Abstract
Nanotechnology holds the promise of revolutionizing our society, bringing numerous beneficial innovations to improve structural materials, electronics, energy, medical imaging, and drug delivery, among other applications. However, nanomaterials present potential safety concerns, and there is accumulating evidence to suggest that nanoparticles may exert adverse effects on the lung and other organ systems. This article will overview the potential risks of engineered nanoparticles and nanotechnology on the respiratory system and highlight recent findings related to pulmonary and systemic effects of inhaled nanoparticles. Special emphasis will be given to carbon nanotubes and the possibility that these nanoparticles could represent an emerging risk for environmental and occupational lung disease, especially in individuals with pre-existing respiratory diseases such as asthma.
Keywords: nanotechnology, nanomaterials, carbon nanotubes, fibrosis, pleura
THE EMERGENCE OF NANOTECHNOLOGY AS A NEW HEALTH CONCERN
Humans have been exposed to a multitude of naturally occurring nanosized particles (e.g., ultrafine particulates from volcanic activity or forest fires) throughout our evolution. Only over the past two decades have we had the capability to manipulate matter at the nanoscale level (1). The beneficial applications of nanotechnology in numerous industrial, consumer, and medical applications are extremely promising. These applications include those that may lead to more efficient water purification, stronger and lighter building materials, increased computing power and speed, improved generation and conservation of energy, and new tools for the diagnosis and treatment of disease. However, the outlook for a future improved by nanotechnology should be tempered by the fact that relatively little is known about the adverse effects of nanomaterials on human health and the environment (2). Rapid advances in nanotechnology have created a multitude of new structures, and we cannot precisely predict how these new nanomaterials will interact with biological systems.
The definition of a nanoparticle is generally considered to be a particle with at least one dimension of 100 nm or less. As a result of their small size and unique physicochemical properties, the toxicological profiles of nanoparticles may differ considerably from those of larger particles composed of the same materials (1, 2). Furthermore, nanoparticles of different materials (e.g., gold, silica, titanium, carbon nanotubes, quantum dots) may have their own unique mechanism of toxicity. Therefore, it seems unlikely that the toxic potential and/or mechanisms of nanoparticles can be predicted or explained by any single unifying concept.
The respiratory system represents a unique target for the potential toxicity of nanoparticles due to the fact that in addition to being the portal of entry for inhaled particles, it also receives the entire cardiac output. As such, there is potential for exposure of the lungs to nanoparticles that are introduced to the body via the act of breathing or by any other exposure route that may result in systemic distribution, including dermal and gastrointestinal absorption and direct injection. Interest in the respiratory system as a target for the potential effects, both beneficial and adverse, of nanoparticles is reflected by the steady increase in the number of scientific publications on these subjects during the past decade (3). For the purposes of this article, only intentionally engineered nanoparticles are considered; unintentionally generated (e.g., via combustion engines, grilling, welding) and naturally occurring nanoparticles are not included in this discussion. Special emphasis will be given to carbon nanotubes, which are an emerging focus of occupational and environmental exposures.
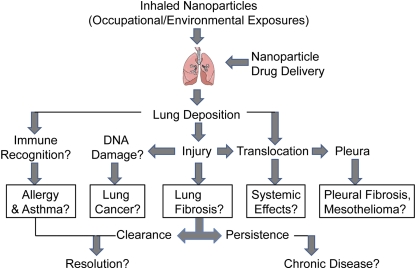
There is the potential for the respiratory system to be exposed to a seemingly countless number of unique nanoparticles, essentially none of which has been sufficiently examined for potential toxicity at this time. A substantial number of nanoparticles are already present in the marketplace in consumer products such as sunscreens, cosmetics, and car wax, just to name a few. A comprehensive list is maintained and updated by the Project on Emerging Technologies at the Woodrow Wilson International Center for Scholars (www.wilsoncenter.org/nano). While many nanoparticles may prove to have little or no toxicity, the fact that there is any potential for adverse effects from nanoparticle exposure suggests that prudence is warranted. Therefore, the potential of nanomaterials to cause disease after occupational or environmental exposures, as well as in drug delivery scenarios, should be carefully evaluated to determine specific outcomes, some of which are illustrated in Figure 1.
Figure 1.
Biologic endpoints that should be evaluated to determine the potential of nanomaterials to cause disease in mice after inhalation to mimic occupational or environmental exposures or by nebulization routes to mimic drug delivery scenarios.
The diversity of nanoparticles include those that are carbon-based (e.g., nanotubes, nanowires, fullerenes), metal-based (e.g., gold, silver, quantum dots, metal oxides such as titanium dioxide and zinc oxide), and those of a biological nature (e.g., liposomes and viruses designed for gene or drug delivery). To demonstrate the complexity of the situation, it is worthwhile to consider the case of carbon nanotubes as an example. Carbon nanotubes can be: (1) produced and/or cleaned using one of several different methods; (2) produced using one of several different metal catalysts; (3) single- or multi-walled; (4) of various lengths; and (5) subjected to numerous surface modifications (3). The result is a vast number of unique carbon nanotube derivatives, all of which fall under the category of “carbon nanotubes.”
CARBON NANOTUBES AND INTERSTITIAL PULMONARY FIBROSIS
Carbon nanotubes (CNT) possess unique characteristics that make them highly desirable for applications in electronics, structural engineering, and medicine due to impressive electrical conductivity, mechanical strength, and ability to be derivatized for custom applications such as drug delivery (3). Given the enormous industrial demand for carbon nanotubes (the global market is predicted to grow to between $1 billion and $2 billion by 2014), there is growing concern that the development and use of engineered CNT might be accompanied by health risks caused by occupational or environmental exposures (4). Structurally, CNT can be described as graphite sheets rolled into cylinders that are one (“single-walled,” SWCNT) or several (“multi-walled,” MWCNT) layers thick. CNT range from one to several nanometers in width and up to several microns in length. This large length to width (aspect) ratio, a property shared with asbestos fibers, has led to concern that inhaled CNT may cause asbestos-like injuries, such as pulmonary fibrosis and lung cancer (5).
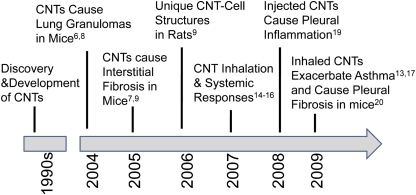
A number of studies over the past five years have shown that CNT, when delivered to the lungs of mice, cause fibrosis and systemic immune responses, form unique structures that bridge macrophages, and exacerbate allergic responses. A timeline of these discoveries is shown in Figure 2. Liquid suspensions of CNT cause pulmonary inflammation and fibrosis when administered to the lung by intratracheal instillation or pharyngeal aspiration (6–10). These studies show that CNT, which aggregate into micron-sized bundles in aqueous media, stimulate the formation of inflammatory foci known as granulomas and fibrotic reactions within the lung parenchyma. Pulmonary fibrosis and/or granulomas have been reported in mice or rats within days after intratracheal instillation of SWCNT (6–9) or MWCNT (10). Moreover, SWCNT delivered to the lung by instillation increases production of platelet-derived growth factor (PDGF) and transforming growth factor (TGF)-β1, two growth factors that mediate the pathogenesis of fibrosis (7–9). Granulomas are associated with large CNT aggregates in the lung that are easily visible by light microscopy, while interstitial pulmonary fibrosis appears to be associated with dispersed CNT that are detected by electron microscopy (7). A recent study showed that SWCNT dispersed with organic solvents to disrupt Van der Waals forces resulted in less deposition in the large airways but more in the interstitium, where the fibrotic response was significantly increased (11). While instillation or aspiration experiments demonstrate that CNT are fibrogenic, these methods do not accurately model deposition patterns of CNT, and much emphasis has been placed on the need for inhalation studies.
Figure 2.
Timeline of selected reports in the literature that indicate toxic effects of carbon nanotubes in mice or rats. Superscripted numbers indicate references for published studies that support these observations.
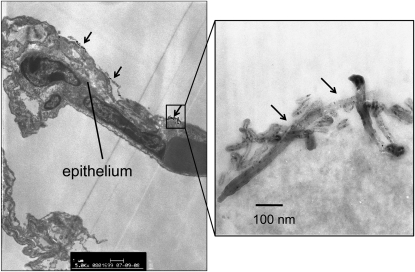
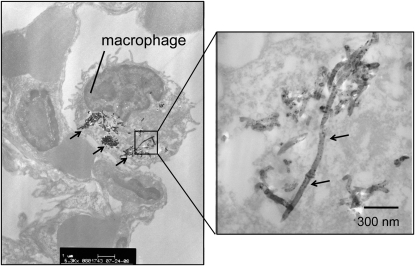
There is recent evidence from whole-body inhalation studies in rodents that the method of pulmonary administration of MWCNT affects deposition patterns and pathology. A study that compared instillation with inhalation indicated that MWCNT inhaled by mice showed a more diffuse pattern of deposition with less severe pathologic lesions than those that were observed after intratracheal instillation of a bolus dose (12). Inhalation work from our laboratory shows that MWCNT cause little inflammation or interstitial fibrosis in the lungs of mice 2 weeks after a single 6-hour exposure, except under conditions of pre-existing inflammation (13). In our hands, aggregates of MWCNT are deposited at the alveolar level in the lungs of mice and are taken up primarily by alveolar macrophages (Figures 3 and 4). Mitchell and coworkers also showed that inhaled MWCNT do not cause significant interstitial lung inflammation or fibrosis in mice, but do cause systemic immunosuppression and splenic oxidative stress (14). The mechanism of splenic immunosuppression involves the release of TGF-β1 from the lungs of MWCNT-exposed mice; TGF-β1 then enters the bloodstream to signal COX-2–mediated increases in prostaglandin-E2 and IL-10 in the spleen, both of which play a role in suppressing T cell proliferation (15). This result greatly differed from earlier work that showed MWCNT administered by intratracheal instillation resulted in pulmonary inflammation and fibrosis (9). Therefore, inhaled MWCNT appear to cause less interstitial fibrosis when delivered by inhalation, but significantly more injury, inflammation, and fibrosis when administered to the lungs by intratracheal or pharyngeal routes. However, even relatively low concentrations of inhaled MWCNT appear to have significant effects on the systemic immune system (14, 15). Erdely and coworkers also reported that SWCNT or MWCNT deposited into the lung induced acute lung and systemic effects, suggesting that the systemic response, if chronic and persistent, could trigger or exacerbate cardiovascular dysfunction and disease (16).
Figure 3.
Transmission electron micrograph showing deposition of multi-walled carbon nanotubes (MWCNT) on the surface of the alveolar epithelium in C57BL6 mice 24 hours after a single 6-hour exposure to 30 mg/m3 MWCNT. Arrows indicate aggregates of MWCNT. Right-hand panel is a high-magnification image of one of these aggregates.
Figure 4.
Transmission electron micrograph showing MWCNT within an alveolar macrophage in the lung of a C57BL6 mouse 24 hours after a single 6-hour exposure to 30 mg/m3 MWCNT. Arrows indicate aggregates of MWCNT. Right-hand panel is a high-magnification image of one of these aggregates.
CARBON NANOTUBES AND ALLERGIC ASTHMA
Increasing evidence indicates that the adverse effects of inhaled MWCNT are increased under conditions of pre-existing inflammation such as allergic asthma. Recent studies have shown that MWCNT exacerbate ovalbumin-induced allergic airway inflammation in mice (13, 17). Ryman-Rasmussen and coworkers demonstrated that exposure of mice to ovalbumin before inhalation of MWCNT caused significant airway fibrosis, whereas ovalbumin or MWCNT alone did not significantly increase airway fibrosis (13). Inoue and colleagues reported that MWCNT administered intratracheally significantly increased ovalbumin-induced T-lymphocyte proliferation and amplified lung Th2 cytokines and chemokines compared with ovalbumin exposure alone (17). Work by Park and coworkers suggests that MWCNT delivered to the lungs of mice may induce allergic responses through B cell activation and production of IgE in the absence of any allergen pre-exposure (18). Collectively, these mouse studies suggest that individuals with allergic asthma are most susceptible to immune responses and airway remodeling caused by carbon nanotube exposure, and that CNT alone might serve as allergens. However, it is unknown whether carbon nanotubes will cause or exacerbate asthma in humans.
CARBON NANOTUBES AND PLEURAL DISEASE
Since carbon nanotubes possess a length to width aspect ratio similar to that of asbestos fibers, there is concern that nanotubes may cause asbestos-related diseases, such as fibrosis of the lung interstitium and pleura, or mesothelioma (cancer of the pleura). Work by Poland and colleagues showed that injection of long MWCNT into the peritoneal cavity of mice (a surrogate for the pleura) induced inflammation, suggesting that MWCNT have asbestos-like pathogenicity (19). This study was important because it demonstrated that long MWCNT caused granuloma formation at the pleura similar to long asbestos fibers. However, a major caveat of the study is that it was not performed in the lung and MWCNT exposure was not by inhalation. More relevant inhalation studies in mice show that MWCNT reach the pleura (the anatomic site of mesothelioma) and cause significant increases in pleural fibrosis (20). However, no indication of mesothelioma was found in mice that inhaled MWCNT. Therefore, it is unknown whether carbon nanotubes represent a new cancer risk factor similar to asbestos. While carbon nanotubes cause lung fibrotic reactions in the interstitium and pleura of mice, their carcinogenic potential has not been adequately addressed.
CONCLUSIONS
Engineered nanomaterials represent new potential risks for environmental and occupational lung diseases. However, because of the infancy of the field of nanotechnology, there are no epidemiologic data to indicate health hazards for the majority of nanomaterials. Some nanoparticles, such as nanosized metals (e.g., nickel, cobalt, vanadium) should clearly represent risk factors for lung diseases, as many of these metals in their native form are known to have fibrogenic or carcinogenic effects in humans. Other nanomaterials, such as fullerenes and carbon nanotubes, show inflammatory, immune, and/or fibrogenic effects in mice or rats, indicating that caution should be taken in the handling of these materials. As the field of nanotechnology continues to emerge, researchers in the field of environmental lung disease are in a unique position to address the toxicity of nanomaterials and prevent occupational and environmental exposures.
Supported by National Institutes of Health Grants R21 ES015801–01 and RC2 ES018772–01 (J.C.B.), The Hamner Institutes for Health Sciences, and North Carolina State University's College of Agricultural and Life Sciences.
Conflict of Interest Statement: J.C.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Borm PJ, Robbins D, Haubold S, Kuhlbusch T, Fissan H, Donaldson K, Schins R, Stone V, Kreyling W, Lademann J, et al. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol 2006;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science 2006;311:622–627. [DOI] [PubMed] [Google Scholar]
- 3.Card JW, Zeldin DC, Bonner JC, Nestmann ER. Pulmonary applications and toxicity of engineered nanoparticles. Am J Physiol Lung Cell Mol Physiol 2008;295:L400–L411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, Alexander A. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci 2006;92:5–22. [DOI] [PubMed] [Google Scholar]
- 5.Jaurand MC, Renier A, Daubriac J. Mesothelioma: do asbestos and carbon nanotubes pose the same health risk? Part Fibre Toxicol 2009;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci 2004;77:126–134. [DOI] [PubMed] [Google Scholar]
- 7.Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol 2005;289:L698–L708. [DOI] [PubMed] [Google Scholar]
- 8.Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci 2004;77:117–125. [DOI] [PubMed] [Google Scholar]
- 9.Mangum JB, Turpin EA, Antao-Menezes A, Cesta MF, Bermudez E, Bonner JC. Single-walled carbon nanotube (SWCNT)-induced interstitial fibrosis in the lungs of rats is associated with increased levels of PDGF mRNA and the formation of unique intercellular carbon structures that bridge alveolar macrophages in situ. Part Fibre Toxicol 2006;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, Arras M, Fonseca A, Nagy JB, Lison D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol 2005;207:221–231. [DOI] [PubMed] [Google Scholar]
- 11.Mercer RR, Scabilloni J, Wang L, Kisin E, Murray AR, Schwegler-Berry D, Shvedova AA, Castranova V. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am J Physiol Lung Cell Mol Physiol 2008;294:L87–L97. [DOI] [PubMed] [Google Scholar]
- 12.Li JG, Li WX, Xu JY, Cai XQ, Liu RL, Li YJ, Zhao QF, Li QN. Comparative study of pathological lesions induced by multiwalled carbon nanotubes in lungs of mice by intratracheal instillation and inhalation. Environ Toxicol 2007;22:415–421. [DOI] [PubMed] [Google Scholar]
- 13.Ryman-Rasmussen JP, Tewksbury EW, Moss OR, Cesta MF, Wong BA, Bonner JC. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am J Respir Cell Mol Biol 2009;40:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell LA, Gao J, Wal RV, Gigliotti A, Burchiel SW, McDonald JD. Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicol Sci 2007;100:203–214. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell LA, Lauer FT, Burchiel SW, McDonald JD. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat Nanotechnol 2009;4:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erdely A, Hulderman T, Salmen R, Liston A, Zeidler-Erdely PC, Schwegler-Berry D, Castranova V, Koyama S, Kim YA, Endo M, et al. Cross-talk between lung and systemic circulation during carbon nanotube respiratory exposure: potential biomarkers. Nano Lett 2009;9:36–43. [DOI] [PubMed] [Google Scholar]
- 17.Inoue K, Koike E, Yanagisawa R, Hirano S, Nishikawa M, Takano H. Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicol Appl Pharmacol 2009;237:306–316. [DOI] [PubMed] [Google Scholar]
- 18.Park EJ, Cho WS, Jeong J, Yi J, Choi K, Park K. Pro-inflammatory and potential allergic responses resulting from B cell activation in mice treated with multi-walled carbon nanotubes by intratracheal instillation. Toxicology 2009;259:113–121. [DOI] [PubMed] [Google Scholar]
- 19.Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, Stone V, Brown S, MacNee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol 2008;3:423–428. [DOI] [PubMed] [Google Scholar]
- 20.Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt J, Tewksbury EW, Moss OR, Wong BA, Dodd DE, Andersen ME, et al. Inhaled multi-walled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol 2009;4:747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]