Abstract
Rationale: Mechanical ventilation is known to induce ventilator-induced diaphragm dysfunction. Patients submitted to mechanical ventilation often receive massive doses of corticosteroids that may cause further deterioration of diaphragm function.
Objectives: To examine whether the combination of 24 hours of controlled mechanical ventilation with corticosteroid administration would exacerbate ventilator-induced diaphragm dysfunction.
Methods: Rats were randomly assigned to a group submitted to 24 hours of controlled mechanical ventilation receiving an intramuscular injection of saline or 80 mg/kg methylprednisolone, a group submitted to 24 hours of spontaneous breathing receiving saline, or methylprednisolone and a control group.
Measurements and Main Results: The diaphragm force–frequency curve was shifted downward in the mechanical ventilation group, but this deleterious effect was prevented when corticosteroids were administered. Diaphragm cross-sectional area of type I fibers was similarly decreased in both mechanical ventilation groups while atrophy of type IIx/b fibers was attenuated after corticosteroid administration. The mechanical ventilation-induced reduction in diaphragm MyoD and myogenin protein expression was attenuated after corticosteroids. Plasma cytokine levels were unchanged while diaphragm lipid hydroperoxides were similarly increased in both mechanical ventilation groups. Diaphragmatic calpain activity was significantly increased in the mechanical ventilation group, but calpain activation was abated with corticosteroid administration. Inverse correlations were found between calpain activity and diaphragm force.
Conclusions: A single high dose of methylprednisolone combined with controlled mechanical ventilation protected diaphragm function from the deleterious effects of controlled mechanical ventilation. Inhibition of the calpain system is most likely the mechanism by which corticosteroids induce this protective effect.
Keywords: respiratory muscles, steroids, proteolysis, calpain
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Mechanical ventilation is known to induce ventilator-induced diaphragm dysfunction. Patients submitted to mechanical ventilation often receive massive doses of corticosteroids that may cause further deterioration of diaphragm function, but such interactions are not well delineated.
What This Study Adds to the Field
A single high dose of methylprednisolone concomitantly with 24 hours of controlled mechanical ventilation protects against ventilator-induced diaphragm dysfunction by inhibiting activation of the calpain system.
Mechanical ventilation is an essential life-saving therapy for patients with respiratory failure. Difficulties in discontinuing ventilatory support are frequent, time consuming, and remain a serious problem (1). Although weaning failure may be due to a variety of factors, ventilator-induced diaphragmatic dysfunction probably plays an important role in this process. Animal models of mechanical ventilation have consistently shown that controlled mechanical ventilation (CMV) leads to reduced force generated by the diaphragm in vitro (2–6) but also in vivo (5, 7–9). These effects develop in a time-dependent manner and are associated with a reduction in diaphragm fiber dimensions (2, 10). Decreased protein synthesis and increased diaphragmatic proteolysis are known to be important contributors to this muscle dysfunction (10, 11). All three major proteolytic systems (lysosomal proteases, proteasomes, and calpains) are activated in the diaphragm secondary to CMV (12). Studies indicate that prolonged CMV promotes increased diaphragmatic 20S proteasome activity (10), increased mRNA expression levels of two muscle specific E3 ligases, Murf-1 and Mafbx (13, 14), and increased cathepsin B and calpain activity (15). In addition, caspase-3 activation was shown to be a requirement for mechanical ventilator–induced myofiber atrophy and myonuclear apoptosis (16). The important role of proteolysis in the development of ventilator-induced diaphragm dysfunction was further confirmed by a microarray performed in the diaphragm after 18 hours of CMV showing changes in the gene expression profile of several proteolytic enzymes (12).
Note that patients submitted to mechanical ventilation are often treated acutely with corticosteroids for underlying lung or systemic diseases. This is significant because glucocorticoid treatment is associated with a steroid-induced myopathy affecting both respiratory and peripheral muscles. Indeed, acute treatment of animals with massive doses of corticosteroids has been shown to induce severe respiratory and limb muscle wasting, causing alterations in diaphragm function together with a predominantly type IIx/b atrophy (17). In humans, a reduction in peripheral and respiratory muscle strength, an elevation of urinary creatine excretion, and selective type IIb atrophy is observed after short-term high-dose corticosteroid administration (18). In mechanically ventilated patients, numerous cases of steroid-induced myopathy have been reported in the intensive care unit (19–24). Importantly, this myopathy seems to be associated with increased time of mechanical ventilation duration and length of hospital stay (25). The extent to which corticosteroids may contribute to diaphragm weakness in mechanically ventilated patients is not known. Therefore, to investigate this important issue, we studied the effects of corticosteroid administration on diaphragm function in an animal model of CMV. We hypothesized that administration of a corticosteroid (methylprednisolone) would exacerbate CMV-induced diaphragmatic weakness developed during 24 hours of CMV. Surprisingly, our results revealed that corticosteroid administration provided protection against CMV-induced diaphragmatic atrophy and contractile dysfunction. Therefore, we performed another series of experiments aimed at determining the mechanism(s) responsible for the beneficial effect of corticosteroids on diaphragm function. Our results suggest that the protective action of corticosteroids appears to be associated with inhibition of the calpain protease system. Some of the results of this study have been previously reported in the form of an abstract (26).
METHODS
Experimental Procedures, Study Design
Adult male Wistar rats were randomly assigned to one of four groups: (1) an acutely anesthetized control group (C, n = 8), (2) an anesthetized group submitted to 24 hours of spontaneous breathing receiving an injection of saline (SB, n = 7), (3) an anesthetized group submitted to 24 hours of controlled mechanical ventilation receiving an injection of saline (CMV-Saline, n = 10), (4) an anesthetized group submitted to 24 hours of controlled mechanical ventilation receiving an injection of 80 mg/kg methylprednisolone (CMV-Methylprednisolone, n = 14). In addition, a spontaneous-breathing group receiving a single intramuscular injection of 80 mg/kg of methylprednisolone (SB-Methylprednisolone, n = 6) was added to examine whether treatment with an acute high dose of corticosteroids in spontaneous breathing rats would affect diaphragmatic contractile properties and fiber type dimensions. This was not the case. Therefore, only the SB group treated with saline was subsequently used for further analysis. The study was approved by the animal Experiments Committee of the Medical Faculty of the Katholieke Universiteit Leuven.
Rats were anesthetized with sodium pentobarbital, tracheotomized, and either mechanically ventilated for 24 hours (control mode; tidal volume, ± 0.5 ml/100 g; frequency of breathing, 55–60 breath/min) with a volume-driven small-animal ventilator (Harvard Apparatus model 665A, Holliston, MA) or were breathing spontaneously for 24 hours. Rats breathed humidified air, maintained at 37°C and enriched with O2. An intramuscular injection of either saline or methylprednisolone was given at the beginning of the study period.
After 24 hours, segments of the costal diaphragm were removed for measurement of in vitro contractile properties as previously described (27). Diaphragm samples were stored for further histological examination and fiber typing and for biochemical analysis. Blood samples were collected for subsequent measurement of plasma cytokine levels.
Histological Procedure and Fiber Type Analysis
Serial sections of the costal diaphragm were cut and stained with Hematoxylin and Eosin and with adenosine triphosphatase to determine CSA and fiber type proportions of the different fiber types.
Plasma Cytokine Levels
Plasma levels of IL-1α, IL-1β, IL-2, IL-4, IL-10, tumor necrosis factor (TNF)-α, and IFN-γ were measured using a custom SearchLight rat cytokine proteome array (Perbio Science, Erembodegem, Belgium). Plasma of lipopolysaccharide-treated rats was used as a positive control.
Diaphragm Lipid Peroxidation Measurement
Diaphragm levels of total hydroperoxides were assessed by using the modifications of the ferrous oxidation/xylenol orange technique described by Hermes-Lima and colleagues (28), as previously described (29).
Diaphragm Protein Oxidation
Diaphragmatic protein oxidation was measured by assaying the levels of protein carbonyls using the Oxyblot procedure (Biognost, Heule, Belgium).
Reverse Transcriptase Polymerase Chain Reaction
Total RNA was isolated from the diaphragm. MyoD and myogenin mRNA levels were determined with reverse transcriptase–polymerase chain reaction. QuantumRNA 18S (Applied Biosystems, Lennik, Belgium) was used as an internal standard.
Western Blot Analysis
Proteins were separated via polyacrylamide gel electrophoresis and transferred to a polyvinyldifluoride membrane. Membranes were incubated with primary antibodies against MyoD, myogenin, exhaled nitric oxide synthase (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS) (see online supplement for details) and with horseradish peroxidase (HRP)-conjugated secondary antibodies. Quantification by densitometric scanning was normalized to protein levels determined by PonceauS staining (see Figures E3, E4, and E5 in the online supplement).
Diaphragm Calpain Activity
Indirect assessment of calpain activity was performed by measuring the 145/150-kD cleavage products of αII-spectrin by Western blotting. In addition, the 190-kD calpain specific degradation product of talin was also measured by Western blotting. Mouse monoclonal primary antibodies and HRP-conjugated secondary antibodies were used.
Statistical Analysis
Statistical analysis was performed using the SAS Statistical package (SAS Institute, Cary, NC). Comparisons between the four groups were performed using one-way analysis of variance. Differences between means were assessed with a Newman-Keuls post hoc test. Data are means ± SD. Correlations were determined with Pearson's correlation coefficient.
RESULTS
Effect of Methylprednisolone in Spontaneously Breathing Animals
The mortality rate in the SB-Methylprednisolone group was 25%. Blood gas data and arterial blood pressure were similar in the two SB groups (data not shown). Diaphragm force generated at all stimulation frequencies was similar in the spontaneous-breathing groups receiving either methylprednisolone or saline (e.g., 1 Hz: 3.85 ± 0.25 vs. 3.82 ± 0.26 N/cm2; 160 Hz: 20.2 ± 2.4 vs. 20.6 ± 1.7 N/cm2, respectively). In addition, diaphragm CSA of the different fiber types and fiber type proportions did not differ between the two groups. See the online supplement for figures E1 and E2 depicting diaphragmatic force and CSA, respectively. Diaphragm fiber-type proportions are shown in the online data supplement, Table E1.
Response to Mechanical Ventilation
The mortality rate in the SB, CMV-Saline and CMV-Methylprednisolone groups was 21, 28, and 15%, respectively. Blood gas data and arterial blood pressure were not different among the mechanical-ventilation rats and were maintained within the normal range (Table 1). Blood Pco2 levels were higher in SB animals in comparison with the other groups.
TABLE 1.
BLOOD GAS DATA, DIAPHRAGM TETANIC FORCE, TWITCH FORCE AND ITS CHARACTERISTICS, I.E., TIME TO PEAK TENSION AND HALF-RELAXATION TIME IN CONTROLS, SPONTANEOUS BREATHING, AND CONTROLLED MECHANICAL VENTILATION RATS TREATED WITH SALINE OR METHYLPREDNISOLONE
| C | SB | CMV-Saline | CMV-Methylprednisolone | |
|---|---|---|---|---|
| Po2 | — | 148 ± 33 | 137 ± 51 | 134 ± 54 |
| Pco2 | — | 49 ± 12* | 32 ± 13 | 37 ± 14 |
| pH | — | 7.357 ± 0.04 | 7.479 ± 0.09 | 7.410 ± 0.16 |
| Po (N/cm2) | 22.9 ± 1.9 | 20.2 ± 1.4 | 15.1 ± 2.3† | 17.8 ± 2.6‡ |
| Pt (N/cm2) | 4.0 ± 0.2 | 3.9 ± 0.3 | 3.4 ± 0.8 | 4.2 ± 0.8§ |
| TPT (ms) | 19 ± 3 | 20 ± 3 | 20 ± 2 | 20 ± 3 |
| 1/2 RT (ms) | 22 ± 3 | 24 ± 3 | 22 ± 6 | 25 ± 5 |
Definition of abbreviations: C = controls; CMV = controlled mechanical ventilation; Po = tetanic force; Pt = twitch force; RT = relaxation time; SB = spontaneous breathing; TPT = time to peak tension.
Values are mean ± SD.
P < 0.05 vs. CMV-Saline and CMV-Methylprednisolone;
P < 0.05 vs. others;
P < 0.01 vs. C;
P < 0.05 vs. CMV-Saline.
Diaphragm Contractile Properties
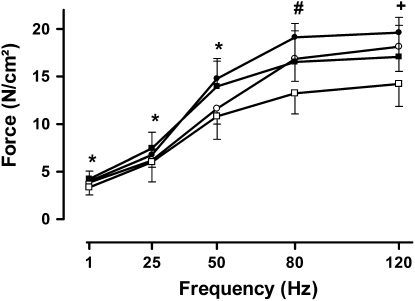
The diaphragm force–frequency curve of the CMV-Saline group was shifted downward, indicating a reduction in force generation at all stimulation frequencies (Figure 1). By contrast, the forces generated by the CMV-Methylprednisolone group were similar to those of the control group (Figure 1). Therefore, significant differences were present between the CMV-Saline and CMV-Methylprednisolone groups (P < 0.05). Maximal tetanic tension was significantly reduced in both mechanical-ventilation groups. However, when mechanical ventilation was combined with methylprednisolone, maximal tetanic tension was decreased less (P < 0.05) than in the CMV-Saline group (Table 1). Neither time to peak tension, nor one-half relaxation time were altered by CMV-Saline or CMV-Methylprednisolone (Table 1).
Figure 1.
Diaphragm force generated during the force–frequency relationship in control rats (C, closed circles, n = 5), spontaneous breathing rats (SB, open circles, n = 5), and controlled mechanical ventilation rats treated either with saline (CMV-Saline, open squares, n = 10) or with methylprednisolone (CMV-Methylprednisolone, closed squares, n = 9). Values are means ± SD, expressed in absolute values. *P < 0.05 CMV-Saline versus CMV-Methylprednisolone, #P < 0.01 CMV-Saline versus others, +P < 0.05 CMV-Saline versus others.
Diaphragm Fiber Type Analysis
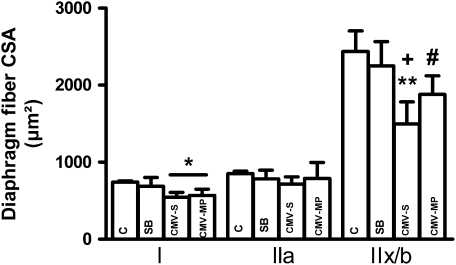
There were no significant differences in diaphragm fiber proportion between the four groups (pooled values type I: 42 ± 1, type IIa: 30 ± 1, type IIx/b: 28 ± 1%). Type I cross-sectional area was similarly decreased in the diaphragm of CMV-Saline and CMV-Methylprednisolone (−26%) groups compared with controls (P < 0.05) whereas dimensions of the diaphragm type IIa fibers were similar between the four groups (Figure 2). Finally, the decrease in diaphragm type IIx/b fibers was more severe in the CMV-Saline group (−39%, P < 0.001 vs. C and SB) than in the CMV-Methylprednisolone group (−23%, P < 0.05 vs. C and SB) such that a significant difference between CMV-Saline and CMV-Methylprednisolone was found (P < 0.05).
Figure 2.
Diaphragm cross-sectional area (CSA) of the type I, type IIa, and type IIx/b fibers in the control rats (C, n = 4), spontaneous-breathing rats (SB, n = 5), and controlled mechanical-ventilation rats treated either with saline (CMV-S, n = 6) or with methylprednisolone (CMV-MP, n = 7). Values are means ± SD. *P < 0.005 versus C, **P < 0.001 versus C and SB, +P < 0.05 versus CMV-MP, #P < 0.05 versus C and SB.
Diaphragm Histological Analysis
Qualitative examination of the diaphragm sections stained with hematoxylin and eosin did not reveal any abnormalities in diaphragm histology with the exception that diaphragm fiber dimensions were smaller in the mechanically ventilated groups.
Plasma Cytokine Levels
None of the studied cytokines (IL-1α, IL-1β, IL-2, IL-4, IL-10, TNF-α, and IFN-γ) were detected after controlled mechanical ventilation combined with saline or methylprednisolone administration.
Diaphragm Lipid Peroxidation Measurement
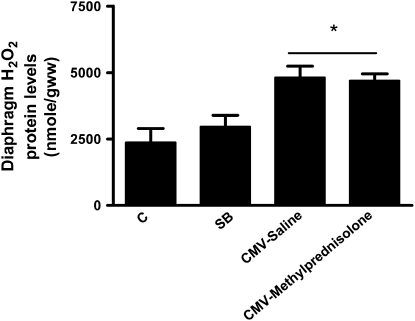
Diaphragm levels of total hydroperoxides were similar in control and SB rats and were significantly increased in the CMV-Saline and CMV-Methylprednisolone groups (Figure 3). The magnitude of this increase was similar between the two mechanically ventilated groups (CMV-Saline: +102% and CMV-Methylprednisolone: +98%).
Figure 3.
Diaphragm lipid hydroperoxide levels in control rats (C, n = 7), spontaneous breathing rats (SB, n = 6), and controlled mechanical-ventilation rats treated either with saline (CMV-Saline, n = 9) or with methylprednisolone (CMV-Methylprednisolone, n = 9). Values are means ± SD. *P < 0.05 versus C and SB.
Diaphragm Protein Oxidation
Quantification of the amount of oxidation was done while using the oxidative index, as previously described (30, 31). The oxidative index is the ratio between densitometric values of the oxidized proteins and the total proteins. The oxidative index was significantly increased (+40%) in the CMV-Saline group (P < 0.05 vs. C and SB) and to a similar extent in the CMV-Methylprednisolone group.
Western Blot Analysis of Nitric Oxide Synthase Isoforms
Diaphragmatic protein levels of eNOS were similar between the four groups (see Figure E3 in the online supplement). Furthermore, iNOS and nNOS were not detected in the diaphragm of the different groups (see Figure E4 in the online supplement).
Western Blot Analysis of MyoD and Myogenin
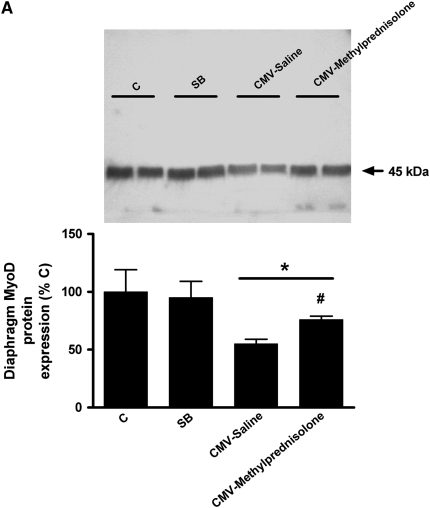
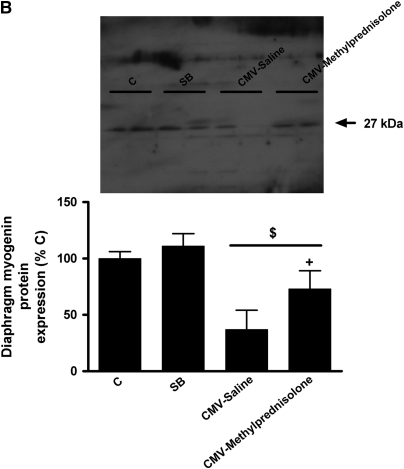
Diaphragm MyoD protein levels were significantly decreased in the CMV-Saline group (−45%, P < 0.05 vs. C and SB), but to a lesser extent in the CMV-Methylprednisolone group (−23%, P < 0.05 vs. C and SB); the latter was significantly different from the CMV-Saline group (P < 0.05) (Figure 4A). Similarly, diaphragm myogenin protein content was severely decreased in the CMV-Saline group (−63%, P < 0.01 vs. C and SB) but significantly less in the CMV-Methylprednisolone group (−27%, P < 0.01 vs. C, SB and CMV-Saline) (Figure 4B).
Figure 4.
Western blot densitometric analysis of (A) MyoD and (B) myogenin for control rats (C, n = 5), spontaneous breathing rats (SB, n = 5), and controlled mechanical-ventilation rats treated either with saline (CMV-Saline, n = 6) or with methylprednisolone (CMV-Methylprednisolone, n = 7). Values are means ± SD and are expressed as a percentage of C. *P < 0.05 versus C and SB, #P < 0.05 versus CMV-Saline, $ P < 0.01 versus C and SB, + P < 0.01 versus CMV-saline.
Strong positive correlations were found between diaphragm MyoD and myogenin protein levels and diaphragm force (Table 2). Diaphragm type IIx/b fiber dimensions were also positively correlated with MyoD (r = 0.70, P < 0.001) and myogenin (r = 0.80, P < 0.0001) protein levels.
TABLE 2.
CORRELATIONS BETWEEN DIAPHRAGM FORCE GENERATED AT DIFFERENT FREQUENCIES AND DIAPHRAGM MYOD AND MYOGENIN PROTEIN LEVELS AND CALPAIN ACTIVITY
| Indirect Measure of Calpain Activity |
||||
|---|---|---|---|---|
| Hz | MyoD Protein | Myogenin Protein | Talin | αII-Spectrin |
| 50 | r = 0.49 | r = 0.48 | r = −0.60 | r = −0.48 |
| P < 0.05 | P < 0.05 | P < 0.005 | P < 0.05 | |
| 80 | r = 0.72 | r = 0.68 | r = −0.54 | r = −0.40 |
| P < 0.005 | P < 0.001 | P < 0.01 | P < 0.05 | |
| 120 | r = 0.66 | r = 0.68 | r = −0.51 | r = −0.46 |
| P < 0.005 | P < 0.005 | P < 0.01 | P < 0.05 | |
| 160 | r = 0.72 | r = 0.73 | r = −0.46 | r = −0.54 |
| P < 0.0005 | P < 0.0005 | P < 0.05 | P < 0.005 | |
mRNA Expression of MyoD and Myogenin
Diaphragm MyoD mRNA expression levels were decreased in the CMV-Saline group (−50%, P < 0.05 vs. C and SB.) and in the CMV-Methylprednisolone group (−49% P < 0.05 vs. C and SB). Myogenin mRNA expression levels were significantly increased in all groups compared with the controls.
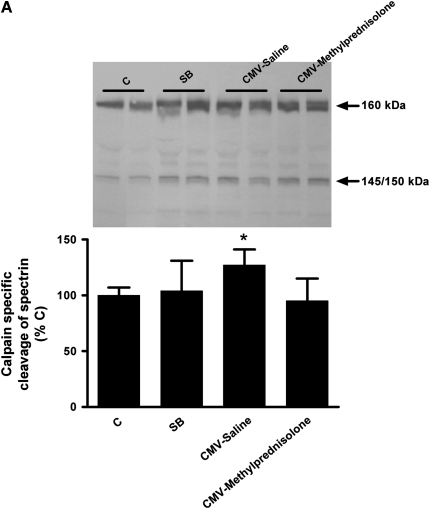
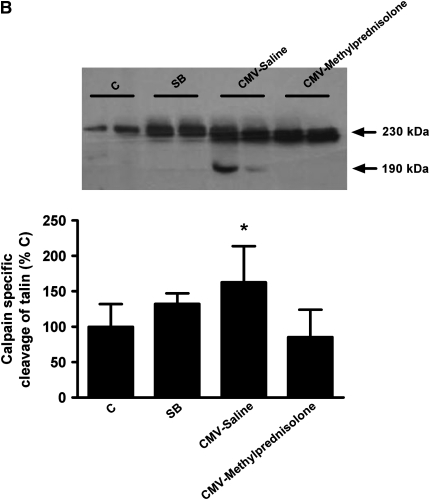
Calpain Activity
As previously mentioned, calpain activity was indirectly evaluated by measuring the 145/150-kD cleavage products of αII-spectrin but also the 190-kD calpain-specific degradation product of talin. Both measurements showed that calpain activity was increased after CMV-Saline when compared with other groups (P < 0.05) (Figure 5). When mechanical ventilation was combined with corticosteroid administration calpain activity was similar as in the control and SB groups, meaning that calpain activity was significantly higher after mechanical ventilation alone (P < 0.05) (Figure 5). Moreover, negative correlations were observed between calpain activity and the force produced by the diaphragm at all stimulation frequencies (Table 2).
Figure 5.
Western blot densitometric analysis of (A) αII-spectrin degradation and (B) talin degradation in the diaphragm of control rats (C, n = 6), spontaneous breathing rats (SB, n = 7), and controlled mechanical-ventilation rats with saline injection (CMV-Saline, n = 7) or with methylprednisolone (CMV-Methylprednisolone, n = 6). Values are means ± SD and are expressed as percentage of C. * P < 0.05 versus others.
DISCUSSION
The present study reveals that whereas 24 hours of controlled mechanical ventilation (CMV) resulted in diaphragmatic force reduction, fiber atrophy, and reduction in MyoD and myogenin expression, administration of an acute high dose of corticosteroids concomitantly with CMV maintained diaphragmatic force, fiber dimensions, and MyoD and myogenin protein levels. This important finding suggests that a high dose of corticosteroids protects the diaphragm against the deleterious effects of CMV. Moreover, this protective effect was neither due to a reduction of oxidative stress nor to the antiinflammatory actions of corticosteroids. We postulate that the mechanism behind this protective effect is inhibition of the calpain protease system as the increase in diaphragm calpain activity was suppressed when CMV was combined with corticosteroids. Moreover, corticosteroids preserved MyoD and myogenin protein expression in the diaphragm during prolonged CMV and may also contribute to the corticosteroid-induced protective effect on the diaphragm.
The dose of 80 mg/kg of methylprednisolone as used in the present study is large compared with some clinical applications of steroids. Indeed, in the treatment of life-threatening asthma or lung transplant rejection, doses may amount to 1,000 mg methylprednisolone per day. Hence, the dose of 80 mg/kg is approximately five times higher than the doses described in these patients. Nonetheless, the dose used in the current study is clearly less than that recommended for treating acute spinal cord injury, 10.8 grams per day for the first 24 hours and 9.1 grams per day for those receiving methylprednisolone for 48 hours (32). These doses hence far exceed the dose used in the present study. The dose of 80 mg/kg of methylprednisolone was selected for study because we previously showed that the same dose administered for 5 days induced severe respiratory muscle weakness (17). Therefore, we predicted that a single injection of this dose would be sufficient to worsen ventilator-induced diaphragm dysfunction in our model of mechanical ventilation.
In agreement with prior studies (2–6), the present study showed that 24 hours of CMV resulted in reduced in vitro diaphragmatic force production. Interestingly, the administration of corticosteroids during controlled mechanical ventilation protected the diaphragm against CMV-induced contractile dysfunction at submaximal stimulation frequencies. Nonetheless, maximal diaphragmatic tetanic tension was still significantly decreased compared with controls but to a lesser extent than that observed during CMV alone (−22% vs. −34%).
As previously reported (2, 6, 10), CMV resulted in diaphragm type I and type IIx/b atrophy, whereas diaphragm fiber proportions did not change. Importantly, compared with CMV with a saline treatment, administration of a high dose of corticosteroids during CMV significantly attenuated the atrophy of diaphragm type IIx/b fibers. This preservation of diaphragmatic fiber size may be due to the corticosteroid-mediated prevention of mechanical ventilation-induced activation of the protease calpain.
Protein levels of the regulatory factors MyoD and myogenin were significantly reduced in the diaphragm after 24 hours of CMV, which is in agreement with previous findings (33). When corticosteroids were administered acutely during CMV, the decrease in diaphragm MyoD and myogenin protein levels was attenuated and significantly less than in the diaphragm of CMV-Saline rats. The diaphragmatic mRNA expression levels of MyoD followed the changes observed at the protein level. In contrast, myogenin mRNA levels did not parallel the changes seen at the protein level, as myogenin mRNA levels significantly increased after mechanical ventilation. Although the role of these muscle-specific transcription factors is not fully established in mature adult skeletal muscles, MyoD has been shown to be essential for normal diaphragmatic contractile function in adult rodents as its deletion is associated with a downward shift of the diaphragm force–frequency relationship and a decrease in tetanic tension (34). Therefore, the decreased expression of diaphragm MyoD after 24 hours of controlled mechanical ventilation may contribute to the decrease in diaphragm force seen in these animals. Likewise, the acute administration of corticosteroids with CMV, while limiting diaphragm MyoD reduction, may play a role in preserving diaphragm force. The fact that diaphragm MyoD did not return to control levels after corticosteroid treatment may explain why diaphragmatic tetanic tension remained slightly decreased. The role of MyoD in maintaining diaphragm force is further supported by the strong relationship between MyoD levels and diaphragm force production (33).
Similarly to MyoD, diaphragm myogenin protein levels in the present study were also maintained after corticosteroid treatment, and relationships between diaphragm force and fiber dimensions were also found. The implication of preserved myogenin protein levels in maintaining diaphragm force is not known because the role of myogenin in mature muscles has been principally investigated during denervation and regeneration or after muscle injury (35). However, in another rat model of mechanical ventilation during which rats were allowed to breathe spontaneously for intermittent periods, the same observation between diaphragm myogenin protein levels and diaphragm force was observed (33). Indeed, intermittent spontaneous breathing during the course of CMV minimized the decrease in diaphragm MyoD and myogenin observed during CMV and resulted in a preservation of diaphragm force and fiber dimension. Significant relationships between diaphragm MyoD and myogenin with diaphragm-force production were also present in this model. Our data suggest that the effects of corticosteroids observed in the present study on diaphragm force may result, at least in part, from an effect on diaphragm MyoD and myogenin protein levels, which in turn may help to limit fiber atrophy and may also preserve diaphragm force.
In an effort to further elucidate the mechanism(s), responsible for the corticosteroids-mediated diaphragmatic protection against CMV-induced atrophy and contractile dysfunction, we explored several other potential venues of protection. Although it has been reported that ventilator-induced diaphragm dysfunction is associated with several changes in the diaphragm, including atrophy, oxidative stress, proteolysis, and decreased expression of growth factors or myogenic regulatory factors, the exact contribution of each of these factors to diaphragmatic contractile dysfunction is not known. In the present study, we focused on those factors that may be involved in the protective effect of corticosteroids on diaphragm function during CMV.
First, we hypothesized that ventilation would lead to lung injury and inflammation. This inflammatory response would be associated with the production of muscle-damaging cytokines that would leak into the systemic circulation and therefore could be responsible for the observed effects on diaphragm function after CMV. Because circulating cytokines did not increase during prolonged CMV, the protective effect of corticosteroids does not appear to be related to its antiinflammatory action on the lung. In addition, local diaphragmatic inflammation was not observed after controlled mechanical ventilation as neutrophils and ED1+ and ED2+ macrophages were absent around the diaphragm fibers (36). Moreover, unpublished data (S. K. Powers and colleagues) revealed no evidence of cytokine elevation in the diaphragm from mechanically ventilated animals. Hence the beneficial action of steroids in the present study is not related to their antiinflammatory action.
Second, several studies have shown that glucocorticoids have antioxidant properties, as they can inhibit lipid peroxidation. In vitro high doses of corticosteroids have, indeed, been shown to counterbalance the effects of oxygen radicals on lipid peroxidation (37). Moreover, the antioxidant properties of corticosteroids have been previously reported in rats, in which plasma lipid peroxidation levels induced by lipopolysaccharide were suppressed by administration of methylprednisolone (38). Also, in patients with spinal cord injury, a neuroprotective effect of high doses of corticosteroids was shown to result from an inhibition of lipid peroxidation (39, 40). Because evidence indicates that oxidative stress is an important contributor to ventilator-induced diaphragm dysfunction (41), it is possible that corticosteroids may have acted by retarding CMV-induced lipid peroxidation in the diaphragm. In accordance with previous reports (10, 42), the present study showed that diaphragmatic lipid hydroperoxide levels were increased after 24 hours of CMV. Nonetheless, acute corticosteroid administration during CMV did not depress lipid hydroperoxide levels. Moreover, the level of protein oxidation in the diaphragm after mechanical ventilation was also not affected by administration of corticosteroids. We interpret this finding as evidence that the protective effect of corticosteroids was not due to a reduction in diaphragmatic lipid peroxidation or protein oxidation.
Third, because corticosteroids can inhibit the expression of NOS, we measured the diaphragmatic expression of the different NOS isoforms. As previously shown (43), the expression of NOS isoforms in the diaphragm were not elevated after controlled mechanical ventilation, nor did corticosteroid administration impact the protein abundance of any NOS isoform. Therefore, the protective effect of corticosteroids is not due to the inhibition of NOS.
Finally, we investigated the effect of corticosteroid administration on calpain activation in the diaphragm during prolonged CMV. A number of in vitro (44–46) and in vivo (47–49) studies have reported inhibition of calpain by corticosteroids. For example, degradation of neurofilament proteins in spinal cord homogenates by calpain was inhibited by methylprednisolone (45). In another study, treatment of mdx myotubes with methylprednisolone reduced calpain substrate hydrolysis to nearly normal levels (44). Glucocorticoid pretreatment of rabbits subjected to hypoxia prevented the increase in αII-spectrin breakdown that occurred in untreated animals during hypoxia, indicating impairment of calpain activation (47).
Nonetheless, evidence also exists that glucocorticoids can promote muscle proteolysis in some experimental models (50, 51). Why corticosteroids protect against muscle proteolysis in some cases is not known, but it was shown that inhibition of calpain by methylprednisolone was concentration dependent, being minimal at lower concentrations (45). It is, however, interesting to speculate on how corticosteroids might affect calpain activity in the present study. The calpain activity in muscle fibers is regulated by the endogenous calpain inhibitor, calpastatin, and by increasing the cytosolic calcium concentrations required for its proteolytic action (52). Indirect evidence suggests that intracellular calcium levels are increased in the diaphragm after CMV (12). Inhibition of calpain activity by methylprednisolone might have been driven by suppressing increased intracellular calcium levels directly or indirectly by eliminating the generation of extracellular mediators resulting in an inhibition of the rise in intracellular calcium. Indeed, evidence exists that corticosteroids might protect against calpain activation by preventing an increase in cytosolic calcium levels (49). On the other hand, recent evidence indicates that corticosteroids can retard calpain activation by preserving calpastatin levels (48). Indeed, because calpastatin is the endogenous inhibitor of calpain, preservation of calpastatin in cells could retard calpain activation.
Growing evidence indicates that the calpain system plays an important role in muscle proteolysis and is probably the earliest proteolytic event leading to muscle atrophy (52). In this regard, we previously demonstrated that inhibition of calpain activity by administration of leupeptin, a calpain inhibitor, completely abolished CMV-induced diaphragmatic and contractile dysfunction (15). In the present study, diaphragmatic calpain activity, assessed indirectly by measuring spectrin and talin degradation, was elevated following 24 hours of CMV when compared with C and SB but when corticosteroids were administered acutely during CMV, the increase in diaphragmatic calpain activity was abolished. This finding suggests that a reduction in diaphragmatic calpain activity induced by corticosteroids may lead to a decreased proteolysis of muscle proteins, including the contractile proteins, and thereby results in a preservation of diaphragm force–generating capacity in the corticosteroid-treated rats. Therefore, the corticosteroid-induced inhibition of the calpain system in the present study is a possible mechanism to explain why corticosteroid administration protects against CMV-induced atrophy and contractile dysfunction following 24 hours of CMV. Note, however, that it is unclear whether the positive effect of corticosteroids on diaphragm function would also be noted after longer periods of CMV.
In conclusion, the present study revealed that a single high dose of methylprednisolone during CMV prevented diaphragm dysfunction induced by CMV. This strategy minimized diaphragm atrophy and preserved MyoD and myogenin protein levels, which could have contributed to protection of the diaphragm against contractile dysfunction. Finally, our data also indicate that inhibition of calpain activity is a likely mechanism to explain, at least in part, the protective effect of corticosteroids against CMV-induced diaphragmatic atrophy and contractile dysfunction.
Supplementary Material
Acknowledgments
The authors thank Mrs. Petra Weckx for cutting and staining the histological sections.
Supported by FWO-Vlaanderen, Belgium, grant #G.0389.03, KUL Research Foundation, grant #OT06/52 and AstraZeneca Pharmaceuticals.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.200702-296OC on October 10, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Valverdu I, Fernandez R, de la Cal MA, Benito S, Tomas R. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med 1995;332:345–350. [DOI] [PubMed] [Google Scholar]
- 2.Gayan-Ramirez G, de Paepe K, Cadot P, Decramer M. Detrimental effects of short-term mechanical ventilation on diaphragm function and IGF-I mRNA in rats. Intensive Care Med 2003;29:825–833. [DOI] [PubMed] [Google Scholar]
- 3.Le Bourdelles G, Viires N, Boczkowski J, Seta N, Pavlovic D, Aubier M. Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am J Respir Crit Care Med 1994;149:1539–1544. [DOI] [PubMed] [Google Scholar]
- 4.Powers SK, Shanely RA, Coombes JS, Koesterer TJ, McKenzie M, Van Gammeren D, Cicale M, Dodd SL. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol 2002;92:1851–1858. [DOI] [PubMed] [Google Scholar]
- 5.Sassoon CS, Caiozzo VJ, Manka A, Sieck GC. Altered diaphragm contractile properties with controlled mechanical ventilation. J Appl Physiol 2002;92:2585–2595. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Luo J, Bourdon J, Lin MC, Gottfried SB, Petrof BJ. Controlled mechanical ventilation leads to remodeling of the rat diaphragm. Am J Respir Crit Care Med 2002;166:1135–1140. [DOI] [PubMed] [Google Scholar]
- 7.Anzueto A, Peters JI, Tobin MJ, de Los SR, Seidenfeld JJ, Moore G, Cox WJ, Coalson JJ. Effects of prolonged controlled mechanical ventilation on diaphragmatic function in healthy adult baboons. Crit Care Med 1997;25:1187–1190. [DOI] [PubMed] [Google Scholar]
- 8.Capdevila X, Lopez S, Bernard N, Rabischong E, Ramonatxo M, Martinazzo G, Prefaut C. Effects of controlled mechanical ventilation on respiratory muscle contractile properties in rabbits. Intensive Care Med 2003;29:103–110. [DOI] [PubMed] [Google Scholar]
- 9.Radell PJ, Remahl S, Nichols DG, Eriksson LI. Effects of prolonged mechanical ventilation and inactivity on piglet diaphragm function. Intensive Care Med 2002;28:358–364. [DOI] [PubMed] [Google Scholar]
- 10.Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D, Belcastro A, Powers SK. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med 2002;166:1369–1374. [DOI] [PubMed] [Google Scholar]
- 11.Shanely RA, Van Gammeren D, DeRuisseau KC, Zergeroglu AM, McKenzie MJ, Yarasheski KE, Powers SK. Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am J Respir Crit Care Med 2004;170:994–999. [DOI] [PubMed] [Google Scholar]
- 12.DeRuisseau KC, Shanely RA, Akunuri N, Hamilton MT, Van Gammeren D, Zergeroglu AM, McKenzie M, Powers SK. Diaphragm unloading via controlled mechanical ventilation alters the gene expression profile. Am J Respir Crit Care Med 2005;172:1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Testelmans D, Maes K, Wouters P, Gosselin N, Deruisseau K, Powers S, Sciot R, Decramer M, Gayan-Ramirez G. Rocuronium exacerbates mechanical ventilation-induced diaphragm dysfunction in rats. Crit Care Med 2006;34:3018–3023. [DOI] [PubMed] [Google Scholar]
- 14.Zhu E, Sassoon CS, Nelson R, Pham HT, Zhu L, Baker MJ, Caiozzo VJ. Early effects of mechanical ventilation on isotonic contractile properties and MAF-box gene expression in the diaphragm. J Appl Physiol 2005;99:747–756. [DOI] [PubMed] [Google Scholar]
- 15.Maes K, Testelmans D, Powers S, Decramer M, Gayan-Ramirez G. Leupeptin inhibits ventilator-induced diaphragm dysfunction in rats. Am J Respir Crit Care Med 2007;175:1134–1138. [DOI] [PubMed] [Google Scholar]
- 16.McClung JM, Kavazis AN, DeRuisseau KC, Falk DJ, Deering MA, Lee Y, Sugiura T, Powers SK. Caspase-3 regulation of diaphragm myonuclear domain during mechanical ventilation-induced atrophy. Am J Respir Crit Care Med 2007;175:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nava S, Gayan-Ramirez G, Rollier H, Bisschop A, Dom R, de Bock V, Decramer M. Effects of acute steroid administration on ventilatory and peripheral muscles in rats. Am J Respir Crit Care Med 1996;153:1888–1896. [DOI] [PubMed] [Google Scholar]
- 18.Dekhuijzen PN, Decramer M. Steroid-induced myopathy and its significance to respiratory disease: a known disease rediscovered. Eur Respir J 1992;5:997–1003. [PubMed] [Google Scholar]
- 19.Bachmann P, Gaussorgues P, Piperno D, Fussy A, Jaboulay JM, Robert D. [Acute myopathy after status asthmaticus]. Presse Med 1987;16:1486. [PubMed] [Google Scholar]
- 20.Behbehani NA, Al Mane F, D'yachkova Y, Pare P, FitzGerald JM. Myopathy following mechanical ventilation for acute severe asthma: the role of muscle relaxants and corticosteroids. Chest 1999;115:1627–1631. [DOI] [PubMed] [Google Scholar]
- 21.Bercker S, Weber-Carstens S, Deja M, Grimm C, Wolf S, Behse F, Busch T, Falke KJ, Kaisers U. Critical illness polyneuropathy and myopathy in patients with acute respiratory distress syndrome. Crit Care Med 2005;33:711–715. [DOI] [PubMed] [Google Scholar]
- 22.Goh AY, Chan PW. Acute myopathy after status asthmaticus: steroids, myorelaxants or carbon dioxide? Respirology 1999;4:97–99. [DOI] [PubMed] [Google Scholar]
- 23.Lacomis D, Giuliani MJ, Van Cott A, Kramer DJ. Acute myopathy of intensive care: clinical, electromyographic, and pathological aspects. Ann Neurol 1996;40:645–654. [DOI] [PubMed] [Google Scholar]
- 24.MacFarlane IA, Rosenthal FD. Severe myopathy after status asthmaticus. Lancet 1977;2:615. [DOI] [PubMed] [Google Scholar]
- 25.Amaya-Villar R, Garnacho-Montero J, Garcia-Garmendia JL, Madrazo-Osuna J, Garnacho-Montero MC, Luque R, Ortiz-Leyba C. Steroid-induced myopathy in patients intubated due to exacerbation of chronic obstructive pulmonary disease. Intensive Care Med 2005;31:157–161. [DOI] [PubMed] [Google Scholar]
- 26.Maes K, Gayan-Ramirez G, Testelmans D, Deruisseau K, Powers S, Decramer M. High dose corticosteroids may protect the rat diaphragm from mechanical ventilation effects [abstract]. Proc Am Thorac Soc 2005;2:A161. [Google Scholar]
- 27.Dekhuijzen PN, Gayan-Ramirez G, de Bock V, Dom R, Decramer MTriamcinolone and prednisolone affect contractile properties and histopathology of rat diaphragm differently. J Clin Invest 1993;92:1534–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermes-Lima M, Willmore WG, Storey KB. Quantification of lipid peroxidation in tissue extracts based on Fe(III)xylenol orange complex formation. Free Radic Biol Med 1995;19:271–280. [DOI] [PubMed] [Google Scholar]
- 29.Powers SK, Demirel HA, Vincent HK, Coombes JS, Naito H, Hamilton KL, Shanely RA, Jessup J. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am J Physiol 1998;275:R1468–R1477. [DOI] [PubMed] [Google Scholar]
- 30.Canton M, Neverova I, Menabo R, Van EJ, Di LF. Evidence of myofibrillar protein oxidation induced by postischemic reperfusion in isolated rat hearts. Am J Physiol Heart Circ Physiol 2004;286:H870–H877. [DOI] [PubMed] [Google Scholar]
- 31.Vescovo G, Ravara B, Dalla LL. Skeletal muscle myofibrillar protein oxidation and exercise capacity in heart failure. Basic Res Cardiol 2008;103:285–290. [DOI] [PubMed] [Google Scholar]
- 32.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW, Marshall LF, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 1997;277:1597–1604. [PubMed] [Google Scholar]
- 33.Gayan-Ramirez G, Testelmans D, Maes K, Racz GZ, Cadot P, Zador E, Wuytack F, Decramer M. Intermittent spontaneous breathing protects the rat diaphragm from mechanical ventilation effects. Crit Care Med 2005;33:2804–2809. [DOI] [PubMed] [Google Scholar]
- 34.Staib JL, Swoap SJ, Powers SK. Diaphragm contractile dysfunction in MyoD gene-inactivated mice. Am J Physiol Regul Integr Comp Physiol 2002;283:R583–R590. [DOI] [PubMed] [Google Scholar]
- 35.Mehiri SN, Barreiro E, Hayot M, Voyer M, Comtois AS, Grassino AE, Czaika G. Time-based gene expression programme following diaphragm injury in a rat model. Eur Respir J 2005;25:422–430. [DOI] [PubMed] [Google Scholar]
- 36.Van Gammeren D, Falk DJ, DeRuisseau KC, Sellman JE, Decramer M, Powers SK. Reloading the diaphragm following mechanical ventilation does not promote injury. Chest 2005;127:2204–2210. [DOI] [PubMed] [Google Scholar]
- 37.Uhler TA, Frim DM, Pakzaban P, Isacson O. The effects of megadose methylprednisolone and U-78517F on toxicity mediated by glutamate receptors in the rat neostriatum. Neurosurgery 1994;34:122–127. [PubMed] [Google Scholar]
- 38.Kouno T, Egashira T, Takayama F, Kudo Y, Yamanaka Y. Effect of methylprednisolone on plasma lipid peroxidation induced by lipopolysaccharide. Jpn J Pharmacol 1994;64:163–169. [DOI] [PubMed] [Google Scholar]
- 39.Hilton G, Frei J. High-dose methylprednisolone in the treatment of spinal cord injuries. Heart Lung 1991;20:675–680. [PubMed] [Google Scholar]
- 40.Marzatico F, Gaetani P, Buratti E, Messina AL, Ferlenga P, Baena R. Effects of high-dose methylprednisolone on Na(+)-K+ ATPase and lipid peroxidation after experimental subarachnoid hemorrhage. Acta Neurol Scand 1990;82:263–270. [DOI] [PubMed] [Google Scholar]
- 41.Betters JL, Criswell DS, Shanely RA, Van Gammeren D, Falk D, DeRuisseau KC, Deering M, Yimlamai T, Powers SK. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am J Respir Crit Care Med 2004;170:1179–1184. [DOI] [PubMed] [Google Scholar]
- 42.Zergeroglu MA, McKenzie MJ, Shanely RA, Van Gammeren D, DeRuisseau KC, Powers SK. Mechanical ventilation-induced oxidative stress in the diaphragm. J Appl Physiol 2003;95:1116–1124. [DOI] [PubMed] [Google Scholar]
- 43.Van Gammeren D, Falk DJ, Deering MA, DeRuisseau KC, Powers SK. Diaphragmatic nitric oxide synthase is not induced during mechanical ventilation. J Appl Physiol 2007;102:157–162. [DOI] [PubMed] [Google Scholar]
- 44.Alderton JM, Steinhardt RA. Calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. J Biol Chem 2000;275:9452–9460. [DOI] [PubMed] [Google Scholar]
- 45.Banik NL, Matzelle D, Terry E, Hogan EL. A new mechanism of methylprednisolone and other corticosteroids action demonstrated in vitro: inhibition of a proteinase (calpain) prevents myelin and cytoskeletal protein degradation. Brain Res 1997;748:205–210. [DOI] [PubMed] [Google Scholar]
- 46.Sur P, Sribnick EA, Patel SJ, Ray SK, Banik NL. Dexamethasone decreases temozolomide-induced apoptosis in human gliobastoma T98G cells. Glia 2005;50:160–167. [DOI] [PubMed] [Google Scholar]
- 47.Ostwald K, Hayashi M, Nakamura M, Kawashima S. Subcellular distribution of calpain and calpastatin immunoreactivity and fodrin proteolysis in rabbit hippocampus after hypoxia and glucocorticoid treatment. J Neurochem 1994;63:1069–1076. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz SM, Duffy JY, Pearl JM, Goins S, Wagner CJ, Nelson DP. Glucocorticoids preserve calpastatin and troponin I during cardiopulmonary bypass in immature pigs. Pediatr Res 2003;54:91–97. [DOI] [PubMed] [Google Scholar]
- 49.Wang M, Sakon M, Umeshita K, Okuyama M, Shiozaki K, Nagano H, Dohno K, Nakamori S, Monden M. Prednisolone suppresses ischemia-reperfusion injury of the rat liver by reducing cytokine production and calpain mu activation. J Hepatol 2001;34:278–283. [DOI] [PubMed] [Google Scholar]
- 50.Tiao G, Fagan J, Roegner V, Lieberman M, Wang JJ, Fischer JE, Hasselgren PO. Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J Clin Invest 1996;97:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Luo GJ, Wang JJ, Hasselgren PO. Dexamethasone stimulates proteasome- and calcium-dependent proteolysis in cultured L6 myotubes. Shock 1998;10:298–306. [DOI] [PubMed] [Google Scholar]
- 52.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev 2003;83:731–801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.