Abstract
Molecular imaging (MI) may be defined as imaging in vivo using molecules that report on biologic function. This review will focus on the clinical use of radioactive tracers (nonpharmacologic amounts of compounds labeled with a radioactive substance) that permit external imaging using single photon emission computed tomography (planar, SPECT) or positron emission tomography (PET) imaging. Imaging of lung cancer has been revolutionized with the use of fluorine-18–labeled fluorodeoxyglucose (18F-FDG), an analog of glucose that can be imaged using PET. The ability to carry out whole body imaging after intravenous injection of 18F-FDG allows accurate staging of disease, helping to determine regional and distant nodal and other parenchymal involvement. Glycolysis is increased in nonmalignant conditions, including inflammation (e.g., sarcoidosis), and 18F-FDG PET is a sensitive method for evaluation of active inflammatory disease. Inflammatory disease has been imaged, even before the advent of PET, with planar and SPECT imaging using gallium-67, a radiometal that binds to transferrin. Metabolic alteration in pulmonary pathology is currently being studied, largely in lung cancer, primarily with PET, with a variety of other radiotracers. Prominent among these is thymidine; fluorine-18–labeled thymidine PET is being increasingly used to evaluate proliferation rate in lung and other cancers. This overview will focus on the clinical utility of 18F-FDG PET in the staging and therapy evaluation of lung cancer as well as in imaging of nonmalignant pulmonary conditions. PET and SPECT imaging with other radiotracers of interest will also be reviewed. Future directions in PET imaging of pulmonary pathophysiology will also be explored.
Keywords: SPECT, PET, radiolabeled ligands, cancer, infection
Molecular imaging reveals the functional characteristics of disease. Hence it is important to remember that molecular characteristics may reflect a variety of pathophysiologic conditions; thus, patient characteristics need to be borne in mind to enable optimal utilization of these tremendously useful imaging modalities. Glycolysis is elevated in a multitude of cancers as well as in nonmalignant disorders. Conversely, glycolysis may not be elevated in some malignancies, including some aggressive cancers that may use other metabolic substrates (e.g., glutamine) for energy. Similarly, other radiotracers also detect underlying molecular processes: gallium uptake occurs not only in neoplasms (e.g., high grade lymphoma), but also in inflammatory conditions, such as Pneumocystis carinii pneumonia. This review will therefore address each molecular imaging modality and assess its role in both malignant and inflammatory pulmonary conditions. Finally, references will be selected for their relevance as well as their comprehensiveness: recent reviews rather than studies will be preferred.
POSITRON EMISSION TOMOGRAPHY
Positron emission tomography (PET) enables in vivo imaging of the distribution of fluorodeoxyglucose (FDG) (and other positron-emitting ligands) with high(< 1 cm) resolution and high (nanomolar) sensitivity. The addition of transmission computed tomography (CT) to PET instruments results in images that provide both functional/molecular information and structural images, and PET/CT has consequently become the most rapidly developing medical imaging modality.
Deoxyglucose is an analog of glucose that enters the cell by facilitated transport mediated by glucose transporters. While it is phosphorylated by hexokinase in a manner identical to glucose, it does not proceed further down the glycolysis pathway and thus accumulates in the cell. Accumulation of deoxyglucose in the cell is therefore proportional to its glycolytic rate. The attachment to deoxyglucose of fluorine-18, a positron emitter with an approximately 110-minute half-life, resulting in fluorodeoxyglucose or FDG, permits external imaging using PET. Cancer cells typically have high rates of glycolysis; cancers therefore appear as areas of tracer accumulation on FDG PET/CT.
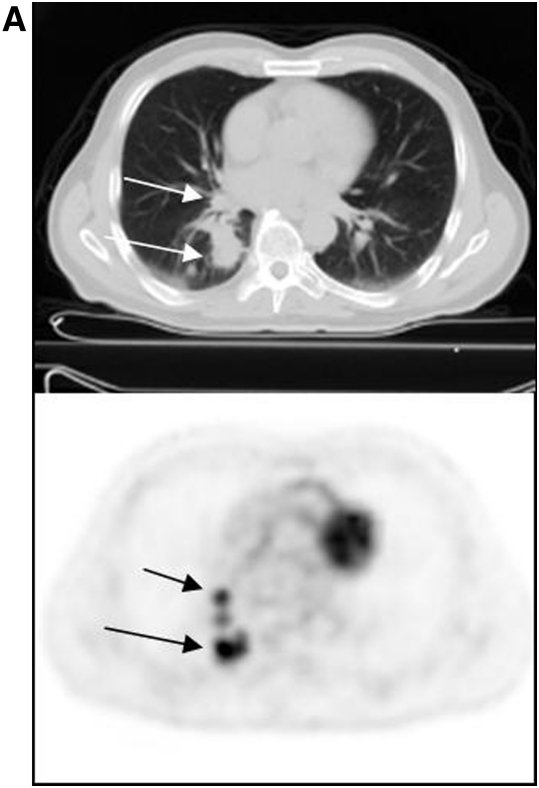
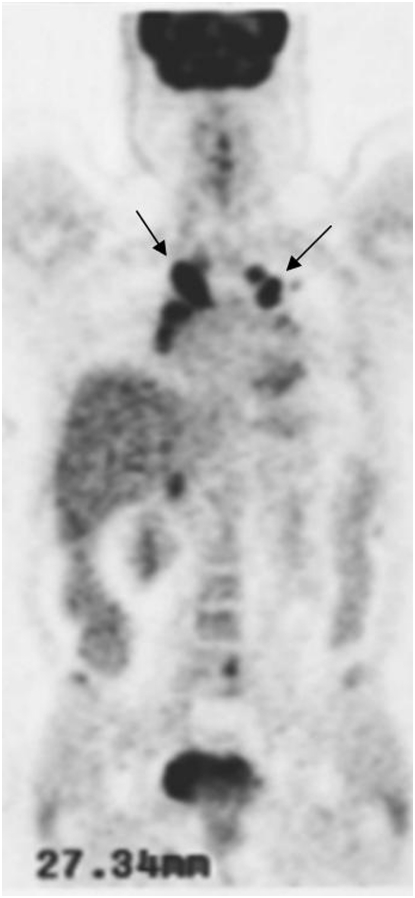
PET/CT with 18F-FDG is being used to stage a variety of epithelial and other neoplasms. The first FDA-approved application of 18F-FDG was in the evaluation of solitary pulmonary nodules (1). The accuracy of detection of malignant pulmonary lesions using FDG PET is very high, typically more than 90% (2). Moreover, the ability to image the entire body enables evaluation of nodal status as well as presence of distant metastases. Figure 1 is an example of a patient with a lung cancer, with additional foci of accumulation in the nodes and bones, indicative of (previously unsuspected) distant disease, and altering patient management.
Figure 1.
Patient with lung cancer, imaged with fluorodeoxyglucose (FDG) positron emission tomorgraphy (PET)/computed tomography (CT). The patient had known right lung cancer and hilar metastasis (A, arrows); right supraclavicular nodal metastasis (B, arrow) and left femoral osseous metastasis (C, arrow) were revealed on FDG PET/CT, changing management.
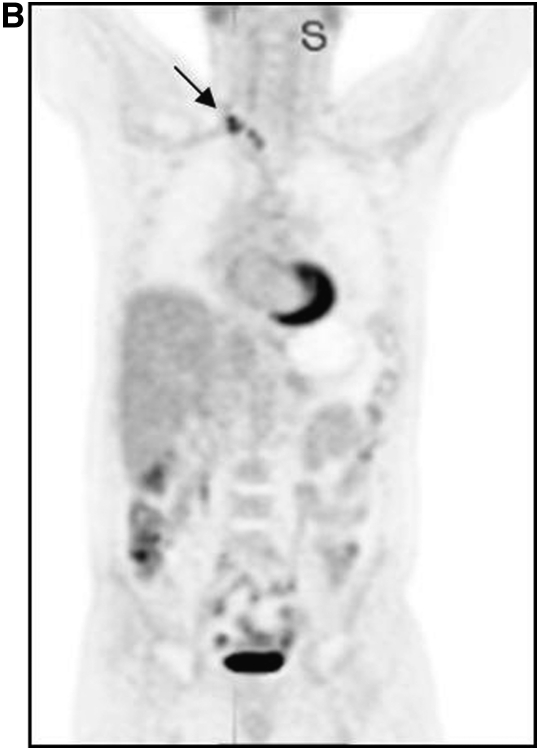
In addition to aiding accurate staging of disease, FDG PET/CT is also very useful in the evaluation of response to therapy. Figure 2 is one such example. The same patient as depicted in Figure 1 was imaged 3 months after systemic therapy. The previously visualized lesions are far less intense after therapy, indicative of response. Note that the structural abnormality in this patient has not altered—as may be intuitive, changes in molecular and functional characteristics occur before structural change is apparent. Most studies have demonstrated the utility of FDG PET in the evaluation of response to therapy after completion of therapy; an increasing number of studies have suggested that such evaluation is also useful soon after initiation of therapy (i.e., after the first course of chemotherapy). Validation of this promising approach is ongoing.
Figure 2.
Same patient as in Figure 1, before (left) and after (right) therapy. The bone lesion (arrows) is much less glycolytically active after therapy, though it appears unchanged on companion CT.
It must be remembered that glycolysis may be altered in conditions other than lung cancer. Thus, FDG PET/CT will detect pulmonary lesions in other malignancies, most commonly lymphoma (1, 3). Finally, some malignancies (e.g., bronchioloalveolar carcinoma) are not glycolytically active and thus are frequently negative on FDG PET/CT.
Other metabolic tracers have also been studied in patients with lung cancer. The most actively studied has been radiolabeled thymidine. Thymidine is a nucleoside that is incorporated into DNA, and uptake of radiolabeled (with chromium or tritium) thymidine is used in vitro to measure cell proliferation. Carbon-11 is a positron emitter with a half-life of approximately 20 minutes, and 11C-labeled thymidine was used to image cell proliferation in a variety of cancers; subsequently, 18F-l-thymidine (FLT) has been used to image proliferation in lung and other cancers (4). Glycolytically active lesions such as tuberculosis, with minimal cell proliferation, may be distinguished from highly proliferative lung cancers using this method.
The utility of FDG PET to delineate metabolically viable tumor has found increasing application in the identification of appropriate tumor volumes for external beam radiotherapy (5). Frequently, structural imaging is inadequate for this purpose, since lung cancer may be indistinguishable structurally from contiguous processes such as collapse, or neighboring normal mediastinal structures. Molecular imaging is invaluable in such instances for accurate identification of tumor; it can also aid in the delineation of tumor volumes for therapy (appropriate methods for delineation are currently being evolved, though there is currently a lack of consensus on standardization of the technique) (6).
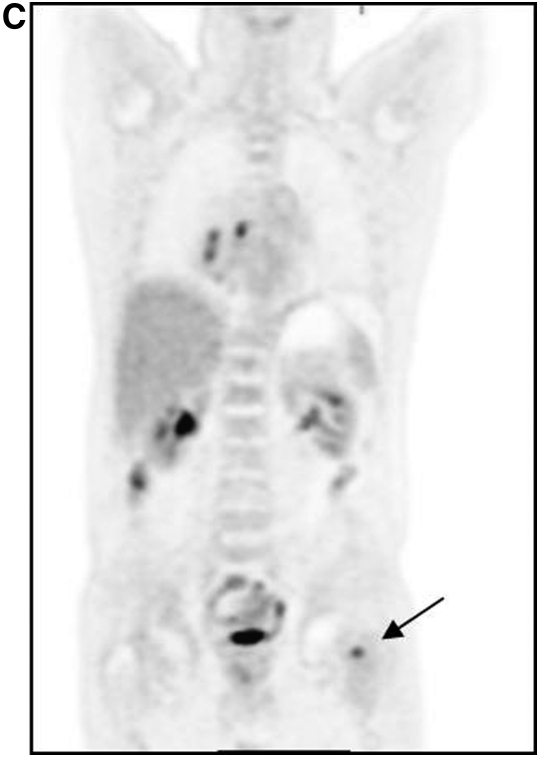
Increased glycolysis is also a function of many inflammatory conditions. Thus, FDG PET/CT frequently detects active inflammatory conditions such as sarcoidosis (7). The imaging modality has been used to assess the presence of active disease as well as disease response to therapy. Figure 3 is an example of a patient with sarcoidosis. Note that the bilateral glycolytically active adenopathy is indistinguishable from that in lymphoma (though the symmetric distribution is more characteristic of sarcoid). Other infectious conditions (such as active tuberculosis) will also result in foci of increased FDG accumulation, and may confound image interpretation. Tuberculosis is in fact usually indistinguishable from primary malignancy, and biopsy confirmation may be necessary. This has limited the utility of FDG PET in the identification of lung malignancy in regions where tuberculosis is prevalent.
Figure 3.
Chest FDG PET of a patient with active sarcoidosis. Bilateral inflamed mediastinal nodes (arrows) are a characteristic feature of this disease.
IMAGING THE CANCER PHENOTYPE
In addition to PET imaging of metabolic processes, there has been increasing interest in noninvasive in vivo evaluation of features of the cancer phenotype, particularly those that have an impact on outcome. Hypoxia has been the best studied of these features. Several studies have shown that patients with hypoxic tumors respond less well to therapy and have shortened disease-free and overall survival. 2-nitroimidazole compounds accumulate in hypoxic tissue, and the ability to attach positron emitters to many such compounds led to their study as PET imaging agents of hypoxia (8), with the observation that these tracers accumulated in hypoxic tumors, and that a positive PET image correlated negatively with response and survival end-points. Another agent, Cu(II)-diacetyl-bis(N4)-methylthiosemicarbazone (Cu-ATSM), has also been studied with comparable results (9).
Tumor vascularity is another feature of the cancer phenotype that has implications for prognosis as well as for development of appropriate therapies. Imaging of neovasculature in tumor has important implications for design of appropriate therapies that adversely impact tumor vascularity. PET agents have been developed and are being studied in the clinic (10), as are agents labeled with technetium-99m (99mTc), a single photon emitting agent that is widely available (11).
SINGLE PHOTON IMAGING
Planar γ camera imaging of pulmonary function predates PET imaging by decades. The most widely used planar imaging has been with particles (typically macro-aggregated albumin, MAA) labeled with a single photon emitter. These particles lodge in the first pre-arteriolar bed. Thus, when injected intravenously, they are trapped in the pulmonary vasculature. The most widely used single photon emitter, 99mTc, has a half-life of 6 hours and decays exclusively by emission of a low-energy (140 KeV) photon, with consequent excellent imaging characteristics for γ camera imaging. 99mTc-labeled MAA is the most widely used agent for single photon imaging of the pulmonary vasculature, and is the standard for evaluation of relative (segmental and individual) pulmonary perfusion. Such imaging is being found to be increasingly useful in avoidance of normal lung during external beam therapy in patients with lung cancer (5). Comparable methods have also been developed for evaluation of pulmonary ventilation, typically using inert gases (xenon-133, krypton-81m), as well as radioaerosols (12).
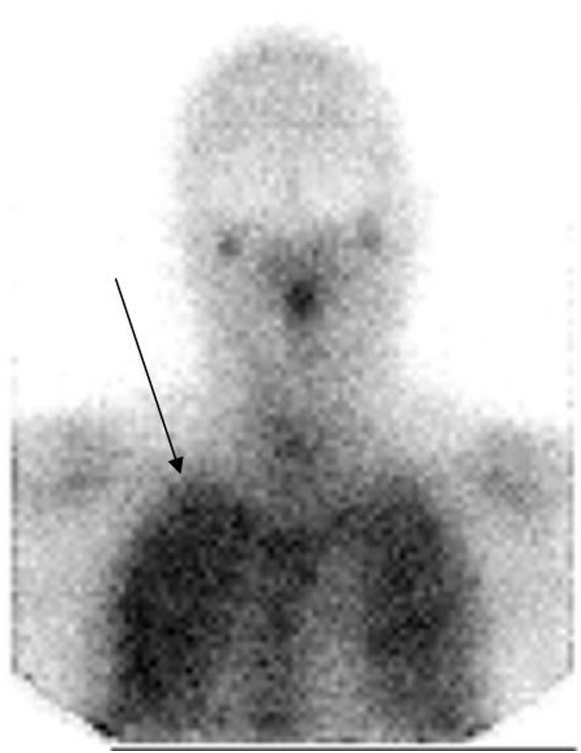
Single photon imaging—planar and SPECT—has also been used in evaluation of pulmonary infections. The most widely used tracer for this purpose has been gallium-67 citrate, which has very high accuracy in the detection of this condition in the appropriate clinical setting (13). Positive images may be obtained as soon as 4 hours after administration of the radiotracer. A positive image is characterized by diffuse uptake of tracer throughout both lungs (Figure 4), though the distribution may be bibasilar, especially in patients who have been receiving pentamidine aerosol prophylaxis.
Figure 4.
An anterior chest planar image of a patient with HIV and symptoms of Pneumocystis carinii pneumonia. There is uptake of gallium-67 in both lungs (arrow).
In contrast to positron emitters, most of which are relatively short-lived, half-lives of single photon emitters are longer. This enables the evaluation of agents with relatively long biologic half-lives, typically macromolecules, that permit appropriate radionuclide attachment. Radiolabeled antibodies have been studied to evaluate organ biodistribution and tumor targeting of the native molecule. A study (14) with an antibody targeting epidermal growth factor receptor (EGFr) demonstrated that at small mass amounts, most of the antibody went to normal EGFr sites in the liver, precluding targeting to squamous lung cancer. Only at higher doses was there targeting to tumor.
Targeting of antibody to tumor was also demonstrated using indium-111 (111In, a single photon emitter with an ∼3-d half-life) labeled to an antibody that recognizes Lewis-y, an antigen known to be overexpressed in the majority of small cell lung cancers (15). Small cell lung cancer is sensitive to both radiotherapy and immunotherapy, and this study has led to the exploration of radioimmunotherapy in this disease. Imaging with this agent will also provide a noninvasive method for identification of antigen-positive disease, thus selecting patients for appropriate therapy.
SUMMARY AND FUTURE
Molecular imaging of lung cancer will play an increasingly important role in staging of disease as well as in evaluation of treatment response. FDG PET is being increasingly used for the evaluation of solitary pulmonary nodules; for staging of patients with lung cancer, both for mediastinal involvement and distant metastases, and for response to therapy (16). FDG PET/CT permits identification of treatment volume for radiation therapy (16, 17), though it may not be as useful for adaptive radiotherapy, since glycolysis may also be increased in normal contiguous lung during radiotherapy, particularly as a consequence of inflammatory change.
The limitations of FDG have led to the development of other positron-emitting radiotracers for the evaluation of lung cancer (18). Radiolabeled thymidine has been described earlier in this review; PET with 18FlT promises to become an established method for the in vivo evaluation of tumor proliferation. Other metabolic tracers that are currently being studied include radiolabeled (C-11 or F-18) choline and acetate, to study membrane integrity and fatty acid synthesis, respectively; amino acids (e.g., methionine) have been studied in other cancers and may have applicability in lung cancers, particularly those tumors that obtain their energy through mechanisms other than glycolysis.
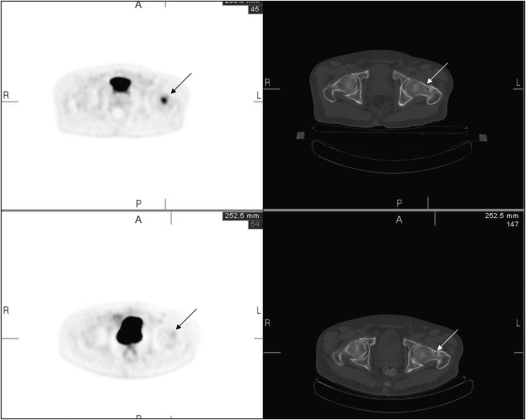
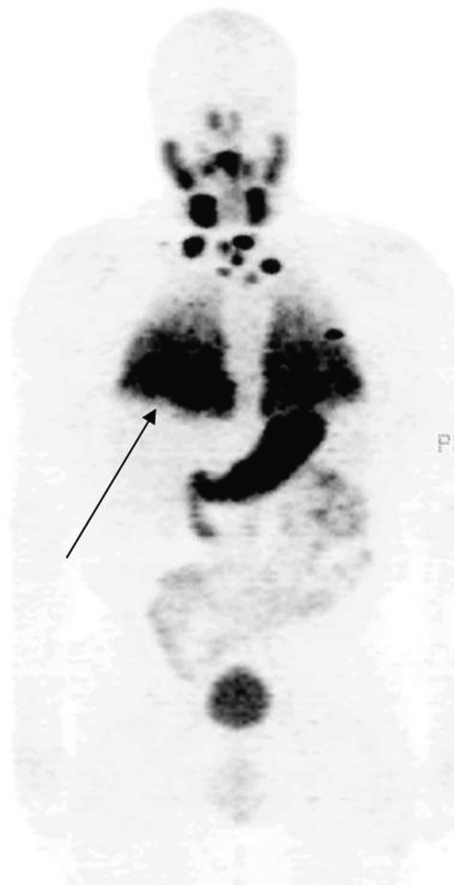
Radiation-absorbed dose to lung cancer as well as normal parenchyma may be calculated using SPECT and PET. Figure 5 is an example of a patient with metastatic thyroid cancer studied with iodine-124, an isotope with an approximately 4-day half-life. Targeting of the isotope to diffuse lung metastases (Figure 5, arrow) is apparent. The quantitative nature of PET permits identification of disease as well as calculation of radiation-absorbed dose, permitting calculation of safe amounts of I-131 for therapy. The development of hybrid SPECT/CT machines, which provide structural volumetric information to single photon imaging, will further enable quantification of radioactivity and permit accurate quantitation of radiation doses after systemic radiotherapy.
Figure 5.
A chest FDG PET three-dimensional projection of patient with metastatic thyroid cancer, imaged 3 days after oral iodine-124. Diffuse lung metastases (arrow) are clearly visualized.
Features of the cancer phenotype including hypoxia and neovascularity have been studied in lung cancer, and systematic validation studies are planned. The ability to attach radionuclides to the growing array of molecular therapies of promise in lung cancer should considerably aid in the identification of molecules with desirable targeting and biodistribution characteristics.
While the focus of molecular imaging research has been cancer, imaging of inflammatory and other pulmonary conditions has proceeded apace; it is beyond the scope of this essentially clinical review.
Conflict of Interest Statement: C.R.D. served as a consultant for Wilex AG and served on the Board or Advisory Board for IBA-M $5,001–$10,000. He received lecture fees from GlaxoSmithKline $1,001–$5,000 and received grant support from the NIH and the FDA $100,001–more.
References
- 1.Castell F, Cook GJ. Quantitative techniques in 18FDG PET scanning in oncology. Br J Cancer 2008;98:1597–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bar-Shalom R, Kagna O, Israel O, Guralnik L. Noninvasive diagnosis of solitary pulmonary lesions in cancer patients based on 2-fluoro-2-deoxy-D-glucose avidity on positron emission tomography/computed tomography. Cancer 2008;113:3213–3221. [DOI] [PubMed] [Google Scholar]
- 3.Schöder H, Moskowitz C. PET imaging for response assessment in lymphoma: potential and limitations. Radiol Clin North Am 2008;46:225–241. [DOI] [PubMed] [Google Scholar]
- 4.Bading JR, Shields AF. Imaging of cell proliferation: status and prospects. J Nucl Med 2008;49:64S–80S. [DOI] [PubMed] [Google Scholar]
- 5.Chang JY, Dong L, Liu H, Starkschall G, Balter P, Mohan R, Liao Z, Cox JD, Komaki R. Image-guided radiation therapy for non-small cell lung cancer. J Thorac Oncol 2008;3:177–186. [DOI] [PubMed] [Google Scholar]
- 6.Mac Manus MP, Hicks RJ. Impact of PET on radiation therapy planning in lung cancer. Radiol Clin North Am 2007;45:627–638. [DOI] [PubMed] [Google Scholar]
- 7.Braun JJ, Kessler R, Constantinesco A, Imperiale A. 18F-FDG PET/CT in sarcoidosis management: review and report of 20 cases. Eur J Nucl Med Mol Imaging 2008;35:1537–1543. [DOI] [PubMed] [Google Scholar]
- 8.Lee FT, Scott AM. Hypoxia positron emission tomography imaging with 18F-fluoromisonidazole. Semin Nucl Med 2007;37:451–461. [DOI] [PubMed] [Google Scholar]
- 9.Chen DL, Dehdashti F. Advances in positron emission tomographic imaging of lung cancer. Proc Am Thorac Soc 2005;2:541–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beer AJ, Lorenzen S, Metz S, Herrmann K, Watzlowik P, Wester HJ, Peschel C, Lordick F, Schwaiger M. Comparison of integrin αvβ3 expression and glucose metabolism in primary and metastatic lesions in cancer patients: a PET study using 18F-galacto-RGD and 18F-FDG. J Nucl Med 2008;49:22–29. [DOI] [PubMed] [Google Scholar]
- 11.Decristoforo C, Faintuch-Linkowski B, Rey A, von Guggenberg E, Rupprich M, Hernandez-Gonzales I, Rodrigo T, Haubner R. [99mTc]HYNIC-RGD for imaging integrin αvβ3 expression. Nucl Med Biol 2006;33:945–952. [DOI] [PubMed] [Google Scholar]
- 12.Zhang G, Dilling TJ, Stevens CW, Forster KM. Functional lung imaging in thoracic cancer radiotherapy. Cancer Control 2008;15:112–119. [DOI] [PubMed] [Google Scholar]
- 13.Kroe DM, Kirsch CM, Jensen WA. Diagnostic strategies for Pneumocystis carinii pneumonia. Semin Respir Infect 1997;12:70–78. [PubMed] [Google Scholar]
- 14.Divgi CR, Welt S, Kris M, Real FX, Yeh SD, Gralla R, Merchant B, Schweighart S, Unger M, Larson SM, et al. Phase I and imaging trial of indium 111-labeled anti-epidermal growth factor receptor monoclonal antibody 225 in patients with squamous cell lung carcinoma. J Natl Cancer Inst 1991;83:97–104. [DOI] [PubMed] [Google Scholar]
- 15.Krug LM, Milton DT, Jungbluth AA, Chen LC, Quaia E, Pandit-Taskar N, Nagel A, Jones J, Kris MG, Finn R, et al. Targeting Lewis Y (Le(y)) in small cell lung cancer with a humanized monoclonal antibody, hu3S193: a pilot trial testing two dose levels. J Thorac Oncol 2007;2:947–952. [DOI] [PubMed] [Google Scholar]
- 16.Mac Manus M, Hicks RJ. The use of positron emission tomography (PET) in the staging/evaluation, treatment, and follow-up of patients with lung cancer: a critical review. Int J Radiat Oncol Biol Phys 2008;72:1298–1306. [DOI] [PubMed] [Google Scholar]
- 17.Macapinlac HA. Clinical applications of positron emission tomography/computed tomography treatment planning. Semin Nucl Med 2008;38:137–140. [DOI] [PubMed] [Google Scholar]
- 18.Miele E, Spinelli GP, Tomao F, Zullo A, De Marinis F, Pasciuti G, Rossi L, Zoratto F, Tomao S. Positron emission tomography (PET) radiotracers in oncology–utility of 18F-Fluoro-deoxy-glucose (FDG)-PET in the management of patients with non-small-cell lung cancer (NSCLC). J Exp Clin Cancer Res 2008;27:52. [DOI] [PMC free article] [PubMed] [Google Scholar]