (Fig. 4B). In accordance with our functional data, an intact p53 binding consensus is only found in the CGB7 promoter.
Figure 4.
CGB7 promoter activity is induced by p53. (A) Dual luciferase-reporter assays were performed in SaOS-2 cells after cotransfection of either CGB1 (B1), CGB5 (B5), CGB7 (B7) or LHB promoter-reporter constructs together with plasmids expressing wild-type or mutant p53 and a Renilla luciferase transfection control plasmid. Firefly luciferase was measured and normalized to Renilla luciferase activity. Fold induction by p53 is displayed as the ratio of promoter activities obtained with wild-type compared with DNA-binding mutant p53 protein. The empty reporter vector served as control (mean ± SD, n ≥ 3, **p < 0.01). (B) The p53 binding site in the CGB7 promoter is not conserved in the other CGB genes. Alignment of CGB and LHB promoter regions around the p53 binding site (framed) of the CGB7 promoter. Positions relative to the transcriptional start sites are given. Identical nucleotides are presented by dots. Spaces are indicated by lines.
Figure 5.
Identification of a p53 response element in the CGB7 promoter. Dual luciferase-reporter assays were performed in SaOS-2 cells after cotransfection of wild-type, shortened or mutant CGB7 promoter-reporter constructs together with plasmids expressing wild-type or mutant p53 and a Renilla luciferase transfection control plasmid. Fold induction by p53 is displayed in each case. The CGB7 promoter constructs are numbered relative to the transcriptional start site. Empty firefly reporter vector served as control. Both half-sites of the p53 binding site (indicated in bold) in the CGB7 promoter construct −1,200/365 were mutated separately (mut 1 or mut 2) or together (mut 1 + 2). Mutant bases are in lower case italics (mean ± SD, n = 3, *p < 0.05, **p < 0.01).
p53 directly binds to the CGB7 promoter.
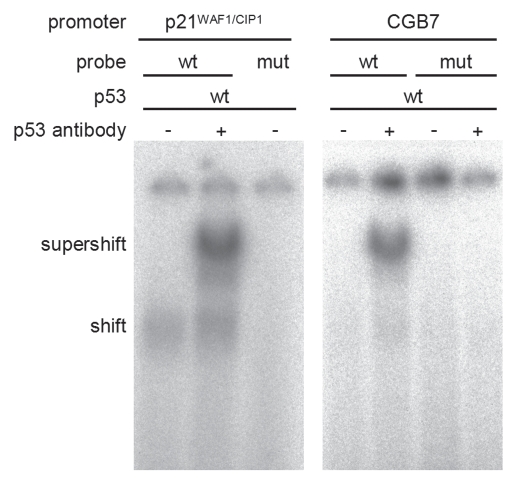
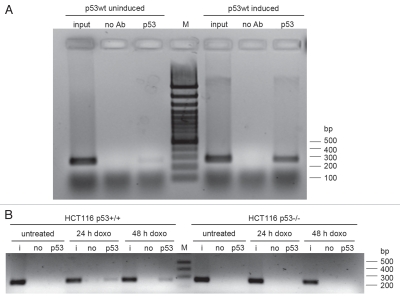
We subsequently demonstrated binding of p53 to the identified p53 site in the CGB7 promoter in vitro by performing electrophoretic mobility shift assays (EMSAs). The established p53 response element in the p21WAF1/CIP1 promoter34 served as positive control. Band migration was reduced after addition of p53 to the wild-type p21WAF1/CIP1 promoter probe (shift) and after addition of anti-p53 antibody a supershift was observed. Using the CGB7 promoter probe a p53 supershift is detected, which is absent when the identified p53 binding site is mutated (Fig. 6). The poor shifts of p53 protein with DNA, which are considerably enhanced upon addition of a p53-specific antibody, have been noted earlier.35 Finally, chromatin immunoprecipitation (ChIP) results confirmed that p53 is also bound to this region in the CGB7 promoter in vivo (Fig. 7). In the tet-off inducible DLD-1 cell system binding of p53 to the CGB7 promoter was detected after induction of the p53 wild-type transgene (Fig. 7A). Furthermore, using another cell system p53 bound to the CGB7 promoter in HCT116 cells carrying wild-type p53 after doxorubicin treatment whereas no binding was detected in HCT p53−/− cells after treatment with the chemotherapeutic agent (Fig. 7B).
Figure 6.
p53 binds to the p53 response element in the CGB7 promoter. Electrophoretic mobility shift assays (EMSA) using wild-type CGB7 promoter probes or probes in which the p53 response element was mutated were performed with in vitro-transcribed and -translated p53 protein and anti-p53 antibody. p21 probes (left) served as positive control.
Figure 7.
In vivo p53 binding to a p53 response element in the CGB7 promoter. (A) Chromatin immunoprecipitation (ChIP) experiments were performed in tet-off inducible DLD-1 cells, carrying a p53 wild-type (p53wt) transgene, before and after induction of p53wt expression. (B) ChIP experiments in HCT116 cells with wild-type p53 (HCT116 p53+/+) or with targeted deletions in both p53 alleles (HCT116 p53−/−) 0 h, 24 h or 48 h after doxorubicin (doxo) treatment. CGB7 promoter fragments were amplified in the input (i), in a control preparation without antibody (no) and after immunoprecipitation with p53 antibody (p53) and separated on an agarose gel.
Taken together, these observations demonstrate that CGB7 is a new direct transcriptional target gene of the tumor suppressor p53.
Discussion
The p53 protein has previously been reported to support reproduction in mice, in which it has been shown to regulate LIF expression in the maternal endometrium.5 The observations reported here suggest that p53 may contribute to reproduction in humans, too, by inducing the expression of CGB7, one of a family of chorionic gonadotropin proteins specific to primates.36
The dimeric glycoprotein hormone hCG is one of the earliest and most important trophoblast signals required for the successful establishment of pregnancy in humans.12 In previous reports functions of hCG independent of the α-subunit and the LH/CG receptor have been reported.17–20 Furthermore, CGA was described to be generally expressed in excess of β-subunits.15 Therefore, as heterodimer or as free CGB having functions independent from either CGA or the LH/CG receptor, transcriptional regulation of CGB subunit genes could be critical to determine the stage and tissue specific patterns of hCG activity. The various CGB subunits are nearly identical and distinct functions have not so far been reported. In this respect, the selective induction of CGB7 by p53 is particularly remarkable because of the strong sequence homology between the genes and promoter regions of the CGB isoforms. We clearly demonstrate that p53 exclusively binds to a well-defined p53 response element in the CGB7 promoter upregulating CGB7 expression. Importantly, this p53-binding site is absent in the other CGB promoters as well as the LHB promoter although generally these promoters are largely conserved in their nucleotide sequence. Interestingly, the p53 response element in the CGB7 promoter at position −394 to −375 relative to the transcriptional start site is placed outside the previously published CGB core promoter (from −315 to −188) which contains the basal transcriptional activation sites responsible for placenta-specific expression of CGB3/5/8.37,38 However, it had already been speculated that more regulatory sequences may be found up to −700 bp upstream from the transcriptional start site.37
In addition to the selective p53-dependent expression described here, differential expression patterns of CGB3/5/8 and CGB7 have been described earlier indicating that CGB7 is expressed in small amounts in several non-malignant, non-trophoblastic tissues.26 These obvious differences in the spatiotemporal expression of the CGB subunits suggest a model in which the differences in promoter activity constitute the decisive feature of the CGB family allowing the tight, independent regulation of functionally redundant isoforms under different conditions. Moreover, differential regulation could also be a hint to yet unknown functional differences between the CGB subunits.
The relative amounts of the CGB isoforms were determined on the mRNA level in first trimester trophoblasts,23 showing that CGB7 accounts for a minor percentage of the total CGB mRNA produced by the embryo. However, the relative expression of CGB7 and CGB3/5/8 in very early pregnancy around the implantation window has not been and for ethical reasons cannot be investigated. Since CGB3, 5 and 8 account for most of the CGB in the placenta, it was implicated that CGB7 plays only a minor role also in other tissues or at other times in pregnancy. However, very recently it was reported that CGB7 is the only CGB isoform produced by the secretory endometrium, shedding new light on the importance of this neglected isoform.39 It was suggested that CGB7 may be important for preparing the endometrium for embryo implantation.39 Furthermore, p53 was also found to be expressed in normal cycling endometrium.40,41
The hypothesis that p53 participates in the regulation of human reproduction by inducing CGB7 is in accordance with previous reports showing the association between SNPs in the human p53 pathway and reduced fertility.10,42 Also, it was suggested that p53 regulates the efficiency of human implantation through modulating LIF expression after testing the human p53 codon 72 SNP in Hupki mice.43 Furthermore, p53 was shown to mediate trophoblast invasion by inducing MMP-2 expression.44 Recently, a role of p53 also in spermatogenesis was described in reference 45. In this report p53 is found to directly transactivate the spermatogenesis-associated SPATA18 gene through a binding site in the first intron. These new findings together with previous results from invertebrate species imply that the original function of the p53 family may be found in the context of fertility and development rather than in tumor suppression.42
In summary, our results identify CGB7 as direct transcriptional target of the tumor suppressor p53, suggesting that p53 might play a role in human embryo implantation via CGB7. Generally, the observations further expand the functional spectrum of p53 as a transcription factor in humans by describing a role of p53 very different from its established functions in cell cycle arrest, apoptosis and senescence.
Materials and Methods
Plasmids.
Human p53 expression plasmids pCMV-p53wt and pCMV-p53mut (R175H) were made available by Bert Vogelstein32 and cDNAs were subcloned into pcDNA3.1-HisC (Invitrogen, V38520). The pGL4.70 hRluc plasmid (Promega, E6881) encodes Renilla luciferase and served as transfection control in reporter-luciferase assays.
Full-length CGB and LHB promoter constructs were amplified from human genomic DNA and cloned as KpnI/NcoI (CGB5, CGB7, LHB) or KpnI/HindIII (CGB1) fragments into the pGL4.10 firefly-luciferase reporter-gene vector (Promega, E6651) using the following isoform-specific primers: CGB5 for 5′-CGG GGT ACC CCG GAC CGC TGT GGA CTC-3′, CGB7 for 5′-CGG GGT ACC AGC AAC AAC CAC AGT TCA GAC CC-3′, CGB5 + 7 rev 5′-CAT GCC ATG GTT GGT GCG TCC CCT GCC-3′, LHB for 5′-CGG GGT ACC GCA GCA AGA ACC AAA GTT CAG G-3′, LHB rev 5′-CAT GCC ATG GTT GGT GCA TCC CCT GCC-3′, CGB1 for 5′-CGG GGT ACC GTT GAA TAG GAA CTC TCC CG-3′ and CGB1 rev 5′-CCC AAG CTT ACA TGG AAA GTA AAT TGA GTC TC-3′. Truncated CGB7 promoter constructs −736/365, −475/365, −357/365 and 66/365 were created using restriction enzyme sites for XhoI, SacI, DpnI and Eco147I, respectively. The nomenclature uses the first and last nucleotide positions relative to the transcriptional start site, the translational start ATG being at position +366. CGB7 promoter constructs carrying mutations in the p53 binding site at position −394 to −375 were created by site-directed mutagenesis employing standard methods.
CGB and LHB promoter sequences were submitted to the GenBank databases under accession numbers EU526020, EU526021, EU526022 and EU526023. Entrez Gene IDs for the CGB subunits are 114335 (CGB1), 114336 (CGB2), 1082 (CGB3), 93659 (CGB5), 94027 (CGB7) and 94115 (CGB8).
Cell culture and doxorubicin treatment.
Human primary first trimester trophoblasts were obtained and cultured as described in reference 46, and generously provided by Ana Claudia Zenclussen (Magdeburg, Germany). Collection and use of the cells was approved by the Ethical Review Board at the University of Magdeburg and written informed consent was obtained. Human foreskin fibroblasts HFF (ATCC, SCRC-1041) were cultured in a humidified atmosphere with 10% CO2 at 37°C in DMEM (PAA, E15-892) supplemented with 10% fetal calf serum (Lonza, DE14-801F). SaOS-2 cells (DSMZ, ACC243) were cultured as described previously in reference 31.
Parental human colon carcinoma HCT116 cells and HCT116 cells with targeted deletions in both p53 alleles33 as well as inducible DLD-1 cell lines carrying p53wt or p53R175H transgenes regulated by a modified tetracycline (tet)-regulated gene-expression system32 were generously provided by Bert Vogelstein and cultured as described previously in reference 31. Induced p53 protein expression is achieved by doxycycline removal. Cells were harvested 9 h after p53 induction.
Doxorubicin was used at a final concentration of 0.2 µg/ml for 48 h.
Transfections, dual-luciferase reporter-gene assays.
siRNA transfections were performed as described previously in reference 47, using validated Stealth™ p53 siRNA (Invitrogen, VHS40367) at a final concentration of 100 nM with DharmaFECT 1 transfection reagent (Thermo Fisher Scientific, T-2001-01) at a final dilution of 1:1,000 according to the manufacturer's instructions. Cells were treated with doxorubicin 48 h after siRNA transfection.
Plasmid transfections and promoter-reporter assays were performed as described previously in reference 47 and 48 pGL4.10 empty vector served as control.
RNA and protein extraction, real-time RT-PCR.
Total RNA and protein were isolated using Trizol reagent (Invitrogen, 15596026). One-step real-time RT-PCR was performed as described previously in reference 48. To distinguish between the different CGB isoforms specific primers were employed and the amplification products were confirmed by sequencing. Semi-quantitative analyses were performed by normalization to expression levels of β-actin or L7 mRNA. All primers used for real-time RT-PCR are listed in Table 1.
Table 1.
Primers used for real-time RT-PCR
| Forward | Reverse | |
| CGB7 | CCT CCC TGG CCT TGT CTA CTT CTC | GCA CCG TGG CCG AAG CAT |
| CGB3/5/8 | TGA GCC ACT CCT GCG CCC | CAG CCC CTG GAA CAT CTC CA |
| CGB1/2 | CCC CAG GGC CAG TGA GG | GAC ATG GAA AGT AAA TTG AGT CTC CG |
| LHβ | GCT ACT GCC CCA CCA TGA TG | GGC CTG AGA GTT GGG GGT GG |
| GPHα | ATG GAT TAC TAC AGA AAA TAT GC | TTA AGA TTT GTG ATA ATA ACA AGT AC |
| cyclin B2 | AAA GTT GGC TCC AAA GGG TCC TT | GAA ACT GGC TGA ACC TGT AAA AAT |
| β-actin | CCT TCA ACA CCC CAG CCA TGT ACG | GCC GTG GCC ATC TCT TGC TCG AAG |
| L7 | GCA CTA TCA CAA GGA ATA TAG GCA G | CCC ATG CAA TAT ATG GCT CTA C |
hCG protein measurement and protein gel blotting.
Determination of CGB protein in the cell culture supernatants was performed employing the HCG+β Elecsys electrochemoluminescence immunoassay (Roche, 03271749190) which detects all CGB isoforms.
Protein gel blotting was performed as described previously using 30 µg total protein separated on a 12% SDS-polyacrylamide gel.48 Monoclonal mouse anti-p53 DO-1 (Calbiochem, OP43L) and anti-β-actin AC-15 (Sigma, A1978) antibodies were employed at 1:2,000 or 1:5,000 dilutions, respectively.
Electrophoretic mobility shift assays (EMSA).
CGB7 and p21 probes were generated as described previously in reference 49, using oligonucleotide duplexes with the following sense sequences: CGB7 wt, 5′-GGG GGA CAG GAC TAG CCC AGA CAC GCC CAC CAG C-3′; CGB7 mut, 5′-GGG GGA CAG GAC CGT CCC AGA AAA TCC CAC TCA GC-3′; p21 wt, 5′-GGC CAT CAG GAA CAT GTC CCA ACA TGT TGA GCT CT-3′; p21 mut, 5′-GGC CAT CAG GAA TAT ATC CCA ATA TAT TGA GCT CT-3′. p53wt protein was synthesized employing the TNT® T7/SP6 Coupled Reticulocyte Lysate System (Promega, L5020). 2.5 µl reaction solution with p53wt protein were incubated with the DNA probe, 1 µg polydAdT, 1 µg salmon sperm DNA, 1 µl 12.5% deoxycholate, 1 µl 10% NP-40 and 7 µl binding buffer (25 mM Tris pH 7.5, 130 mM NaCl, 3 mM KCl, 5% BSA, 12% glycerol, 1 mM DTT) for 20 min at room temperature. For supershifts p53 protein was preincubated with 200 ng of mouse anti-p53 pAB421 antibody (Biomol, P-603) for 15 min at room temperature before adding the DNA probe. Electrophoresis was performed in 1xTBE as described before in references 50 and 51.
Chromatin immunoprecipitation (ChIP).
ChIP experiments were performed as previously described using 1 µg of monoclonal anti-p53 antibody (DO-1) for precipitation.52,53 Products were amplified by PCR using the primers ChIP CGB7 for, 5′-AAG CCC CTG CCG GGC ATA C-3′ and ChIP CGB7 rev, 5′-CCT TCC CCG CGA TCC AG-3′.
Statistical analysis.
Statistical significance relative to the respective control was analyzed applying the Student t-test and expressed as p value with *p < 0.05 and **p < 0.01 with n indicating the number of independent experiments.
Acknowledgments
We thank A.C. Zenclussen for providing trophoblasts, B. Vogelstein for cell lines and plasmids, and M. Cross and G.A. Müller for comments on the manuscript. This work was supported by a junior research grant awarded by the Medical School at the University of Leipzig (to S. Sohr) and grants from the Interdisciplinary Center for Clinical Research (IZKF) Leipzig (to K.E.).
Abbreviations
- CGA
human chorionic gonadotropin alpha subunit
- CGB
human chorionic gonadotropin beta subunit
- ChIP
chromatin immunoprecipitation
- hCG
human chorionic gonadotropin
- IVF
in vitro fertilization
- LHB
luteinizing hormone beta subunit
- LIF
leukemia inhibitory factor
- SNP
single nucleotide polymorphism
- TGFβ
transforming growth factor beta
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vigneron A, Vousden KH. p53, ROS and senescence in the control of aging. Aging (Albany NY) 2010;2:471–474. doi: 10.18632/aging.100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.el-Deiry WS, Kern SE, Pietenpol JA, Kinzler KW, Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992;1:45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- 4.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 5.Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450:721–724. doi: 10.1038/nature05993. [DOI] [PubMed] [Google Scholar]
- 6.Hu W, Feng Z, Atwal GS, Levine AJ. p53: a new player in reproduction. Cell Cycle. 2008;7:848–852. doi: 10.4161/cc.7.7.5658. [DOI] [PubMed] [Google Scholar]
- 7.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 8.Hu W, Feng Z, Levine AJ. The regulation of human reproduction by p53 and its pathway. Cell Cycle. 2009;8:3621–3622. doi: 10.4161/cc.8.22.9938. [DOI] [PubMed] [Google Scholar]
- 9.Su MT, Lin SH, Chen YC. Genetic association studies of angiogenesis- and vasoconstriction-related genes in women with recurrent pregnancy loss: a systematic review and meta-analysis. Hum Reprod Update. 2011 doi: 10.1093/humupd/dmr027. [DOI] [PubMed] [Google Scholar]
- 10.Kang HJ, Feng Z, Sun Y, Atwal G, Murphy ME, Rebbeck TR, et al. Single-nucleotide polymorphisms in the p53 pathway regulate fertility in humans. Proc Natl Acad Sci USA. 2009;106:9761–9766. doi: 10.1073/pnas.0904280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berndt S, Perrier d'Hauterive S, Blacher S, Pequeux C, Lorquet S, Munaut C, et al. Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. FASEB J. 2006;20:2630–2632. doi: 10.1096/fj.06-5885fje. [DOI] [PubMed] [Google Scholar]
- 12.Tsampalas M, Gridelet V, Berndt S, Foidart JM, Geenen V, Perrier d'Hauterive S. Human chorionic gonadotropin: a hormone with immunological and angiogenic properties. J Reprod Immunol. 2010;85:93–98. doi: 10.1016/j.jri.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Licht P, Losch A, Dittrich R, Neuwinger J, Siebzehnrubl E, Wildt L. Novel insights into human endometrial paracrinology and embryo-maternal communication by intrauterine microdialysis. Hum Reprod Update. 1998;4:532–538. doi: 10.1093/humupd/4.5.532. [DOI] [PubMed] [Google Scholar]
- 14.Motta EL, Smith GD, Serafini PC, Coslovsky M, Hassun P, Rocha AM, et al. Human choriogonadotropin prior to controlled ovarian stimulation and in vitro fertilization improves implantation and pregnancy rates. J Assist Reprod Genet. 2009;26:305–311. doi: 10.1007/s10815-009-9322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagirnaja L, Rull K, Uuskula L, Hallast P, Grigorova M, Laan M. Genomics and genetics of gonadotropin beta-subunit genes: Unique FSHB and duplicated LHB/CGB loci. Mol Cell Endocrinol. 2010;329:4–16. doi: 10.1016/j.mce.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maston GA, Ruvolo M. Chorionic gonadotropin has a recent origin within primates and an evolutionary history of selection. Mol Biol Evol. 2002;19:320–335. doi: 10.1093/oxfordjournals.molbev.a004085. [DOI] [PubMed] [Google Scholar]
- 17.Iles RK. Ectopic hCGbeta expression by epithelial cancer: malignant behaviour, metastasis and inhibition of tumor cell apoptosis. Mol Cell Endocrinol. 2007;260:264–270. doi: 10.1016/j.mce.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Kane N, Kelly R, Saunders PT, Critchley HO. Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor. Endocrinology. 2009;150:2882–2888. doi: 10.1210/en.2008-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler SA, Iles RK. The free monomeric beta subunit of human chorionic gonadotrophin (hCGbeta) and the recently identified homodimeric beta-beta subunit (hCGbetabeta) both have autocrine growth effects. Tumour Biol. 2004;25:18–23. doi: 10.1159/000077719. [DOI] [PubMed] [Google Scholar]
- 20.Cole LA. New discoveries on the biology and detection of human chorionic gonadotropin. Reprod Biol Endocrinol. 2009;7:8. doi: 10.1186/1477-7827-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henke A, Gromoll J. New insights into the evolution of chorionic gonadotrophin. Mol Cell Endocrinol. 2008;291:11–19. doi: 10.1016/j.mce.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Policastro PF, Daniels-McQueen S, Carle G, Boime I. A map of the hCG beta-LH beta gene cluster. J Biol Chem. 1986;261:5907–5916. [PubMed] [Google Scholar]
- 23.Bo M, Boime I. Identification of the transcriptionally active genes of the chorionic gonadotropin beta gene cluster in vivo. J Biol Chem. 1992;267:3179–3184. [PubMed] [Google Scholar]
- 24.Hallast P, Rull K, Laan M. The evolution and genomic landscape of CGB1 and CGB2 genes. Mol Cell Endocrinol. 2007;260-262:2–11. doi: 10.1016/j.mce.2005.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parrott AM, Sriram G, Liu Y, Mathews MB. Expression of type II chorionic gonadotropin genes supports a role in the male reproductive system. Mol Cell Biol. 2011;31:287–299. doi: 10.1128/MCB.00603-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellet D, Lazar V, Bieche I, Paradis V, Giovangrandi Y, Paterlini P, et al. Malignant transformation of nontrophoblastic cells is associated with the expression of chorionic gonadotropin beta genes normally transcribed in trophoblastic cells. Cancer Res. 1997;57:516–523. [PubMed] [Google Scholar]
- 27.Jurisicova A, Antenos M, Kapasi K, Meriano J, Casper RF. Variability in the expression of trophectodermal markers beta-human chorionic gonadotrophin, human leukocyte antigen-G and pregnancy specific beta-1 glycoprotein by the human blastocyst. Hum Reprod. 1999;14:1852–1858. doi: 10.1093/humrep/14.7.1852. [DOI] [PubMed] [Google Scholar]
- 28.Ramu S, Acacio B, Adamowicz M, Parrett S, Jeyendran RS. Human chorionic gonadotropin from day 2 spent embryo culture media and its relationship to embryo development. Fertil Steril. 2011;96:615–617. doi: 10.1016/j.fertnstert.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed AG, Klopper A. Diagnosis of early pregnancy by assay of placental proteins. Br J Obstet Gynaecol. 1983;90:604–611. doi: 10.1111/j.1471-0528.1983.tb09275.x. [DOI] [PubMed] [Google Scholar]
- 30.Haidacher S, Blaschitz A, Desoye G, Dohr G. Immunohistochemical evidence of p53 protein in human placenta and choriocarcinoma cell lines. Hum Reprod. 1995;10:983–988. doi: 10.1093/oxfordjournals.humrep.a136082. [DOI] [PubMed] [Google Scholar]
- 31.Krause K, Wasner M, Reinhard W, Haugwitz U, Langezu Dohna C, Mössner J, et al. The tumour suppressor protein p53 can repress transcription of cyclin B. Nucleic Acids Res. 2000;28:4410–4418. doi: 10.1093/nar/28.22.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Zhang L, Hwang PM, Rago C, Kinzler KW, Vogelstein B. Identification and classification of p53-regulated genes. Proc Natl Acad Sci USA. 1999;96:14517–14522. doi: 10.1073/pnas.96.25.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 34.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-P. [DOI] [PubMed] [Google Scholar]
- 35.Löhr K, Möritz C, Contente A, Dobbelstein M. p21/CDKN1A mediates negative regulation of transcription by p53. J Biol Chem. 2003;278:32507–32516. doi: 10.1074/jbc.M212517200. [DOI] [PubMed] [Google Scholar]
- 36.Hallast P, Saarela J, Palotie A, Laan M. High divergence in primate-specific duplicated regions: human and chimpanzee chorionic gonadotropin beta genes. BMC Evol Biol. 2008;8:195. doi: 10.1186/1471-2148-8-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otani T, Otani F, Krych M, Chaplin DD, Boime I. Identification of a promoter region in the CG beta gene cluster. J Biol Chem. 1988;263:7322–7329. [PubMed] [Google Scholar]
- 38.Johnson W, Jameson JL. AP-2 (activating protein 2) and Sp1 (selective promoter factor 1) regulatory elements play distinct roles in the control of basal activity and cyclic adenosine 3′,5′-monophosphate responsiveness of the human chorionic gonadotropin-beta promoter. Mol Endocrinol. 1999;13:1963–1975. doi: 10.1210/me.13.11.1963. [DOI] [PubMed] [Google Scholar]
- 39.Zimmermann G, Ackermann W, Alexander H. Expression and production of human chorionic gonadotropin (hCG) in the normal secretory endometrium: evidence of CGB7 and/or CGB6beta hCG subunit gene expression. Biol Reprod. 2011 doi: 10.1095/biolreprod.111.092429. [DOI] [PubMed] [Google Scholar]
- 40.Maia H, Jr, Maltez A, Studart E, Athayde C, Coutinho EM. Proliferation kinetics in adenomyosis during the menstrual cycle and during oral contraceptive use. Gynecol Endocrinol. 2004;18:101–106. doi: 10.1080/09513590310001652982. [DOI] [PubMed] [Google Scholar]
- 41.Maia H, Jr, Maltez A, Studart E, Athayde C, Coutinho EM. Ki-67, Bcl-2 and p53 expression in endometrial polyps and in the normal endometrium during the menstrual cycle. BJOG. 2004;111:1242–1247. doi: 10.1111/j.1471-0528.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- 42.Hu W. The role of p53 gene family in reproduction. Cold Spring Harb Perspect Biol. 2009;1:1073. doi: 10.1101/cshperspect.a001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Z, Zhang C, Kang HJ, Sun Y, Wang H, Naqvi A, et al. Regulation of female reproduction by p53 and its family members. FASEB J. 2011;25:2245–2255. doi: 10.1096/fj.10-180166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staun-Ram E, Goldman S, Shalev E. Ets-2 and p53 mediate cAMP-induced MMP-2 expression, activity and trophoblast invasion. Reprod Biol Endocrinol. 2009;7:135. doi: 10.1186/1477-7827-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bornstein C, Brosh R, Molchadsky A, Madar S, Kogan-Sakin I, Goldstein I, et al. SPATA18, a spermatogenesis-associated gene, is a novel transcriptional target of p53 and p63. Mol Cell Biol. 2011;31:1679–1689. doi: 10.1128/MCB.01072-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schumacher A, Brachwitz N, Sohr S, Engeland K, Langwisch S, Dolaptchieva M, et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J Immunol. 2009;182:5488–5497. doi: 10.4049/jimmunol.0803177. [DOI] [PubMed] [Google Scholar]
- 47.Böhlig L, Friedrich M, Engeland K. p53 activates the PANK1/miRNA-107 gene leading to downregulation of CDK6 and p130 cell cycle proteins. Nucleic Acids Res. 2011;39:440–453. doi: 10.1093/nar/gkq796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sohr S, Engeland K. RHAMM is differentially expressed in the cell cycle and downregulated by the tumor suppressor p53. Cell Cycle. 2008;7:3448–3460. doi: 10.4161/cc.7.21.7014. [DOI] [PubMed] [Google Scholar]
- 49.Haugwitz U, Wasner M, Wiedmann M, Spiesbach K, Rother K, Mössner J, et al. A single cell cycle genes homology region (CHR) controls cell cycle-dependent transcription of the cdc25C phosphatase gene and is able to cooperate with E2F or Sp1/3 sites. Nucleic Acids Res. 2002;30:1967–1976. doi: 10.1093/nar/30.9.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirschner RD, Rother K, Müller GA, Engeland K. The retinal dehydrogenase/reductase retSDR1/DHRS3 gene is activated by p53 and p63 but not by mutants derived from tumors or EEC/ADULT malformation syndromes. Cell Cycle. 2010;9:2177–2188. doi: 10.4161/cc.9.11.11844. [DOI] [PubMed] [Google Scholar]
- 51.Friedrich M, Böhlig L, Kirschner RD, Engeland K, Hauschildt S. Identification of two regulatory binding sites which confer myotube specific expression of the mono-ADP-ribosyltransferase ART1 gene. BMC Mol Biol. 2008;9:91. doi: 10.1186/1471-2199-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirschner RD, Sänger K, Müller GA, Engeland K. Transcriptional activation of the tumor suppressor and differentiation gene S100A2 by a novel p63-binding site. Nucleic Acids Res. 2008;36:2969–2980. doi: 10.1093/nar/gkn132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wasner M, Haugwitz U, Reinhard W, Tschöp K, Spiesbach K, Lorenz J, et al. Three CCAAT-boxes and a single cell cycle genes homology region (CHR) are the major regulating sites for transcription from the human cyclin B2 promoter. Gene. 2003;312:225–237. doi: 10.1016/S0378-1119(03)00618-8. [DOI] [PubMed] [Google Scholar]