Abstract
Phage display of random peptide libraries is a powerful, unbiased method frequently used to discover ligands for virtually any protein of interest and to reveal functional protein–protein interaction partners. Moreover, in vivo phage display permits selection of peptides that bind specifically to different vascular beds without any previous knowledge pertaining to the nature of their corresponding receptors. Vascular targeting exploits molecular differences inherent in blood vessels within given organs and tissues, as well as diversity between normal and angiogenic blood vessels. Over the years, our group has identified phage capable of homing to lung blood vessels based on screenings using immortalized lung endothelial cells combined with in vivo selections after intravenous administration of combinatorial libraries. Peptides targeting lung vasculature have been extensively characterized and a lead homing peptide has shown interesting biological properties, bringing novel insights as to the implications of lung endothelial cell apoptosis in the pathogenesis of emphysema. We have also designed and developed targeted nanoparticles with imaging capabilities and/or drug delivery functions by combining phage display technology and elemental gold (Au) nanoparticles, constituting a promising platform for the development of therapeutic agents for imaging and treatment of lung disorders. Given the important role of the endothelium in the pathogenesis and progression of several diseases associated with the airways, ligand-directed discovery of lung vascular markers is an important milestone toward the development of future targeted therapies.
Keywords: vascular targeting, lung, phage display
To allow for an efficient exchange of gases between the blood stream and the air, the lungs constitute the largest external surface area in a human body and, in turn, contain approximately half of the body's total endothelial cell surface. Given this extensive circulatory bed, realizing and recognizing the molecular diversity in healthy and diseased lungs is an important concept required for the development of targeted therapies and novel diagnostic methods of lung disease. Millions of people suffer from some form of lung disorder, which, if grouped together, would represent the third leading cause of death in the United States, affecting nearly 1 in 7 people. The development of novel targeted agents for diagnosing, imaging, and treatment of these diseases would greatly improve both health care and patient survival.
Vascular heterogeneity is a relatively new concept supported by a growing body of evidence generated over the past decades (1–4). The antiquated view that blood vessels are a seemingly homogenous wall of almost identical endothelial cells has been replaced by the idea that blood vessels are, in fact, an intricate population of cells that differ among each other in organs and even within the same tissue. Such heterogeneity is already evident in the early stages of organ development, including the lung. Moreover, endothelial cells have an intimate connection with adjacent tissue, participating in organogenesis in an organ-specific manner. A lack of endothelial cells or a disruption in blood vessel formation interrupts lung development, and signals from the airways epithelial tissue are necessary for lung vasculogenesis and endothelial cell formation (5, 6). In the pancreas, an absence of endothelial cells prevents islet development and results in a halt of insulin production (7) while, in the liver, they are important for tissue morphogenesis but their absence does not interfere with hepatocyte differentiation (8). Hence, blood vessels are essential not only in supplying tissues and cells with oxygen and nutrients but also in modulating and shaping tissue and organ formation. Such close association between endothelium and tissue is likely to be maintained throughout adulthood and to play an important role in tissue homeostasis.
The existence of lung vascular heterogeneity has been proposed and has given rise to the identification of two distinct populations of endothelial cells: artery-derived and microvascular endothelial cells (9, 10). Interestingly, the current hypothesis for lung development proposes that the vasculature is formed by a combination of vasculogenesis (peripheral microvasculature) and angiogenesis (proximal macrovasculature) (reviewed in Reference 6). This hypothesis supports the notion that the two different endothelial cell populations might have arisen as a result of these two embryonic processes. These cells display distinctive lectin-binding and E-cadherin expression profiles and respond differently to permeability-inducing drugs, reflecting some of the intricacies of the lung vascular bed (Table 1). Notably, endothelial cell heterogeneity does not seem to be limited to vascular beds from different organs and tissues; it can also be found within the same vessel branch or segment (9, 11). Other lung vascular ligands have also been identified on the basis ofntheir association with cancer metastasis: dipeptidyl peptidase (CD26) and Ca2+-activated chloride channel (human CLCA-2 and mouse CLCA-1) (Table 1), both selectively expressed in the lung vasculature, serve as adhesion molecules for lung metastatic cancer cells (12, 13).
TABLE 1.
LIST OF SELECTED MOLECULAR LIGANDS PREFERENTIALLY EXPRESSED IN THE LUNG VASCULATURE IDENTIFIED THROUGH DIFFERENT METHODOLOGIES
| Lung Vascular Receptor | Ligand | Localization | Reference |
|---|---|---|---|
| Cell Culture Studies | |||
| α-galactose and α-N-actyl-galactosamine | Griffonia simplicifolia lectin | Microvascular endothelial cells | 9, 10 |
| α- and β-N-actyl-galactosamine | Helix pomatia lectin | Pulmonary artery derived endothelial cells | 9, 10 |
| E-cadherin | N.D. | Microvascular endothelial cells (not expressed by artery endothelial cells) | 9 |
| Phage Display | |||
| Membrane dipeptidyl peptidase (CD26) | GFE peptide | Lung endothelial cells | 2, 48 |
| Receptor unknown | CGSPGWVRC peptide | Lung endothelial cells | 31 |
| cDNA Array | |||
| Phospholipase A2 group XII | N.D. | Lung endothelial cells | 49 |
| Secreted frizzled related protein 1 (sFRP1) | N.D. | Lung endothelial cells | 49 |
| Osteoglycin | N.D. | Lung endothelial cell, smooth muscle cell, around cartilage and alveoli | 49, 50 |
| Other Approaches | |||
| Ca2+-activated chloride channels (human CLCA-2/mouse CLCA-1/Lu-ECAM-1) | α6β4 integrin | Endothelia of the aorta and pulmonary venules | 12 |
| Dipeptidyl peptidase IV (CD26) | Fibronectin | Lung endothelium | 13 |
| Angiotensin converting enzyme (ACE) (lung selective marker) | Antibody anti-angiotensin converting enzyme | Endothelial luminal surface | 52 |
| Platelet-endothelial adhesion molecule-1 (PECAM-1)/CD31 (lung selective marker) | Antibody anti-platelet-endothelial adhesion molecule-1 | Intercellular borders of the endothelial monolayer | 52 |
| Aminopeptidase P (APP) | Antibody anti-aminopeptidase P | Caveola of lung endothelium | 53 |
Definition of abbreviation: N.D. = not determined.
Overall, more than 40 different cell types have been identified and characterized as components of human lungs and respiratory airways (14) and as our understanding of the cellular diversity of the vasculature expands, we anticipate that other lung-derived endothelial cell populations will be recognized. In fact, the 2008 National Heart, Lung, and Blood Institute (NHLBI) workshop recommended further studies for the characterization of obscure and undefined cellular components of the lungs and the discovery of new and more efficient molecular markers for these various cellular types, including pulmonary vascular cells (14). These recommendations, if accomplished, would represent important milestones in improving our knowledge of the diversity present in the airways' vascular bed and its implication in health and disease; however, these are not trivial tasks. To accomplish these goals, it is important to probe the surface of the vascular bed using functional assays that detect the availability and accessibility of receptors expressed on the cell surface to binding probes, without the limitations imposed by preconceived biases or assumptions about their nature. While different methodologies have been used toward this goal (Table 1), phage display stands out as a technique that is well-suited to assist in accomplishing such milestones.
PHAGE DISPLAY
In vivo phage display has proven to be a valuable tool in isolating probes that home selectively to different vascular beds and targeting receptors expressed exclusively on certain blood vessels. Both tissue-specific and angiogenesis-related vascular ligand–receptor pairs have been identified with this technology (15, 16). These organ- or tumor-specific peptides can subsequently be used for targeted delivery of cytotoxic drugs, proapoptotic peptides, fluorophores, or cytokines to the vasculature and can generally improve selectivity and/or therapeutic windows in animal models (3, 19–23). Vascular receptors are also attractive targets for systemic delivery of viruses in gene therapy, particularly because such receptors are readily accessible through circulation and can often promote ligand-mediated particle uptake by cells (24). We have used innovative biotechnology concepts for the direct assembly of nanoparticles homing to receptors expressed selectively in vasculature associated with cardiovascular and pulmonary diseases. Based on in vivo phage display technology, we have uncovered a vascular address system that allows organ-specific targeting to normal blood vessels and angiogenesis-related targeting to tumor blood vessels (αV integrin–binding motif RGD and cell adhesion motif NGR) (15–19, 25–27). We have also developed imaging technology for determining the distribution of such targeted probes in vivo, their organ specificity, and their cellular receptors (28, 29). The body of our previous work—among others—suggests that (1) the vascular endothelium of organs is indeed modified in a tissue-specific manner and (2) the development of diseases with a vascular component is accompanied by specific abnormalities in the cells constituting blood vessels. The later is an important concept for the development of future therapies directed at the early detection of lung diseases.
Over the past decade, we were the first to report on a phage display library screening that was performed in a cancer patient followed by a large-scale survey of motifs that localized to different organs. This report confirmed the idea that tissue distribution of circulating peptides is nonrandom (15). A high-throughput analysis of selected motifs using pattern recognition software to analyze short amino acid residues in silico revealed similarities to ligands of differentially expressed cell surface proteins, leading to the validation of several candidate ligand–receptor pairs in the vasculature of normal and diseased human tissues. These candidates include bone morphogenetic protein in bone marrow, interleukin 11-receptor α in prostate, and a novel peptide targeting white fat vasculature (15, 21, 24, 25). Results obtained from in silico methods required further validation, including immunostaining to ensure tissue specificity and alanine scanning to ensure binding specificity (15, 21, 24, 25). The methodology used by our group represents a major step toward the ultimate goal of outlining a molecular map of the human vasculature, which has been widely noted, commented upon, and editorialized. In addition, we have also proposed a framework of ethical guidelines to allow new types of patient-oriented research (30). In the future, in vivo phage display will permit researchers to probe the endothelium in an unbiased manner, allowing for the building of an organ- and tissue-directed receptor-ligand map of their corresponding vascular beds. This map can be further exploited for the development of targeted therapies.
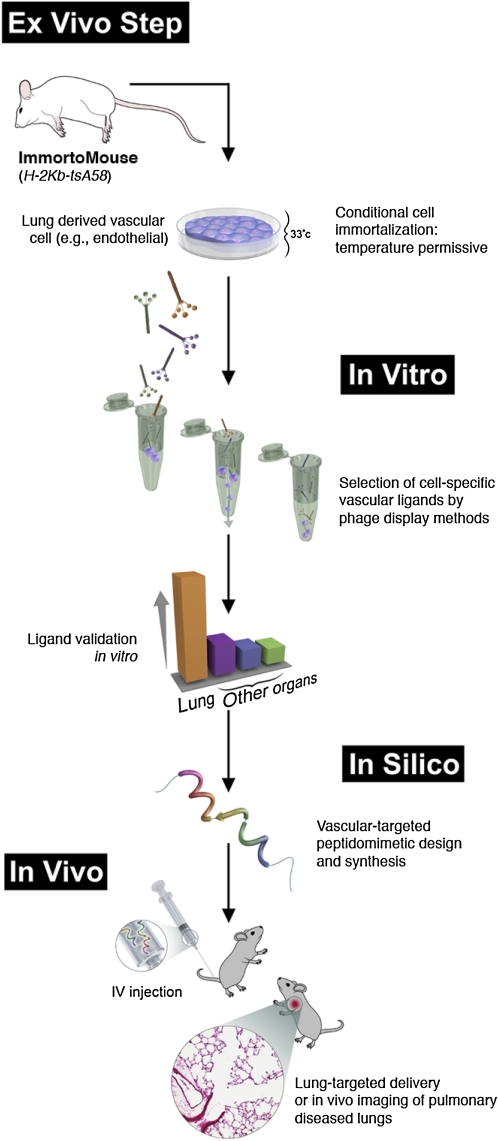
TARGETING PULMONARY EMPHYSEMA
A logical extension of our work is to isolate motifs that home in vivo to the blood vessels of selective tissues affected by cardiovascular and lung disorders. With this purpose in mind, we have developed a new strategy for targeting different organs by combining in vivo, in vitro, and ex vivo phage display screenings (Figure 1). Probes specific to the lung vasculature identified using this strategy can then be used for the development of novel therapeutic and imaging agents for the treatment of pulmonary diseases as well as disease model management. Performing a combinatorial screening on an immortalized lung endothelial cell line recently confirmed this methodology. The screening led to the identification of a lung endothelial cell–binding peptide (CGSPGWVRC) which, once injected into mice, preferentially homed to lung blood vessels (31). These results support the observation that lung endothelial cells in vitro retain at least some of their molecular and physiologic properties (9, 10, 32). Peptide homing was further validated by ex vivo immunostaining in lung endothelium cells as well as endothelial cells from other organs to ensure lung specificity and no biased distribution toward the lung (31).
Figure 1.
Framework for discovery and validation of organ-specific vascular markers. In the ex vivo steps, endothelial cells isolated from different organs of the H-2Kb-tsA58 mouse (termed ImmortoMouse; see Reference 51) are maintained in culture at permissive temperature. Using the in vitro phage display method BRASIL (biopanning and rapid analysis of selective interactive ligands; see Reference 40), peptides targeting these cell populations are selected, validated, and prioritized based on their binding profile. Peptide ligands are further validated by phage in vivo assays in which, after tail-vein injection, phage homing to the different vascular beds are quantified by colony count or immunostaining with anti-bacteriophage specific antibodies. The peptides targeting organ-specific vascular ligands then serve as a template for the design (in silico) and synthesis of organ targeting-agents. These designed agents can be delivered in vivo for targeted therapy applications, generation of animal models of degenerative diseases, or in vivo imaging of organ specific diseases.
When the lung-targeting peptide identified in our screening was fused to a pro-apoptotic motif (D(KLAKLAK)) and injected into mice, it specifically induced programmed cell death of lung endothelial cells in vivo with all the hallmarks of human emphysema. Emphysema or chronic pulmonary obstructive disease is the fourth leading cause of mortality and morbidity in the United States alone, and is caused (in most cases) by chronic cigarette smoking, with an estimated 10 to 16 million affected individuals. Our results corroborate the concept that alveolar endothelial cells have a central role in the maintenance of lung structure: recent findings have shown that alveolar capillary endothelial cell apoptosis predominates over epithelial cell death in the lungs of mice chronically exposed to cigarette smoke (33) and that trophic factors, such as vascular endothelial growth factor (VEGF), are required for both the apoptosis and the development of emphysema (33–37). We reasoned that ligand-directed programmed endothelial cell death may constitute a convenient, nongenetic means of generating pulmonary emphysema in mice or other small animals. Nongenetic rodent models of pulmonary emphysema (such as exogenous administration of nontargeted chemical agents or of cigarette smoke) are often time-consuming, labor-intensive, and not entirely reflective of human pulmonary emphysema (38). Hence, this new rodent model of emphysema offers an alternative to overcome some of these limitations—most importantly, the contribution of the lung vasculature to the progression of emphysema. In fact, the integration of our recent understanding of the role of lung endothelium in emphysema pathophysiology (33–37, 39) to new technologies such as combinatorial peptide targeting (15–17, 40) and lung-derived endothelial cell immortalization (32) offers an excellent opportunity to revisit this challenge and illustrates how combinatorial screenings on immortalized cells followed by in vivo targeting can be used as an experimental framework for the discovery and validation of additional ligand-directed pharmacodelivery systems in lung disorders (Figure 1).
Finally, the combination of nanotechnology and phage display offers unique opportunities to improve the diagnosis and treatment of cardiovascular and pulmonary diseases. The design and development of targeted nanoparticles with imaging capabilities and/or drug delivery functions is a significant and innovative concept being developed in our laboratory—combining phage display technology and gold (Au) nanoparticles (41, 42)—and will likely aid in overcoming some of the difficulties associated with targeted nanoparticles. Also known as colloidal gold, or nanogold, Au-nanoparticles have been the subject of extensive research and manipulation to achieve exquisite molecular, optical, and electronic properties. Au-nanoparticles are considered safe, which makes them unique candidates for several applications in medicine and biology. Phage can be selected based on their cell binding and internalization properties, and the Au-phage nonoparticles we have studied have been developed based on characterized ligand–receptor pairs selectively accessible upon intravenous and local administration. This is a unique and attractive feature of in vivo phage display screening: it detects targets on the basis of their cell surface expression and access to a binding probe (43). These important properties can be subsequently integrated into a nanoparticle assembly platform based on Au-phage targeted delivery systems. The combination of in vivo phage display and nanoparticle assembly constitutes a promising platform for the development of innovative therapeutic agents for the imaging and treatment of lung-associated disorders.
FUTURE PERSPECTIVES
Angiogenesis is an important component in cancer and in a wide range of nonneoplastic diseases. As proposed by the late Dr. Judah Folkman, “by viewing the process of angiogenesis as an ‘organizing principle’ in biology, intriguing insights into the molecular mechanisms of seemingly unrelated phenomena might be gained” (44). This concept is likely to find important applications in the development of novel therapeutic agents and imaging tools for lung diseases. In asthma and chronic inflammation, an increase in the number of blood vessels (angiogenesis) and the remodeling of the airways have been reported and introduce the prospect for innovative approaches in therapeutic targeting of these diseases (45, 46). The impact of chronic pulmonary disease on HIV-infected patients has also been recently pointed out, and pulmonary emphysema is now recognized as a significant cause of mortality for these patients (47). Angiogenesis and endothelial cell apoptosis are also important components of chronic pulmonary disorders during AIDS progression. Therefore, the identification of vascular markers associated with these disorders will uncover novel targets for in vivo imaging, facilitating earlier diagnosis and improving life expectancy for many patients. A more thorough knowledge of how the vascular components of the lungs interact with each other and with surrounding tissues is, therefore, an important step for the development of new therapeutic interventions in pulmonary diseases.
Conflict of Interest Statement: R.J.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.K.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.M.T. received grant support from the NHLBI $100,001 or more and from the Alpha 1 Foundation $50,001–$100,000. W.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature 1996;380:364–366. [DOI] [PubMed] [Google Scholar]
- 2.Rajotte D, Arap W, Hagedorn M, Koivunen E, Pasqualini R, Ruoslahti E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J Clin Invest 1998;102:430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trepel M, Arap W, Pasqualini R. In vivo phage display and vascular heterogeneity: implications for targeted medicine. Curr Opin Chem Biol 2002;6:399–404. [DOI] [PubMed] [Google Scholar]
- 4.Aird WC. Mechanisms of endothelial cell heterogeneity in health and disease. Circ Res 2007;100:174–190. [DOI] [PubMed] [Google Scholar]
- 5.Gebb SA, Shannon JM. Tissue interactions mediate early events in pulmonary vasculogenesis. Dev Dyn 2000;217:159–169. [DOI] [PubMed] [Google Scholar]
- 6.Pauling MH, Vu TH. Mechanisms and regulation of lung vascular development. Curr Top Dev Biol 2004;64:73–99. [DOI] [PubMed] [Google Scholar]
- 7.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science 2001;294:564–567. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 2001;294:559–563. [DOI] [PubMed] [Google Scholar]
- 9.Stevens T, Phan S, Frid MG, Alvarez D, Herzog E, Stenmark KR. Lung vascular cell heterogeneity: endothelium, smooth muscle, and fibroblasts. Proc Am Thorac Soc 2008;5:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 2004;67:139–151. [DOI] [PubMed] [Google Scholar]
- 11.Chetham PM, Babál P, Bridges JP, Moore TM, Stevens T. Segmental regulation of pulmonary vascular permeability by store-operated Ca2+ entry. Am J Physiol 1999;276:L41–L50. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Ghany M, Cheng HC, Elble RC, Pauli BU. The breast cancer beta 4 integrin and endothelial human CLCA2 mediate lung metastasis. J Biol Chem 2001;276:25438–25446. [DOI] [PubMed] [Google Scholar]
- 13.Cheng HC, Abdel-Ghany M, Elble RC, Pauli BU. Lung endothelial dipeptidyl peptidase IV promotes adhesion and metastasis of rat breast cancer cells via tumor cell surface-associated fibronectin. J Biol Chem 1998;273:24207–24215. [DOI] [PubMed] [Google Scholar]
- 14.Franks TJ, Colby TV, Travis WD, Tuder RM, Reynolds HY, Brody AR, Cardoso WV, Crystal RG, Drake CJ, Engelhardt J, et al. Resident cellular components of the human lung: current knowledge and goals for research on cell phenotyping and function. Proc Am Thorac Soc 2008;5:763–766. [DOI] [PubMed] [Google Scholar]
- 15.Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardó-Vila M, Giordano RJ, Mintz PJ, Ardelt PU, Yao VJ, Vidal CI, et al. Steps toward mapping the human vasculature by phage display. Nat Med 2002;8:121–127. [DOI] [PubMed] [Google Scholar]
- 16.Yao VJ, Ozawa MG, Trepel M, Arap W, McDonald DM, Pasqualini R. Targeting pancreatic islets with phage display assisted by laser pressure catapult microdissection. Am J Pathol 2005;166:625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolonin MG, Sun J, Do KA, Vidal CI, Ji Y, Baggerly KA, Pasqualini R, Arap W. Synchronous selection of homing peptides for multiple tissues by in vivo phage display. FASEB J 2006;20:979–981. [DOI] [PubMed] [Google Scholar]
- 18.Trepel M, Pasqualini R, Arap W. Screening phage-display peptide libraries for vascular targeted peptides. Methods Enzymol 2008;445:83–106. [DOI] [PubMed] [Google Scholar]
- 19.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998;279:377–380. [DOI] [PubMed] [Google Scholar]
- 20.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, Krajewski S, Lombardo CR, Rao R, Ruoslahti E, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med 1999;5:1032–1038. [DOI] [PubMed] [Google Scholar]
- 21.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med 2004;6:625–632. [DOI] [PubMed] [Google Scholar]
- 22.Curnis F, Sacchi A, Borgna L, Magni F, Gasparri A, Corti A. Enhancement of tumor necrosis factor α antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13). Nat Biotechnol 2000;18:1185–1190. [DOI] [PubMed] [Google Scholar]
- 23.Tandle A, Hanna E, Lorang D, Hajitou A, Moya CA, Pasqualini R, Arap W, Adem A, Starker E, Hewitt S, et al. Tumor vasculature-targeted delivery of tumor necrosis factor-alpha. Cancer 2009;115:128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zurita AJ, Troncoso P, Cardó-Vila M, Logothetis CJ, Pasqualini R, Arap W. Combinatorial screenings in patients: the interleukin-11 receptor alpha as a candidate target in the progression of human prostate cancer. Cancer Res 2004;64:435–439. [DOI] [PubMed] [Google Scholar]
- 25.Pasqualini R, Arap W. Vascular targeting. In: Encyclopedia of cancer, 2nd ed. Bertino JR, editor. New Jersey: Academic Press, Inc.; 2002. pp. 4.501–507.
- 26.Christianson DR, Ozawa MG, Pasqualini R, Arap W. Techniques to decipher molecular diversity by phage display. Methods Mol Biol 2007;357:385–406. [DOI] [PubMed] [Google Scholar]
- 27.Sergeeva A, Kolonin MG, Molldrem JJ, Pasqualini R, Arap W. Display technologies: application for the discovery of drug and gene delivery agents. Adv Drug Deliv Rev 2006;58:1622–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hajitou A, Trepel M, Lilley CE, Soghomonyan S, Alauddin MM, Marini FC III, Restel BH, Ozawa MG, Moya CA, Rangel R, et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell 2006;125:385–398. [DOI] [PubMed] [Google Scholar]
- 29.Hajitou A, Lev DC, Hannay JA, Korchin B, Staquicini FI, Soghomonyan S, Alauddin MM, Benjamin RS, Pollock RE, Gelovani JG, et al. A preclinical model for predicting drug response in soft-tissue sarcoma with targeted AAVP molecular imaging. Proc Natl Acad Sci USA 2008;105:4471–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pentz RD, Cohen CB, Wicclair M, DeVita MA, Flamm AL, Youngner SJ, Hamric AB, McCabe MS, Glover JJ, Kittiko WJ, et al. Ethics guidelines for research with the recently dead. Nat Med 2005;11:1145–1149. [DOI] [PubMed] [Google Scholar]
- 31.Giordano RJ, Lahdenranta J, Zhen L, Chukwueke U, Petrache I, Langley RR, Fidler IJ, Pasqualini R, Tuder RM, Arap W. Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J Biol Chem 2008;283:29447–29460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ. Tissue-specific microvascular endothelial cell lines from H-2K(b)-tsA58 mice for studies of angiogenesis and metastasis. Cancer Res 2003;63:2971–2976. [PubMed] [Google Scholar]
- 33.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 2004;114:1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 2003;29:88–97. [DOI] [PubMed] [Google Scholar]
- 36.Tuder RM, Petrache I, Elias JA, Voelkel NF, Henson PM. Apoptosis and emphysema: the missing link. Am J Respir Cell Mol Biol 2003;28:551–554. [DOI] [PubMed] [Google Scholar]
- 37.Tang K, Rossiter HB, Wagner PD, Breen EC. Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl Physiol 2004;97:1559–1566. (discussion 1549). [DOI] [PubMed] [Google Scholar]
- 38.Mahadeva R, Shapiro SD. Animal models of pulmonary emphysema. Curr Drug Targets Inflamm Allergy 2005;4:665–673. [DOI] [PubMed] [Google Scholar]
- 39.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005;11:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giordano RJ, Cardo-Vila M, Lahdenranta J, Pasqualini R, Arap W. Biopanning and rapid analysis of selective interactive ligands. Nat Med 2001;7:1249–1253. [DOI] [PubMed] [Google Scholar]
- 41.Souza GR, Christianson DR, Staquicini FI, Ozawa MG, Snyder EY, Sidman RL, Miller JH, Arap W, Pasqualini R. Networks of gold nanoparticles and bacteriophage as biological sensors and cell targeting agents. Proc Natl Acad Sci USA 2006;103:1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Souza GR, Yonel-Gumruk E, Fan D, Easley J, Rangel R, Guzman-Rojas L, Miller JH, Arap W, Pasqualini R. Bottom-up assembly of hydrogels from bacteriophage and Au nanoparticles: the effect of cis- and trans-acting factors. PLoS One 2008;3:e2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozawa MG, Zurita AJ, Dias-Neto E, Nunes DN, Sidman RL, Gelovani JG, Arap W, Pasqualini R. Beyond receptor expression levels: the relevance of target accessibility in ligand-directed pharmacodelivery systems. Trends Cardiovasc Med 2008;18:126–132. [DOI] [PubMed] [Google Scholar]
- 44.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 2007;6:273–286. [DOI] [PubMed] [Google Scholar]
- 45.McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med 2001;164:S39–S45. [DOI] [PubMed] [Google Scholar]
- 46.Warner SM, Knight DA. Airway modeling and remodeling in the pathogenesis of asthma. Curr Opin Allergy Clin Immunol 2008;8:44–48. [DOI] [PubMed] [Google Scholar]
- 47.Petrache I, Diab K, Knox KS, Twigg HL III, Stephens RS, Flores S, Tuder RM. HIV associated pulmonary emphysema: a review of the literature and inquiry into its mechanism. Thorax 2008;63:463–469. [DOI] [PubMed] [Google Scholar]
- 48.Rajotte D, Ruoslahti E. Membrane dipeptidase is the receptor for a lung-targeting peptide identified by in vivo phage display. J Biol Chem 1999;274:11593–11598. [DOI] [PubMed] [Google Scholar]
- 49.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, et al. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA 2003;100:10623–10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernández B, Kampmann A, Pipp F, Zimmermann R, Schaper W. Osteoglycin expression and localization in rabbit tissues and atherosclerotic plaques. Mol Cell Biochem 2003;246:3–11. [PubMed] [Google Scholar]
- 51.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA 1991;88:5096–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muzykantov VR. Biomedical aspects of targeted delivery of drugs to pulmonary endothelium. Expert Opin Drug Deliv 2005;2:909–926. [DOI] [PubMed] [Google Scholar]
- 53.Oh P, Borgström P, Witkiewicz H, Li Y, Borgström BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, et al. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat Biotechnol 2007;25:327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]