Abstract
Rationale: In aging HIV-infected populations comorbid diseases are important determinants of morbidity and mortality. Pulmonary diseases have not been systematically assessed in the combination antiretroviral therapy (ART) era.
Objectives: To determine the incidence of pulmonary diseases in HIV-infected persons compared with HIV-uninfected persons.
Methods: We analyzed data from the Veterans Aging Cohort Study Virtual Cohort, consisting of 33,420 HIV-infected veterans and 66,840 age, sex, race and ethnicity, and site-matched HIV-uninfected veterans. Using Poisson regression, incidence rates and adjusted incidence rate ratios were calculated to determine the association of HIV with pulmonary disease. The Virtual Cohort was merged with the 1999 Veterans Large Health Survey to adjust for self-reported smoking in a nested sample (14%).
Measurements and Main Results: Incident chronic obstructive pulmonary disease, lung cancer, pulmonary hypertension, and pulmonary fibrosis, as well as pulmonary infections, were significantly more likely among HIV-infected patients compared with uninfected patients in adjusted analyses, although rates of asthma did not differ by HIV status. Bacterial pneumonia and chronic obstructive pulmonary disease were the two most common incident pulmonary diseases, whereas opportunistic pneumonias were less common. Absolute rates of most pulmonary diseases increased with age, although the relative differences between those with and without HIV infection were greatest in younger persons. Chronic obstructive pulmonary disease and asthma, as well as pulmonary infections, were less likely in those with lower HIV RNA levels and use of ART at baseline.
Conclusions: Pulmonary diseases among HIV-infected patients receiving care within the Veterans Affairs Healthcare System in the combination ART era reflect a substantial burden of non–AIDS-defining and chronic conditions, many of which are associated with aging.
Keywords: HIV; respiratory tract diseases; lung diseases, obstructive; pneumonia; pneumonia, bacterial
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Pulmonary diseases have been among the most common complications of HIV infection, but the spectrum of infectious and noninfectious pulmonary disease has not been assessed in the current combination antiretroviral therapy era.
What This Study Adds to the Field
In addition to a continued greater risk of infectious pulmonary complications, our findings support a greater risk of chronic obstructive pulmonary disease, lung cancer, pulmonary hypertension, and pulmonary fibrosis in HIV-infected patients compared with HIV-uninfected patients. These data suggest that there may be a shift in the epidemiology of pulmonary diseases afflicting HIV-infected patients in the current era with a significant burden of traditional non–HIV-associated pulmonary diseases, many of which are associated with aging.
Before the introduction and widespread use of combination antiretroviral therapy (ART), pulmonary diseases were among the most frequent complications of HIV infection and were associated with significant morbidity and mortality (1, 2). The multicenter Pulmonary Complications of HIV Infection Study conducted approximately 15 years ago before the combination ART era found that the most common pulmonary conditions encountered were infectious and included acute bronchitis, Pneumocystis pneumonia (PCP), and bacterial pneumonia (3). These complications were significantly more likely in HIV-infected Pulmonary Complications of HIV Infection Study participants compared with control subjects without HIV. Subsequent studies have suggested that chronic obstructive pulmonary disease (COPD) (4, 5), lung cancer (6), and pulmonary hypertension (7) may also occur with greater frequency in populations infected with HIV. The mechanisms for the observed increases in these diseases, however, are not well understood.
In the combination ART era, the incidence of pneumonia and other opportunistic infections has decreased (8–11). Consistent with this, a greater proportion of pulmonary admissions in recent years were attributable to noninfectious conditions in one report (12). However, the spectrum of infectious and noninfectious pulmonary disease has not been systematically assessed in the current ART era in a multisite cohort. In keeping with the increasing importance of non–HIV-associated comorbid diseases on outcomes in patients infected with HIV (13–15) and greater life expectancies as a result of ART, the spectrum of pulmonary diseases in HIV-infected patients has likely changed.
A better contemporary understanding of the burden of pulmonary diseases afflicting HIV-infected persons is important to inform patient care and future research. We determined the incidence of pulmonary diseases in a national Veterans Affairs (VA)–based cohort of HIV-infected participants demographically matched to control subjects without HIV receiving care within the VA in the ART era. We compared how incidence rates varied by HIV status and age. Because non–AIDS-associated cardiovascular, liver, and renal diseases are increasingly recognized as important comorbidities in aging HIV-infected patients (16), we were particularly interested in determining the burden of noninfectious compared with infectious pulmonary complications. Some of the results of these studies have been previously reported in the form of an abstract (17).
METHODS
Cohort
We used data from the Veterans Aging Cohort Study Virtual Cohort that consists of 33,420 HIV-infected subjects and 66,840 HIV-uninfected subjects. The Virtual Cohort has been previously described in detail and is approved by the institutional review boards at participating institutions (18); readers may also find additional details in the online supplement. Each subject was followed from the baseline index date (first documentation of HIV status within the VA Healthcare System for HIV-infected patients or date of matching for HIV-uninfected patients) until the end of July 2007. Because administrative data are generally inaccurate measures of tobacco exposure (19), the Virtual Cohort was merged with data from the 1999 Large Health Survey of Veteran Enrollees (LHS) to adjust analyses for self-reported smoking status (20), yielding a nested sample of 3,707 HIV-infected patients and 9,980 HIV-uninfected patients with data from both sources.
Pulmonary Conditions
Pulmonary conditions were diagnosed based on ICD-9 codes. As in previous studies (21), at least one inpatient or at least two outpatient codes were required to establish a diagnosis, because this improves the accuracy of ICD-9 codes. Prevalence at baseline was defined as the presence of a condition within a window of 12 months before and up to 6 months after the index date. Diagnoses that were documented later than 6 months after the index date were considered incident, because we were interested in comparing the incidence of diseases after HIV infection was recognized and patients were receiving medical care for HIV within the VA.
Clinical Characteristics and Comorbidities
Additional data retrieved from the VA administrative databases included baseline age; sex; race and ethnicity; and ICD-9 diagnoses of alcohol disorders, drug abuse, and hepatitis C infection. For HIV-infected patients, we obtained CD4+ cell count (cells per microliter), HIV-1 RNA (copies per milliliter), and use of ART during the baseline window (22, 23). In the nested sample consisting of patients in the Virtual Cohort and the LHS, smoking status was determined by self-report; as in other studies (24) and based on the LHS questions, patients were classified as ever smokers (>100 lifetime cigarettes) versus those who had never smoked (25).
Statistical Analysis
Baseline characteristics in HIV-infected and uninfected members of the cohort were compared using the t test or Wilcoxon rank-sum test for normally or nonnormally distributed continuous variables, respectively, and the chi-square test for categorical variables. To determine incidence rates, we calculated time at risk (person-years) from the index date until first diagnosis of each pulmonary disease. Incidence rates are expressed per 1,000 person-years. Subjects were censored at date of death or last known activity within the VA until July 2007.
To determine the independent risk associated with HIV infection for each pulmonary disease, incidence rate ratios (IRR) were calculated in Poisson regression models that included all HIV-infected and uninfected subjects. A statistically significant interaction between HIV and age below 50 years was identified for bacterial pneumonia (P < 0.001) and COPD (P = 0.02), with a trend toward significance for lung cancer (P = 0.08). Models were therefore stratified by age less than 50, or 50 years and older.
We also performed analyses restricted to two subsets of patients. To assess for confounding by cigarette smoking on the association of HIV infection with each pulmonary disease, we performed analyses within the restricted cohort of 3,707 HIV-infected patients and 9,980 HIV-uninfected patients from the merged Virtual Cohort/LHS. To examine the impact of HIV-specific variables, we restricted models to the HIV-infected patients in the Virtual Cohort, and adjusted for baseline CD4+ cell count, HIV RNA level, and use of ART. The square root of the CD4+ cell count and the log10 HIV RNA level were used to approximate normally distributed variables for use in multivariate models. P values of less than 0.05 were considered statistically significant. All analyses were performed using Stata 10.0 (Stata Corp., College Station, TX).
RESULTS
Baseline Characteristics and Prevalent Pulmonary Diseases
The HIV-infected and uninfected cohorts were matched for demographic characteristics. The median age was 45 years; most patients were male and over 40% were black (Table 1). Alcohol disorders (21% vs. 19%), drug abuse (23% vs. 15%), and hepatitis C infection (30% vs. 11%) were all significantly more common among HIV-infected patients compared with HIV-uninfected patients (P < 0.001 for all). Most patients were smokers, with 80% of the HIV-infected patients compared with 76% of the HIV-uninfected patients reporting having ever smoked (P < 0.001) in the nested sample of the Virtual Cohort and LHS. At baseline, the median CD4+ cell count among HIV-infected patients was 264 cells/μl (interquartile range 108–451), and 65% were on ART.
TABLE 1.
BASELINE CHARACTERISTICS AND DIAGNOSES OF PULMONARY DISEASE
| Characteristic | HIV-infected (n = 33,420) | HIV-uninfected (n = 66,840) | P Value |
|---|---|---|---|
| Age, median (IQR) | 45 (39–51) | 45 (39–51) | 0.05 |
| Male | 98% | 98% | 1.0 |
| Race | 1.0 | ||
| Black | 43% | 43% | |
| White | 32% | 32% | |
| Hispanic/other | 25% | 25% | |
| Hepatitis C infection | 30% | 11% | <0.001 |
| Alcohol disorders | 21% | 19% | <0.001 |
| Drug abuse | 23% | 15% | <0.001 |
| Ever-smoked* | 80% | 76% | <0.001 |
| HIV-related variables† | |||
| CD4+ cell count (cells/μl), median (IQR) | 264 (108–451) | — | |
| CD4+ <200 cells/μl | 40% | — | |
| HIV RNA level (copies/ml), median (IQR) | 14,815 (1,000–86,400) | — | |
| HIV RNA level <400 copies/ml | 14% | — | |
| ART | 65% | — | |
| Pulmonary disease at baseline | |||
| Any pulmonary disease | 7.0% | 6.0% | 0.01 |
| COPD | 4.6% | 4.0% | <0.001 |
| Asthma | 2.0% | 2.4% | <0.001 |
| Lung cancer | 0.55% | 0.38% | <0.001 |
| Pulmonary hypertension | 0.20% | 0.16% | 0.13 |
| Pulmonary fibrosis | 0.37% | 0.10% | <0.001 |
| Bacterial pneumonia | 7.5% | 1.1% | <0.001 |
| PCP | 5.3% | 0 | <0.001 |
| TB | 2.0% | 0.14% | <0.001 |
Definition of abbreviations: ART = antiretroviral therapy; COPD = chronic obstructive pulmonary disease; IQR = interquartile range; PCP = Pneumocystis pneumonia; TB = tuberculosis.
Smoking data were from the merged Virtual Cohort and Veterans Large Health Survey datasets and include 3,707 patients infected with HIV and 9,890 patients uninfected with HIV.
Baseline values of CD4+ cell count were available in 66%, HIV RNA levels in 64%, and ART use in 78% of patients infected with HIV.
Overall, 7% of HIV-infected patients and 6% of HIV-uninfected patients were diagnosed with one or more pulmonary conditions at baseline (P = 0.01). When considering individual conditions, the HIV-infected patients were significantly more likely to have diagnoses of COPD, lung cancer, pulmonary fibrosis, bacterial pneumonia, PCP, and tuberculosis (TB). In contrast, HIV-uninfected patients were significantly more likely to have a baseline diagnosis of asthma. There was no difference in the prevalence of pulmonary hypertension between groups.
Unadjusted Incidence of Pulmonary Diseases in HIV-infected Patients Compared with HIV-uninfected Patients
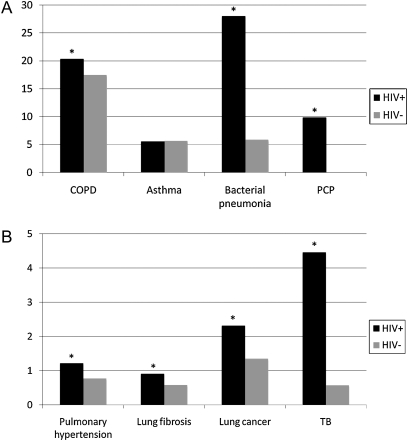
In unadjusted analyses, HIV-infected patients were more likely to have incident diagnoses of all infectious and noninfectious pulmonary diseases compared with HIV-uninfected patients, with the exception of asthma (Figure 1). Bacterial pneumonia and COPD were the two most commonly diagnosed pulmonary conditions. The incidence rate of bacterial pneumonia was 28.0 (95% confidence interval [CI], 27.2–28.8) per 1,000 person-years (PY) among HIV-infected patients compared with 5.8 (95% CI, 5.6–6.0) per 1,000 PY among HIV-uninfected patients (P < 0.001). The incidence of COPD was 20.3 (95% CI, 19.7–21.0) per 1,000 PY among HIV-infected patients compared with 17.5 (95% CI, 17.1–17.9) per 1,000 PY among HIV-uninfected patients (P < 0.001).
Figure 1.
(A and B) Unadjusted incidence per 1,000 person-years of pulmonary disease by HIV status. Incidence rates of each pulmonary condition are shown per 1,000 person-years on the y axis. Rates for HIV-infected patients (HIV+) are shown in the solid bars, and for HIV-uninfected patients (HIV−) are shown in the shaded bars. *P < 0.05 for HIV+ compared with HIV− patients. COPD = chronic obstructive pulmonary disease; PCP = Pneumocystis pneumonia; TB = tuberculosis.
Overall, COPD was the most common noninfectious pulmonary disease in HIV-infected patients, with 16% carrying a baseline or incident diagnosis of COPD. Asthma was the second most common incident pulmonary diagnosis in both groups, with a similar incidence of 5.6 (95% CI, 5.2–5.9) and 5.6 (95% CI, 5.4–5.8) per 1,000 PY among HIV-infected and uninfected patients, respectively (P = 0.6). Although less frequent, lung cancer, pulmonary hypertension, and pulmonary fibrosis were also significantly more likely in HIV-infected patients compared with HIV-uninfected patients. Lung cancer occurred with an incidence rate per 1,000 PY of 2.3 (95% CI, 2.1–2.5) in HIV-infected patients compared with 1.3 (95% CI, 1.2–1.5) in HIV-uninfected patients (P < 0.001). The incidence of pulmonary hypertension per 1,000 PY was 1.2 (95% CI, 1.1–1.4) compared with 0.8 (95% CI, 0.7–0.8) (P < 0.001), and that of pulmonary fibrosis was 0.9 (95% CI, 0.8–1.0) versus 0.6 (95% CI, 0.5–0.7) (P < 0.001) in HIV-infected and uninfected patients, respectively.
Other opportunistic pulmonary infections were less frequently encountered compared with bacterial pneumonia. The incidence of PCP among HIV-infected patients was 9.9 per 1,000 PY (95% CI, 9.4–10.3) and 0.02 (95% CI, 0.009–0.03) among HIV-uninfected patients (P < 0.001). The incidence of TB was 4.5 (95% CI, 4.2–4.8) per 1,000 PY among HIV-infected patients compared with 0.6 (95% CI, 0.5–0.6) among HIV-uninfected patients (P < 0.001).
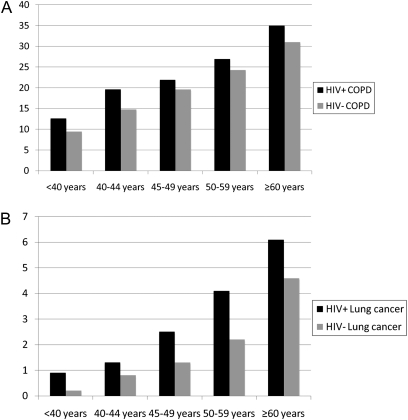
Incidence of Pulmonary Diseases Stratified by Age
To examine the impact of age on incidence of pulmonary diseases, we stratified patients into age groups from less than 40 years to greater than 60 years. With the exception of PCP and asthma, the incidence of pulmonary diseases increased in both HIV-infected and uninfected patients, with highest absolute rates in the oldest age groups (see Table E1 in the online supplement), as illustrated in Figure 2 for COPD and lung cancer. In general, the absolute difference between the incidence rates of pulmonary disease in HIV-infected patients compared with HIV-uninfected patients tended to increase with age, whereas the relative incidence tended to decrease with age for all conditions except for TB and asthma.
Figure 2.
Incidence of chronic obstructive pulmonary disease and lung cancer by age and HIV status. Incidence rates of chronic obstructive pulmonary disease (A) and lung cancer (B) are shown per 1,000 person-years on the y axis. Rates for HIV-infected patients (HIV+) are shown in the solid bars, and for HIV-uninfected patients (HIV−) are shown in the shaded bars. The incidence of chronic obstructive pulmonary disease and of lung cancer both increase significantly according to age (P trend < 0.001 for HIV-infected and uninfected patients for each condition).
Adjusted IRRs for Pulmonary Diseases
To identify independent risk factors for pulmonary diseases, we adjusted IRRs for age as a continuous variable, sex, race and ethnicity, alcohol disorders, drug abuse, and hepatitis C in models stratified by age less than 50 or 50 years and older, given the significant interaction of HIV with age less than 50 years for several conditions. HIV infection was independently associated with a significantly higher risk for all of the pulmonary diseases examined, except for asthma (Table 2, Tables E2 and E3). Although infectious pulmonary complications were most strongly associated with HIV, noninfectious pulmonary diseases were also significantly increased among HIV-infected patients. Importantly, the association with HIV infection remained essentially unchanged for most diseases when adjusted for ever smoking (Table 2) in the nested sample of the Virtual Cohort merged with the LHS.
TABLE 2.
INCIDENCE RATE RATIO FOR THE ASSOCIATION OF HIV INFECTION AND PULMONARY DISEASES STRATIFIED BY AGE IN MULTIVARIATE POISSON REGRESSION MODELS WITH AND WITHOUT ADJUSTING FOR SMOKING
| Incidence Rate Ratio (95% confidence interval) for HIV Infection |
||||
|---|---|---|---|---|
| Virtual Cohort |
Nested Sample of Virtual Cohort Merged with LHS, Adjusting For Having Ever Smoked |
|||
| Condition | Age <50 yr | Age ≥50 yr | Age <50 yr | Age ≥50 yr |
| COPD | 1.17 (1.11–1.24)* | 1.08 (1.01–1.15)* | 1.25 (1.08–1.43)* | 1.11 (0.96–1.29) |
| Asthma | 0.97 (0.89–1.06) | 0.93 (0.80–1.08) | 0.90 (0.64–1.27) | 0.87 (0.62–1.24) |
| Lung cancer | 2.13 (1.78–2.57)* | 1.73 (1.47–2.04)* | 2.28 (1.29–4.02)* | 1.76 (1.23–2.50)* |
| Pulmonary hypertension | 1.53 (1.21–1.93)* | 1.57 (1.23–2.00)* | 1.00 (0.48–2.10) | 1.66 (1.03–2.68)* |
| Pulmonary fibrosis | 1.59 (1.22–2.08)* | 1.45 (1.10–1.94)* | 1.78 (0.91–3.48) | 1.79 (1.01–3.14)* |
| Bacterial pneumonia | 5.09 (4.77–5.42)* | 2.84 (2.61–3.10)* | 4.24 (3.55–5.05)* | 2.44 (2.02–2.96)* |
| TB | 5.97 (4.98–7.16)* | 5.43 (4.03–7.30)* | 4.96 (2.96–8.29)* | 8.89 (4.07–19.4)* |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; LHS = 1999 Veterans Large Health Survey; TB = tuberculosis.
All models are adjusted for age as a continuous variable, race and ethnicity, sex, alcohol disorders, drug abuse, and hepatitis C infection. Models using the nested sample of the Virtual Cohort merged with the LHS are additionally adjusted for having ever smoked. Full models with incidence rate ratios for all predictors are shown in Tables E2 and E3. The incidence rate ratio for Pneumocystis pneumonia is >100 in all models.
P < 0.05.
When comparing the results of the multivariable models across the age strata of less than 50 or 50 years and older, HIV infection was associated with a greater IRR in younger patients particularly for bacterial pneumonia and lung cancer. COPD was also associated with a greater IRR among younger patients, although this difference was less substantial.
Incidence of Pulmonary Diseases by CD4+ Cell Count, HIV RNA Level, and ART among HIV-infected Patients
In analyses restricted to HIV-infected patients only, we determined the incidence of pulmonary diseases stratified by baseline CD4+ count, HIV RNA level, or use of ART (Table E4). Consistent with previous studies, patients with higher CD4+ cell counts or HIV RNA levels less than 400 copies/ml at baseline had the lowest rates of bacterial pneumonia, PCP, and TB. Patients on ART at baseline were also less likely to have incident bacterial pneumonia or TB. The incidence of COPD and asthma was also significantly lower in patients on ART, and COPD was less likely in those with baseline HIV RNA levels less than 400 copies/ml.
In multivariate Poisson regression models, we examined the independent impact of HIV-specific variables on incident pulmonary diseases (Tables 3 and 4). As expected, infectious pulmonary complications remained significantly less likely in patients with higher CD4+ cell counts, suppressed HIV viral load, and use of ART at baseline. Interestingly, COPD and asthma remained significantly less likely in patients with lower HIV RNA levels and in those on ART at baseline in multivariate models. Point estimates for the IRR remained similar in models restricted to HIV-infected patients when adjusted for having ever smoked, but were not significant in this smaller sample derived from the merged Virtual Cohort and LHS (Table 4).
TABLE 3.
ADJUSTED INCIDENCE RATE RATIOS FOR RISK FACTORS ASSOCIATED WITH PULMONARY DISEASES AMONG HIV-INFECTED PATIENTS IN MULTIVARIATE POISSON REGRESSION MODELS: VIRTUAL COHORT, WITHOUT ADJUSTING FOR SMOKING
| Incidence Rate Ratio (95% confidence interval) |
|||
|---|---|---|---|
| Condition | Square Root of CD4+ Count (cells/μl) | Log10 HIV RNA (copies/ml) | ART |
| COPD | 1.00 (0.99–1.01) | 1.08 (1.03–1.13)* | 0.90 (0.82–0.99)* |
| Asthma | 1.01 (1.00–1.02) | 1.10 (1.01–1.20)* | 0.77 (0.64–0.91)* |
| Lung cancer | 0.99 (0.97–1.01) | 0.95 (0.84–1.06) | 0.99 (0.76–1.29) |
| Pulmonary hypertension | 0.98 (0.96–1.01) | 0.99 (0.84–1.18) | 0.97 (0.66–1.43) |
| Pulmonary fibrosis | 0.99 (0.95–1.02) | 0.89 (0.74–1.08) | 0.80 (0.53–1.21) |
| Bacterial pneumonia | 0.97 (0.97–0.98)* | 1.18 (1.14–1.22)* | 0.84 (0.78–0.91)* |
| PCP | 0.92 (0.91–0.92)* | 1.28 (1.20–1.35)* | 0.87 (0.77–0.99)* |
| TB | 0.97 (0.95–0.98)* | 1.28 (1.17–1.41)* | 0.71 (0.59–0.86)* |
Definition of abbreviations: ART = antiretroviral therapy; COPD = chronic obstructive pulmonary disease; PCP = Pneumocystis pneumonia; TB = tuberculosis.
All models are adjusted for age as a continuous variable, race and ethnicity, sex, alcohol disorders, drug abuse, and hepatitis C infection in addition to CD4+ cell count, HIV viral level, and ART. The square root of the CD4+ cell count and log of the HIV viral load were modeled as continuous variables. Full models including incidence rate ratios for all predictors are shown in Table E5.
P < 0.05.
TABLE 4.
ADJUSTED INCIDENCE RATE RATIOS FOR RISK FACTORS ASSOCIATED WITH PULMONARY DISEASES AMONG HIV-INFECTED PATIENTS IN MULTIVARIATE POISSON REGRESSION MODELS: MERGED VIRTUAL COHORT AND LHS, ADJUSTING FOR SMOKING
| Incidence Rate Ratio (95% confidence interval) |
||||
|---|---|---|---|---|
| Condition | Square Root of CD4+ Count (cells/μl) | Log10 HIV RNA (copies/ml) | ART | Ever smoked |
| COPD | 1.00 (0.98–1.01) | 1.08 (0.97–1.21) | 0.93 (0.73–1.18) | 2.41 (1.70–3.42)* |
| Asthma | 1.00 (0.96–1.04) | 1.19 (0.94–1.51) | 0.72 (0.45–1.17) | 0.81 (0.48–1.37) |
| Lung cancer | 0.96 (0.92–1.01) | 0.97 (0.72–1.31) | 0.73 (0.38–1.40) | 6.22 (1.49–25.91)* |
| Pulmonary hypertension | 1.01 (0.93–1.08) | 0.94 (0.59–1.50) | 0.87 (0.31–2.43) | 1.43 (0.41–5.04) |
| Pulmonary fibrosis | 0.99 (0.92–1.06) | 0.79 (0.52–1.19) | 1.75 (0.60–5.08) | 1.69 (0.48–-5.91) |
| Bacterial pneumonia | 0.97 (0.95–0.98)* | 1.11 (0.99–1.22) | 0.84 (0.67–1.05) | 1.25 (0.96–1.62) |
| PCP | 0.91 (0.88–0.93)* | 1.24 (1.06–1.46)* | 0.76 (0.53–1.08) | 1.14 (0.78–1.69) |
| TB | 1.02 (0.98–1.07) | 1.24 (0.93–1.65) | 0.96 (0.53–1.74) | 1.12 (0.56–2.26) |
Definition of abbreviations: ART = antiretroviral therapy; COPD = chronic obstructive pulmonary disease; LHS = 1999 Veterans Large Health Survey; PCP = Pneumocystis pneumonia; TB = tuberculosis.
All models are adjusted for age as a continuous variable, race and ethnicity, sex, alcohol disorders, drug abuse, and hepatitis C infection in addition to CD4+ cell count, HIV viral level, and ART. Models using the nested sample of the Virtual Cohort merged with the LHS are additionally adjusted for having ever smoked. The square root of the CD4+ cell count and log of the HIV viral load were modeled as continuous variables. Full models including incidence rate ratios for all predictors are shown in Table E5.
P < 0.05.
DISCUSSION
In this study, we sought to determine the incidence of pulmonary diseases in HIV-infected patients using a VA-based, multisite cohort with demographically matched HIV-uninfected control subjects. We found that patients infected with HIV were more likely to have incident diagnoses of noninfectious chronic diseases including COPD, lung cancer, pulmonary hypertension, and pulmonary fibrosis, as well as pulmonary infections including bacterial pneumonia, PCP, and TB when compared with HIV-uninfected patients. In contrast, the incidence of asthma (a disease that often develops at younger ages that may precede HIV infection) did not vary according to HIV status. Bacterial pneumonia and COPD were the two most commonly diagnosed pulmonary conditions in our cohort. This notably contrasts with the previous Pulmonary Complications of HIV Infection Study conducted in the precombination ART era in which the two most common pulmonary complications were PCP followed by bacterial pneumonia (3). Moreover, rates of pulmonary infection in our study were substantially lower than in several precombination ART era studies, although such comparisons must be interpreted cautiously given differences in patient populations and diagnostic and reporting methods (3, 26).
Fewer infectious complications and a greater frequency of non–HIV-associated pulmonary diseases, particularly COPD in our cohort, seems to parallel the greater burden of chronic, noninfectious comorbid diseases afflicting many aging HIV-infected patients (27). Our findings are important for health care providers to consider when evaluating HIV-infected patients, particularly those who may present with respiratory symptoms or impairment. Whether persons infected with HIV may be at sufficient risk to benefit from screening tests to detect chronic lung disease has not been investigated. Although the magnitude of increased risk associated with HIV infection for COPD is modest, the incidence of COPD and the clinical significance of these findings are substantial. Rates of COPD are unlikely to be exclusive to a veteran population, because the prevalence of smoking among other populations infected with HIV is equally high (28–33). How COPD and other chronic underlying lung diseases influence the risk for morbidity, pulmonary infections, and mortality among HIV-infected patients requires further investigation.
Our results demonstrate that HIV infection confers an independent risk for a number of infectious and noninfectious pulmonary diseases after adjusting for potential confounders. The IRRs for the association of HIV infection with both infectious and noninfectious pulmonary diseases are robust in nested models adjusting for smoking. These findings are consistent with previous work that has clearly demonstrated an increased risk of bacterial pneumonia, PCP, and TB among HIV-infected patients; although decreased, we demonstrate that these remain significant complications in the current ART era. Our analyses strengthen previous work to support an increased risk for COPD (4, 5), lung cancer (6), and pulmonary hypertension (7) among HIV-infected patients. The association of HIV infection with asthma or airway hyperresponsiveness has been conflicting in previous studies (34–36). We did not find an association between HIV infection and incident asthma, and in fact asthma was more likely to be present at baseline among HIV-uninfected patients compared with HIV-infected patients. Ours is the first report of an increased risk for pulmonary fibrosis associated with HIV infection in the ART era. However, we are unable from these data to ascribe the development of pulmonary fibrosis to a specific interstitial lung disease or more generically as a consequence of scarring from prior infection or other predisposing conditions.
We also examined the relationship between HIV-specific variables, measured at study baseline, and incidence of pulmonary disease. Among HIV-infected patients, rates of infectious pulmonary diseases were significantly greater among those who were not on ART at baseline and who had lower CD4+ cell counts and higher HIV RNA levels; these findings are consistent with prior studies and suggest that baseline values of these variables are important predictors of subsequent disease. Interestingly, we found that HIV-infected patients on ART and those with lower HIV RNA levels at baseline were less likely to have incident COPD and asthma. These findings are consistent with supportive evidence from other studies. Continuous ART is associated with a decreased risk overall of AIDS and non-AIDS events (37), and ART has been suggested to have a protective effect on pulmonary function (38). Within the alveolar space, ART also decreases pulmonary HIV viral load and decreases nonspecific pulmonary inflammation (39, 40). However, current data on the impact of ART on lung function and chronic pulmonary disease are conflicting, because another study has found that airflow obstruction was more likely in patients on ART (41). Further investigations are needed to clarify these findings.
Our study supports the likelihood that as HIV-infected patients age, the burden of many infectious and noninfectious pulmonary diseases will increase. We found that age is an important risk factor for many pulmonary diseases among HIV-infected patients. We also found that the relative incidence rate comparing the HIV-infected and uninfected patients generally decreased with advancing age. One explanation for this narrowing relative incidence may be a competing risk for death among the HIV-infected patients resulting in misleadingly low incidence rates in older patients when compared with HIV-uninfected patients. HIV-infected patients may not survive to develop the diseases that occur in older HIV-uninfected patients given that veterans infected with HIV have a death rate that is approximately two fold higher than veterans without HIV (42). This could explain the significant positive interaction in statistical models between HIV infection and age less than 50 for several pulmonary conditions.
The greater incidence of pulmonary diseases normally associated with aging and their presentation at younger ages in HIV-infected patients also raise the possibility that HIV infection accelerates aging-related decline in lung health, in parallel to what has been described for the development of other comorbid diseases (16). HIV infection seems to confer a phenotype of premature frailty (43) and has been associated with aging-related changes in the immune system (44–46). As a result, HIV infection may render the lung more susceptible to the earlier presentation of diseases, such as COPD, which has been suggested as a disease of accelerated lung aging (47, 48). Smoking, which is prevalent among populations infected with HIV, further exacerbates cellular senescence (49).
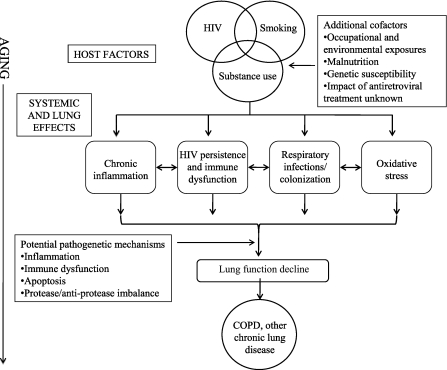
The pathogenesis of COPD and other chronic lung diseases in HIV remains unclear. Multiple interacting factors including increased systemic and lung oxidative stress (50–52) and recurrent respiratory tract infections and colonization (41, 53) in the setting of aging and HIV are likely to be involved (Figure 3). The role of HIV persistence and low level replication, immune activation, and chronic inflammation on the development of chronic lung diseases require further investigation.
Figure 3.
Model for accelerated progression of chronic lung diseases among patients infected with HIV. The intersection of HIV, smoking, substance use, and other host-related factors may lead to systemic and lung-specific effects, including infections and colonization (especially in the setting of HIV-related immune incompetence); oxidative stress induced by HIV infection, smoking, and other exposures; and systemic immune dysfunction characterized by persistent immune activation and chronic inflammation. These may lead to lung inflammation and injury via a variety of mechanisms and subsequent lung function decline, culminating in the clinical manifestation of chronic obstructive pulmonary disease and other chronic lung diseases. COPD = chronic obstructive pulmonary disease.
Our study has a number of strengths. This is the largest, multicenter nationwide cohort study to date describing the incidence and risk factors for pulmonary disease in HIV-infected patients in the combination ART era. The Virtual Cohort is nationally representative of HIV-infected veterans receiving care within the VA health care system, which is the largest single provider of health care to HIV-infected patients within the United States (54). Although predominantly male, our cohort is racially and ethnically diverse. An additional strength is the inclusion of HIV-uninfected control subjects who were demographically matched to the HIV-infected patients.
Our study has certain limitations. Diagnoses based on ICD-9 codes are likely to be accurate but may underestimate incidence, because ICD-9 codes have a high specificity but moderate sensitivity for a variety of conditions (55–58). We may also underestimate incidence if events occur outside of the VA. However, this may be more likely to underestimate the incidence of acute events rather than chronic diseases. Detection bias caused by access to care is less likely to explain the increased rates of pulmonary disease among HIV-infected patients because HIV-uninfected control subjects are required to be active within the VA in the same fiscal year as their HIV-infected counterparts.
In summary, we found that infectious and noninfectious pulmonary diseases are substantially increased among HIV-infected veterans compared with HIV-uninfected veterans. The two most common incident pulmonary diseases were bacterial pneumonia and COPD. Our data suggest that there may be a shift in the epidemiology of pulmonary diseases among HIV-infected patients, with a greater burden of non–HIV-associated pulmonary diseases in the current era, particularly among populations aging on successful ART. In addition to a continued increased risk of infectious pulmonary complications, our findings also support a greater risk of COPD, lung cancer, pulmonary hypertension, and a previously unrecognized increased incidence of pulmonary fibrosis associated with HIV infection. Whether the increased rates of chronic, noninfectious pulmonary diseases are related to prior infections, senescence of the lung in the setting of HIV infection, a direct pathogenetic effect of HIV, ART, or other mechanisms is still unclear; additional studies are needed to confirm and further investigate these findings.
Supplementary Material
Supported by NIH/NCRR 1KL2 RR024138 and NIH/NHLBI 1R01 HL090342 (K.C.); NIH K24 HL087713–04 (L.H.); NIH/NIA K23AG024896 (K.K.O.); and NIH/NIAAA 3 U01AA13566 (A.C.J.). Funding for this publication was made possible in part by CTSA Grant Number KL2 RR024138 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
All authors have met authorship criteria. Participated in study conception and design (K.C., L.H., J.L.G., M.B.G., S.T.B., M.C.R.-B., K.K.O., D.R., C.L.G., A.A.B., A.C.J.); acquisition of data (A.C.J.); analysis and interpretation of data (K.C., L.H., J.L.G., A.C.J.); drafting the article (K.C.) and revising it critically for important intellectual content (L.H, J.L.G., M.B.G., S.T.B., M.C.R.-B., K.K.O., D.R., C.L.G., A.A.B., A.C.J.); and final approval of the version to be published (K.C., L.H., J.L.G., M.B.G., S.T.B., M.C.R.-B., K.K.O., D.R., C.L.G., A.A.B., A.C.J.). K.C. has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Part of this work was presented in abstract form at the American Thoracic Society International Conference in 2006.
Originally Published in Press as DOI: 10.1164/rccm.201006-0836OC on October 1, 2010
Author Disclosure: K.C. received grant support from the National Institutes of Health (NIH) (more than $100,001). L.H. received grant support from the NIH (more than $100,001). J.L.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.B.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.T.B. received grant support (institutional) from Merck ($10,001–$50,000) and the NIH (more than $100,001). He owns stocks or options of Pfizer ($5,001–$10,000). M.C.R.-B. received grant support (institutional) from Pfizer ($1,001–$5,000). K.K.O. received lecture fees from Johns Hopkins University (up to $1,000) and grant support from the NIH ($50,001–$100,000 and $10,001–$50,000). D.R. received grant support from Pfizer ($1,001–$5,000) and the CDC (more than $100,001). C.L.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.A.B. received grant support from Valeant Pharma ($10,001–$50,000). A.C.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rosen MJ, Clayton K, Schneider RF, Fulkerson W, Rao AV, Stansell J, Kvale PA, Glassroth J, Reichman LB, Wallace JM, et al. Intensive care of patients with HIV infection: utilization, critical illnesses, and outcomes. Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med 1997;155:67–71. [DOI] [PubMed] [Google Scholar]
- 2.Murray JF, Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part I. Am Rev Respir Dis 1990;141:1356–1372. [DOI] [PubMed] [Google Scholar]
- 3.Wallace JM, Hansen NI, Lavange L, Glassroth J, Browdy BL, Rosen MJ, Kvale PA, Mangura BT, Reichman LB, Hopewell PC. Respiratory disease trends in the Pulmonary Complications of HIV Infection Study cohort. Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med 1997;155:72–80. [DOI] [PubMed] [Google Scholar]
- 4.Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Nagaraja HN, Drake J, Clanton TL. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med 2000;132:369–372. [DOI] [PubMed] [Google Scholar]
- 5.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest 2006;130:1326–1333. [DOI] [PubMed] [Google Scholar]
- 6.Kirk GD, Merlo C, Mehta SH, Galai N, Vlahov D, Samet J, Engels EA. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis 2007;45:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta NJ, Khan IA, Mehta RN, Sepkowitz DA. HIV-related pulmonary hypertension: analytic review of 131 cases. Chest 2000;118:1133–1141. [DOI] [PubMed] [Google Scholar]
- 8.Feikin DR, Feldman C, Schuchat A, Janoff EN. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet Infect Dis 2004;4:445–455. [DOI] [PubMed] [Google Scholar]
- 9.Kohli R, Lo Y, Homel P, Flanigan TP, Gardner LI, Howard AA, Rompalo AM, Moskaleva G, Schuman P, Schoenbaum EE. Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (HER) study. Clin Infect Dis 2006;43:90–98. [DOI] [PubMed] [Google Scholar]
- 10.Le Moing V, Rabaud C, Journot V, Duval X, Cuzin L, Cassuto JP, Al Kaied F, Dellamonica P, Chene G, Raffi F. Incidence and risk factors of bacterial pneumonia requiring hospitalization in HIV-infected patients started on a protease inhibitor-containing regimen. HIV Med 2006;7:261–267. [DOI] [PubMed] [Google Scholar]
- 11.Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338:853–860. [DOI] [PubMed] [Google Scholar]
- 12.Grubb JR, Moorman AC, Baker RK, Masur H. The changing spectrum of pulmonary disease in patients with HIV infection on antiretroviral therapy. AIDS 2006;20:1095–1107. [DOI] [PubMed] [Google Scholar]
- 13.Braithwaite RS, Justice AC, Chang CC, Fusco JS, Raffanti SR, Wong JB, Roberts MS. Estimating the proportion of patients infected with HIV who will die of comorbid diseases. Am J Med 2005;118:890–898. [DOI] [PubMed] [Google Scholar]
- 14.Krentz HB, Kliewer G, Gill MJ. Changing mortality rates and causes of death for HIV-infected individuals living in Southern Alberta, Canada from 1984 to 2003. HIV Med 2005;6:99–106. [DOI] [PubMed] [Google Scholar]
- 15.Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, Armstrong AW, Fraser S, Wallace MR. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr 2006;41:194–200. [DOI] [PubMed] [Google Scholar]
- 16.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 2009;338:a3172. [DOI] [PubMed] [Google Scholar]
- 17.Crothers K, Justice AC, Rimland D, Gibert CL, Rodriguez-Barradas M, Brown S, Goetz M, Butt AA, Huang L. Increased infectious and non-infectious pulmonary diseases among HIV positive compared to HIV negative veterans. Proc Am Thorac Soc 2006;3:A477. [Google Scholar]
- 18.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, Justice AC. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care 2006;44:S25–S30. [DOI] [PubMed] [Google Scholar]
- 19.McAfee T, Grossman R, Dacey S, McClure J. Capturing tobacco status using an automated billing system: steps toward a tobacco registry. Nicotine Tob Res 2002;4:S31–S37. [DOI] [PubMed] [Google Scholar]
- 20.Kazis LE, Selim A, Rogers W, Ren XS, Lee A, Miller DR. Dissemination of methods and results from the veterans health study: final comments and implications for future monitoring strategies within and outside the veterans healthcare system. J Ambul Care Manage 2006;29:310–319. [DOI] [PubMed] [Google Scholar]
- 21.McGinnis KA, Fine MJ, Sharma RK, Skanderson M, Wagner JH, Rodriguez-Barradas MC, Rabeneck L, Justice AC. Understanding racial disparities in HIV using data from the Veterans Aging Cohort 3-Site Study and VA administrative data. Am J Public Health 2003;93:1728–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, Goulet J, Simberkoff M, Butt AA, Rimland D, et al. Veterans Aging Cohort Study (VACS): overview and description. Med Care 2006;44:S13–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Justice AC, Erdos J, Brandt C, Conigliaro J, Tierney W, Bryant K. The Veterans Affairs Healthcare System: a unique laboratory for observational and interventional research. Med Care 2006;44:S7–S12. [DOI] [PubMed] [Google Scholar]
- 24.Cigarette smoking among adults—United States, 2006. MMWR Morb Mortal Wkly Rep 2007;56:1157–1161. [PubMed] [Google Scholar]
- 25.Perlin J, Kazis LE, Skinner K, Ren XS, Lee A, Rogers WH, Spiro A, Selim A, Miller D. Health status and outcomes of veterans: physical and mental component summary scores. Veterans SF-36 and 1999 Large Health Survey of Veteran Enrollees. Executive Report. Washington, D.C., Department of Veterans Affairs, Veterans Health Administration, Office of Quality and Performance. May 2000.
- 26.Hirschtick RE, Glassroth J, Jordan MC, Wilcosky TC, Wallace JM, Kvale PA, Markowitz N, Rosen MJ, Mangura BT, Hopewell PC. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl J Med 1995;333:845–851. [DOI] [PubMed] [Google Scholar]
- 27.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS 2008;22:2409–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns DN, Hillman D, Neaton JD, Sherer R, Mitchell T, Capps L, Vallier WG, Thurnherr MD, Gordin FM. Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. Terry Beirn Community Programs for Clinical Research on AIDS. J Acquir Immune Defic Syndr Hum Retrovirol 1996;13:374–383. [DOI] [PubMed] [Google Scholar]
- 29.Page-Shafer K, Delorenze GN, Satariano WA, Winkelstein W Jr. Comorbidity and survival in HIV-infected men in the San Francisco Men's Health Survey. Ann Epidemiol 1996;6:420–430. [DOI] [PubMed] [Google Scholar]
- 30.Galai N, Park LP, Wesch J, Visscher B, Riddler S, Margolick JB. Effect of smoking on the clinical progression of HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol 1997;14:451–458. [DOI] [PubMed] [Google Scholar]
- 31.Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clin Infect Dis 2000;31:808–812. [DOI] [PubMed] [Google Scholar]
- 32.Turner J, Page-Shafer K, Chin DP, Osmond D, Mossar M, Markstein L, Huitsing J, Barnes S, Clemente V, Chesney M. Adverse impact of cigarette smoking on dimensions of health-related quality of life in persons with HIV infection. AIDS Patient Care STDS 2001;15:615–624. [DOI] [PubMed] [Google Scholar]
- 33.Diaz PT, Wewers MD, Pacht E, Drake J, Nagaraja HN, Clanton TL. Respiratory symptoms among HIV-seropositive individuals. Chest 2003;123:1977–1982. [DOI] [PubMed] [Google Scholar]
- 34.O'Donnell CR, Bader MB, Zibrak JD, Jensen WA, Rose RM. Abnormal airway function in individuals with the acquired immunodeficiency syndrome. Chest 1988;94:945–948. [DOI] [PubMed] [Google Scholar]
- 35.Wallace JM, Stone GS, Browdy BL, Tashkin DP, Hopewell PC, Glassroth J, Rosen MJ, Reichman LB, Kvale PA. Nonspecific airway hyperresponsiveness in HIV disease. Pulmonary Complications of HIV Infection Study Group. Chest 1997;111:121–127. [DOI] [PubMed] [Google Scholar]
- 36.Poirier CD, Inhaber N, Lalonde RG, Ernst P. Prevalence of bronchial hyperresponsiveness among HIV-infected men. Am J Respir Crit Care Med 2001;164:542–545. [DOI] [PubMed] [Google Scholar]
- 37.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283–2296. [DOI] [PubMed] [Google Scholar]
- 38.Kristoffersen US, Lebech A, Gerstoft J. Changes in lung function in an optimally treated population: a 4.5 year follow-up study. Presented at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). September 12–15, 2009. San Francisco, CA. Abstract H-1561.
- 39.Twigg HL, Knox KS. HIV-related lung disorders. Drug Discov Today Dis Mech 2007;4:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Twigg HL, Soliman DM, Day RB, Knox KS, Anderson RJ, Wilkes DS, Schnizlein-Bick CT. Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. Am J Respir Crit Care Med 1999;159:1439–1444. [DOI] [PubMed] [Google Scholar]
- 41.George MP, Kannass M, Huang L, Sciurba FC, Morris A. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS ONE 2009;4:e6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crothers K, Goulet JL, Rodriguez-Barradas MC, Gibert CL, Oursler KA, Goetz MB, Crystal S, Leaf DA, Butt AA, Braithwaite RS, et al. Impact of cigarette smoking on mortality in HIV-positive and HIV-negative veterans. AIDS Educ Prev 2009;21:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, Margolick JB. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci 2007;62:1279–1286. [DOI] [PubMed] [Google Scholar]
- 44.Cao W, Jamieson BD, Hultin LE, Hultin PM, Effros RB, Detels R. Premature aging of T cells is associated with faster HIV-1 disease progression. J Acquir Immune Defic Syndr 2009;50:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tenorio AR, Spritzler J, Martinson J, Gichinga CN, Pollard RB, Lederman MM, Kalayjian RC, Landay AL. The effect of aging on T-regulatory cell frequency in HIV infection. Clin Immunol 2009;130:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Appay V, Almeida JR, Sauce D, Autran B, Papagno L. Accelerated immune senescence and HIV-1 infection. Exp Gerontol 2007;42:432–437. [DOI] [PubMed] [Google Scholar]
- 47.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest 2009;135:173–180. [DOI] [PubMed] [Google Scholar]
- 48.MacNee W. Accelerated lung aging: a novel pathogenic mechanism of chronic obstructive pulmonary disease (COPD). Biochem Soc Trans 2009;37:819–823. [DOI] [PubMed] [Google Scholar]
- 49.Nyunoya T, Monick MM, Klingelhutz A, Yarovinsky TO, Cagley JR, Hunninghake GW. Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol 2006;35:681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Treitinger A, Spada C, Verdi JC, Miranda AF, Oliveira OV, Silveira MV, Moriel P, Abdalla DS. Decreased antioxidant defense in individuals infected by the human immunodeficiency virus. Eur J Clin Invest 2000;30:454–459. [DOI] [PubMed] [Google Scholar]
- 51.Pacht ER, Diaz P, Clanton T, Hart J, Gadek JE. Alveolar fluid glutathione decreases in asymptomatic HIV-seropositive subjects over time. Chest 1997;112:785–788. [DOI] [PubMed] [Google Scholar]
- 52.Cole SB, Langkamp-Henken B, Bender BS, Findley K, Herrlinger-Garcia KA, Uphold CR. Oxidative stress and antioxidant capacity in smoking and nonsmoking men with HIV/acquired immunodeficiency syndrome. Nutr Clin Pract 2005;20:662–667. [DOI] [PubMed] [Google Scholar]
- 53.Morris AM, Huang L, Bacchetti P, Turner J, Hopewell PC, Wallace JM, Kvale PA, Rosen MJ, Glassroth J, Reichman LB, et al. Permanent declines in pulmonary function following pneumonia in human immunodeficiency virus-infected persons. The Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med 2000;162:612–616. [DOI] [PubMed] [Google Scholar]
- 54.Justice AC, Landefeld CS, Asch SM, Gifford AL, Whalen CC, Covinsky KE. Justification for a new cohort study of people aging with and without HIV infection. J Clin Epidemiol 2001;54:S3–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kieszak SM, Flanders WD, Kosinski AS, Shipp CC, Karp H. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol 1999;52:137–142. [DOI] [PubMed] [Google Scholar]
- 56.Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived from ICD-9-CCM administrative data. Med Care 2002;40:675–685. [DOI] [PubMed] [Google Scholar]
- 57.Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol 2004;57:131–141. [DOI] [PubMed] [Google Scholar]
- 58.Justice AC, Lasky E, McGinnis KA, Skanderson M, Conigliaro J, Fultz SL, Crothers K, Rabeneck L, Rodriguez-Barradas M, Weissman SB, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: a comparison of disease measurement strategies. Med Care 2006;44:S52–S60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.